Abstract
While the worldwide prevalence of cocaine use remains significant, medications, or small molecule approaches, to treat drug addictions have met with limited success. Anti-addiction vaccines, on the other hand, have demonstrated great potential for treating drug abuse using a distinctly different mechanism of eliciting an antibody response that blocks the pharmacological effects of drugs. We provide a review of vaccine-based approaches to treating stimulant addictions; specifically and cocaine addictions. This selective review article focuses on the one cocaine vaccine that has been into clinical trials and presents new data related to pre-clinical development of a methamphetamine (MA) vaccine. We also review the mechanism of action for vaccine induced antibodies to abused drugs, which involves kinetic slowing of brain entry as well as simple blocking properties. We present pre-clinical innovations for MA vaccines including hapten design, linkage to carrier proteins and new adjuvants beyond alum. We provide some new information on hapten structures and linkers and variations in protein carriers. We consider a carrier, outer membrance polysaccharide coat protein (OMPC), that provides some self-adjuvant through lipopolysaccharide components and provide new results with a monophosopholipid adjuvant for the more standard carrier proteins with cocaine and MA. The review then covers the clinical trials with the cocaine vaccine TA-CD. The clinical prospects for advances in this field over the next few years include a multi-site cocaine vaccine clinical trial to be reported in 2013 and phase 1 clinical trials of a MA vaccine in 2014.
Keywords: clinical trial, cocaine, stimulants, vaccines
Introduction
Worldwide, the United Nations Office on Drugs and Crime (UNDOC) estimates between 0.3% to 0.4% of the adult population, between 15–19 million people, to have used cocaine at least once in the previous year. Still, the highest prevalence of cocaine use remains in North America, affecting approximately 2% of adults aged 15–64 years [1]. To date, while there are no FDA approved pharmacotherapies for treating cocaine dependence, of those that are in use, there are multiple limitations. Of these limitations, the most significantly problematic include cost, availability, medication compliance, dependence, diversion of some to illicit use and relapse to addiction after discontinuing their use. Here, immunotherapies using either passive monoclonal antibodies or active vaccines have distinctly different mechanisms and therapeutic utility from small molecule approaches to treatment and have shown distinct promise with demonstrated potential to help the patient achieve and sustain abstinence and have few of the limitations associated with anti-addiction medications. Immunotherapy for addictions also has been steadily growing as an area for new ideas and technologies, although human studies of these therapies have been limited to active vaccines against nicotine and cocaine, and no monoclonals for passive immunization have been tested in humans. Therefore this review will focus on vaccines rather than monoclonals, and focus on the human vaccine issues for cocaine, since a cocaine vaccine has progressed to late phase 2 clinical trials in humans. This review also will cover the pre-clinical vaccine design issues for methamphetamine (MA), because many technical innovations are being deployed for MA vaccines that will be highly relevant to second generation cocaine vaccines. Monoclonal antibodies to MA are also rapidly approaching testing in humans as they have been developed over the past 15 years for passive immunization in humans where the delays of several weeks that are needed to raise antibody responses to the vaccines are unrealistic, such as in reversal of MA overdoses in the emergency setting. However, the translation of monoclonals into practical pharmacotherapies for humans has been hampered by relatively high production costs and by requiring parenteral delivery that is impractical for substance abuse care in all but emergency settings [2].
Antibody actions in blocking abused drugs
Drugs of abuse lead to reward and reinforcement by rapidly entering the brain and attaching to neuronal receptors on very specific brain pathways and antibodies prevent those drugs from accessing these brain pathways. Specifically both cocaine and MA bind to the three monoamine transporters (dopamine, norepinephrine and serotonin) preventing these transporters from removing these neurotransmitters from the synapse. This removal is the main mechanism for inactivation of neurotransmission from these monoamine releasing neurons. The action of MA furthermore reverses the direction of the transporters such that these monoamines are actively pumped out from the cytoplasm of the neuron into the synapse. The specific brain pathway involved with reward is dopaminergic and connects the ventral tegmental area to the nucleus accumbens. The small molecular size and lipophilicity of these abused drugs allows them to traverse the blood–brain barrier rapidly, and to diffuse quickly to their transporter binding sites and markedly prolong dopamine activity at the nucleus accumbens [3].
Antibodies capture the abused drug before it can cross the blood–brain barrier, thereby preventing activation of the brain's reinforcement pathways, but also have an additional pharmacokinetic effect of buffering against rapid transit of abused drugs into the brain [2,4]. Thus the rapid accumulation from the bolus dose is prevented. A critical human observation to illustrate this kinetic point is that smoked cocaine administration sparked an epidemic of abuse when smoked crack cocaine replaced marketing of cocaine powder for intranasal administration due to its very rapid delivery of cocaine to the brain within seconds, which is highly reinforcing [5]. Moreover, oral cocaine requires considerably larger doses to be as reinforcing as the intravenous route of administration [6]. This need for an increased dose in part reflects that oral cocaine reaches the brain relatively slowly, and although high blood and brain concentrations can be attained, much higher concentrations from this route are needed to produce euphoria or other reinforcing effects [7–9]. Antibodies in the circulation have a similar kinetic way of slowing drug entry into the brain and reducing the reinforcing effects of the drug.
The fundamental concept in creating anti-drug antibodies is to create a new macromolecular compound, which the body will recognize as a foreign antigen that requires an immune response. Drugs of abuse by themselves are far too small to elicit such immune responses from the antibody generating B and T white blood cells, so their presentation to the immune processes must be changed through a conjugate vaccine. A conjugate vaccine chemically links the abused drug to a large immunogenic protein such as inactivated tetanus or cholera toxin [10]. Both of these proteins are widely used vaccines, and the concept of linking them to small molecules called haptens in order to produce an antibody response was pioneered in the 1970s as a treatment for digitalis toxicity and as an anti-morphine vaccine [11–13].
Antidrug vaccines are active immunizations, where administration of the vaccine triggers an immunological response against the agent in the human subject resulting in the production of an antigen-specific, immunoglobulin G (IgG)-mediated antibody response as shown in Figure 1 upper part [14–17]. Immunological memory is created, whereby re-exposure to the agent (i.e. through a booster injection) results in amplification of the initial response, with high level production of the anti-drug IgG antibodies. The effectiveness of the vaccine is then measured by its ability to create antibodies with specificity and high binding affinity for the drug of abuse and the robustness of the antibody response, i.e. the concentration of antibody produced as shown in the lower part of Figure 1 [18]. An important complication in clinical studies, which are described below, is that some cocaine abusers spontaneously develop low affinity anti-cocaine antibodies before vaccination, and these low affinity antibodies are a marker for very poor antibody responses to immunization with these vaccines.
Figure 1.
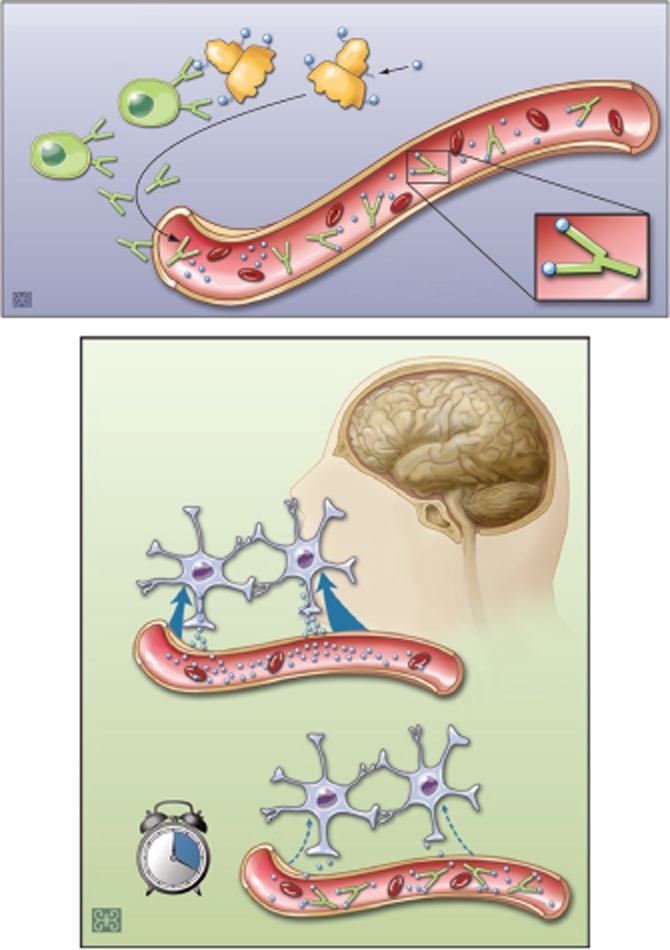
Mechanism of action of a vaccine against cocaine addiction. In the absence of the vaccine, cocaine is readily absorbed at the blood–brain barrier and thereby enters the brain. As shown in the top part of this figure, the vaccine interacts with dendritic blood cells to produce antibodies from B-cells that are secreted into the blood stream. In the brain, the drug causes reinforcement of pleasurable effects, or the ‘high’ associated with cocaine. If a vaccine is administered, it stimulates the production of antibodies against cocaine. Subsequently, if cocaine is taken, the antibodies bind to the drug and sequester it in the blood circulation. This antibody–drug binding prevents the cocaine from rapidly leaving the blood vessels and entering the brain, thereby reducing the drug's euphoric effects
Recent pre-clinical advances in anti-addiction vaccines
We have learned many important properties of this immunological approach to treatment from in vivo studies of monoclonals. For example, passive immunization of rats with a high affinity (Kd = 11 nm) monoclonal MA antibody reduced MA self-administration [19]. With higher MA doses (lower antibody : drug ratio), self-administration paradoxically increased compared with no antibody being present, indicating compensation and suggesting that providing an adequate antibody dose is critical for optimal efficacy. MA antibodies also protect against the increases in blood pressure, heart rate, and locomotor activation (horizontal activity) induced by a high dose of MA [20,21]. Finally, a recent variation on monoclonal usage has been to introduce a viral vector with the DNA for the monoclonal into animals (and for potential use in humans) in order to produce a humanized monoclonal or Fab fragment for a more extended period of time than a single infusion of a monoclonal would allow [22]. However, this gene therapy faces practical hurdles for implementation in essentially healthy humans, as most cocaine and MA abusers do not have the immediately life-threatening illnesses for which the FDA has permitted gene therapy [23].
Vaccines do not face these same practical issues of cost, parenteral administration or use of viral vectors in application to humans, but vaccines have several other challenges involving the carrier protein, the hapten and its linkage to the carrier protein and adjuvant selection, which can include using self-adjuvanting constructs as the carrier. Because both cocaine and MA have low molecular weights, they are too small to elicit an immune response on their own. However, they can become immunogenic by conjugation to an appropriate carrier protein. Periodic booster doses with the complete immunogen, not simply using MA or cocaine alone, are needed to maintain satisfactory antibody titres [14,24]. The conjugation or linking of the drug to the carrier protein has generally been accomplished using 4–6 atom spacers such as succinic acid [25–28]. No structural rule has emerged to predict which linkers will be most effective, nor have systematic comparisons of linkers, linker strategies or haptenation ratios (molar ratio of drug : carrier protein) been carried out. Future studies may need to address these questions systematically for optimal vaccine design, but another practical issue has been the difficulty in determining the actual antibody affinity as well as concentration. The usual ELISA means for determining affinity and concentration can be inaccurate, and equilibrium dialysis has been used to address some of these limitations [29].
Our understanding of the binding affinity and kinetics between cocaine or MA and the antibodies produced has been explored only in buffer systems which, although physiologically relevant in pH and salt concentrations, lack many serum components present in the blood and may not represent the actual binding behaviour inside the body. Since the antibody response to cocaine and to the carrier protein cholera toxin subunit B (CTB) did not closely correlate in many subjects [18], comparison of the binding properties to these different targets may provide insight into the immune response to this and other conjugate vaccines. Recently we have found evidence for ∼15 fold decrease in an anti-cocaine monoclonal antibody's effective affinity for binding to cocaine in 20–50% of human serum compared with that in saline buffer. Furthermore, we also found cocaine has a moderate affinity (KD of about 2 μm) to 20% human serum although it has very little interaction with bovine serum albumin and non-specific IgG. In developing vaccines for cocaine both in preclinical and clinical phases only ELISA and equilibrium dialysis have been explored, resulting in some potential limitations regarding the exact quantitative and qualitative requirements for anti-cocaine antibodies.
The study of binding kinetics is essential to understand fully the molecular interactions between anti-drug antibodies and the drug, which drive the clinical benefit of drug–conjugate vaccines [29]. A simplified in vitro model does not reflect the complicated interactions in live rodents and humans based on the adsorption, distribution, metabolism and elimination properties of these drugs, particularly with cocaine which is metabolized by enzymes such as butyrylcholinesterase, pseudocholinesterase and liver carboxylesterases [30,31]. In earlier studies pharmacological effects were noticed within 2 min of peak plasma cocaine concentrations in the range of 150–500 nm in patients who smoked 10–40 mg of cocaine base [32]. The goal of cocaine immunotherapy is to block cocaine entry to the brain at peak plasma concentration, and based on simulations with these parameters a successful second generation vaccine needs to have an affinity that drops from our current anti-cocaine vaccine affinity of above 100 to the 10 nm range, because this high affinity brings the concentration of free cocaine closer to zero. However, clinical efficacy does not appear to need such a high affinity reflecting the need to block as little as 40% of the cocaine to prevent euphoria, probably due to slowed entry of the drug into the brain as previously mentioned. Nevertheless, we have tried to identify haptens and linkages that might have optimal structural stability and thereby produce more limited variation and a higher antibody quantity and affinity in its polyclonal response. As an example, in constructing a cocaine hapten for linkage to our carrier protein we have found recently that an aliphatic linkage to nor-cocaine has resulted in slow hydrolysis of the hapten, therefore making that hapten construct not desirable for cocaine vaccines.
We have tested a variety of carrier proteins, including bovine serum albumin (BSA), ovalbumin (OVA), keyhole limpet hemocyanin (KLH), cholera toxin B subunit (CTB), tetanus toxoid (TT) and the N mengitidis outer membrane protein complex (OMPC) [14,24–28,33]. The OMPC carrier is self adjuvanting to some extent since it stimulates TLR2 and TLR4 and cytokines (TNFα, IL10, INFγ). However, OMPC works much better when combined with alum, which releases IL-1 [34]. Succinyl MA was conjugated to BSA, OVA and KLH. To test these vaccine constructs, a primary vaccination and a booster vaccination at week 3 were done as a typical experimental design. In these studies with BALB/c mice, antibody levels were measured using ELISA and the patterns of response assessed. In general, significant amounts of antibody were detectable by 4 weeks, peaking at 6–8 weeks and declining after 8–12 weeks. Carriers such as KLH, TT, and OMPC stimulate substantially higher antibody levels than do others we have tested, and the antibodies persist longer with these carriers as well, but particularly OMPC which had anti-MA antibodies at good levels up to 26 weeks after the initial immunization. We have found similar results using a different mouse strain (C57BL/6), and showed a rough equivalence for TT and KLH conjugates with MA.
The critical importance of adjuvants beyond alum is illustrated by succinyl MA conjugated to OMPC which elicited much greater early anti-MA IgG antibody levels than the BSA or OVA, but was also superior to both of these carriers with levels 3–10-fold higher at 26 weeks. Since OMPC contains additional adjuvant properties based on lipopolysaccharide content [35], we therefore explored the adjuvant monophospho-lipid (MPL), which has some characteristics similar to OMPC's adjuvant capability. When combined with the tetanus toxoid conjugate vaccine, the MPL gave initial anti-IgG levels similar to that found with OMPC.
We conducted a similar comparison of succinylnorcocaine conjugated to BSA, OVA, OMPC and CTB, since the human cocaine vaccine uses CTB as a carrier. The differences from CTB were striking. At week 4, OMPC had 5-fold higher levels than CTB and BSA, and 3-fold higher than OVA. At week 16, OMPC continued to have levels 2–3-fold higher than OVA and BSA, and 5-fold higher than CTB. While it is difficult to compare anti-cocaine to anti-MA IgG levels directly, the relative strength of response is clear. The self-adjuvanting OMPC was superior to the other carriers for cocaine, particularly the carrier used in the current human vaccine. Thus, other protein carriers are clearly worthy of investigation, and other adjuvants besides alum may provide important advantages in the peak IgG response produced and the duration of that high IgG response.
We also have identified haptens for MA that might produce a specific antibody binding pocket conformation rather than several different conformations in its polyclonal response. Because MA has three flexible bonds in its linkage to the carrier protein, this could produce several different conformations. We found that a rigid structure could produce a greater total antibody response. To form a rigid structure we synthesized methyltetrahydro-isoquinoline (MIQ) and succinic acid, and mixed that compound with succinic anhydride to form a four carbon chain linked to the MIQ at the amide end of the MA molecule (MIQ-N-succinate) (see Figure 2 for the chemical structures). The linker now had a carboxylic acid end group to attach to the lysine residues on the carrier proteins. This linkage structure is also unique compared with other linker structures, because other linkers are attached to the phenyl ring, which is at the opposite end of MA. This unique hapten-linker structure has led to some challenges in developing standard curves for determining antibody titres and affinities using an ELISA, but the antibody levels have been sufficient to influence behavioural measures of place preference, as independent measures for the potency of our antibody responses. These behavioural results showed that vaccinated mice conditioned with MA had reduced conditioned approach behaviours and decreased conditioned activity levels compared with control groups indicating attenuated MA place conditioning, and separate studies showed reduced and delayed MA locomotor effects [36].
Figure 2.
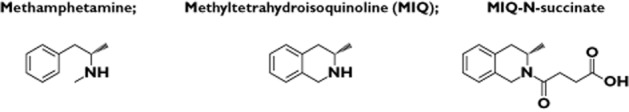
Chemical structures for MIQ-N-succinate synthesis
Cocaine vaccine in clinical trials
In the phase I trial (n = 34), TA-CD based on cholera toxin B induced cocaine-specific antibodies in all vaccinated subjects, and subsequent outpatient (n = 18) and human laboratory studies (n = 12) reported attenuation in their subjective experience and euphoria from smoked cocaine [14,17]. The immunological blockade lasted 2 to 4 months following the final vaccination. The only adverse effects were a few subjects with mild tachycardia, elevated temperature or hypertension with no serious adverse effects [16]. The outpatient trial comparing low to high dose vaccination (100 μg × four injections, or 400 μg × five injections) showed less relapse with the high dose (89% vs 43%).
The phase II trial (n = 115) gave 360 μg for five injections and about one-third attained antibody levels above 43 μg ml1, which was sufficient to block most expected cocaine doses. This high antibody group had more cocaine-free urines than the low antibody (<43 μg ml1) or placebo groups [15]. The antibodies remained elevated for 3 months after the last vaccination, and no safety concerns arose. Non-responding patients often had IgM antibodies to cocaine before vaccination. This poor prognostic marker could represent a response to adduct formation by the drug to native proteins in vivo [37]. Since exposure to cocaine alone will not provoke an increase in antibodies due to lack of cross linking the antibodies expressed on the B cell surface, boosting with this conjugate vaccine is required about every 3 months to maintain high antibody levels.
An ongoing multi-site, phase IIb clinical trial has followed the success of this first cocaine vaccine clinical trial of TA-CD [15]. This 4 month, double-blind, randomized, placebo-controlled, multicentre study includes 300 treatment-seeking, cocaine-dependent individuals receiving five vaccinations. This phase IIb clinical test should be completed in June 2012 with top line results available by 2013. Based on the success of this vaccine in the earlier clinical trials, this cocaine vaccine may be one of the first anti-addiction vaccines. The full clinical registration path for FDA approval of this vaccine will require another multisite phase 3 study replication, if this current study can demonstrate a significantly greater proportion of subjects becoming cocaine-abstinent for at least 3 weeks on the vaccine compared with the placebo. However, it does not appear that a full 1000 vaccinated subjects will be required for a safety assessment, since the CTB carrier has an outstanding safety record, and the clinical trials for this nor-cocaine haptenated conjugate vaccine have been remarkably free of adverse events.
The behavioural challenges for any successful vaccination programme start with the need to have 2 to 3 months where the patient can be brought to a treatment site for the series of vaccinations. While continued drug abuse during the 3 months of vaccination does not interfere with the vaccine's ability to stimulate the required antibody production, the patient needs to get these vaccinations at appropriate times over the 3 months (e.g. 2, 4, 8 and 12 weeks after the initial vaccination) and continued drug abuse may increase the risk of failure to appear for these follow-up visits. Thus, counselling or other treatment efforts will be critical to insure compliance with the schedule of vaccinations. Such interventions could vary from residential substance abuse care to outpatient contingency management, in which patients are paid to come for the vaccinations with an escalating pay schedule for each vaccination obtained.
Future clinical developments
In addition to the clinical trials being conducted on vaccines for cocaine, preclinical development of second generation vaccines for MA and cocaine are ongoing. Future vaccine trials will use more potent adjuvants than alum and include more effective carriers such as OMPC that have adjuvant properties due to their lipopolysaccaride content. Several outstanding adjuvants are commercially available, and nicotine vaccines are the most likely to first benefit from these new adjuvants due to the already ongoing interest in these vaccines of major pharmaceutical companies such as Novartis and GSK which control these novel adjuvants. Another likely focus will be on using booster injections with different adjuvants from the original vaccine that might only use alum. Shifting adjuvants during a series of vaccinations could prolong antibody durations and perhaps strengthen the development of high affinity in the polyclonal antibodies. Some work is also expected outside the United States, in China in particular, for commercializing these vaccines. Chinese companies have the capital needed, as well as the required government support, for moving these vaccines rapidly into the public health sectors where they are most needed.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Research support was provided by VA & NIH grants K05-DA0454 and P50-DA18827; DP1DA033502, U01 DA023898, and R01 DA030338. This material is the result of work supported with resources and the use of facilities at the Michael E. DeBakey VA Medical Center, Houston, Texas. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
References
- 1.United Nations Office on Drugs and Crime. 2010. World Drug Report. Available at http://www.unodc.org/documents/wdr/WDR_2010/2.3_Coca-cocaine.pdf (last accessed 8 March 2012)
- 2.Kosten T, Owens SM. Immunotherapy for the treatment of drug abuse. Pharmacol Ther. 2005;108:76–85. doi: 10.1016/j.pharmthera.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Quantification of behavior Sackler colloquium: addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orson FM, Kinsey BM, Singh RA, Wu Y, Gardner T, Kosten TR. Substance abuse vaccines. Ann N Y Acad Sci. 2008;1141:257–269. doi: 10.1196/annals.1441.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson RA, Boyd SJ, Ziegelstein RC, Herning R, Cadet JL, Henningfield JE, Schuster CR, Contoreggi C, Gorelick DA. Effect of rate of administration on subjective and physiological effects of intravenous cocaine in humans. Drug Alcohol Depend. 2006;82:19–24. doi: 10.1016/j.drugalcdep.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Cornish JW, O'Brien CP. Crack cocaine abuse: an epidemic with many public health consequences. Annu Rev Public Health. 1996;17:259–273. doi: 10.1146/annurev.pu.17.050196.001355. [DOI] [PubMed] [Google Scholar]
- 7.Walsh SL, Stoops WW, Moody DE, Lin SN, Bigelow GE. Repeated dosing with oral cocaine in humans: assessment of direct effects, withdrawal, and pharmacokinetics. Exp Clin Psychopharmacol. 2009;17:205–216. doi: 10.1037/a0016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith BJ, Jones HE, Griffiths RR. Physiological, subjective and reinforcing effects of oral and intravenous cocaine in humans. Psychopharmacology (Berl) 2001;156:435–444. doi: 10.1007/s002130100740. [DOI] [PubMed] [Google Scholar]
- 9.Rush CR, Baker RW, Wright K. Acute physiological and behavioral effects of oral cocaine in humans: a dose-response analysis. Drug Alcohol Depend. 1999;55:1–12. doi: 10.1016/s0376-8716(98)00164-1. [DOI] [PubMed] [Google Scholar]
- 10.Plotkin SA, editor. History of Vaccine Development. New York, Dordrecht, Heidelberg and London: Springer; 2008. [Google Scholar]
- 11.Curd J, Smith TW, Jaton JC, Haber E. The isolation of digoxin-specific antibody and its use in reversing the effects of digoxin. Proc Natl Acad Sci U S A. 1971;68:2401–2406. doi: 10.1073/pnas.68.10.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkowitz B, Spector S. Evidence for active immunity to morphine in mice. Science. 1972;178:1290–1292. doi: 10.1126/science.178.4067.1290. [DOI] [PubMed] [Google Scholar]
- 13.Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. Changes in heroin self-administration by a rhesus monkey after morphine immunization. Nature. 1974;252:708–710. doi: 10.1038/252708a0. [DOI] [PubMed] [Google Scholar]
- 14.Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry. 2005;58:158–164. doi: 10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 15.Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66:1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosten TR, Rosen M, Bond J, Settles M, St. Clair Roberts J, Shields J, Jack L, Fox B. Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine. 2002;20:1196–1204. doi: 10.1016/s0264-410x(01)00425-x. [DOI] [PubMed] [Google Scholar]
- 17.Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol Psychiatry. 2010;67:59–65. doi: 10.1016/j.biopsych.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orson FM, Kinsey BM, Singh RA, Wu Y, Kosten TR. Vaccines for cocaine abuse. Hum Vaccin. 2009;5:194–199. doi: 10.4161/hv.5.4.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMillan DE, Hardwick WC, Li M, Owens SM. Effects of murine-derived anti-methamphetamine monoclonal antibodies on (+)-methamphetamine self-administration in the rat. J Pharmacol Exp Ther. 2004;309:1248–1255. doi: 10.1124/jpet.103.061762. [DOI] [PubMed] [Google Scholar]
- 20.Byrnes-Blake KA, Laurenzana EM, Landes RD, Gentry WB, Owens SM. Monoclonal IgG affinity and treatment time alters antagonism of (+)-methamphetamine effects in rats. Eur J Pharmacol. 2005;521:86–94. doi: 10.1016/j.ejphar.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Gentry WB, Laurenzana EM, Williams DK, Owens SM. Safety and efficiency of an anti-(+)-methamphetamine monoclonal antibody in the protection against cardiovascular and central nervous system effects of (+)-methamphetamine in rats. Int Immunopharmacol. 2006;6:968–977. doi: 10.1016/j.intimp.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Hicks MJ, De Bishnu P, Rosenberg JB, Davidson JT, Moreno AY, Janda KD, Wee S, Koob GF, Hackett NR, Kaminsky SM, Worgall S, Toth M, Mezey JG, Crystal RG. Cocaine analog coupled to disrupted adenovirus: a vaccine strategy to evoke high-titer immunity against addictive drugs. Mol Ther. 2011;19:612–619. doi: 10.1038/mt.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall E. Gene therapy on trial. Science. 2000;288:951–957. doi: 10.1126/science.288.5468.951. [PubMed] [Google Scholar]
- 24.Hatsukami DK, Rennard S, Jorenby D. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin Pharmacol Ther. 2005;78:456–467. doi: 10.1016/j.clpt.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Fox BS, Kantak KM, Edwards MA, Black KM, Bollinger BK, Botka AJ, French TL, Thompson TL, Schad VC, Greenstein JL, Gefter ML, Exley MA, Swain PA, Briner TJ. Efficacy of a therapeutic cocaine vaccine in rodent models. Nat Med. 1996;2:1129–1132. doi: 10.1038/nm1096-1129. [DOI] [PubMed] [Google Scholar]
- 26.Pentel PR, Malin DH, Ennifar S. A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacol Biochem Behav. 2000;65:191–198. doi: 10.1016/s0091-3057(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 27.Hieda Y, Keyler DE, VanDeVoort JT. Immunization of rats reduces nicotine distribution to brain. Psychopharmacology. 1999;143:150–157. doi: 10.1007/s002130050930. [DOI] [PubMed] [Google Scholar]
- 28.Hieda Y, Keyler DE, Vandevoort JT, Pentel PR. Active immunization alters the plasma nicotine concentration in rats. J Pharmacol Exp Ther. 1997;283:1076–1081. [PubMed] [Google Scholar]
- 29.Ramakrishnan M, De Melo FA, Kinsey BM, Ladbury JE, Kosten TR, Orson FM. Probing cocaine-antibody interactions in buffer and human serum. PLoS ONE. 2012;7:e40518. doi: 10.1371/journal.pone.0040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart DJ, Inaba T, Lucassen M, Kalow W. Cocaine metabolism: cocaine and norcocaine hydrolysis by liver and serum esterases. Clin Pharmacol Ther. 1979;25:464–468. doi: 10.1002/cpt1979254464. [DOI] [PubMed] [Google Scholar]
- 31.Kamendulis LM, Brzezinski MR, Pindel EV, Bosron WF, Dean RA. Metabolism of cocaine and heroin is catalyzed by the same human liver carboxylesterases. J Pharmacol Exp Ther. 1996;279:713–717. [PubMed] [Google Scholar]
- 32.Jenkins AJ, Keenan RM, Henningfield JE, Cone EJ. Correlation between pharmacological effects and plasma cocaine concentrations after smoked administration. J Anal Toxicol. 2002;26:382–392. doi: 10.1093/jat/26.7.382. [DOI] [PubMed] [Google Scholar]
- 33.Apicella MA, Murphy TF. 2006. pp. 1569–1572. Haemophilus influenzae type b (Hib) vaccine Meyler's Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions (Fifteenth Edition),
- 34.Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1β and IL-18 release. J Immunol. 2007;178:5271–5276. doi: 10.4049/jimmunol.178.8.5271. [DOI] [PubMed] [Google Scholar]
- 35.Pérez-Melgosa M, Ochs HD, Linsley PS, Laman JD, van Meurs M, Flavell RA, Ernst RK, Miller SI, Wilson CB. Carrier-mediated enhancement of cognate T cell help: the basis for enhanced immunogenicity of meningococcal outer membrane protein polysaccharide conjugate vaccine. Eur J Immunol. 2001;31:2373–2381. doi: 10.1002/1521-4141(200108)31:8<2373::aid-immu2373>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 36.Shen XY, Kosten TA, Lopez AL, Kinsey BM, Kosten TR, Orson FM. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend. 2013;129:41–48. doi: 10.1016/j.drugalcdep.2012.09.007. PMID: 23022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindroth K, Mastache EF, Roos I, Fernández AG, Fernández C. Understanding thymus-independent antigen-induced reduction of thymus-dependent immune responses. Immunology. 2004;112:413–419. doi: 10.1111/j.1365-2567.2004.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


