Abstract
Strategies for assisting smoking cessation include behavioural counselling to enhance motivation and to support attempts to quit and pharmacological intervention to reduce nicotine reinforcement and withdrawal from nicotine. Three drugs are currently used as first line pharmacotherapy for smoking cessation, nicotine replacement therapy, bupropion and varenicline. Compared with placebo, the drug effect varies from 2.27 (95% CI 2.02, 2.55) for varenicline, 1.69 (95% CI 1.53, 1.85) for bupropion and 1.60 (95% CI 1.53, 1.68) for any form of nicotine replacement therapy. Despite some controversy regarding the safety of bupropion and varenicline, regulatory agencies consider these drugs as having a favourable benefit/risk profile. However, given the high rate of psychiatric comorbidity in dependent smokers, practitioners should closely monitor patients for neuropsychiatric symptoms. Second-line pharmacotherapies include nortriptyline and clonidine. This review also offers an overview of pipeline developments and issues related to smoking cessation in special populations such as persons with psychiatric comorbidity and pregnant and adolescent smokers.
Keywords: bupropion, clonidine, mental health, nicotine, pregnancy, varenicline
Introduction
Tobacco addiction is currently considered a chronic disorder that accounts for nearly half a million premature deaths each year in the US alone [1–3] and nearly 6 million people worldwide and causes hundreds of billions of dollars of economic damage [4]. It is predicted that smoking will be responsible for approximately 1 billion smoking-related deaths during the 21st century [5]. Quitting at any age reduces the overall risk of morbidity and mortality [6,7]. Article 14 of the Framework Convention on Tobacco Control states that every country should implement and provide smoking cessation assistance [8]. Seventy percent of smokers report that they would like to quit, and every year, 40% do quit for at least 1 day [9]. Some highly addicted smokers make serious attempts to quit but are unable to stop for longer than several hours [10]. Finally, approximately 80% of smokers who attempt to quit on their own return to smoking within 1 month, and only 3% of smokers quit successfully each year [9]. Despite the continuing debate over the legitimacy of using resources for individual smoking cessation instead of policy measures and interventions [11–14], the cost-effectiveness of smoking cessation programmes has been consistently confirmed [15–17].
Strategies for helping smokers to quit include behavioural counselling to enhance motivation and to support attempts to quit and pharmacological intervention to reduce nicotine reinforcement and the withdrawal symptoms of cessation of tobacco use [18]. Simple advice to stop smoking results in an increased rate of quitting [1,3,19], and counselling increases abstinence rates [20] as a function of time spent with the patient [1]. A minimum intervention for tobacco addiction includes several steps (the 5 As): ask, advise, assess, assist and arrange [3].
Three drugs are currently marketed as first line pharmacotherapy for smoking cessation, nicotine replacement therapy, bupropion hydrochloride (sustained release) and varenicline tartrate [1,3]. These drugs are, on average, rated as satisfactory by users, with a preference for varenicline among those who tried all three medications [21]. Clonidine and nortriptyline have been proposed as second line pharmacotherapies [1,3].
Nicotine replacement therapy
Although the potential role of alkaloids other than nicotine has not been ruled out [22], nicotine is considered to be the main tobacco compound that causes and sustains addiction to tobacco [23]. Nicotine replacement therapy (NRT) was the first proven effective medication for the treatment of tobacco addiction and remains a first line pharmacotherapy in helping smokers quit [24]. Nicotine administration has been shown to reverse nicotine/tobacco withdrawal symptoms and the craving to smoke experienced by smokers in the days and weeks following smoking cessation [25]. NRT has also been shown to reduce the reinforcing effects of smoking [26], and it provides an alternative source for some of its reinforcing and cognitive effects [26]. Depending on the country, NRT is marketed either as a buccal absorption product (gum, lozenge, nasal spray, inhaler or sublingual tablet) or as a transdermal patch [27]. NRT, which can be purchased over the counter in many countries, is the only non-prescription drug shown to be effective for smoking cessation. Other nicotine delivery systems are currently being developed [28–31]. The multiple formulations of NRT offer smokers a choice regarding the route of administration, which may have a positive influence on adherence to treatment [32] (Figure 1).
Figure 1.
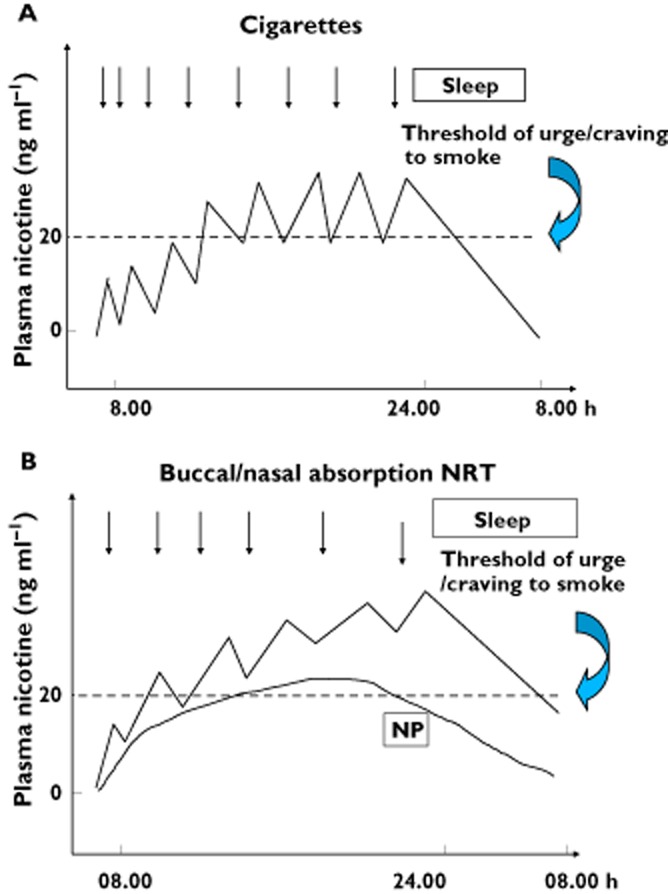
Schematic presentation of plasma nicotine concentrations in venous blood of a smoker over a 24 h period (A) and in an abstinent smoker using nicotine patch (NP) and buccal/nasal absorption nicotine replacement products (B). Combination of NP with rapid, buccal/nasal absorption NRT leads to a higher area under the plasma nicotine concentration curve and peak nicotine concentrations. These result in more time spent above the craving/urge to smoke plasma nicotine concentration threshold and a better mimicking of self-titrated nicotine peaks. (With permission from Journal of Chronic Obstructive Pulmonary Disease, Reference Foulds et al. [26])
Pharmacokinetics of nicotine replacement therapies
The bioavailability of nicotine by cigarette smoke inhalation is close to 100% due to the large absorption surface of the lungs and to nicotine's direct delivery to the brain through the pulmonary arteries and heart-carotid circulation. The bioavailability of nicotine by NRTs is much less than that of cigarette smoke [27]. Depending on the brand, the transdermal patch system offers a continuous release of nicotine over 16 or 24 h (Figure 1). By contrast, oral (i.e. buccal absorption) formulations are short acting, the dose can be self-titrated and, thus, time-adjusted according to the patient's needs [32]. Hence, oral NRTs provide smokers with a coping strategy when cigarette cravings occur [26]. Because the available delivery systems do not reproduce the rapid increase and high arterial plasma and brain concentration of nicotine achieved through inhalation of cigarette smoke, NRTs only partially eliminate withdrawal symptoms [32].
Therapeutic efficacy of nicotine replacement therapies
In terms of tobacco abstinence, the efficacy of NRTs varies depending on the formulation, duration and dosage. Risk ratios range from 1.43 (95% CI 1.33, 1.43) to 2.02 (95% CI 1.49, 3.73) (see Table 1) [1,3,33]. Improvement of the efficacy of NRT seems possible by combining the transdermal patch with an oral formulation that permits ad libitum nicotine delivery [34]. Combined NRT formulations have been shown to result in higher abstinence rates than single NRT in some, [1,3] though not all [35], meta-analyses. NRT is usually started the day of the programmed quit date. Although considered safe, nicotine preloading (i.e. NRT before a quit date) showed conflicting efficacy results [36–38]. Some evidence suggests that NRT can be used with the goal of smoking reduction as an intermediate step for complete and long term abstinence [39]. A recent study by Alpert et al. [41], which received attention from numerous media outlets, concluded that NRT is no more effective in helping people to stop smoking long term – that is, after having stopped its use – than trying to quit on their own. Hughes et al. [40] argued convincingly that the conclusions of Alpert et al. [41] were largely overstated and that the study had an inherent methodological bias. In particular, testing the efficacy of NRT during a relapse that occurs years after its use is not an appropriate evaluation of the effectiveness of NRT [40].
Table 1.
Efficacy of monotherapies for smoking cessation. Summary of Cochrane reviews' findings on the efficacy of medications for smoking cessation
| Medication | Number of studies (subjects) | Relative risk (95% CI) |
|---|---|---|
| Varenicline (2 mg day−1) vs. placebo | 15 | 2.27 (2.02, 2.55) |
| Varenicline (2 mg day−1) vs. bupropion | 3 | 1.52 (1.22, 1.88) |
| Varenicline (2 mg day−1) vs. NRT | 2 | 1.13 (0.94, 1.35) |
| NRT (any form) vs. control | NA | 1.58 (1.50, 1.66) |
| Nicotine gum vs. control | 53 | 1.43 (1.33, 1.53) |
| Nicotine patch vs. control | 41 | 1.66 (1.53, 1.81) |
| Nicotine inhaler vs. control | 4 | 1.90 (1.36, 2.67) |
| Oral tablet/lozenges vs. control | 6 | 2.00 (1.63, 2.45) |
| Nicotine nasal spray vs. control | 4 | 2.02 (1.49, 3.73) |
| Bupropion SR vs. placebo | 36 | 1.69 (1.53, 1.85) |
| Nortriptyline vs. placebo | 6 (975) | 2.03 (1.48, 2.78) |
| Clonidine vs. placebo | 6 | 1.63 (1.22, 2.18) |
Smoking cessation is frequently associated with weight increase [42]. NRT has been shown to limit post-cessation weight gain during the active treatment phase, an effect that does not persist after the termination of treatment [43].
Safety and tolerability of nicotine replacement therapies
The safety profile of NRT is favourable and probably the best among the three first line medications for smoking cessation. The possible adverse effects of night time transdermal nicotine administration, including nightmares, insomnia or EEG changes, have been debated [44,45]. Transdermal patches can cause mild skin irritation at the placement site; nicotine gum may cause mouth soreness, dyspepsia, hiccups, and jaw pain; the nicotine inhaler may cause mouth and throat irritation and coughing; the nicotine lozenge can lead to mouth and throat irritation and hiccups; and the nicotine nasal spray may cause throat and nasal irritation and a runny nose [32].
There is some concern that buccal absorption NRTs are used longer than intended because of persistent nicotine dependence. Persistent use of nicotine gum was first reported in 1988 [46] and later confirmed [47]. However, it noteworthy that after more than 30 years of wordlwide marketing, there has been no post-marketing alert signal regarding the safety of NRT.
Bupropion
Bupropion SR was the first licensed non-nicotinic pharmacological therapy for smoking cessation. Bupropion was first approved as an atypical antidepressant in the USA and other countries. Its development for tobacco addiction treatment was based on the observation that it reduced craving for cigarettes [48]. Bupropion is a beta-phenylethylamine derivative, which explains its stimulant property. It preferentially blocks norepinephrine and dopamine re-uptake in the mesolimbic system and the nucleus accumbens [49] and is also an antagonist of nicotinic receptors. Hence, it blocks the reinforcing effects of nicotine to a certain extent [50,51]. Bupropion is metabolized into three active metabolites by cytochrome 2B6.
Therapeutic efficacy of bupropion
The recommended dosage of bupropion is 150 mg twice daily. Because steady-state plasma concentrations of bupropion are obtained 5 to 8 days after treatment initiation, smokers treated with bupropion should aim to quit approximately 1 week after the start of treatment [52].
Several meta-analyses have confirmed the efficacy of bupropion [1,3,53]. The Cochrane review has shown a risk ratio over placebo of 1.69 (95% CI 1.53, 1.85) [53] (see Table 1). Bupropion has been shown to decrease nicotine/tobacco withdrawal symptoms and cigarette cravings [54] and to reduce post-cessation weight gain, at least until the end of treatment [43]. One meta-analysis has shown a significant increase of efficacy when bupropion is added to standard nicotine patch therapy [3], a result not clearly reported in the Cochrane review [53].
Safety and tolerability of bupropion
The most common adverse effects associated with the use of bupropion SR at the recommended dosage of 150 mg twice daily in clinical trials included insomnia, headache, dry mouth, nausea and anxiety [55]. Insomnia and anxiety are also recognized symptoms of nicotine/tobacco withdrawal. Only insomnia and dry mouth occurred significantly more frequently with bupropion SR than with placebo. Infrequent but clinically important adverse reactions to bupropion SR include seizures and hypersensitivity reactions. The most common adverse effects (insomnia and dry mouth) are generally transient and often resolve quickly without therapeutic intervention. They can be managed, if necessary, by dose reduction. Although data are limited, bupropion is considered safe for use in patients with cardiovascular disease [56] or COPD [57]. Bupropion SR is contraindicated in smokers with known hypersensitivity to the drug or its excipients, those with current or previous seizure disorders, bulimia, anorexia nervosa and those taking monoamine oxidase inhibitors. Furthermore, bupropion SR should not be administered while patients are undergoing abrupt withdrawal from alcohol or benzodiazepines, as it carries a risk of seizures [55].
It should be noted, that soon after the marketing of bupropion SR for smoking cessation, a high rate of neuropsychiatric adverse reactions was reported. Using post-marketing spontaneous reporting data, a recent study showed an increased risk of reported depression and suicidal/self-injurious behaviour with bupropion compared with NRT [58]. However, a meta-analysis of controlled studies did not shown any increase in suicidal behaviour with bupropion vs. placebo [59].
Varenicline
Pharmacology and clinical pharmacology of varenicline
Varenicline is the most recently approved treatment for smoking cessation. Varenicline binds the α4β2 subtype nAChR with subnanomolar affinity and high selectivity. The affinity of varenicline for the α4β2 receptor is approximately three-fold and 16-fold greater than that of cytisine and nicotine (Ki of 0.06 nmol l−1, 0.17 nmol l−1 and 0.95 nmol l−1, respectively) [60,61]. Varenicline is also a full agonist of homomericα7 nAChR [62]. It is hypothesized that varenicline has a dual mechanism of action: (i) it acts as a partial agonist at the α4β2 receptor that reduces the smoking cessation-induced drop in mesolimbic dopamine concentrations, potentially relieving withdrawal symptoms and (ii), consequent to its agonist activity and high affinity, it antagonizes the activity of nicotine at the α4β2 receptor and blocks nicotine-induced dopaminergic activation, potentially reducing the reward from smoking relapse [60,63].
The plasma elimination half-life of varenicline is approximately 24 h. Because varenicline does not undergo significant hepatic metabolism, its pharmacokinetics are unaffected in patients with hepatic insufficiency. Varenicline is excreted in the urine, and its renal clearance is dose-proportional. In patients with mild renal impairment (creatinine clearance >50 ml min−1 and ≤80 ml min−1), pharmacokinetics are unchanged compared with subjects with normal renal function. In patients with moderate renal impairment (creatinine clearance ≥30 ml min−1 and ≤50 ml min−1), varenicline exposure is increased 1.5-fold. Similarly, varenicline exposure increases 2.1-fold in patients with severe renal impairment (creatinine clearance <30 ml min−1). Therefore, caution is warranted with the use of varenicline in subjects with renal impairment, and when renal impairment is severe, dose adjustment is necessary [60].
Therapeutic efficacy of varenicline
The recommended dosage is 1 mg twice daily following a 1 week up-titration. However, it has been shown that a self-regulated, flexible dosing regimen of varenicline is well tolerated, with superior effectiveness vs. placebo [64]. Smokers treated with varenicline should normally aim to quit approximately 1 week after the start of the treatment. It has been shown, however, that using a flexible quit-date paradigm had efficacy and safety similar to those in the previous fixed quit-date paradigm [65]. Meta-analyses have confirmed the increased efficacy of varenicline on smoking quit rates at the dosage of 2 mg day−1 during a 12 week treatment compared with placebo and also to bupropion (see Table 1) [3,66,67]. For instance, the Cochrane review has shown a risk ratio over placebo of 2.27 (95% CI 2.02, 2.55). Only two published studies have compared varenicline with NRT [68,69]. In an open but randomized study, varenicline performed better than the transdermal nicotine patch [68]. Varenicline has been shown to result in higher abstinence rates than combined or high dose NRT in some [35], but not all [1], meta-analyses. A 12 week treatment extension yields better cessation rates for smokers who quit successfully for at least 1 week at the end of the first 12 weeks of treatment [70]. The combination of varenicline with NRT or bupropion seems to be safe [71,72], but no published, randomized controlled data exist as to the superiority of this co-administration over one or the other alone.
Like NRT and bupropion, varenicline has been shown to limit post-cessation weight gain during the active treatment phase, an effect that does not persist after treatment has ended [43]. In addition, some data suggest that varenicline may help reduce alcohol consumption in smokers who drink heavily [73,74].
Safety and tolerability of varenicline
Phase I reports have shown that varenicline is tolerated after single doses up to 3 mg in smokers and 1 mg in non-smokers. Nausea and vomiting at doses above 3.0 mg in smokers and 1 mg in non-smokers are dose limiting [75]. Recent reviews of the safety profile of varenicline concluded that the most frequent adverse event was nausea, occurring in 30–40% of users [76–78]. The nausea was generally reported as mild to moderate and diminishing over time, and it was associated with low attributable discontinuation rates. Other common adverse effects included insomnia, abnormal dreams and headaches. In the randomized, controlled phase III studies, serious adverse events were rare, with no treatment-related deaths during the treatment or follow-up phases. There are currently no known contraindications to varenicline [79].
Post-marketing surveillance reports have suggested an increased risk of reported depression and suicidal/self-injurious behaviour with varenicline (and bupropion) compared with NRT [58,80–82]. However, a pooled analysis of more than 5000 smokers without current psychiatric history who participated in one of 10 randomized, placebo-controlled clinical trials found that there was no significant increase in overall psychiatric adverse events aside from sleep disorders [83]. A study that assessed neuropsychiatric adverse effects failed to find any difference in measurements of depressive symptoms, anxiety, or aggression/hostility between varenicline and placebo among smokers without psychiatric disorders [84]. Furthermore, a retrospective analysis of 80 600 adults prescribed varenicline, using records from the UK General Practice Research Database, found that the incidence of depression and suicide was not greater with varenicline than with NRT or bupropion [85]. The post-marketing reports led the US Food and Drug Administration (FDA) and the European Medicine Agency to add a ‘black box warning’ to the product labelling for both varenicline and bupropion SR. Recently, two FDA-sponsored epidemiological studies that evaluated the risk of neuropsychiatric adverse events associated with smoking cessation drugs found no difference in risk of neuropsychiatric hospitalizations between varenicline and NRT [86]. Smoking and/or smoking cessation are frequently associated with neuropsychiatric symptoms and suicide-related outcomes [87]. Therefore, drug regulatory agencies acknowledge that distinguishing between drug-related adverse effects/events and the neuropsychiatric effects related to smoking and/or smoking cessation is difficult, and they strongly recommend that patients be closely monitored for neuropsychiatric symptoms [88].
A meta-analysis suggested an increased rate of cardiovascular events with varenicline [89]. However, a more recent meta-analysis that accounted for the major biases not taken into account in the previous work concluded that treatment with varenicline is not associated with increased rates of cardiovascular events compared with placebo [90].
Nortriptyline and clonidine have been proposed as second line pharmacotherapies by the US Clinical Practice Guidelines for treating tobacco use and dependence [1,3].
Nortriptyline, a tricyclic antidepressant, is believed to block the re-uptake of norepinephrine and serotonin and, by this mechanism, reduce tobacco withdrawal symptoms [91]. Although nortriptyline has been confirmed as a smoking cessation drug (see Tables 1 and 2) [1,3,53], its unfavourable adverse effect profile prevented the drug from receiving FDA approval for smoking cessation. A well-powered, randomized, placebo-controlled study [92] and a meta-analysis [53] demonstrated that combining nortriptyline with NRT is no more effective than NRT alone.
Table 2.
Efficacy of combined pharmacotherapies for smoking cessation. U.S. Public Health Service clinical practice guideline meta-analysis
| Combination therapies | Number of arms | Estimated OR (95% CI) |
|---|---|---|
| Nicotine patch (long term, >14 weeks) + ad lib NRT | 3 | 3.6 (2.5, 5.2) |
| Nicotine patch + bupropion SR | 3 | 2.5 (1.9, 3.4) |
| Nicotine patch + nortriptyline | 2 | 2.3 (1.3, 4.2) |
| Nicotine patch + inhaler | 2 | 2.2 (1.3, 3.6) |
Adapted from The Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff [3]. CI, confidence interval; NRT, nicotine replacement therapy; OR, odds ratio; SR, sustained release.
Clonidine is an α2-adrenergic agonist antihypertensive medication that decreases sympathetic outflow [32]. It is not approved for smoking cessation, but early studies demonstrated some efficacy, only among women, as an aid in smoking cessation (see Tables 1 and 2) [1,3,93]. Its unfavourable, dose-dependent adverse effect profile (dry mouth and sedation) and limited efficacy preclude its widespread use [93].
Special populations
Psychiatric comorbidity
Smoking prevalence among individuals with psychiatric disorders is 2-to 4-fold higher than in the general population [94], with even higher smoking prevalence rates among certain diagnostic groups, such as persons with bipolar disorder (60%) [95] or schizophrenia (65–90%) [96–98. Individuals with a mental illness are also more likely to be heavy smokers [95] and to suffer more severe withdrawal symptoms [99]. Smoking may substantially increase cardiovascular morbidity and mortality among individuals with mental illness in association with atypical antipsychotics-induced weight increase and the consequent incidence of type 2 diabetes mellitus [100]. Despite increasing evidence that individuals with psychiatric disorders are motivated to quit [101], tobacco addiction remains an under-treated and under-recognized problem within this patient population [102].
The efficacy of NRT seems to be modest in smokers with schizophrenia, but abstinence rates seems to be higher among schizophrenics treated with NRT in combination with atypical antipsychotics compared with standard antipsychotics [103]. A recent meta-analysis showed that bupropion increased the rate of smoking abstinence in smokers with schizophrenia (RR = 2.78, 95% CI 1.02, 7.58) without jeopardizing their mental state [104]. Accumulating evidence shows that varenicline is also effective and safe in schizophrenic patients [105–110].
Several studies suggest that pharmacological treatments for tobacco addiction used in the general population can be similarly effective among those with depressive comorbidity. A secondary analysis of a smoking cessation study demonstrated that the smoking cessation rate among smokers with depression who used nicotine gum was more than twice the rate among those who used placebo gum [111]. However, to our knowledge, no specific study has assessed the efficacy of NRT in smokers with major depression. A meta-analysis of bupropion SR and nortriptyline found that these treatments are effective in increasing long-term cessation rates in smokers with a history of depression (OR = 3.42, 95% CI 1.70, 6.84) [1]. Assumptions that all antidepressants have comparable effects owing to certain similarities in mechanisms of action have proven to be incorrect. Although both bupropion and nortriptyline are effective for smoking cessation, several studies have shown that selective serotonin re-uptake inhibitors do not increase abstinence rates [53].
Finally, the US clinical guideline for treating tobacco use and dependence recommends that smokers with psychiatric or addictive comorbidity use any medication proven effective in the general population of smokers, except when use of the medication is contraindicated [1].
Pregnancy
In addition to increasing pregnancy and perinatal hazards, maternal smoking during pregnancy is an independent risk factor for obesity, type 2 diabetes mellitus, smoking and tobacco addiction, and psychiatric disorders, all of which cause mortality up to age 20 years in the offspring [112]. Despite the risks associated with smoking during pregnancy, the number of pregnant women who smoke until delivery remains high. For example, it has been reported that during pregnancy 13% of women smoke in the US [113] and 18% of women in France [114]. NRT use during pregnancy is approved in some countries but not in others [115]. The use of NRT during pregnancy is controversial because nicotine has been shown to have foetotoxicity in animal models. In addition, previous randomized controlled trials have been inconclusive with respect to the efficacy and safety of NRT on cessation rates among pregnant smokers [113]. A very recent, sufficiently powered, randomized, placebo-controlled trial with a maximum of 2 months of nicotine patch exposure during the entire pregnancy did not find either an increased abstinence rate or higher birth weight with the nicotine patch as compared with a placebo patch [116]. Similarly, there was no difference in safety [116]. Because of the lack of randomized, sufficiently powered, controlled studies among pregnant smokers, neither bupropion nor varenicline is indicated for smoking cessation during pregnancy.
Adolescents
Given that that early smoking initiation is strongly associated with premature death [117] and that more than 80% of dependent smokers start smoking before the age of 18 years, tobacco addiction can be regarded as a youth disorder extending into adulthood [118]. Unfortunately, the burden of the disease does not always wait until midlife to manifest. For instance, smoking has been shown to independently predict suicide in adolescents and young adults [119]. A recent meta-analysis and systematic review detected no significant efficacy of pharmacological therapy in adolescents [120,121]. Consequently, no definitive recommendations can be made regarding the use of pharmacotherapy for smoking cessation in this population of smokers, who are future candidates for smoking-related illnesses [1].
Pipeline developments
New galenic formulations of varenicline under development include a controlled-release formulation (ClinicalTrials.gov Identifiers: NCT00741884 andNCT005227150), a free base patch (ClinicalTrials.gov Identifiers: NCT00741884, NCT01234142 and NCT01013454) and a free base solution (ClinicalTrials.gov Identifier: NCT00774605). The α4β2 nAchR partial agonist cytisine has been used for decades as a smoking cessation drug in Eastern European countries and has been shown to be effective and safe as an aid for smoking cessation [122–125]. Other α4β2nAchR partial agonists have been tested, with little success [126].
The finding that cigarette smoking is associated with inhibition of both monoamine oxidase (MAO) A and B subtypes has led to the hypothesis that MAO subtype-selective inhibitors may represent a novel class of medications that could be developed for smoking cessation [127–129]. Although early trials were promising [130,131], subsequent trials yielded disappointing results. Selective MAO B inhibitors, either alone [132,133] or in association with the nicotine patch [134], did not sufficiently increase the abstinence rate in powered, randomized, placebo-controlled trials.
Nicotine vaccines are designed to stimulate to the production of antibodies by the immune system that bind to nicotine in the bloodstream and prevent it from entering the brain by crossing the blood–brain barrier. With a reduced amount of nicotine reaching the brain, it is anticipated that the reinforcing effects of nicotine are diminished, thereby making it easier to quit smoking [135]. Three anti-nicotine vaccines are currently in advanced stages of clinical evaluation [136], including phase III (ClinicalTrials.gov Identifiers: NCT01178346, NCT001102114). None of the vaccine studies carried out to date has reported major side effects [136]. Optimism has steeply decreased with the announcement that the vaccines did not increase abstinence rate in two of the phase III trials [137].
Medications that affect GABA or NMDA neurotransmission may decrease the reinforcing properties of nicotine, and thus may be useful as pharmacological treatments of tobacco addiction [28,138]. Topiramate inhibits glutamatergic neurotransmission while simultaneously enhancing GABAergic tone [139]. Topiramate has been shown to produce gender-specific effects on smoking cessation. In a randomized placebo-controlled trial, men (but not women) were approximately four times more likely to quit smoking when treated with topiramate as compared with placebo [140]. Topiramate has also been shown to be effective in reducing smoking in alcoholic smokers [141,142]. This drug has currently entered phase III of its development as an aid for smoking cessation (ClinicalTrials.gov Identifiers: NCT00755716 and NCT00280839). Baclofen, a selective GABA-B agonist, has shown some clinical and preclinical evidence for treating tobacco addiction [28]. A recent randomized placebo-controlled pilot trial demonstrated a significant reduction in craving and smoking [143], and another randomized placebo-controlled trial is underway (ClinicalTrials.gov Identifier: NCT01228994). Two small scale trials have shown little promise of gabapentin treating smoking cessation [144–146]. Three drugs affecting NMDA are currently being investigated in phase I trials: memantine (ClinicalTrials.gov Identifier: NCT00136786), GW468816 [147] and cycloserine (ClinicalTrials.gov Identifier: NCT01062932). Finally, a pilot study exploring the effect of N-acetylcysteine has shown promising trends [148].
Modafinil is marketed as a wakefulness-promoting agent for excessive sleepiness associated with narcolepsy, obstructive sleep apnoea, and shift work sleep disorder [149]. Because of its efficacy in cocaine dependence [150] and its potential to alleviate nicotine/tobacco withdrawal symptoms [151], modafinil has been investigated as a treatment for smoking cessation [152]. However, the trial has been discontinued owing to the detrimental effect of the drug on smoking cessation. Dopamine D3-receptor antagonists have shown promise in animal models [153]. GSK598809, a selective D3 antagonist, is currently being investigated in a phase II randomized placebo-controlled trial (ClinicalTrials.gov Identifier: NCT01188967).
Relapse prevention
Although behavioural support, nicotine replacement therapy, bupropion, and varenicline are all effective smoking cessation treatments, many smokers who stop after using these treatments subsequently relapse to smoking [154]. Consequently, any interventions that reduce relapse rates among abstinent smokers could have a substantial impact on maintaining long term abstinence [155]. Two recent meta-analyses propose different conclusions as a result of the different types of analysis performed [155,156]. The Cochrane review concludes that extended treatment with varenicline may prevent relapse, that extended treatment with bupropion is unlikely to have a clinically important effect, and that studies of extended treatment with nicotine replacement are needed [156]. By contrast, the review by Agboola et al. concludes that all three medications, NRT, bupropion and varenicline, are effective in preventing relapse following an initial period of abstinence [155].
Conclusion
This review confirms that effective first and second line medications help smokers to quit. These treatments are effective across a broad range of populations, and clinicians should encourage and offer counselling and prescribe pharmacotherapy to any patient willing to attempt quitting. Some critics have suggested that smoking cessation medications have no useful applications in ‘real-world’ settings. However, systematic biases in cross-sectional community studies are likely to underestimate the effectiveness of smoking cessation medications [157].
Despite the relative efficacy of these treatments, many smokers relapse after a quit attempt, and alternative pharmacotherapies are needed [158] to increase cessation rates and to prevent relapses.
It is conceivable that tobacco use and addiction necessitate long term pharmacological treatments not only to induce abstinence but also to maintain it. If this is true, treatments that maintain long term smoking cessation and abstinence would contribute to the reduction of smoking-related morbidity and mortality. Because smoking is the main cause of premature death, morbidity-mortality studies related to smoking cessation medications should be implemented in the future.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, HJA and IB had a board membership, received payment for lectures including service on speakers bureau with Pfizer in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN, Heyman RB, Koh HK, Kottke TE, Lando HA, Mecklenburg RE, Mermelstein RJ, Mullen PD, Orleans CT, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. updated May 2008. Available at http://www.ncbi.nlm.nih.gov.gate2.inist.fr/books/NBK12193 (last accessed 1 October 2010) [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Annual smoking-attributable mortality, years of potential life lost, and productivity losses−United States, 1997–2001. MMWR Morb Mortal Wkly Rep. 2005;54:625–628. [PubMed] [Google Scholar]
- 3.The Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med. 2008;35:158–176. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.2011. WHO report on the global tobacco epidemic: warning about the dangers of tobacco Available at http://whqlibdoc.who.int/hq/2011/WHO_NMH_TFI_11.3_eng.pdf (last accessed 27 June 2012)
- 5.Jha P. Avoidable global cancer deaths and total deaths from smoking. Nat Rev Cancer. 2009;9:655–664. doi: 10.1038/nrc2703. [DOI] [PubMed] [Google Scholar]
- 6.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years'observation on male British doctors. Br Med J. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjartveit K, Tverdal A. Health consequences of sustained smoking cessation. Tob Control. 2009;18:197–205. doi: 10.1136/tc.2008.026898. [DOI] [PubMed] [Google Scholar]
- 8.Raw M. Framework Convention on Tobacco Control (FCTC) Article 14 guidelines: a new era for tobacco dependence treatment. Addiction. 2011;106:2055–2057. doi: 10.1111/j.1360-0443.2011.03536.x. [DOI] [PubMed] [Google Scholar]
- 9.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, Clark DB. Analyzing milestones in smoking cessation: illustration in a nicotine patch trial in adult smokers. J Consult Clin Psychol. 2006;74:276–285. doi: 10.1037/0022-006X.74.2.276. [DOI] [PubMed] [Google Scholar]
- 11.West R, McNeill A, Britton J, Bauld L, Raw M, Hajek P, Arnott D, Jarvis M, Stapleton J. Should smokers be offered assistance with stopping? Addiction. 2010;105:1867–1869. doi: 10.1111/j.1360-0443.2010.03111.x. [DOI] [PubMed] [Google Scholar]
- 12.Chapman S, Wakefield M. Smoking cessation strategies. BMJ. 2012;344:e1732. doi: 10.1136/bmj.e1732. [DOI] [PubMed] [Google Scholar]
- 13.Chapman S, MacKenzie R. The global research neglect of unassisted smoking cessation: causes and consequences. PLoS Med. 2010;7:e1000216. doi: 10.1371/journal.pmed.1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapman S. The inverse impact law of smoking cessation. Lancet. 2009;373:701–703. doi: 10.1016/S0140-6736(09)60416-5. [DOI] [PubMed] [Google Scholar]
- 15.Richard P, West K, Ku L. The return on investment of a Medicaid tobacco cessation program in Massachusetts. PLoS ONE. 2012;7:e29665. doi: 10.1371/journal.pone.0029665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor M, Leonardi-Bee J, Agboola S, McNeill A, Coleman T. Cost effectiveness of interventions to reduce relapse to smoking following smoking cessation. Addiction. 2011;106:1819–1826. doi: 10.1111/j.1360-0443.2011.03493.x. [DOI] [PubMed] [Google Scholar]
- 17.Mahmoudi M, Coleman CI, Sobieraj DM. Systematic review of the cost-effectiveness of varenicline vs. bupropion for smoking cessation. Int J Clin Pract. 2012;66:171–182. doi: 10.1111/j.1742-1241.2011.02877.x. [DOI] [PubMed] [Google Scholar]
- 18.Aubin HJ, Karila L, Reynaud M. Pharmacotherapy for smoking cessation: present and future. Curr Pharm Des. 2011;17:1343–1350. doi: 10.2174/138161211796150837. [DOI] [PubMed] [Google Scholar]
- 19.Stead LF, Bergson G, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2008;(2) doi: 10.1002/14651858.CD000165.pub3. ): CD000165. [DOI] [PubMed] [Google Scholar]
- 20.Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2005;(2) doi: 10.1002/14651858.CD001292.pub2. CD001292. [DOI] [PubMed] [Google Scholar]
- 21.Etter JF, Schneider NG. An internet survey of use, opinions and preferences for smoking cessation medications: nicotine, varenicline, and bupropion. Nicotine Tob Res. 2013;15:59–68. doi: 10.1093/ntr/nts084. [DOI] [PubMed] [Google Scholar]
- 22.Fant RV, Buchhalter AR, Buchman AC, Henningfield JE. Pharmacotherapy for tobacco dependence. Handb Exp Pharmacol. 2009;192:487–510. doi: 10.1007/978-3-540-69248-5_17. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Department of Health and Human Services. The Health Consequences of Smoking: Nicotine Addiction: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Health Promotion and Education, Office on Smoking and Health; 1988. Available at http://profiles.nlm.nih.gov/NN/B/B/Z/D/_/nnbbzd.pdf(last accessed 1 October 2010) [Google Scholar]
- 24.Edens E, Massa A, Petrakis I. Novel pharmacological approaches to drug abuse treatment. Curr Top Behav Neurosci. 2010;3:343–386. doi: 10.1007/7854_2009_29. [DOI] [PubMed] [Google Scholar]
- 25.West RJ, Jarvis MJ, Russell MA, Carruthers ME, Feyerabend C. Effect of nicotine replacement on the cigarette withdrawal syndrome. Br J Addict. 1984;79:215–219. doi: 10.1111/j.1360-0443.1984.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 26.Foulds J, Burke M, Steinberg M, Ziedonis DM, Williams JM. Advances in pharmacotherapy for tobacco dependence. Expert Opin Emerg Drugs. 2004;9:39–53. doi: 10.1517/eoed.9.1.39.32951. [DOI] [PubMed] [Google Scholar]
- 27.Berlin I. Therapeutic strategies to optimize the efficacy of nicotine replacement therapies. COPD. 2009;6:272–276. doi: 10.1080/15412550903049116. [DOI] [PubMed] [Google Scholar]
- 28.Buchhalter AR, Fant RV, Henningfield JE. Novel pharmacological approaches for treating tobacco dependence and withdrawal: current status. Drugs. 2008;68:1067–1088. doi: 10.2165/00003495-200868080-00005. [DOI] [PubMed] [Google Scholar]
- 29.Tonnesen P, Lauri H, Perfekt R, Mann K, Batra A. Efficacy of a nicotine mouth spray in smoking cessation: a randomised, double blind trial. Eur Respir J. 2012;40:548–554. doi: 10.1183/09031936.00155811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Orlando KJ, Fox BS. Tolerability and pharmacokinetics of single and repeated doses of nicotine with The Straw, a novel nicotine replacement product. Nicotine Tob Res. 2004;6:63–70. doi: 10.1080/14622200310001656876. [DOI] [PubMed] [Google Scholar]
- 31.Westman EC, Tomlin KF, Perkins CE, Rose JE. Oral nicotine solution for smoking cessation: a pilot tolerability study. Nicotine Tob Res. 2001;3:391–396. doi: 10.1080/14622200126973. [DOI] [PubMed] [Google Scholar]
- 32.Nides M. Update on pharmacologic options for smoking cessation treatment. Am J Med. 2008;121(4 Suppl. 1):S20–31. doi: 10.1016/j.amjmed.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(1) doi: 10.1002/14651858.CD000146.pub3. CD000146. [DOI] [PubMed] [Google Scholar]
- 34.Sweeney CT, Fant RV, Fagerstrom KO, McGovern JF, Henningfield JE. Combination nicotine replacement therapy for smoking cessation: rationale, efficacy and tolerability. CNS Drugs. 2001;15:453–467. doi: 10.2165/00023210-200115060-00004. [DOI] [PubMed] [Google Scholar]
- 35.Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO. Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta-analysis. Ann Med. 2012;44:588–589. doi: 10.3109/07853890.2012.705016. [DOI] [PubMed] [Google Scholar]
- 36.Bullen C, Howe C, Lin RB, Grigg M, Laugesen M, McRobbie H, Glover M, Walker N, Wallace-Bell M, Whittaker R, Rodgers A. Pre-cessation nicotine replacement therapy: pragmatic randomized trial. Addiction. 2010;105:1474–1483. doi: 10.1111/j.1360-0443.2010.02989.x. [DOI] [PubMed] [Google Scholar]
- 37.Shiffman S, Ferguson SG. Nicotine patch therapy prior to quitting smoking: a meta-analysis. Addiction. 2008;103:557–563. doi: 10.1111/j.1360-0443.2008.02138.x. [DOI] [PubMed] [Google Scholar]
- 38.Lindson N, Aveyard P. An updated meta-analysis of nicotine preloading for smoking cessation: investigating mediators of the effect. Psychopharmacology (Berl) 2011;214:579–592. doi: 10.1007/s00213-010-2069-3. [DOI] [PubMed] [Google Scholar]
- 39.Lingford-Hughes AR, Welch S, Peters L, Nutt DJ. BAP updated guidelines: evidence-based guidelines for the pharmacological management of substance abuse, harmful use, addiction and comorbidity: recommendations from BAP. J Psychopharmacol. 2012;26:899–952. doi: 10.1177/0269881112444324. [DOI] [PubMed] [Google Scholar]
- 40.Hughes JR, Cummings KM, Foulds J, Shiffman S, West R. Effectiveness of nicotine replacement therapy-a rebuttal. Addiction. 2012;107:1527–1528. doi: 10.1111/j.1360-0443.2012.03925.x. [DOI] [PubMed] [Google Scholar]
- 41.Alpert HR, Connolly GN, Biener L. A prospective cohort study challenging the effectiveness of population-based medical intervention for smoking cessation. Tob Control. 2013;22:32–37. doi: 10.1136/tobaccocontrol-2011-050129. [DOI] [PubMed] [Google Scholar]
- 42.Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ. 2012;345:e4439. doi: 10.1136/bmj.e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farley AC, Hajek P, Lycett D, Aveyard P. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst Rev. 2012;(1) doi: 10.1002/14651858.CD006219.pub3. CD006219. [DOI] [PubMed] [Google Scholar]
- 44.Aubin HJ, Luthringer R, Demazieres A, Dupont C, Lagrue G. Comparison of the effects of a 24-h nicotine patch and a 16-h nicotine patch on smoking urges and sleep. Nicotine Tob Res. 2006;8:193–201. doi: 10.1080/14622200500489989. [DOI] [PubMed] [Google Scholar]
- 45.Staner L, Luthringer R, Dupont C, Aubin HJ, Lagrue G. Sleep effects of a 24-h versus a 16-h nicotine patch: a polysomnographic study during smoking cessation. Sleep Med. 2006;7:147–154. doi: 10.1016/j.sleep.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Hajek P, Jackson P, Belcher M. Long-term use of nicotine chewing gum. Occurrence, determinants, and effect on weight gain. JAMA. 1988;260:1593–1596. [Research Support, Non-U.S. Gov't]. Sep 16; [PubMed] [Google Scholar]
- 47.Etter JF. Dependence on the nicotine gum in former smokers. Addict Behav. 2009;34:246–251. doi: 10.1016/j.addbeh.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 48.Ferry LH. Non-nicotine pharmacotherapy for smoking cessation. Prim Care. 1999;26:653–669. doi: 10.1016/s0095-4543(05)70122-6. [Review]. Sep; [DOI] [PubMed] [Google Scholar]
- 49.Wilkes S. The use of bupropion SR in cigarette smoking cessation. Int J Chron Obstruct Pulmon Dis. 2008;3:45–53. doi: 10.2147/copd.s1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E, Sulser F. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995;56:395–401. [PubMed] [Google Scholar]
- 51.Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther. 2000;295:321–327. [PubMed] [Google Scholar]
- 52.Aubin HJ, Lebargy F, Berlin I, Bidaut-Mazel C, Chemali-Hudry J, Lagrue G. Efficacy of bupropion and predictors of successful outcome in a sample of French smokers: a randomized placebo-controlled trial. Addiction. 2004;99:1206–1218. doi: 10.1111/j.1360-0443.2004.00814.x. [DOI] [PubMed] [Google Scholar]
- 53.Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007;(1) doi: 10.1002/14651858.CD000031.pub3. CD000031. [DOI] [PubMed] [Google Scholar]
- 54.Mooney ME, Sofuoglu M. Bupropion for the treatment of nicotine withdrawal and craving. Expert Rev Neurother. 2006;6:965–981. doi: 10.1586/14737175.6.7.965. [DOI] [PubMed] [Google Scholar]
- 55.Aubin HJ. Tolerability and safety of sustained-release bupropion in the management of smoking cessation. Drugs. 2002;62(Suppl. 2):45–52. doi: 10.2165/00003495-200262002-00005. [DOI] [PubMed] [Google Scholar]
- 56.Tonstad S, Farsang C, Klaene G, Lewis K, Manolis A, Perruchoud AP, Silagy C, van Spiegel PI, Astbury C, Hider A, Sweet R. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicentre, randomised study. Eur Heart J. 2003;24:946–955. doi: 10.1016/s0195-668x(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 57.Tashkin D, Kanner R, Bailey W, Buist S, Anderson P, Nides M, Gonzales D, Dozier G, Patel MK, Jamerson B. Smoking cessation in patients with chronic obstructive pulmonary disease: a double-blind, placebo-controlled, randomised trial. Lancet. 2001;357:1571–1575. doi: 10.1016/s0140-6736(00)04724-3. [DOI] [PubMed] [Google Scholar]
- 58.Moore TJ, Furberg CD, Glenmullen J, Maltsberger JT, Singh S. Suicidal behavior and depression in smoking cessation treatments. PLoS ONE. 2011;6:e27016. doi: 10.1371/journal.pone.0027016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wightman DS, Foster VJ, Krishen A, Richard NE, Modell JG. Meta-analysis of suicidality in placebo-controlled clinical trials of adults taking bupropion. Prim Care Companion J Clin Psychiatry. 2010;12 doi: 10.4088/PCC.09m00894blu. pii: PCC.09m00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jimenez-Ruiz C, Berlin I, Hering T. Varenicline: a novel pharmacotherapy for smoking cessation. Drugs. 2009;69:1319–1338. doi: 10.2165/00003495-200969100-00003. [DOI] [PubMed] [Google Scholar]
- 61.Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, Williams KE. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 62.Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- 63.Tonstad S, Rollema H. Varenicline in smoking cessation. Expert Rev Respir Med. 2010;4:291–299. doi: 10.1586/ers.10.27. [DOI] [PubMed] [Google Scholar]
- 64.Niaura R, Hays JT, Jorenby DE, Leone FT, Pappas JE, Reeves KR, Williams KE, Billing CB., Jr The efficacy and safety of varenicline for smoking cessation using a flexible dosing strategy in adult smokers: a randomized controlled trial. Curr Med Res Opin. 2008;24:1931–1941. doi: 10.1185/03007990802177523. [DOI] [PubMed] [Google Scholar]
- 65.Rennard S, Hughes J, Cinciripini PM, Kralikova E, Raupach T, Arteaga C, Aubin LB, Russ C. A randomized placebo-controlled trial of varenicline for smoking cessation allowing flexible quit dates. Nicotine Tob Res. 2011;14:343–350. doi: 10.1093/ntr/ntr220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nides M, Glover ED, Reus VI, Christen AG, Make BJ, Billing CB, Jr, Williams KE. Varenicline versus bupropion SR or placebo for smoking cessation: a pooled analysis. Am J Health Behav. 2008;32:664–675. doi: 10.5555/ajhb.2008.32.6.664. [DOI] [PubMed] [Google Scholar]
- 67.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4) doi: 10.1002/14651858.CD006103.pub2. CD006103. [DOI] [PubMed] [Google Scholar]
- 68.Aubin HJ, Bobak A, Britton JR, Oncken C, Billing CB, Jr, Gong J, Williams KE, Reeves KR. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax. 2008;63:717–724. doi: 10.1136/thx.2007.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stapleton J, Watson L, Spirling LI, Smith R, Milbrandt A, Ratcliffe M, Sutherland G. Varenicline in the routine treatment of tobacco dependence: a pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction. 2007;103:146–154. doi: 10.1111/j.1360-0443.2007.02083.x. [DOI] [PubMed] [Google Scholar]
- 70.Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation. JAMA. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- 71.Ebbert JO, Burke MV, Hays JT, Hurt RD. Combination treatment with varenicline and nicotine replacement therapy. Nicotine Tob Res. 2009;11:572–576. doi: 10.1093/ntr/ntp042. [DOI] [PubMed] [Google Scholar]
- 72.Ebbert JO, Croghan IT, Sood A, Schroeder DR, Hays JT, Hurt RD. Varenicline and bupropion sustained-release combination therapy for smoking cessation. Nicotine Tob Res. 2009;11:234–239. doi: 10.1093/ntr/ntn031. [DOI] [PubMed] [Google Scholar]
- 73.Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl) 2012;223:299–306. doi: 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O'Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology (Berl) 2011;215:655–663. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faessel HM, Smith BJ, Gibbs MA, Gobey JS, Clark DJ, Burstein AH. Single-dose pharmacokinetics of varenicline, a selective nicotinic receptor partial agonist, in healthy smokers and nonsmokers. J Clin Pharmacol. 2006;46:991–998. doi: 10.1177/0091270006290669. [DOI] [PubMed] [Google Scholar]
- 76.Cahill K, Stead L, Lancaster T. A preliminary benefit-risk assessment of varenicline in smoking cessation. Drug Saf. 2009;32:119–135. doi: 10.2165/00002018-200932020-00005. [DOI] [PubMed] [Google Scholar]
- 77.Ebbert JO, Wyatt KD, Hays JT, Klee EW, Hurt RD. Varenicline for smoking cessation: efficacy, safety, and treatment recommendations. Patient Prefer Adherence. 2010;4:355–362. doi: 10.2147/ppa.s10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leung LK, Patafio FM, Rosser WW. Gastrointestinal adverse effects of varenicline at maintenance dose: a meta-analysis. BMC Clin Pharmacol. 2011;11:15. doi: 10.1186/1472-6904-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Potts LA, Garwood CL. Varenicline: the newest agent for smoking cessation. Am J Health Syst Pharm. 2007;64:1381–1384. doi: 10.2146/ajhp060428. [DOI] [PubMed] [Google Scholar]
- 80.Moore TJ, Glenmullen J, Furberg CD. Prescription drugs associated with reports of violence towards others. PLoS ONE. 2010;5:e15337. doi: 10.1371/journal.pone.0015337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuehn BM. New reports examine psychiatric risks of varenicline for smoking cessation. JAMA. 2012;307:129–130. doi: 10.1001/jama.2011.1924. [DOI] [PubMed] [Google Scholar]
- 82.Harrison-Woolrych M, Ashton J. Psychiatric adverse events associated with varenicline: an intensive postmarketing prospective cohort study in New Zealand. Drug Saf. 2011;34:763–772. doi: 10.2165/11594450-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 83.Tonstad S, Davies S, Flammer M, Russ C, Hughes J. Psychiatric adverse events in randomized, double-blind, placebo-controlled clinical trials of varenicline: a pooled analysis. Drug Saf. 2010;33:289–301. doi: 10.2165/11319180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 84.Garza D, Murphy M, Tseng LJ, Riordan HJ, Chatterjee A. A double-blind randomized placebo-controlled pilot study of neuropsychiatric adverse events in abstinent smokers treated with varenicline or placebo. Biol Psychiatry. 2011;69:1075–1082. doi: 10.1016/j.biopsych.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 85.Gunnell D, Irvine D, Wise L, Davies C, Martin RM. Varenicline and suicidal behaviour: a cohort study based on data from the General Practice Research Database. BMJ. 2009;339:b3805. doi: 10.1136/bmj.b3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.FDA. 2011. FDA drug safety communication: safety review update of Chantix (varenicline) and risk of neuropsychiatric adverse events Available at http://www.fda.gov/Drugs/DrugSafety/ucm276737.htm(last accessed 29 March 2013)
- 87.Covey LS, Berlin I, Hu MC, Hakes JK. Smoking and suicidal behaviours in a sample of US adults with low mood: a retrospective analysis of longitudinal data. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-000876. pii: e000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.FDA. The smoking cessation aids varenicline (marketed as Chantix) and bupropion (marketed as Zyban and generics): suicidal ideation and behavior. 2009. FDA Drug Safety Newsletter; 2; updated 2009. Available at http://www.fda.gov/Drugs/DrugSafety/DrugSafetyNewsletter/ucm110235.htm(last accessed 1 October 2010)
- 89.Singh S, Loke YK, Spangler JG, Furberg CD. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359–1366. doi: 10.1503/cmaj.110218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856. doi: 10.1136/bmj.e2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.George TP, O'Malley SS. Current pharmacological treatments for nicotine dependence. Trends Pharmacol Sci. 2004;25:42–48. doi: 10.1016/j.tips.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 92.Aveyard P, Johnson C, Fillingham S, Parsons A, Murphy M. Nortriptyline plus nicotine replacement versus placebo plus nicotine replacement for smoking cessation: pragmatic randomised controlled trial. BMJ. 2008;336:1223–1227. doi: 10.1136/bmj.39545.852616.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gourlay SG, Stead LF, Benowitz NL. Clonidine for smoking cessation. Cochrane Database Syst Rev. 2008;(3) doi: 10.1002/14651858.CD000058.pub2. CD000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lising-Enriquez K, George TP. Treatment of comorbid tobacco use in people with serious mental illness. J Psychiatry Neurosci. 2009;34:E1–2. [PMC free article] [PubMed] [Google Scholar]
- 95.Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 96.Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14:106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McCreadie RG. Use of drugs, alcohol and tobacco by people with schizophrenia: case-control study. Br J Psychiatry. 2002;181:321–325. doi: 10.1192/bjp.181.4.321. [DOI] [PubMed] [Google Scholar]
- 98.Williams JM, Ziedonis D. Addressing tobacco among individuals with a mental illness or an addiction. Addict Behav. 2004;29:1067–1083. doi: 10.1016/j.addbeh.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 99.Aubin HJ. Management of emergent psychiatric symptoms during smoking cessation. Curr Med Res Opin. 2009;25:519–525. doi: 10.1185/03007990802707600. [DOI] [PubMed] [Google Scholar]
- 100.Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150:1115–1121. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 101.Siru R, Hulse GK, Tait RJ. Assessing motivation to quit smoking in people with mental illness: a review. Addiction. 2009;104:719–733. doi: 10.1111/j.1360-0443.2009.02545.x. [DOI] [PubMed] [Google Scholar]
- 102.Fagerstrom K, Aubin HJ. Management of smoking cessation in patients with psychiatric disorders. Curr Med Res Opin. 2009;25:511–518. doi: 10.1185/03007990802707568. [DOI] [PubMed] [Google Scholar]
- 103.George TP, Ziedonis DM, Feingold A, Pepper WT, Satterburg CA, Winkel J, Rounsaville BJ, Kosten TR. Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. Am J Psychiatry. 2000;157:1835–1842. doi: 10.1176/appi.ajp.157.11.1835. [DOI] [PubMed] [Google Scholar]
- 104.Tsoi DT, Porwal M, Webster AC. Efficacy and safety of bupropion for smoking cessation and reduction in schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2010;196:346–353. doi: 10.1192/bjp.bp.109.066019. [DOI] [PubMed] [Google Scholar]
- 105.Williams JM, Anthenelli RM, Morris CD, Treadow J, Thompson JR, Yunis C, George TP. A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73:654–660. doi: 10.4088/JCP.11m07522. [DOI] [PubMed] [Google Scholar]
- 106.Yousefi MK, Folsom TD, Fatemi SH. A review of varenicline's efficacy and tolerability in smoking cessation studies in subjects with schizophrenia. J Addict Res Ther. 2011;S4(1) doi: 10.4172/2155-6105.S4-001. pii: 3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weiner E, Buchholz A, Coffay A, Liu F, McMahon RP, Buchanan RW, Kelly DL. Varenicline for smoking cessation in people with schizophrenia: a double blind randomized pilot study. Schizophr Res. 2011;129:94–95. doi: 10.1016/j.schres.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shim JC, Jung DU, Jung SS, Seo YS, Cho DM, Lee JH, Lee SW, Kong BG, Kang JW, Oh MK, Kim SD, McMahon RP, Kelly DL. Adjunctive varenicline treatment with antipsychotic medications for cognitive impairments in people with schizophrenia: a randomized double-blind placebo-controlled trial. Neuropsychopharmacology. 2011;37:660–668. doi: 10.1038/npp.2011.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu ME, Tsai SJ, Jeang SY, Peng SL, Wu SL, Chen MC, Tsai YL, Yang ST. Varenicline prevents affective and cognitive exacerbation during smoking abstinence in male patients with schizophrenia. Psychiatry Res. 2011;190:79–84. doi: 10.1016/j.psychres.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 110.McClure JB, Swan GE, Catz SL, Jack L, Javitz H, McAfee T, Deprey M, Richards J, Zbikowski SM. Smoking outcome by psychiatric history after behavioral and varenicline treatment. J Subst Abuse Treat. 2010;38:394–402. doi: 10.1016/j.jsat.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kinnunen T, Doherty K, Militello FS, Garvey AJ. Depression and smoking cessation: characteristics of depressed smokers and effects of nicotine replacement. J Consult Clin Psychol. 1996;64:791–798. doi: 10.1037//0022-006x.64.4.791. [DOI] [PubMed] [Google Scholar]
- 112.Aubin HJ, Berlin I, Reynaud M. Early life origins of adult disease and maternal smoking during pregnancy. Am J Public Health. 2012;102:e12. doi: 10.2105/AJPH.2012.300650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oncken CA, Kranzler HR. What do we know about the role of pharmacotherapy for smoking cessation before or during pregnancy? Nicotine Tob Res. 2009;11:1265–1273. doi: 10.1093/ntr/ntp136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grange G, Vayssiere C, Borgne A, Ouazana A, L'Huillier JP, Valensi P, Peiffer G, Aubin HJ, Renon D, Thomas D, Lebargy F. Description of tobacco addiction in pregnant women. Eur J Obstet Gynecol Reprod Biol. 2005;120:146–151. doi: 10.1016/j.ejogrb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 115.Coleman T, Chamberlain C, Cooper S, Leonardi-Bee J. Efficacy and safety of nicotine replacement therapy for smoking cessation in pregnancy: systematic review and meta-analysis. Addiction. 2011;106:52–61. doi: 10.1111/j.1360-0443.2010.03179.x. [DOI] [PubMed] [Google Scholar]
- 116.Coleman T, Cooper S, Thornton JG, Grainge MJ, Watts K, Britton J, Lewis S. A randomized trial of nicotine-replacement therapy patches in pregnancy. N Engl J Med. 2012;366:808–818. doi: 10.1056/NEJMoa1109582. [DOI] [PubMed] [Google Scholar]
- 117.Pirie K, Peto R, Reeves GK, Green J, Beral V. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2012;381:133–141. doi: 10.1016/S0140-6736(12)61720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Herman AI, Sofuoglu M. Comparison of available treatments for tobacco addiction. Curr Psychiatry Rep. 2010;12:433–440. doi: 10.1007/s11920-010-0134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bronisch T, Hofler M, Lieb R. Smoking predicts suicidality: findings from a prospective community study. J Affect Disord. 2008;108:135–145. doi: 10.1016/j.jad.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 120.Kim Y, Myung SK, Jeon YJ, Lee EH, Park CH, Seo HG, Huh BY. Effectiveness of pharmacologic therapy for smoking cessation in adolescent smokers: meta-analysis of randomized controlled trials. Am J Health Syst Pharm. 2011;68:219–226. doi: 10.2146/ajhp100296. [DOI] [PubMed] [Google Scholar]
- 121.Bailey SR, Crew EE, Riske EC, Ammerman S, Robinson TN, Killen JD. Efficacy and tolerability of pharmacotherapies to aid smoking cessation in adolescents. Paediatr Drugs. 2012;14:91–108. doi: 10.2165/11594370-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Etter JF. Cytisine for smoking cessation. Arch Intern Med. 2006;166:1553–1559. doi: 10.1001/archinte.166.15.1553. [DOI] [PubMed] [Google Scholar]
- 123.Tutka P, Zatonski W. Cytisine for the treatment of nicotine addiction: from a molecule to therapeutic effcacy. Pharmacol Rep. 2005;58:777–798. [PubMed] [Google Scholar]
- 124.West R, Zatonski W, Cedzynska M, Lewandowska D, Pazik J, Aveyard P, Stapleton J. Placebo-controlled trial of cytisine for smoking cessation. N Engl J Med. 2011;365:1193–1200. doi: 10.1056/NEJMoa1102035. [DOI] [PubMed] [Google Scholar]
- 125.Walker N, Howe C, Bullen C, McRobbie H, Glover M, Parag V, Williman J, Veale R, Nosa V, Barnes J. Study protocol for a non-inferiority trial of cytisine versus nicotine replacement therapy in people motivated to stop smoking. BMC Public Health. 2011;11:880. doi: 10.1186/1471-2458-11-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tonstad S, Holme I, Tonnesen P. Dianicline, a novel {alpha}4{beta}2 nicotinic acetylcholine receptor partial agonist, for smoking cessation: a randomized placebo-controlled clinical trial. Nicotine Tob Res. 2010;13:1–6. doi: 10.1093/ntr/ntq191. [DOI] [PubMed] [Google Scholar]
- 127.George TP, Weinberger AH. Monoamine oxidase inhibition for tobacco pharmacotherapy. Clin Pharmacol Ther. 2008;83:619–621. doi: 10.1038/sj.clpt.6100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Berlin I, Anthenelli RM. Monoamine oxidase and tobacco smoking. Int J Neuropsychopharmacol. 2001;4:33–42. doi: 10.1017/S1461145701002188. [DOI] [PubMed] [Google Scholar]
- 129.Berlin I, Said S, Spreux-Varoquaux O, Olivares R, Launay JM, Puech AJ. Monoamine oxidase A and B activities in heavy smokers. Biol Psychiatry. 1995;38:756–761. doi: 10.1016/0006-3223(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 130.Berlin I, Said S, Spreux-Varoquaux O, Launay JM, Olivares R, Millet V, Lecrubier Y, Puech AJ. A reversible monoamine oxidase A inhibitor (moclobemide) facilitates smoking cessation and abstinence in heavy, dependent smokers. Clin Pharmacol Ther. 1995;58:444–452. doi: 10.1016/0009-9236(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 131.Berlin I, Aubin HJ, Pedarriosse AM, Rames A, Lancrenon S, Lagrue G. Lazabemide, a selective, reversible monoamine oxidase B inhibitor, as an aid to smoking cessation. Addiction. 2002;97:1347–1354. doi: 10.1046/j.1360-0443.2002.00258.x. [DOI] [PubMed] [Google Scholar]
- 132.Weinberger AH, Reutenauer EL, Jatlow PI, O'Malley SS, Potenza MN, George TP. A double-blind, placebo-controlled, randomized clinical trial of oral selegiline hydrochloride for smoking cessation in nicotine-dependent cigarette smokers. Drug Alcohol Depend. 2010;107:188–195. doi: 10.1016/j.drugalcdep.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Killen JD, Fortmann SP, Murphy GM, Jr, Hayward C, Fong D, Lowenthal K, Bryson SW, Killen DT, Schatzberg AF. Failure to improve cigarette smoking abstinence with transdermal selegiline + cognitive behavior therapy. Addiction. 2010;105:1660–1668. doi: 10.1111/j.1360-0443.2010.03020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Berlin I, Hunneyball IM, Greiling D, Jones SP, Fuder H, Stahl HD. A selective reversible monoamine oxidase B inhibitor in smoking cessation: effects on its own and in association with transdermal nicotine patch. Psychopharmacology (Berl) 2012;223:89–98. doi: 10.1007/s00213-012-2692-2. [DOI] [PubMed] [Google Scholar]
- 135.Raupach T, van Schayck CP. Pharmacotherapy for smoking cessation: current advances and research topics. CNS Drugs. 2011;25:371–382. doi: 10.2165/11590620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 136.Cerny EH, Cerny T. Vaccines against nicotine. Hum Vaccin. 2009;5:200–205. doi: 10.4161/hv.5.4.7310. [DOI] [PubMed] [Google Scholar]
- 137.2011. Nabi biopharmaceuticals announces results of second NicVAX(R) phase III clinical trial. Available at http://phx.corporate-ir.net/phoenix.zhtml?c=100445&p=irol-newsArticle&ID=1626882&highlight=(last accessed 27 June 2012)
- 138.Jackson A, Nesic J, Groombridge C, Clowry O, Rusted J, Duka T. Differential involvement of glutamatergic mechanisms in the cognitive and subjective effects of smoking. Neuropsychopharmacology. 2009;34:257–265. doi: 10.1038/npp.2008.50. [DOI] [PubMed] [Google Scholar]
- 139.Shinn AK, Greenfield SF. Topiramate in the treatment of substance-related disorders: a critical review of the literature. J Clin Psychiatry. 2010;71:634–648. doi: 10.4088/JCP.08r04062gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Anthenelli RM, Blom TJ, McElroy SL, Keck PE., Jr Preliminary evidence for gender-specific effects of topiramate as a potential aid to smoking cessation. Addiction. 2008;103:687–694. doi: 10.1111/j.1360-0443.2008.02148.x. [DOI] [PubMed] [Google Scholar]
- 141.Baltieri DA, Daro FR, Ribeiro PL, Andrade AG. Effects of topiramate or naltrexone on tobacco use among male alcohol-dependent outpatients. Drug Alcohol Depend. 2009;105:33–41. doi: 10.1016/j.drugalcdep.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 142.Johnson BA, Ait-Daoud N, Akhtar FZ, Javors MA. Use of oral topiramate to promote smoking abstinence among alcohol-dependent smokers. Arch Intern Med. 2005;165:1600–1605. doi: 10.1001/archinte.165.14.1600. [DOI] [PubMed] [Google Scholar]
- 143.Franklin TR, Harper D, Kampman K, Kildea-McCrea S, Jens W, Lynch KG, O'Brien CP, Childress AR. The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study. Drug Alcohol Depend. 2009;103:30–36. doi: 10.1016/j.drugalcdep.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.White WD, Crockford D, Patten S, El-Guebaly N. A randomized, open-label pilot comparison of gabapentin and bupropion SR for smoking cessation. Nicotine Tob Res. 2005;7:809–813. doi: 10.1080/14622200500259887. [DOI] [PubMed] [Google Scholar]
- 145.Sood A, Ebbert JO, Wyatt KD, Croghan IT, Schroeder DR, Sood R, Hays JT. Gabapentin for smoking cessation. Nicotine Tob Res. 2010;12:300–304. doi: 10.1093/ntr/ntp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sood A, Ebbert JO, Schroeder DR, Croghan IT, Sood R, Vander Weg MW, Wong GY, Hays JT. Gabapentin for smoking cessation: a preliminary investigation of efficacy. Nicotine Tob Res. 2007;9:291–298. doi: 10.1080/14622200601080307. [DOI] [PubMed] [Google Scholar]
- 147.Evins AE, Pachas G, Mischoulon D, Urbanoski K, Carlini S, Sousa J, Bentley K, Rigotti NA, Nino-Gomez J, Loebl T, Janes AC, Kaufman MJ, Fava M. A double-blind, placebo-controlled trial of the NMDA glycine site antagonist, GW468816, for prevention of relapse to smoking in females. J Clin Psychopharmacol. 2011;31:597–602. doi: 10.1097/JCP.0b013e31822bb390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schmaal L, Berk L, Hulstijn KP, Cousijn J, Wiers RW, van den Brink W. Efficacy of N-acetylcysteine in the treatment of nicotine dependence: a double-blind placebo-controlled pilot study. Eur Addict Res. 2011;17:211–216. doi: 10.1159/000327682. [DOI] [PubMed] [Google Scholar]
- 149.Ballon JS, Feifel D. A systematic review of modafinil: potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–566. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- 150.Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- 151.Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu A, Niaura R, Epstein L. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alcohol Depend. 2002;67:219–223. doi: 10.1016/s0376-8716(02)00067-4. [DOI] [PubMed] [Google Scholar]
- 152.Schnoll RA, Wileyto EP, Pinto A, Leone F, Gariti P, Siegel S, Perkins KA, Dackis C, Heitjan DF, Berrettini W, Lerman C. A placebo-controlled trial of modafinil for nicotine dependence. Drug Alcohol Depend. 2008;98:86–93. doi: 10.1016/j.drugalcdep.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Le Foll B, Sokoloff P, Stark H, Goldberg SR. Dopamine D3 receptor ligands block nicotine-induced conditioned place preferences through a mechanism that does not involve discriminative-stimulus or antidepressant-like effects. Neuropsychopharmacology. 2005;30:720–730. doi: 10.1038/sj.npp.1300622. [DOI] [PubMed] [Google Scholar]
- 154.McNeill A, Raw M, Whybrow J, Bailey P. A national strategy for smoking cessation treatment in England. Addiction. 2005;100(Suppl. 2):1–11. doi: 10.1111/j.1360-0443.2005.01022.x. [DOI] [PubMed] [Google Scholar]
- 155.Agboola S, McNeill A, Coleman T, Leonardi Bee J. A systematic review of the effectiveness of smoking relapse prevention interventions for abstinent smokers. Addiction. 2010;105:1362–1380. doi: 10.1111/j.1360-0443.2010.02996.x. [DOI] [PubMed] [Google Scholar]
- 156.Hajek P, Stead LF, West R, Jarvis M, Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev. 2009;(1) doi: 10.1002/14651858.CD003999.pub3. CD003999. [DOI] [PubMed] [Google Scholar]
- 157.Borland R, Partos TR, Cummings KM. Systematic biases in cross-sectional community studies may underestimate the effectiveness of stop-smoking medications. Nicotine Tob Res. 2012;14:1483–1487. doi: 10.1093/ntr/nts002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pollock JD, Koustova E, Hoffman A, Shurtleff D, Volkow ND. Treatments for nicotine addiction should be a top priority. Lancet. 2009;374:513–514. doi: 10.1016/S0140-6736(09)60352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


