Abstract
Antagonists of the type 1 cysteinyl leukotriene receptor (CysLT1R) are efficacious for bronchoconstriction in humans with bronchial asthma; however, the clinical response to these drugs is heterogeneous. In particular, how CysLT1R expression and function are constitutively regulated in vivo is not known. Here we show that a 7-transmembrane receptor GPR17 negatively regulates the CysLT1R-mediated inflammatory cell accumulation in the bronchoalveolar lavage fluid and lung, the levels of IgE and specific IgG1 in serum, and Th2/Th17 cytokine expression in the lung after intranasal sensitization and challenge with house dust mite (Df) in mice. Sensitization of naïve wild-type recipients with Df-pulsed bone marrow-derived dendritic cells (BMDCs) of each genotype or sensitization of each genotype with Df-pulsed wild-type BMDCs and Df challenge revealed markedly increased pulmonary inflammatory and serum IgE responses for GPR17-deficient mice as compared to wild-type mice and reduced responses in the genotypes lacking CysLT1R. These findings reveal a constitutive negative regulation of CysLT1R functions by GPR17 in both the antigen presentation and downstream phases of allergic pulmonary inflammation.
Keywords: Knockout Mice, Lipid Mediators, Allergy, Inflammation, Lung
INTRODUCTION
The cysteinyl leukotrienes (cys-LTs), leukotriene (LT) C4, LTD4 and LTE4, are proinflammatory lipid mediators (1, 2), particularly implicated in human bronchial asthma for their potent bronchoconstrictive activity (3, 4) via their type 1 receptor, CysLT1R (5). CysLT1R-selective antagonists, termed “lukasts”, are the only mechanism-targeted small molecules that have been approved and widely used for the treatment of asthma (6, 7). However their efficacy is most apparent among subsets of patients with asthma (8, 9). One might speculate that CysLT1R expression and function are tightly regulated and thus in some patients, CysLT1R function is already turned off or obscured. Although in vitro studies have addressed ligand-initiated internalization of CysLT1R and regulation by protein kinase C (10, 11), there is little information on constitutive regulation of CysLT1R function in vivo.
Studies of mouse models with pharmacologic inhibition and targeted gene disruption of the cys-LT pathway revealed that the cys-LTs/CysLT1R axis plays a role not only in mast cell-mediated smooth muscle constriction in the microvasculature (12-14) but also in immune inflammatory cell functions in OVA-induced allergic pulmonary inflammation (15-17). Indeed, the CysLT1R is expressed not only on smooth muscle cells of airways and microvasculature but also on bone marrow-derived cells of the innate and adaptive immune systems, such as mast cells, macrophages, eosinophils, basophils, and dendritic cells (DCs) (18-20). Thus, a potential involvement of the cys-LT/CysLT1R pathway in the infiltrating cells of the allergic inflammatory response has been suggested, but how the cys-LT/CysLT1R pathway is regulated at the antigen-presenting phase and/or at the downstream responses remains to be elucidated.
Human, rat, and mouse GPR17, which are modestly homologous to CysLT1R, were identified as dual receptors for uracil nucleotides and the cys-LTs, LTC4 and LTD4, based on [35S]GTPγS binding assays and single-cell calcium imaging with transfectants (21, 22). Neither we (23) nor others (24, 25) found binding or agonist function for the cys-LTs in GPR17-transfected cell lines. In contrast, we observed that mouse GPR17 is a negative regulator of CysLT1R. Cotransfection of GPR17 with CysLT1R in several cell lines suppressed CysLT1R-mediated calcium flux in response to LTD4, and knockdown of GPR17 in mouse bone marrow-derived macrophages markedly increased the calcium flux in response to LTD4 in a dose-dependent manner (23).
We hypothesized that Gpr17−/− mice would exhibit increased CysLT1R-dependent responses beyond the microvasculature and reveal cellular targets of the cys-LTs in complex models such as antigen-induced pulmonary inflammation. We generated a mouse strain lacking both GPR17 and CysLT1R (Gpr17/Cysltr1−/−) to determine the extent to which any cellular signal in the Gpr17−/− mice was attributable to CysLT1R. In addition, we turned to sensitization and challenge with house dust mite, Dermatophagoides farinae, extract (Df) to examine the role of cys-LTs and their receptors in pulmonary inflammation elicited by an allergen most relevant to human asthma and dominant in homes (26). Finally, we used not only active sensitization and challenge but also a passive approach to sensitization by administering Df-pulsed bone marrow-derived dendritic cells (BMDCs) that allowed us to assess both the afferent and efferent aspects of the CysLT1R-mediated response to challenge in the presence and absence of GPR17. Here we show in both the active and passive sensitization and challenge models that in the absence of GPR17, CysLT1R mediates expansion of the draining lymph node (LN), inflammatory cell infiltration in the bronchoalveolar lavage (BAL) fluid and the bronchovascular bundles of lung tissue, marked increases in total IgE and Df-specific IgG1, and Th2/Th17 cytokine expression in the lung. In WT mice, these responses are modest but again dependent on CysLT1R. These findings reveal the role of CysLT1R in WT C57BL/6 mice and emphasize the separate negative regulatory effects of GPR17 on CysLT1R functions in DCs and in the downstream cellular and humoral responses of allergic pulmonary inflammation.
MATERALS AND METHODS
Mice
Cysltr1−/− mice were originally generated from embryonic stem cells from C57BL/6 mice (12), were maintained by breeding with C57BL/6 mice, and N15 generations were used. Gpr17−/− mice (Deltagen, San Mateo, CA) were established from 129-derived embryonic stem cells, were backcrossed onto the C57BL/6 background for 7-8 generations, and N7 and N8 generations were used (23). To generate GPR17/CysLT1R double-deficient (Gpr17/Cysltr1−/−) mice, Gpr17−/− males (N7) and Cysltr1−/− females were bred to obtain Gpr17+/−Cysltr1− males and Gpr17+/− Cysltr1+/− females. The Gpr17+/−Cysltr1− males and Gpr17+/−Cysltr1+/− females were further bred to obtain Gpr17−/−Cysltr1− males and Gpr17−/−Cysltr1−/− females. The Gpr17/Cysltr1−/− mice were viable and had no apparent abnormalities up to at least 12 months of age. WT littermates from breeding for Cysltr1−/−, Gpr17−/−, and Gpr17/Cysltr1−/− strains were used. 8- to 12-wk-old male and female mice were used. All animal studies were approved by the Animal Care and Use Committee of the Dana-Farber Cancer Institute.
Active Sensitization and Challenge with Repeated Intranasal Injections of Df
Mice received 1 μg of Df (Greer Laboratories, Lenoir, NC) intranasally twice per wk for 3 wks as described (27). 2 d after the last injection, mice were killed by i.p. injection of pentobarbital.
BAL Fluid Cell Analysis
2 d after the last intranasal injection, the trachea was cannulated and BAL fluid was obtained by three repeated lavages with 0.75 ml of Ca2+- and Mg2+-free PBS with 1 mM EDTA. The BAL fluid was centrifuged at 500 x g for 5 min. Cells were resuspended in 0.2 ml of PBS with 1% BSA, and the total cells were counted manually with a hemocytometer. For the differential cell counts of macrophages, neutrophils, eosinophils, and lymphocytes, the cells were cytospun onto a glass slide and stained with Diff-Quik, and cell types in a total of 200 cells were identified by morphologic criteria.
Histology
The lung tissues were excised, and the left lung was fixed and stained as described previously (16). For general morphology, tissue sections were stained with hematoxylin and eosin (H&E). The extent of cellular infiltration in the bronchovascular bundles was assessed in a blinded manner. Congo red staining was used to identify eosinophils, and periodic acid-Schiff staining was used to assess mucus and goblet cells. To assess vascular smooth muscle hyperplasia, some tissue sections were stained with mouse anti-smooth muscle actin mAb (clone 1A4, Covance, Princeton, NJ) and with LSAB+ System-alkaline phosphatase (Dako, Carpinteria, CA) according to the manufacturer’s instructions. The total smooth muscle cell numbers and the thickness of arteriolar medial muscular layer (tunica media) were quantified by Image J (National Institutes of Health image analysis software) on smooth muscle actin-stained sections as previously described (27). Briefly, digital pictures of 5 medium-sized arterioles with a diameter of 30- to 50-μm were taken from each slide, and the readouts are presented as the mean number of smooth muscle cells per 100-μm basement membrane and the mean thickness of the medial arteriolar walls in 5 arterioles from 3-5 mice per group.
For immunohistochemistry to detect CysLT1R, mouse lung tissues were embedded in Tissue-Tek® OCT compound and cut into 5-μm-thick sections. Frozen sections were fixed with 4% paraformaldehyde, washed, and incubated with polyclonal rabbit anti-CysLT1R IgG (RB34, against the conserved sequence DEKNNTKCFEPPQNN of extracellular loop 3 of both mouse and human CysLT1R, 30 μg/ml) (23). Immunoreactivities were visualized with the rabbit ABC-peroxidase staining system (Santa Cruz Biotechnology, Santa Cruz, CA) according to the manufacturer’s instructions. The slides were analyzed with a Leica DM LB2 microscope (Leica Microsystems, Germany). The pictures were taken by a Nikon digital camera DXM 1200 with Nikon ACT-1 (version 2.70) image acquisition software.
Measurement of Total IgE and Df-specific IgG1
Sera were collected by cardiac puncture 2 d after the last intranasal injection. Total IgE was determined with an ELISA kit (BD Biosciences, San Jose, CA). Df-specific IgG1 was measured as described (28). Briefly, 96-well plates were coated with a 5 μg/ml solution of Df and incubated with diluted serum followed by alkaline phosphatase-conjugated anti-mouse IgG1 (SouthernBiotech, Birmingham, AL) and p-nitrophenyl phosphate substrate (Sigma-Aldrich, St. Louis, MO).
Measurement of Cytokine mRNA Expression in the Lung
Total RNA was isolated from the right lungs with TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer’s protocol. Quantities of mRNA for IL-4, IL-5, IL-13, IL-17A, and IFN-γ were measured relative to GAPDH using the Mx3000P Real-Time PCR System (Agilent Technologies, Santa Clara, CA) with gene-specific primers.
Flow Cytometry
Macrophages were harvested by peritoneal lavage with ice-cold PBS, washed, and incubated in RPMI medium containing 10% FBS for 3 h at 37°C in a humidified atmosphere with 5% CO2. Adherent cells were detached with 4 mg/ml lidocaine and 5 mM EDTA in PBS and were incubated with polyclonal rabbit anti-CysLT1R IgG (RB34, 5 μg/ml) and allophycocyanin-conjugated donkey anti-rabbit IgG. Nonspecific rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) was used as a control. Analyses were performed on a FACSCanto flow cytometer (BD Biosciences), and data were analyzed with the FlowJo software (Tree Star, Ashland, OR).
Cytokine Production by Parabronchial LN cells after ex vivo Restimulation with Df
2 d after the last intranasal injection, three parabronchial LNs were excised from each mouse and homogenized. The cell suspensions were filtered through a 70-μm cell strainer, centrifuged at 300 x g for 5 min at room temperature, and resuspended in RPMI1640 medium containing heat-inactivated 10% FBS. After the total number of cells was counted for each mouse, cells were cultured at 2 × 106 cells/ml (100 μl) in the presence of 20 μg/ml Df in a 96-well plate for 72 h. The concentrations of IL-4, IL-5, IL-17A, and IFN-γ in the supernatants were measured with ELISA kits (eBiosciences, San Diego, CA).
Transfer of Df-pulsed BMDCs into Mice and Df Challenge
Adoptive transfer of Df-pulsed BMDCs into naïve mice was carried out as described (29) with modifications. Bone marrow cells were harvested from femurs and tibiae of each mouse and cultured in RPMI medium supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, 50 μM 2-ME, and recombinant mouse GM-CSF as described (30). Floating cells were harvested on day 8 and pulsed with either PBS or 100 μg/ml of Df at a concentration of 1 × 106 cells/ml in a 35-mm culture dish (Sumilon Celltight X, Sumitomo Bakelite, Japan) for 24 h. The next day, the cells were washed twice with PBS and resuspended in PBS. 1 × 104 cells in 50 μl were transferred intranasally to recipients that were briefly anesthetized with isoflurane. At days 10 and 14 after DC transfer, recipient mice were challenged with 1 μg Df intranasally. 2 d after the last challenge, mice were killed by i.p. injection of pentobarbital. BAL fluid analysis, lung histology, and assessment of cytokine production in the LN cells were performed as described above.
Statistical Analysis
Results were expressed as means ± SEM. Student’s unpaired, two-tailed t test was used for the statistical analysis in cases in which the variance was homogeneous, and Welch’s test was used when the variance was heterogeneous. A value of p < 0.05 was considered significant.
RESULTS
GPR17 Deficiency Increases CysLT1R-mediated Df-induced Pulmonary Inflammation, Serum Levels of Total IgE and Df-specific IgG1, and Th2/Th17 Cytokine Expression in Lungs
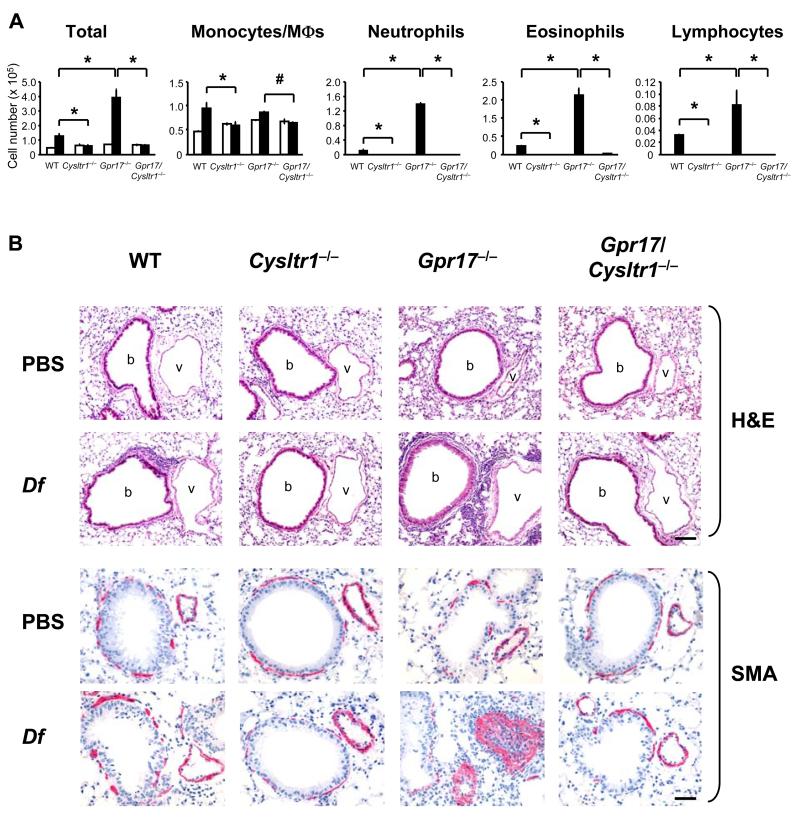
We examined Df-elicited allergic pulmonary inflammation in WT, Cysltr1−/−, Gpr17−/−, and Gpr17/Cysltr1−/− mice to assess the respective roles of CysLT1R and GPR17. Mice received intranasal injections of 1 μg Df or PBS twice per wk for 3 wks and were killed 48 h after the last injection (27). Df-challenged WT mice had an increased cellular infiltration in BAL fluid composed of monocytes/macrophages, neutrophils, eosinophils, and lymphocytes as compared to PBS-injected WT mice. Df-challenged Cysltr1−/− mice showed no increase in any of these cell types in BAL fluid as compared to Df-challenged WT mice. In contrast, Df-challenged Gpr17−/− mice had significant and marked increases in BAL fluid cell numbers of neutrophils, eosinophils, and lymphocytes as compared to Df-challenged WT mice. This exaggerated cellular influx into the BAL fluid of Gpr17−/− mice was completely abrogated in Df-challenged Gpr17/Cysltr1−/− mice (Fig. 1A).
FIGURE 1. GPR17 Deficiency Increases CysLT1R-mediated Df-induced Pulmonary Inflammation.
(A) Inflammatory cell counts in BAL fluid. For active sensitization and challenge, mice received 1 μg of Df (black columns) or PBS (white columns) by intranasal injection twice per wk for 3 wks, and BAL was performed 2 d after the last injection. Total and differential cell counts for monocytes/macrophages (MΦs), neutrophils, eosinophils, and lymphocytes are shown. Values are the mean ± SEM (n = 8-10) combined from 3 independent experiments. *P < 0.01, #P < 0.05. (B) Histologic analyses of the lung. After BAL, lung tissues were fixed with paraformaldehyde and stained with H&E. b, bronchi, v, vessels. Immunohistochemistry was performed with mouse mAb to α-smooth muscle actin (SMA) and ABC-alkaline phosphatase reagent, visualized in red. Scale bars = 100 μm for HE, 50 μm for SMA.
Histologic analysis of the bronchovascular bundles in the lung reflected the cellular influx in the BAL fluid. There was a modest increase in cellular infiltration in WT mice that was largely absent in Cysltr1−/− mice, and there was a marked increase in the cellular infiltration in Gpr17−/− mice that was abolished in the Gpr17/Cysltr1−/− mice (Fig. 1B, H&E). Congo red staining showed that the cellular infiltration in WT and especially in Gpr17−/− mice was enriched with eosinophils as compared to the infiltration in the absence of CysLT1R (Supplemental Fig. 1A, CR). Goblet cell metaplasia with mucus hyperproduction was particularly prominent in Df-challenged Gpr17−/− mice compared to similarly challenged WT mice as assessed by periodic acid-Schiff staining and was abolished in the Cysltr1−/− and Gpr17/Cysltr1−/− mice (Supplemental Fig. 1A, PAS). Unexpectedly, Df-challenged Gpr17−/− mice had strikingly increased smooth muscle cells in the vasculature as assessed by immunohistochemical staining for α-smooth muscle actin (Fig. 1B, SMA). The numbers of α-smooth muscle actin-positive cells as well as thickness of the α-smooth muscle actin-staining layer were significantly increased in Df-challenged Gpr17−/− mice as compared to Df-challenged WT mice (Supplemental Fig. 1B).
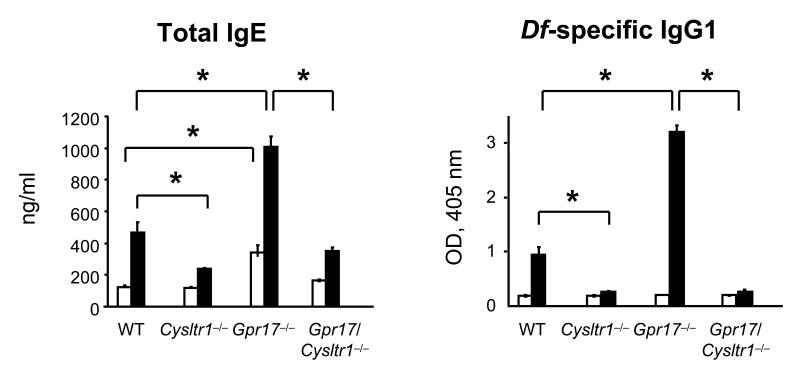
After sensitization and challenge with Df, Cysltr1−/− mice had significantly less total serum IgE and Df-specific IgG1 as compared to similarly treated WT mice (Fig. 2). In contrast, Df-challenged Gpr17−/− mice had significantly increased levels of total IgE and Df-specific IgG1 as compared to WT controls, and these increases were completely abrogated in Df-challenged Gpr17/Cysltr1−/− mice. PBS-injected Gpr17−/− mice also had a significantly increased total IgE level at baseline as compared to WT controls. The levels of Df-specific IgG2a in all serum samples were below the detection limit of the ELISA.
FIGURE 2. GPR17 Deficiency Increases Serum Levels of Total IgE and Df-specific IgG1.
Total IgE and Df-specific IgG1 in serum of PBS-injected (white columns) or Df-challenged (black columns) mice depicted in Figure 1 are shown. Values are the mean ± SEM (n = 8-10) combined from 3 independent experiments. *P < 0.01.
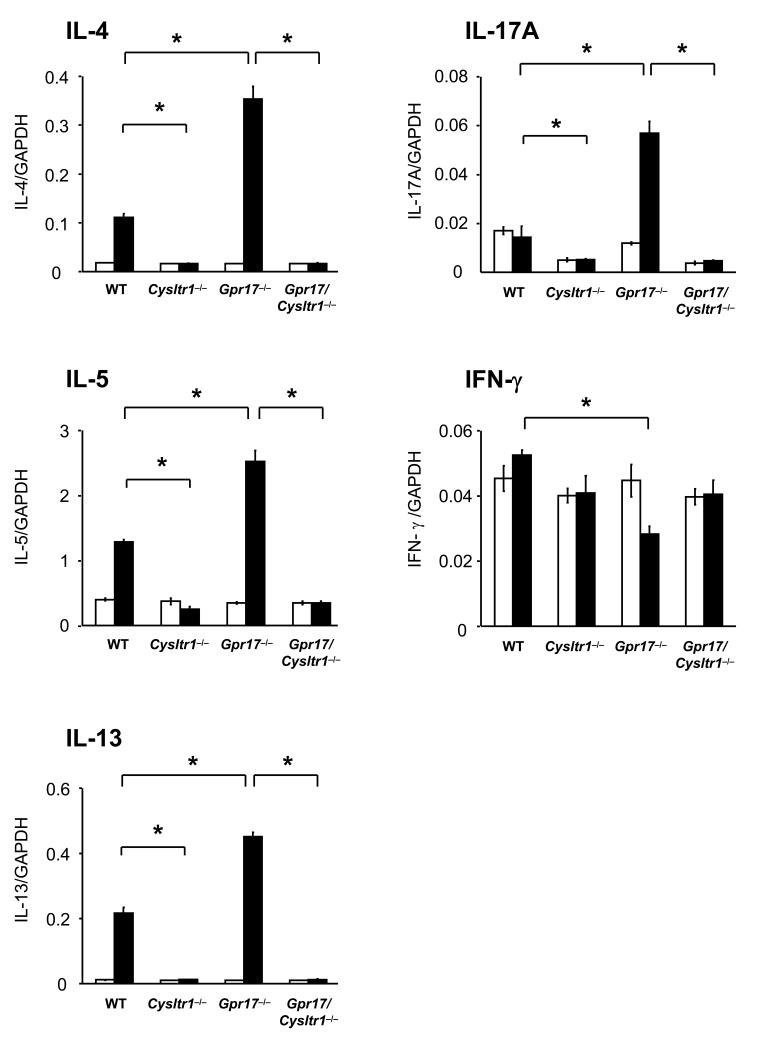
To assess the Th2/Th17/Th1 cell cytokine expression in the lungs after sensitization and challenge with Df, total RNA was isolated from the right lungs of WT, Cysltr1−/−, Gpr17−/−, and Gpr17/Cysltr1−/− mice, and quantitative RT-PCR was performed. In WT mice, sensitization and challenge with Df significantly increased the expression of mRNAs for IL-4, IL-5, and IL-13, but not of mRNAs for IL-17A or IFN-γ as compared to PBS-injected controls (Fig. 3). Df-challenged Cysltr1−/− mice had no increase in mRNA expression of IL-4, IL-5, IL-13, IL-17A, and IFN-γ as compared to PBS-injected controls. Df-challenged Gpr17−/− mice had a significant further increase in expression of IL-4, IL-5, and IL-13, along with a significant increase in IL-17A expression and a decrement in IFN-γ expression as compared to Df-challenged WT mice; each of these changes was completely abrogated in Df-challenged Gpr17/Cysltr1−/− mice (Fig. 3). The aggregate findings for pulmonary inflammation, for levels of serum total IgE and Df-specific IgG1, and for cytokine expression in lungs with Df sensitization and challenge reveal a core role of CysLT1R in an integrated Th2 type response of WT mice that is markedly exaggerated and includes IL-17A in the absence of regulation by GPR17.
FIGURE 3. GPR17 Deficiency Increases Th2/Th17 Cytokine Generation in the Lung.
Relative expression of mRNA for IL-4, IL-5, IL-13, IL-17A, and IFN-γ to GAPDH in lungs of PBS-injected (white columns) or Df-challenged (black columns) mice from 2 of the experiments depicted in Figure 1 was assessed by quantitative RT-PCR. Values are the mean ± SEM (n = 5-7). *P < 0.01.
GPR17 Deficiency Upregulates Df-induced CysLT1R Expression in Inflammatory Cells in Lungs
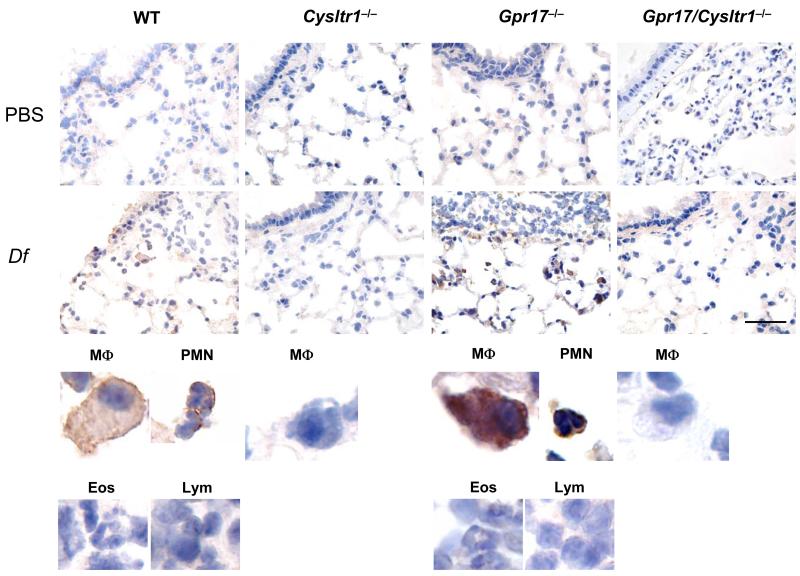
As we had previously observed increased expression of CysLT1R by flow cytometry as well as increased function in in vitro bone marrow-derived macrophages subjected to GPR17 knockdown (23), we used flow cytometry to identify CysLT1R expression on resting peritoneal macrophages. There was significant CysLT1R expression in the macrophages from WT mice but no expression in the cells from Cysltr1−/− mice (Supplemental Fig. 2A), thereby showing the specificity of the antibody. There was further increased CysLT1R expression in the macrophages from Gpr17−/− mice as compared to those from WT mice (Supplemental Fig. 2B).
We next performed immunohistochemical staining for CysLT1R in the lungs from PBS-injected or Df-sensitized and challenged WT, Cysltr1−/−, Gpr17−/−, and Gpr17/Cysltr1−/− mice. Many macrophages and PMNs, but not eosinophils or lymphocytes, in the lung tissue of Df-challenged WT mice stained positively for CysLT1R as compared to the lack of staining in lung tissue from PBS-injected WT mice or from Df-challenged Cysltr1−/− or Gpr17/Cysltr1−/− mice. Furthermore, there was stronger positive staining for CysLT1R in macrophages and PMNs in Df-challenged Gpr17−/− mice (Fig. 4) as well as slight but definite staining in bronchial smooth muscle cells in lung of this genotype (data not shown). The fact that the eosinophils and lymphocytes were not stained in Df-challenged lungs of WT or Gpr17−/− mice suggests that their presence is part of an integrated cytokine/chemokine-directed inflammatory response rather than cys-LT-dependent migration. We were not able to detect CysLT1R expression on the alveolar walls or vasculature of the bronchovascular bundles of any genotype.
FIGURE 4. GPR17 Deficiency Upregulates Df-induced CysLT1R Expression in Inflammatory Cells in Lungs.
Immunohistochemistry for CysLT1R in lungs of WT, Cysltr1−/−, Gpr17−/−, and Gpr17/Cysltr1−/− mice sensitized and challenged with PBS or Df. Lungs were stained with polyclonal rabbit anti-CysLT1R IgG and developed with ABC-peroxidase reagent visualized in brown. Scale bar = 50 μm. Data are representative of results from 2 of the experiments depicted in Figure 1. Magnified pictures of representative macrophages (MΦ), PMNs, eosinophils (Eos), and lymphocytes (Lym) from Df-challenged groups are shown in lower panels.
Sensitization of WT Mice with Df-pulsed GPR17-deficient BMDCs Enhances Pulmonary, LN, and Ig Responses upon Challenge
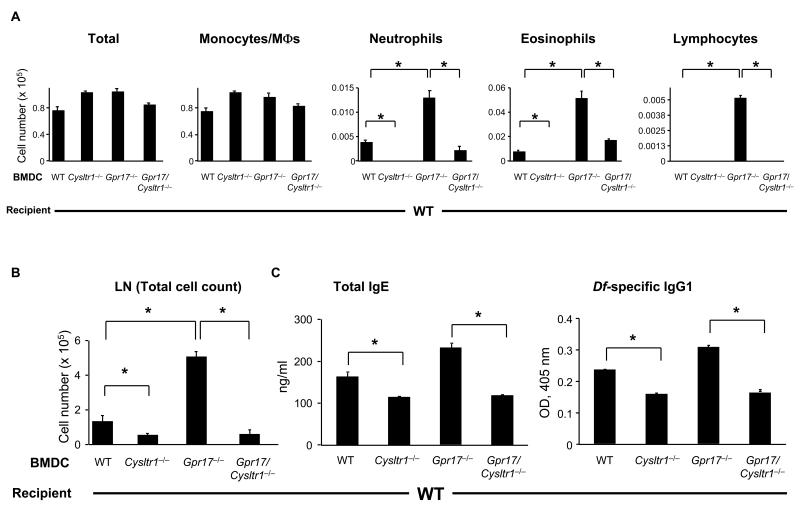
To determine whether the exaggerated inflammatory phenotype in the lungs of Gpr17−/− mice involved the Df-presenting function and afferent aspect of the integrated response, we adoptively transferred 1 × 104 Df-pulsed BMDCs from the four genotypes (WT, Cysltr1−/−, Gpr17−/−, and Gpr17/Cysltr1−/−) to WT recipients, challenged them with 1 μg of Df at days 10 and 14, and killed them for assessment at day 16 (29). The numbers of neutrophils and eosinophils in BAL fluid with Df challenge were increased in WT mice sensitized with Df-pulsed WT BMDCs (WT BMDCs→WT mice), whereas these inflammatory cells were absent with Df challenge of WT mice receiving PBS-treated WT BMDCs (data not shown) or Df-pulsed BMDCs from Cysltr1−/− mice (Cysltr1−/− BMDCs→WT mice) (Fig. 5A). The mere sensitization of WT mice with Df-pulsed BMDCs from Gpr17−/− mice slightly increased the numbers of neutrophils and eosinophils in the BAL fluid (data not shown), and these numbers rose 2- to 3-fold with Df challenge in association with lymphocytes (Gpr17−/− BMDCs→WT mice) (Fig. 5A). This augmentation of cell recruitment to BAL fluid was lost with sensitization by Df-pulsed BMDCs from Gpr17/Cysltr1−/− mice (Gpr17/Cysltr1−/− BMDCs→WT mice). These results suggest that GPR17 is a negative regulator for CysLT1R-dependent, DC-mediated allergic pulmonary inflammation.
FIGURE 5. Sensitization of WT Mice with Df-pulsed GPR17-deficient BMDCs Enhances Pulmonary, LN, and Ig Responses upon Challenge.
(A) Inflammatory cell counts in BAL fluid. BMDCs from each genotype of mice were pulsed with Df at 100 μg/ml for 24 h, and 104 cells were administered intranasally for sensitization into WT recipients. Recipients were challenged with 1 μg of Df intranasally at days 10 and 14 and were killed at day 16 for analyses. Total and differential cell counts for BAL fluid monocytes/macrophages (MΦs), neutrophils, eosinophils, and lymphocytes are shown. (B) Parabronchial LN cell numbers. Parabronchial LN cells were harvested and counted, and total cell numbers are shown. (C) Levels of total IgE and Df-specific IgG1 in serum. Total IgE and Df-specific IgG1 in sera of Df-challenged mice were measured by ELISA. Values are the mean ± SEM (n = 12-15) combined from 3 independent experiments. *P < 0.01.
We harvested the lungs for histology and the LNs for cell counts and restimulation from these mice after BAL. Histologic analysis of the bronchovascular bundles revealed that the cellular infiltrates were moderate in the Gpr17−/− BMDCs→WT mice (data not shown). The LN cells were dissociated, counted, and restimulated at a concentration of 2 × 106 cells/ml with 20 μg/ml of Df for 72 h, and the cytokine concentrations in the supernatant were measured with ELISAs. The total number of LN cells was decreased in Cysltr1−/− BMDCs→WT mice and significantly increased in Gpr17−/− BMDCs→WT mice as compared to WT BMDCs→WT mice (Fig. 5B). The total numbers of LN cells from Gpr17/Cysltr1−/− BMDCs→WT mice were minimal and comparable to the numbers for Cysltr1−/− BMDCs→WT mice (Fig. 5B). Df restimulation of LN cells from WT mice that received PBS-treated BMDCs from any genotype of donor mice did not yield any detectable cytokines (data not shown). LN cells of WT BMDCs→WT mice responded to ex vivo restimulation with Df with increased levels of IL-17A and IFN-γ, and minimal IL-5. IL-5 was selectively increased in ex vivo Df-restimulated LN cells from Gpr17−/− BMDCs→WT mice (Supplemental Fig. 3). We did not detect IL-4 production. Of note was the finding that restimulation of LN cells of Cysltr1−/− BMDCs→WT mice or Gpr17/Cysltr1−/− BMDCs→WT mice failed to produce any detectable cytokines even though the cell numbers for restimulation were equivalent.
We assessed for humoral Th2 immune responses in this cohort by measuring total IgE and Df-specific IgG1. As shown in Figure 5C, Cysltr1−/− BMDCs→WT mice had significantly less total IgE and Df-specific IgG1 as compared to WT BMDCs→WT mice. In contrast, Gpr17−/− BMDCs→WT mice had slightly increased levels of total IgE and Df-specific IgG1 as compared to WT BMDCs→WT mice, and these increases were significantly reduced in Gpr17/Cysltr1−/− BMDCs→WT mice (Fig. 5C). Thus, the contribution of CysLT1R to the function of Df-pulsed BMDCs in mediating sensitization for effective Df challenge is apparent by the cell number of the draining LNs and the levels of humoral IgE/IgG1 and the cellular influx into the BAL fluid and lung on the efferent side.
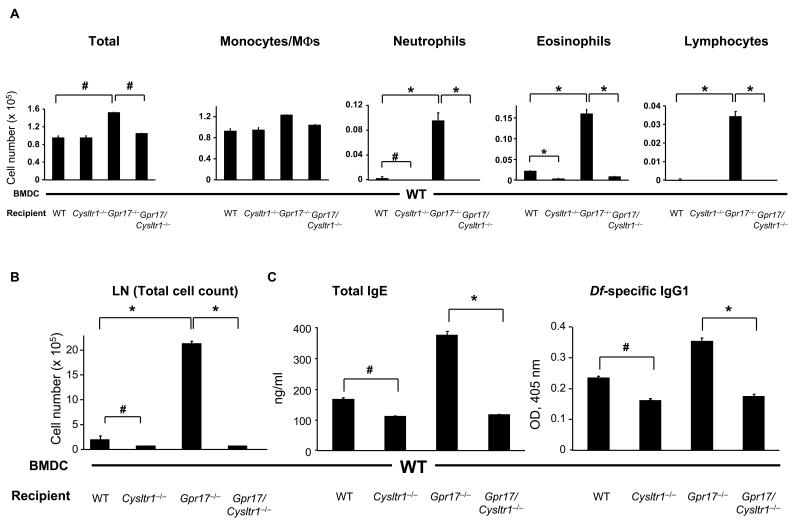
Sensitization of GPR17-deficient Mice with Df-pulsed WT BMDCs Enhances Pulmonary, LN, and Ig Responses upon Challenge
To determine whether the CysLT1R-mediated recipient effector functions are also increased in the absence of GPR17, we sensitized WT, Cysltr1−/−, Gpr17−/−, and Gpr17/Cysltr1−/− mice with Df-pulsed BMDCs from WT mice, challenged them with Df, and assessed for pulmonary and associated LN and Ig responses. As compared to WT recipients, the numbers of neutrophils and eosinophils in BAL fluid were significantly decreased in Cysltr1−/− recipients (Fig. 6A). In contrast, the numbers of neutrophils and eosinophils along with lymphocytes were significantly increased in Gpr17−/− recipients as compared to WT recipients, and this response was abolished in Gpr17/Cysltr1−/− recipients. Histologic analysis of the bronchovascular bundles revealed that the cellular infiltrates were minimal in WT recipients, absent in Cysltr1−/− and Gpr17/Cysltr1−/− mice, and significantly increased in Gpr17−/− mice (data not shown).
FIGURE 6. Sensitization of GPR17-deficient Mice with Df-pulsed WT BMDCs Enhances Pulmonary, LN, and Ig Responses upon Challenge.
(A) Inflammatory cell counts in BAL fluid. BMDCs from WT mice were pulsed with Df at 100 μg/ml for 24 h, and 104 cells were administered intranasally for sensitization into recipients of 4 genotypes. Recipients were challenged with 1 μg of Df intranasally at days 10 and 14 and were killed at day 16 for analyses. Total and differential cell counts for BAL fluid monocytes/macrophages (MΦs), neutrophils, eosinophils, and lymphocytes are shown. (B) Parabronchial LN cell numbers. Parabronchial LN cells were harvested and counted, and total cell numbers are shown. (C) Levels of total IgE and Df-specific IgG1 in serum. Total IgE and Df-specific IgG1 in sera of Df-challenged mice were measured by ELISA. Values are as the mean ± SEM (n = 5-12) combined from 3 independent experiments. *P < 0.01, #P < 0.05.
Moreover, as compared to WT recipients, the total number of LN cells from Cysltr1−/− recipients was significantly decreased, and that from Gpr17−/− recipients was strikingly increased. This increased LN cell number was absent in Gpr17/Cysltr1−/− recipients (Fig. 6B). The restimulated LN cells from WT recipients generated IL-5, IL-17A, and IFN-γ, and the amounts of these cytokines were 4- to 10-fold higher per cell in Gpr17−/− recipients. There was no generation of cytokines with Df restimulation of LN cells from Cysltr1−/− and Gpr17/Cysltr1−/− recipients, again on a per cell basis (Supplemental Fig. 4).
As shown in Figure 6C, Cysltr1−/− recipients had significantly less total IgE and Df-specific IgG1 as compared to WT recipients. In contrast, Gpr17−/− recipients had about 2-fold and 1.5-fold increases in total IgE and Df-specific IgG1, respectively, as compared to WT recipients, and these increases were significantly reduced in Gpr17/Cysltr1−/− recipients (Fig. 6C). Thus, these results reveal additional GPR17-regulated CysLT1R functions that are downstream of the DC antigen presentation phase but yet also influence the inflammatory response in lungs, the draining LNs, and Th2-regulated Igs.
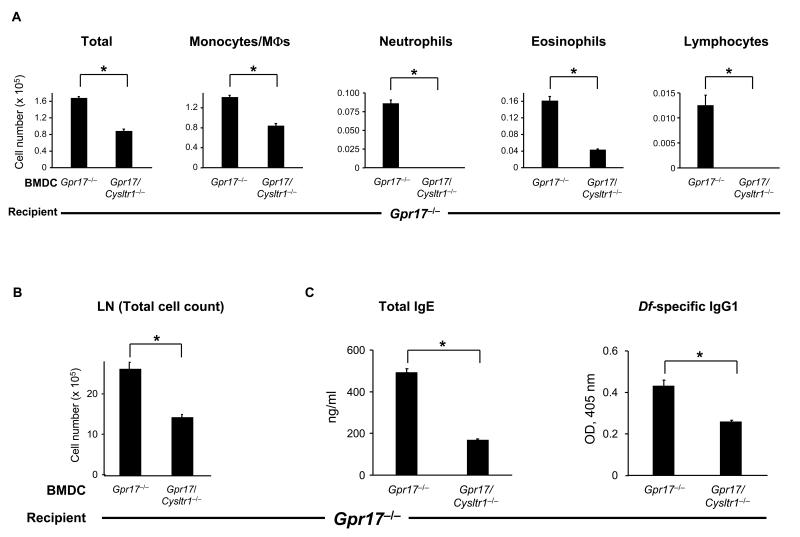
GPR17-deficient Mice Sensitized with Df-pulsed GPR17-deficient BMDCs
Finally, we adoptively transferred Df-pulsed Gpr17−/− or Gpr17/Cysltr1−/− BMDCs into Gpr17−/− recipients to see if DC CysLT1R functions can be apparent in Gpr17−/− recipients. The BAL fluid in Gpr17−/− BMDCs→Gpr17−/− mice was enriched for eosinophils, neutrophils, and lymphocytes, and this augmented response of Gpr17−/− recipients was abrogated in Gpr17/Cysltr1−/− BMDCs→Gpr17−/− mice (Fig. 7A). Histologic analysis revealed that the cellular infiltrates were markedly increased in Gpr17−/− BMDCs→ Gpr17−/− mice, and the increase was reduced in Gpr17/Cysltr1−/− BMDCs→Gpr17−/− mice (Supplemental Fig. 5A). The increased total LN cell number of Gpr17−/− BMDCs→Gpr17−/− mice was significantly reduced in Gpr17/Cysltr1−/− BMDCs→Gpr17−/− mice (Fig. 7B). The levels of IL-5, IL-17A, and IFN-γ with restimulation of LN cells (Supplemental Fig. 5B) and of total IgE and Df-specific IgG1 in serum in Gpr17−/− BMDCs→Gpr17−/− mice was also reduced in Gpr17/Cysltr1−/− BMDCs→Gpr17−/− mice (Fig. 7C). On inspection, the magnitude of the cellular infiltrate in the BAL fluid, the cell numbers in the draining LNs, and the levels of serum IgE/IgG1 were comparable to the findings for Df- pulsed WT BMDCs adoptively transferred into Gpr17−/− recipients (Fig. 6) and greater than those for Gpr17−/− BMDCs adoptively transferred into WT recipients (Fig. 5).
FIGURE 7. GPR17-deficient Mice Sensitized with Df-pulsed GPR17-deficient BMDCs.
(A) Inflammatory cell count in BAL fluid. BMDCs from Gpr17−/− and Gpr17/Cysltr1−/− mice were pulsed with Df at 100 μg/ml for 24 h, and 104 cells were administered intranasally for sensitization of Gpr17−/− recipients. Recipients were challenged with 1 μg of Df intranasally at days 10 and 14 and killed at day 16 for analysis. Total and differential cell counts for BAL fluid monocytes/macrophages (MΦs), neutrophils, eosinophils, and lymphocytes are shown. (B) Parabronchial LN cell numbers. Parabronchial LN cells were harvested and counted, and total cell numbers are shown. (C) Levels of total IgE and Df-specific IgG1 in serum. Total IgE and Df-specific IgG1 in sera of Df-challenged mice were measured by ELISA. Values are the mean ± SEM (n = 10) combined from 2 independent experiments. *P < 0.01.
DISCUSSION
We find that the absence of GPR17 allows a constitutive increased expression of CysLT1R that is further markedly upregulated with an inflammatory response that follows sensitization and challenge with Df. With sensitization and challenge, the phenotype of WT mice includes both local cellular infiltration of the lung and BAL fluid, a systemic further increase in total IgE with appearance of Df-specific IgG1, and increased Th2 cytokine transcripts in the lung. In the Gpr17−/− mice, there is a further significant increase in cellular infiltration of lung, in total IgE and Df-specific IgG1 in serum, and in Th2 cell cytokine transcripts along with an increase in transcript for IL-17A and a decrement of transcript for IFN-γ. The concomitant increased expression of CysLT1R is evident in the infiltrating pulmonary macrophages and neutrophils in WT mice and exaggerated in the lungs of Gpr17−/− mice. The remarkable distribution of CysLT1R effects to both the Df presentation step and the downstream cellular responses were recognized when the 4 genotypes of mice were sensitized by Df-pulsed BMDCs and Df challenged. When the Df-pulsed BMDCs lacked GPR17, the response of WT recipients was marked but abolished if CysLT1R was also absent. Similarly, when the Df-pulsed BMDCs were WT, the local and systemic responses of GPR17-deficient recipients were again marked but abolished if CysLT1R was also absent. When the GPR17-regulated, CysLT1R-mediated functions were re-integrated by sensitizing Gpr17−/− mice with Df-pulsed Gpr17−/− BMDCs and challenging them with Df, the magnitude of responses resembled that of WT BMDCs→Gpr17−/− mice. These findings reveal that the downstream regulatory functions of GPR17 are profound but under the control of the gate-opening sensitization function of CysLT1R on DCs.
A systemic phenotype in naïve mice lacking GPR17 is observed as a significant increase in total serum IgE, reminiscent of our previous observation that total serum IgE was reduced in naïve BALB/c mice lacking LTC4 synthase, the critical enzyme for cys-LT biosynthesis (16). An in vitro study has shown that stimulation of IL-4 and CD40-activated human B cells with LTD4 induces mature epsilon transcripts and increases IgE production (31). Thus, in the absence of GPR17, cys-LTs/CysLT1R might promote the generation of IgE-secreting B cells in vivo.
There was slightly increased expression of CysLT1R in bronchial smooth muscle cells of Df-challenged Gpr17−/− mice but not WT mice. Increased expression of CysLT1R in bronchial smooth muscle cells had been noted with OVA-induced chronic pulmonary inflammation with remodeling in the BALB/c strain (17). Previous reports on molecular characterization of the human CysLT1R showed that the CysLT1R transcripts and proteins are expressed in human lung smooth muscle cells and lung macrophages as assessed by in situ hybridization (5) and by immunohistochemistry (18), respectively. The same group further identified the expression of CysLT1R transcripts and proteins in monocytes/macrophages, eosinophils, and a subset of neutrophils in nasal lavage fluid from patients with seasonal allergic rhinitis (19).
We were surprised to observe that Df restimulation of lung-draining LN cells from mice presenting an induced Th2-like pulmonary and systemic response revealed an unselective cytokine response that was, however, entirely CysLT1R-dependent. In a pharmacologic study in BALB/c mice sensitized by adoptive transfer of Df-pulsed BMDCs and challenged with Df, the recruitment of eosinophils and the levels of IL-5 in BAL fluid were increased by co-stimulation with LTD4 and suppressed by the presence of a CysLT1R antagonist (29). Another pharmacologic study showed that the treatment of BALB/c mice with a CysLT1R antagonist during OVA sensitization reduced the production of IL-4, IL-5, and IFN-γ in BAL fluid after OVA challenge without affecting the levels of OVA-specific IgE and IgG in serum (32). Whereas these pharmacologic studies suggest that CysLT1R activation may augment DC function in both Th2- and Th1-biased immune responses, our findings reveal a role in Th2 and Th17 immune responses in the absence of GPR17. CysLT1R can be upregulated after TCR activation on mouse T cells in vitro and mediates LTD4-elicited calcium flux and migration towards LTD4 (33). An Affymetrix GeneChip analysis with various human T cell subsets has revealed that CysLT1R expression is upregulated in Th2 and effector memory T cells as compared to Th1 and central memory T cells (34). Thus, our future studies may find that Th2 cells as well as Th17 cells preferentially migrate from the regional LNs to lung after Df challenge in Gpr17−/− mice.
Another surprising finding was the appearance of smooth muscle cell hyperplasia in the pulmonary vasculature of the Gpr17−/− mice. The abrogation of this finding in the Gpr17/Cysltr1−/− mice indicates a critical mitogenic role for CysLT1R in vascular smooth muscle cells when GPR17 dampening is lacking. Vascular remodeling is one of the key features in allergen-induced pulmonary inflammation in mice, such as intense inhalation challenge with OVA (35) or Df (36). In vitro studies have shown that LTD4 can potentiate epidermal growth factor-driven human airway smooth muscle cell proliferation (37) and that LTD4 can promote TGF-β- or IL-13-primed human airway smooth muscle cell proliferation in a CysLT1R-dependent manner (38). Recently, Lundequist et al. reported in a similar Df active sensitization and challenge protocol that mice lacking microsomal prostaglandin E synthase-1 show an enhanced vascular smooth muscle hyperplasia (27). Thus, both the anti-proliferative function of prostaglandin E2 and the GPR17 downregulation of CysLT1R can mitigate the action of mitogenic factors for smooth muscle cells in allergic pulmonary inflammation.
Our findings that C57BL/6 Cysltr1−/− and Gpr17/Cysltr1−/− mice were protected from pulmonary inflammation both in active Df sensitization and challenge and in Df-pulsed BMDC transfer models with challenge reveal a broad and dose-related role for CysLT1R in antigen presentation and downstream responses. These Th2-like pulmonary and systemic responses mediated by CysLT1R are profoundly downregulated by GPR17 expression. It is conceivable that the homologous human GPR17 may contribute to the heterogeneous responses to CysLT1R antagonists in patients with bronchial asthma due to variation in GPR17 regulation of the CysLT1R at particular or multiple cell sites.
Supplementary Material
ACKNOWLEDGMENTS
We thank Lena Liu and Juying Lai for technical assistance.
This work was supported in part by National Institutes of Health grants P01HL36110, R01HL82695, and R01HL090630.
Abbreviations used in this paper
- BAL
bronchoalveolar lavage
- BMDC
bone marrow-derived dendritic cell
- cys-LT
cysteinyl leukotriene
- CysLT1R
type 1 receptor for cys-LTs
- Df
extract of Dermatophagoides farinae
- DC
dendritic cell
- LN
lymph node
- LT
leukotriene
- WT
wild-type
REFERENCES
- 1.Austen KF, Maekawa A, Kanaoka Y, Boyce JA. The leukotriene E4 puzzle: finding the missing pieces and revealing the pathobiologic implications. J. Allergy Clin. Immunol. 2009;124:406–414. doi: 10.1016/j.jaci.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters-Golden M, Henderson WR., Jr. Leukotrienes. N. Engl. J. Med. 2007;357:1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 3.Dahlen SE, Hedqvist P, Hammarstrom S, Samuelsson B. Leukotrienes are potent constrictors of human bronchi. Nature. 1980;288:484–486. doi: 10.1038/288484a0. [DOI] [PubMed] [Google Scholar]
- 4.Weiss JW, Drazen JM, Coles N, McFadden ER, Jr., Weller PF, Corey EJ, Lewis RA, Austen KF. Bronchoconstrictor effects of leukotriene C in humans. Science. 1982;216:196–198. doi: 10.1126/science.7063880. [DOI] [PubMed] [Google Scholar]
- 5.Lynch KR, O’Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, Coulombe N, Abramovitz M, Figueroa DJ, Zeng Z, Connolly BM, Bai C, Austin CP, Chateauneuf A, Stocco R, Greig GM, Kargman S, Hooks SB, Hosfield E, Williams DL, Jr., Ford-Hutchinson AW, Caskey CT, Evans JF. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 6.O’Byrne PM, Gauvreau GM, Murphy DM. Efficacy of leukotriene receptor antagonists and synthesis inhibitors in asthma. J. Allergy Clin. Immunol. 2009;124:397–403. doi: 10.1016/j.jaci.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 7.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat. Rev. Immunol. 2008;8:218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 8.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, Zeiger RS, Larsen G, Spahn JD, Bacharier LB, Bloomberg GR, Guilbert TW, Heldt G, Morgan WJ, Moss MH, Sorkness CA, Taussig LM. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J. Allergy Clin. Immunol. 2005;115:233–242. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Pineiro A, Wei LX, Seidenberg BC, Reiss TF. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann. Intern. Med. 1999;130:487–495. doi: 10.7326/0003-4819-130-6-199903160-00005. [DOI] [PubMed] [Google Scholar]
- 10.Naik S, Billington CK, Pascual RM, Deshpande DA, Stefano FP, Kohout TA, Eckman DM, Benovic JL, Penn RB. Regulation of cysteinyl leukotriene type 1 receptor internalization and signaling. J. Biol. Chem. 2005;280:8722–8732. doi: 10.1074/jbc.M413014200. [DOI] [PubMed] [Google Scholar]
- 11.Deshpande DA, Pascual RM, Wang SW, Eckman DM, Riemer EC, Funk CD, Penn RB. PKC-dependent regulation of the receptor locus dominates functional consequences of cysteinyl leukotriene type 1 receptor activation. FASEB J. 2007;21:2335–2342. doi: 10.1096/fj.06-8060com. [DOI] [PubMed] [Google Scholar]
- 12.Maekawa A, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of cysteinyl leukotriene 1 receptor in the enhanced vascular permeability of mice undergoing acute inflammatory responses. J. Biol. Chem. 2002;277:20820–20824. doi: 10.1074/jbc.M203163200. [DOI] [PubMed] [Google Scholar]
- 13.Maekawa A, Kanaoka Y, Xing W, Austen KF. Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. Proc. Natl. Acad. Sci. USA. 2008;105:16695–16700. doi: 10.1073/pnas.0808993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanaoka Y, Maekawa A, Penrose JF, Austen KF, Lam BK. Attenuated zymosan-induced peritoneal vascular permeability and IgE-dependent passive cutaneous anaphylaxis in mice lacking leukotriene C4 synthase. J. Biol. Chem. 2001;276:22608–22613. doi: 10.1074/jbc.M103562200. [DOI] [PubMed] [Google Scholar]
- 15.Henderson WR, Jr., Tang LO, Chu SJ, Tsao SM, Chiang GK, Jones F, Jonas M, Pae C, Wang H, Chi EY. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am. J. Respir. Crit. Care Med. 2002;165:108–116. doi: 10.1164/ajrccm.165.1.2105051. [DOI] [PubMed] [Google Scholar]
- 16.Kim DC, Hsu FI, Barrett NA, Friend DS, Grenningloh R, Ho IC, Al-Garawi A, Lora JM, Lam BK, Austen KF, Kanaoka Y. Cysteinyl leukotrienes regulate Th2 cell-dependent pulmonary inflammation. J. Immunol. 2006;176:4440–4448. doi: 10.4049/jimmunol.176.7.4440. [DOI] [PubMed] [Google Scholar]
- 17.Henderson WR, Jr., Chiang GK, Tien YT, Chi EY. Reversal of allergen-induced airway remodeling by CysLT1 receptor blockade. Am. J. Respir. Crit. Care Med. 2006;173:718–728. doi: 10.1164/rccm.200501-088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Figueroa DJ, Breyer RM, Defoe SK, Kargman S, Daugherty BL, Waldburger K, Liu Q, Clements M, Zeng Z, O’Neill GP, Jones TR, Lynch KR, Austin CP, Evans JF. Expression of the cysteinyl leukotriene 1 receptor in normal human lung and peripheral blood leukocytes. Am. J. Respir. Crit. Care Med. 2001;163:226–233. doi: 10.1164/ajrccm.163.1.2003101. [DOI] [PubMed] [Google Scholar]
- 19.Figueroa DJ, Borish L, Baramki D, Philip G, Austin CP, Evans JF. Expression of cysteinyl leukotriene synthetic and signalling proteins in inflammatory cells in active seasonal allergic rhinitis. Clin. Exp. Allergy. 2003;33:1380–1388. doi: 10.1046/j.1365-2222.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- 20.Parameswaran K, Liang H, Fanat A, Watson R, Snider DP, O’Byrne PM. Role for cysteinyl leukotrienes in allergen-induced change in circulating dendritic cell number in asthma. J. Allergy Clin. Immunol. 2004;114:73–79. doi: 10.1016/j.jaci.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 21.Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, Ferrario S, Parravicini C, Capra V, Gelosa P, Guerrini U, Belcredito S, Cimino M, Sironi L, Tremoli E, Rovati GE, Martini C, Abbracchio MP. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25:4615–4627. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lecca D, Trincavelli ML, Gelosa P, Sironi L, Ciana P, Fumagalli M, Villa G, Verderio C, Grumelli C, Guerrini U, Tremoli E, Rosa P, Cuboni S, Martini C, Buffo A, Cimino M, Abbracchio MP. The recently identified P2Y-like receptor GPR17 is a sensor of brain damage and a new target for brain repair. PLoS ONE. 2008;3:e3579. doi: 10.1371/journal.pone.0003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maekawa A, Balestrieri B, Austen KF, Kanaoka Y. GPR17 is a negative regulator of the cysteinyl leukotriene 1 receptor response to leukotriene D4. Proc. Natl. Acad. Sci. USA. 2009;106:11685–11690. doi: 10.1073/pnas.0905364106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heise CE, O’Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, Stocco R, Bellefeuille JN, Abramovitz M, Cheng R, Williams DL, Jr., Zeng Z, Liu Q, Ma L, Clements MK, Coulombe N, Liu Y, Austin CP, George SR, O’Neill GP, Metters KM, Lynch KR, Evans JF. Characterization of the human cysteinyl leukotriene 2 receptor. J. Biol. Chem. 2000;275:30531–30536. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- 25.Benned-Jensen T, Rosenkilde MM. Distinct expression and ligand-binding profiles of two constitutively active GPR17 splice variants. Br. J. Pharmacol. 2010;159:1092–1105. doi: 10.1111/j.1476-5381.2009.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Platts-Mills TA, Wheatley LM. The role of allergy and atopy in asthma. Curr. Opin. Pulm. Med. 1996;2:29–34. [PubMed] [Google Scholar]
- 27.Lundequist A, Nallamshetty SN, Xing W, Feng C, Laidlaw TM, Uematsu S, Akira S, Boyce JA. Prostaglandin E2 exerts homeostatic regulation of pulmonary vascular remodeling in allergic airway inflammation. J. Immunol. 2010;184:433–441. doi: 10.4049/jimmunol.0902835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cates EC, Fattouh R, Wattie J, Inman MD, Goncharova S, Coyle AJ, Gutierrez-Ramos JC, Jordana M. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J. Immunol. 2004;173:6384–6392. doi: 10.4049/jimmunol.173.10.6384. [DOI] [PubMed] [Google Scholar]
- 29.Machida I, Matsuse H, Kondo Y, Kawano T, Saeki S, Tomari S, Obase Y, Fukushima C, Kohno S. Cysteinyl leukotrienes regulate dendritic cell functions in a murine model of asthma. J. Immunol. 2004;172:1833–1838. doi: 10.4049/jimmunol.172.3.1833. [DOI] [PubMed] [Google Scholar]
- 30.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 31.Lamoureux J, Stankova J, Rola-Pleszczynski M. Leukotriene D4 enhances immunoglobulin production in CD40-activated human B lymphocytes. J. Allergy Clin. Immunol. 2006;117:924–930. doi: 10.1016/j.jaci.2005.12.1329. [DOI] [PubMed] [Google Scholar]
- 32.Okunishi K, Dohi M, Nakagome K, Tanaka R, Yamamoto K. A novel role of cysteinyl leukotrienes to promote dendritic cell activation in the antigen-induced immune responses in the lung. J. Immunol. 2004;173:6393–6402. doi: 10.4049/jimmunol.173.10.6393. [DOI] [PubMed] [Google Scholar]
- 33.Prinz I, Gregoire C, Mollenkopf H, Aguado E, Wang Y, Malissen M, Kaufmann SH, Malissen B. The type 1 cysteinyl leukotriene receptor triggers calcium influx and chemotaxis in mouse αβ- and γδ effector T cells. J. Immunol. 2005;175:713–719. doi: 10.4049/jimmunol.175.2.713. [DOI] [PubMed] [Google Scholar]
- 34.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 35.Tormanen KR, Uller L, Persson CG, Erjefalt JS. Allergen exposure of mouse airways evokes remodeling of both bronchi and large pulmonary vessels. Am. J. Respir. Crit. Care Med. 2005;171:19–25. doi: 10.1164/rccm.200406-698OC. [DOI] [PubMed] [Google Scholar]
- 36.Rydell-Tormanen K, Johnson JR, Fattouh R, Jordana M, Erjefalt JS. Induction of vascular remodeling in the lung by chronic house dust mite exposure. Am. J. Respir. Cell Mol. Biol. 2008;39:61–67. doi: 10.1165/rcmb.2007-0441OC. [DOI] [PubMed] [Google Scholar]
- 37.Panettieri RA, Tan EM, Ciocca V, Luttmann MA, Leonard TB, Hay DW. Effects of LTD4 on human airway smooth muscle cell proliferation, matrix expression, and contraction in vitro: differential sensitivity to cysteinyl leukotriene receptor antagonists. Am. J. Respir. Cell Mol. Biol. 1998;19:453–461. doi: 10.1165/ajrcmb.19.3.2999. [DOI] [PubMed] [Google Scholar]
- 38.Espinosa K, Bosse Y, Stankova J, Rola-Pleszczynski M. CysLT1 receptor upregulation by TGF-beta and IL-13 is associated with bronchial smooth muscle cell proliferation in response to LTD4. J. Allergy Clin. Immunol. 2003;111:1032–1040. doi: 10.1067/mai.2003.1451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.