Abstract
New oral anticoagulants to reduce the incidence of thrombosis have recently become available. When compared to the existing therapy, warfarin, these novel agents have similar efficacy with a reduced risk of spontaneous bleeding. However, these novel agents have been associated with significant, even fatal, bleeding following trauma. Reversal agents are being developed that bind and neutralize these oral anticoagulants. However, these are not yet available. Another strategy is to increase thrombin generation by administration of “bypassing” agents such as prothrombin complex concentrates or factor VIIa. Several animal models have been used to model the hemostatic defect induced by the thrombin inhibitor dabigatran. A rat tail injury model, a rabbit cuticle bleeding model, and a rabbit kidney laceration model have all been reported to show increased bleeding, but with supratherapeutic doses of dabigatran. A mouse tail transection model has been reported to reflect increased bleeding at peak therapeutic dabigatran levels. We found that the Whinna saphenous vein hemostasis model reliably reflects a hemostatic defect at therapeutic levels of dabigatran. This model can potentially reflect the effects of reversal or bypassing agents.
Introduction
Thrombosis represents a serious health problem in the US and Europe with more than 1% of the population currently taking medication to reduce the risk of thrombosis. The new oral anticoagulants (dabigatran that targets thrombin and rivaroxaban or apixaban that target factor Xa) have been shown in clinical trials to have good efficacy in preventing thrombosis with a reduced risk of spontaneous bleeding relative to warfarin [1,2]. In particular, fatal bleeds and intracranial hemorrhage were reduced relative to warfarin. Despite the lower risk of spontaneous bleeding, in trauma these oral anticoagulants have been associated with significant bleeding leading to serious adverse events [3,4].
This trauma induced bleeding has led to a search for agents to reverse the effects of the oral anticoagulants. Reversal agents that bind the thrombin and factor Xa inhibitors, effectively removing them from circulation, are being developed [5–7]. Since these reversal agents have not yet been approved, one strategy has been to use factor VIIa and prothrombin complex concentrates in patients presenting with trauma while on oral anticoagulants [8,9]. While not direct neutralizers of the anticoagulants, factor VIIa and prothrombin complex concentrates act as “bypassing” agents to boost factor Xa and thrombin generation sufficiently to stabilize the patients through surgery.
In this context, it would be valuable to have a model to assess in vivo bleeding due to these oral anticoagulants. Such a model could be a tool to assess bypassing and reversal agents. We have previously described a Saphenous vein injury model that has been used to examine bleeding in mice with hemophilia and to assess therapy in those mice [10,11]. We hypothesized that this model might be useful to examine the effects of the novel anticoagulants on hemostasis.
Materials and Methods
Dabigatran (BIBR 953) was purchased from (Selleck, Houston, TX). The dabigatran concentration was determined by adding a high concentration of thrombin (30 nM) to varied dabigatran (0-100 nM). Residual thrombin was detected with a substrate that binds poorly to thrombin (Pefachrome FXa; Pentapharm, Basel, Switzerland). Dabigatran concentration was determined from the intercept of the plot of residual activity vs concentration of dabigatran assuming a one to one molar interaction with thrombin.
All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of North Carolina. C57BL6/J mice were purchased from Charles Rivers Laboratories (Willmington, MA). Bleeding studies were done essentially as previously described [10,11]. Mice were anesthatized with isoflurane throughout all procedures. The hair on the ventral side of both hind limbs was removed. The animals were placed supine on a temperature and ECG monitoring board. The paws were gently restrained by looping soft polyethylene tubing around them and attaching attaching the tubing to the ECG board. The skin on the left and right ventral hind limb was incised which exposes a length of the saphenous neurovascular bundle; the bundle was covered with normal saline to prevent drying. To allow for injection of dabigatran, the left saphenous vein was cannulated. To assess hemostasis, the right saphenous vein was transected by piercing it with a 23-G needle followed by a longitudinal incision made in the distal portion of the vessel. Blood was gently wicked away until hemostasis occurred. The clot was then removed to restart bleeding and the blood was again wicked away until hemostasis occurs again. Clot disruption was repeated after every incidence of hemostasis for 30 minutes. Two parameters were measured: 1) the number of times that hemostasis occurs in a 30 minute period, and 2) the time required for each hemostasis. At the conclusion of hemostasis measurements, blood was withdrawn from the inferior vena cava; 9 parts blood were mixed with 1 part 0.109 M citrate.
Administration of 15 μg/kg dabigatran intravenously produced plasma levels of about 65 ng/mL. Dabigatran levels were assessed using a thrombin time assay in which one part mouse plasma as the source of dabigatran was mixed with one part normal pooled human plasma as a standardized source of fibrinogen. Clotting was initiated by addition of one part plasma to one part 1 NIH U/mL thrombin (without addition of calcium). Clotting times were converted to dabigatran concentration from a standard curve generated using known concentrations of the inhibitor.
Comparison of treated to untreated animals was done by a two sided Student's t-Test assuming equal variance in the samples.
Results
The hemostasis times for an individual mouse were averaged to obtain a single value for each mouse. Wild type mice (n=8) have an average hemostasis time of 86 seconds (+/- 18 seconds) as shown in Figure 1A. This same data can be expressed as the number of disruptions within a thirty minute period. In this study wild type mice had 19.2 (+/- 3.2) disruptions (FIgure 1B). When wild type mice were injected with 15 μg/kg dabigatran (n=11), they had plasma levels of 63 (+/- 18) ng/mL at the conclusion of the bleeding studies. These animals had their average hemostasis time prolonged to 171 (+/−33) seconds and had only 9.7 (+/- 1.7) disruptions; both were significantly different from wild type (p<0.001).
Figure 1.
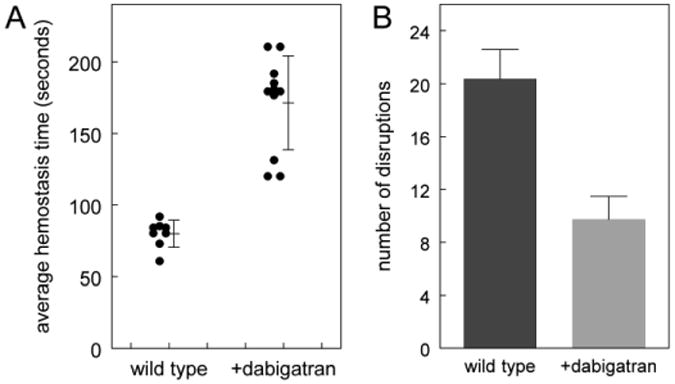
Effect of dabigatran on a saphenous vein bleeding model. A. Bleeding over 30 minutes was measured by disruption of hemostatic clots as described in Methods. Average hemostasis times for wild type C57BL6/J mice (n=8) and C57BL6/J mice injected with 15 μg/kg dabigatran (n=11) are plotted; each dot represents an animal. The mean and standard deviation are shown. Wild type differs from dabigatran treated (p<0.001). B. The data in Figure 1A can be expressed as the number of disruptions in 30 minutes; the error bar shows the standard deviation. Wild type differs from dabigatran treated (p<0.001).
Conclusions
The Whinna saphenous vein hemostasis model was initially developed to assess bleeding in hemophilia [10,11]. One of the features of this model is that it is sensitive to relatively low doses of hemostatic agents; in studies on recombinant factor VIII, average hemostasis time was virtually corrected by administration of only 10 U/kg factor VIII [11]. By contrast, the commonly used model of tail vein transection is a much more significant injury and, unsurprisingly, in hemophilia A mice doses of up to 100 U/kg factor VIII were required to normalize blood loss in >80% of the animals [12].
In preclinical animal studies on the efficacy and safety of dabigatran, one of the initial observations was that the dose required to cause bleeding was much higher than the dose of dabigatran that prevented thrombosis. Wienen et al showed using rats that in a Wessler model of the inferior vena cava, venous thrombus weight could be dose dependently reduced by bolus administration of dabigatran [13]. The ED50 for this reduction of thrombus weight was 33 μg/kg. By contrast, in a model that used a spring loaded device to create a standardized injury on a rat tail, up to 300 μg/kg dabigatran did not show bleeding [13]. This result was consistent with another study that looked at a rabbit cuticle bleeding time as a measure of hemostasis [14]. In the rabbit study, bleeding was only observed at high plasma levels of dabigatran (ED50 of 500 ng/mL (1079 nM)).
In order to study the role of bypassing agents in reversing bleeding, a model of a standardized kidney injury was used [15]. In this model, a defined (15-mm long and 5mm deep) scalpel incision was made at the lateral kidney pole of a rabbit. The time required for this injury to stop bleeding was measured (30 min max). In this study, rabbits were administered 400 μg/kg dose leading to peak dabigatran levels of 800 ng/mL. These are levels that would be expected only in patients after overdose or with impaired clearance due to renal failure. Another study to look at reversal of bleeding used a tail transection model in mice [16]. Mice were given dabigatran etexilate by oral gavage; this resulted in a dose dependent increase in blood loss from 6 minutes in untreated animals to 13 minutes in treated animals. For the reversal study, levels of 200 ng/mL were used. This dabigatran level is similar to peak levels seen in patients taking 300 mg once a day.
In the studies presented here, dabigatran treatment prolonged the average hemostasis times; the dabigatran levels in these mice (63 +/- 18 ng/mL) fall between the peak (89 ng/mL) and trough levels (14 ng/mL) seen in patients taking 150 mg dabigatran etexilate once daily [17] and are close to the steady state trough levels seen in patients who are taking 110 mg dabigatran etexilate twice daily [18]. So in this model, hemostasis is prolonged at dabigatran concentrations within the range of normal therapeutic levels. This study is limited since only a single dose of dabigatran was studied. It is not clear if higher plasma levels of dabigatran would result in more significant bleeding although, by analogy to the tail transection study where there was a dose dependent increase in bleeding [16], we expect the bleeding in this model to show dose dependence.
In summary we have established that a saphenous vein hemostasis model can assess bleeding in mice that have levels of dabigatran comparable to therapeutic levels in patients. Preliminary data shows that this model is useful for testing the in vivo efficacy of bypassing agents [19] and may be useful for testing the efficacy of reversal agents.
Acknowledgments
We would like to thank Jacqueline Brock for performing the bleeding model studies.
Footnotes
This work was presented at the 7th Symposium on Hemostasis: Old System, New Players, New Directions in Chapel Hill, NC on May 16, 2014.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 2.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 3.Baumann Kreuziger LM, Morton CT, Dries DJ. New anticoagulants: A concise review. J Trauma Acute Care Surg. 2012;73:983–92. doi: 10.1097/TA.0b013e318265cf9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parra MW, Zucker L, Johnson ES, Gullett D, Avila C, Wichner ZA, et al. Dabigatran bleed risk with closed head injuries: are we prepared? J Neurosurg. 2013;119:760–5. doi: 10.3171/2013.3.JNS12503. [DOI] [PubMed] [Google Scholar]
- 5.Schiele F, van Ryn J, Canada K, Newsome C, Sepulveda E, Park J, et al. A specific antidote for dabigatran: functional and structural characterization. Blood. 2013;121:3554–62. doi: 10.1182/blood-2012-11-468207. [DOI] [PubMed] [Google Scholar]
- 6.Lu G, DeGuzman FR, Hollenbach SJ, Karbarz MJ, Abe K, Lee G, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19:446–51. doi: 10.1038/nm.3102. [DOI] [PubMed] [Google Scholar]
- 7.Sheffield W, Lambourne M, Bhakta V, Eltringham-Smith L, Arnold M, Crowther M. Active site-mutated thrombin S195A but not active site-blocked thrombin counteracts the anticoagulant activity of dabigatran in plasma. J Thromb Haemost. 2013;11(2):166. abstract OC 36.3. [Google Scholar]
- 8.Lillo-Le Louët A, Wolf M, Soufir L, Galbois A, Dumenil AS, Offenstadt G, et al. Life-threatening bleeding in four patients with an unusual excessive response to dabigatran: implications for emergency surgery and resuscitation. Thromb Haemost. 2012;108:583–5. doi: 10.1160/TH12-03-0149. [DOI] [PubMed] [Google Scholar]
- 9.Warkentin TE, Margetts P, Connolly SJ, Lamy A, Ricci C, Eikelboom JW. Recombinant factor VIIa (rFVIIa) and hemodialysis to manage massive dabigatran-associated postcardiac surgery bleeding. Blood. 2012;119:2172–4. doi: 10.1182/blood-2011-11-393587. [DOI] [PubMed] [Google Scholar]
- 10.Buyue Y, Whinna HC, Sheehan JP. The heparin-binding exosite of factor IXa is a critical regulator of plasma thrombin generation and venous thrombosis. Blood. 2008;112:3234–41. doi: 10.1182/blood-2008-01-136820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pastoft AE, Lykkesfeldt J, Ezban M, Tranholm M, Whinna HC, Lauritzen B. A sensitive venous bleeding model in haemophilia A mice: effects of two recombinant FVIII products (N8 and Advate(®)) Haemophilia. 2012;18:782–8. doi: 10.1111/j.1365-2516.2012.02780.x. [DOI] [PubMed] [Google Scholar]
- 12.Mei B, Pan C, Jiang H, Tjandra H, Strauss J, Chen Y, et al. Rational design of a fully active, long-acting PEGylated factor VIII for hemophilia A treatment. Blood. 2010;116:270–9. doi: 10.1182/blood-2009-11-254755. [DOI] [PubMed] [Google Scholar]
- 13.Wienen W, Stassen JM, Priepke H, Ries UJ, Hauel N. Effects of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate, on thrombus formation and bleeding time in rats. Thromb Haemost. 2007;98:333–8. [PubMed] [Google Scholar]
- 14.Wong PC, Crain EJ, Watson CA, Xin B. Favorable therapeutic index of the direct factor Xa inhibitors, apixaban and rivaroxaban, compared with the thrombin inhibitor dabigatran in rabbits. J Thromb Haemost. 2009;7:1313–20. doi: 10.1111/j.1538-7836.2009.03503.x. [DOI] [PubMed] [Google Scholar]
- 15.Pragst I, Zeitler SH, Doerr B, Kaspereit FJ, Herzog E, Dickneite G, et al. Reversal of dabigatran anticoagulation by prothrombin complex concentrate (Beriplex P/N) in a rabbit model. J Thromb Haemost. 2012;10:1841–8. doi: 10.1111/j.1538-7836.2012.04859.x. [DOI] [PubMed] [Google Scholar]
- 16.Lambourne MD, Eltringham-Smith LJ, Gataiance S, Arnold DM, Crowther MA, Sheffield WP. Prothrombin complex concentrates reduce blood loss in murine coagulopathy induced by warfarin, but not in that induced by dabigatran etexilate. J Thromb Haemost. 2012;10:1830–40. doi: 10.1111/j.1538-7836.2012.04863.x. [DOI] [PubMed] [Google Scholar]
- 17.Ezekowitz MD, Reilly PA, Nehmiz G, Simmers TA, Nagarakanti R, Parcham-Azad K, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study) Am J Cardiol. 2007;100:1419–26. doi: 10.1016/j.amjcard.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 18.Reilly PA, Lehr T, Haertter S, Connolly SJ, Yusuf S, Eikelboom JW, et al. The Effect of Dabigatran Plasma Concentrations and Patient Characteristics on the Frequency of Ischemic Stroke and Major Bleeding in Atrial Fibrillation Patients in the RE-LY Trial. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.07.104. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman M, Volovyk Z, Monroe DM. Reversal Of Dabigatran Anticoagulation By a 4-Factor Prothrombin Complex Concentrate: Correlation Between Effects On Parameters Of Thrombin Generation and Hemostatic Effect In Vivo. Blood. 2013;122 doi: 10.1097/ALN.0000000000000540. abstract 3643. [DOI] [PubMed] [Google Scholar]


