Abstract
Tissue engineering skeletal muscle in vitro is of great importance for the production of tissue-like constructs for treating tissue loss due to traumatic injury or surgery. However, it is essential to find new sources of cells for muscle engineering as efficient in vitro expansion and culture of primary myoblasts are problematic. Mesenchymal stem cells may be a promising source of myogenic progenitor cells and may be harvested in large numbers from adipose tissue. As skeletal muscle is a mechanically dynamic tissue, we have investigated the effect of cyclic mechanical strain on the myogenic differentiation of a coculture system of murine C2C12 myoblasts and human adipose–derived mesenchymal stem cells. Fusion of mesenchymal stem cells with nascent myotubes and expression of human sarcomeric proteins was observed, indicating the potential for myogenic differentiation of human mesenchymal stem cells. Cyclic mechanical strain did not affect the fusion of mesenchymal stem cells, but maturation of myotubes was perturbed.
Keywords: Mechanical strain, myoblasts, mesenchymal stem cells, adipose, myogenesis, fusion
Introduction
There is a need for tissue-engineered skeletal muscle for treatment following severe trauma or surgery. Significant loss of skeletal muscle due to trauma is normally treated using autologous grafts. In this process, a vascularised muscle flap is removed from a donor site and grafted to the site of injury.1 In addition to the formation of significant quantities of scar tissue, such procedures necessarily generate a second site of injury which can lead to further morbidity.1,2 There is therefore a significant need to produce tissue-engineered skeletal muscle as a source of tissue for grafts. Such an approach would avoid the problem of donor-site morbidity and could avoid scar tissue formation that results from the grafting of mature muscle flaps.
Skeletal muscles are hierarchically structured organs that are mainly composed of a tissue consisting of muscle fibres that run almost the entire length of the muscle organ. Despite their length, muscle fibres are in fact single cells formed by the fusion of precursor cells during development. They therefore contain many nuclei within the confines of a single cell membrane (the sarcolemma) surrounding the assembly of filamentous proteins (the sarcomere), which produces the force responsible for muscle contraction. Proliferative precursor cells of a myoblastic phenotype may be induced to fuse to form myotubes in vitro which resemble nascent muscle fibres. Although significant advances have been made in applying myogenic precursors to tissue engineering,3 such approaches are problematic therapeutically due to difficulty in the sub-culture of myoblasts and the lack of availability of donor cells (autologous or otherwise). Mesenchymal stem cells (MSCs) are an attractive alternative source of progenitor cells due to their multipotency, high propensity for self-renewal and the possibility of harvesting large numbers from several different tissues.4,5
There have been several different approaches published to induce myogenic differentiation of MSCs. The earliest such method employed the use of 5-azacytidine, a pharmacological modulator of DNA methylation.6 Others have artificially forced myogenic differentiation by, for example, retroviral transfection to promote expression of PAX3 leading to expression of downstream myogenic regulatory factors (MRFs) and consequent differentiation.7 Such studies, while interesting, perhaps only indicate that MSCs are not prohibited epigenetically from myogenic differentiation. They do not, in themselves, present a useful protocol for culture of skeletal muscle.
Myogenic differentiation of MSCs has also been demonstrated using induction media containing high doses of the steroids dexamethasone and hydrocortisone.8–11 It should be noted that none of these studies employed MSCs from bone marrow. Instead they used adipose tissue or adherent mononuclear cells from cord blood. Some examples of apparently spontaneous differentiation of MSCs have also been reported. MSCs from bone marrow were shown to undergo early stages of myogenic differentiation in response solely to matrix stiffness.12 More recently, this effect has been shown to result in the formation of fused myotubes only from adipose-derived and not bone marrow–derived MSCs.13
Finally, there are a group of studies in which the apparent differentiation of MSCs in coculture with nascent myotubes derived from myoblast cell lines or primary myoblasts was observed. It was shown initially that human MSCs derived from bone marrow were capable of spontaneous fusion with murine C2C12 myoblasts14 and later that such fusion led to expression of human proteins characteristic of skeletal muscle indicating that the MSC nuclei were transcriptionally active post-fusion.15,16 Similar results have also been demonstrated using MSCs derived from adipose tissue.17
Given its role as the principle effector of voluntary motion, skeletal muscle is a mechanically dynamic tissue. Indeed, muscle fibres exert force as a result of contraction and are also subject to the action of external forces.18 It is therefore essential to understand the role of externally applied strain on the development, repair and maturation of muscle in order to develop effective protocols for tissue engineering. The overall effect of cyclic mechanical strain on skeletal myogenesis in vitro is not entirely clear from the literature. Several researchers have reported that cyclic mechanical strain generally increases myogenesis or some aspect of it,19–21 and several others have reported that myogenesis, or at least sarcomeric maturation, is perturbed or hindered by cyclic mechanical strain.22–24 Further investigations are clearly needed in order to clarify the role of mechanical strain and to identify implications for tissue engineering.
In this study, a FlexCell mechanical stimulation device was used to apply cyclic mechanical strain to both monocultures and cocultures of murine C2C12 myoblasts and human adipose–derived MSCs. The coculture system was chosen in order to study the potential of MSCs in tissue engineering skeletal muscle and because the myogenesis in such systems reported in the literature has not been fully characterised. Adipose-derived MSCs were chosen due to their potential for therapeutic use arising from their plentiful source and ease of harvesting. Serum withdrawal only was used in the myogenic differentiation protocols in order to isolate only the effects of the presence of C2C12s and mechanical strain on the MSCs.
Experimental methods
Chemicals and reagents
Dulbecco’s modified Eagle’s medium (DMEM) low glucose variation, Dulbecco’s phosphate buffered saline (PBS), trypsin–ethylenediaminetetraacetic acid (EDTA) preparation and foetal bovine serum (FBS) were obtained from PAA (Pasching, Austria). Adult horse serum, goat serum, paraformaldehyde, Triton X-100, bovine serum albumin and diamidinophenylindole (DAPI) were obtained from Sigma–Aldrich (Dorset, UK). Complete mesenchymal stem cell growth medium was obtained from Promocell (Heidelberg, Germany). ProLong Gold antifade reagent, AlexaFluor-488 conjugated goat-anti-mouse IgG antibody and AlexaFluor-546 goat-anti-rabbit IgG antibody were all purchased from Molecular Probes (Life Technologies, Paisley, UK). DNAse I and DNAse buffer were obtained from Ambion (Life Technologies). Monoclonal mouse-anti-rabbit fast skeletal myosin (myosin heavy chain (MHC)) antibody, monoclonal mouse-anti-mouse (synthetic fragment) MyoD1 antibody and monoclonal rabbit-anti-human lamin A (human only) primary antibody were obtained from Abcam (Cambridge, UK). MicroMACS one-step complementary DNA (cDNA) kit was purchased from Miltenyi Biotec (Surrey, UK). Precision 2X PCR Master Mix with SYBR Green, GeNorm reference gene selection kit and custom designed primers for quantitative reverse transcriptase polymerase chain reaction (RT-PCR) were obtained from Primer Design Ltd (Southampton, UK).
Cell culture
C2C12 myoblasts were obtained from the European Collection of Animal Cell Cultures and maintained in DMEM supplemented with 10% (v/v) FBS. The cells were sub-cultured every 2–3 days or when 80%−90% confluent. Terminal differentiation was induced by changing the growth medium to a low serum formulation containing 2% (v/v) adult horse serum in place of FBS.
Human adipose–derived MSCs were obtained from PromoCell having been isolated from a 26-year-old Caucasian female and enriched and screened for a population positive for CD44 and negative for CD31 and CD45 surface antigens. The cells were maintained in mesenchymal stem cell growth medium (a proprietary, low serum medium) and were seeded for experiments at passage 5 having been purchased at passage 2.
Cocultures of C2C12 myoblasts and MSCs were seeded at a ratio of 1:5 in myoblast growth medium with a total seeding density of 22,222/cm2 in flexible-bottom 6-well plates pre-coated with collagen I (FlexCell, Dunn Labortechnik, Asbach, Germany). The same total seeding density was also used for monocultures of C2C12 myoblasts and MSCs.
Cyclic strain experiments
Cell cultures were subjected to cyclic uniaxial strain using a FlexCell system (Dunn Labortechnik, Asbach, Germany) with UniFlex culture plates and ArcTangle loading posts. The system employs 6-well plates in which the growth surface is a flexible sheet of polymer. A vacuum is employed to deform the flexible growth surface over a cylindrical post machined with truncations, which results in a uniaxial strain field. The vacuum is modulated to give cyclic strain with a choice of waveforms. The waveform employed in this study was a half sine wave with frequency of 1 Hz and amplitude of 12% pulsed in 1-s pulses with a pause of 1 s between each pulse. A plot of the strain waveform is shown in Figure 1.
Figure 1.
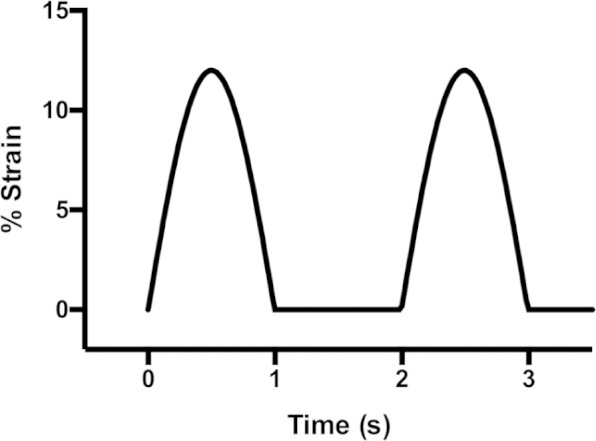
Cyclic strain waveform. Cultures were subjected to 12% strain applied as a half-sinusoidal waveform at a rate of 1 Hz. Strain was applied in pulses of 1 s duration with 1 s rest between each pulse.
Cells were seeded in coculture or monoculture to collagen I–coated UniFlex plates in myoblast growth medium (including 10% v/v foetal calf serum). The cells were allowed to adhere and proliferate for 24 h before changing medium to myoblast differentiation medium (including 2% v/v adult horse serum). The strain regimen was then initiated and continued for 48 h before refreshing the differentiation medium. Cultures were then continued for a further 5 days under static conditions, refreshing the medium once more half way through. The cells were then fixed for immunocytochemistry (ICC) or harvested for gene expression analysis.
ICC
For ICC, cultures were fixed in 3% (w/v) paraformaldehyde in PBS for 10 min at room temperature before rinsing three times with fresh PBS. Cell membranes were partially permeabilised and cultures were blocked against non-specific antibody binding by incubating fixed cultures for 30 min at room temperature in a complete staining buffer containing 0.1% (v/v) Triton X-100, 0.1% (w/v) bovine serum albumin and 1% (v/v) goat serum in PBS. The cultures were then stained with a combination of primary antibodies in staining buffer for 16 h at 4°C. The antibodies against MHC, lamin A and MyoD1 were used at working dilutions of 1/1000, 1/500 and 1/200, respectively. The cultures were then incubated with a mixture of AlexaFluor-546-conjugated goat-anti-rabbit IgG and AlexaFluor-488-conjugated goat-anti-mouse IgG secondary antibodies for 1 h at room temperature. After two rinses in fresh PBS, the cultures were finally incubated with DAPI (300 nM) for 10 min. The cultures were rinsed once and then mounted for microscopy in ProLong Gold antifade reagent. Fluorescence micrographs were captured using a Leica SP5 confocal microscope.
Gene expression analysis by quantitative RT-PCR
For gene expression analysis, the growth medium was aspirated and the cultures rinsed once with PBS before trypsinisation with trypsin-EDTA preparation. The trypsin was removed from the cell suspension by centrifugation, and the cell pellet was lysed in lysis buffer (kit component). Extraction of messenger RNA (mRNA) and reverse transcription to prepare cDNA was carried out using a column-based MicroMACS one-step cDNA kit (Miltenyi Biotec) according to the manufacturer’s instructions. Although the manufacturer stated that the MicroMACS kit removes genomic DNA from samples, an additional DNase treatment was carried out on-column prior to reverse transcription to ensure that no genomic DNA was present in the final cDNA samples. The columns were first equilibrated with DNase I buffer before addition of RNase-free DNase I (two units) in DNase buffer (50 µl). The columns were incubated at room temperature for 2 min before eluting the DNAse with lysis/binding buffer and wash buffer (kit components) prior to continuing with the on-column reverse transcription according to the kit manufacturer’s instructions.
Quantitative RT-PCR (qPCR) was carried out using a StepOne Plus qPCR cycler (Applied Biosystems, Life Technologies). SYBR Green detection chemistry was employed, and cycling conditions were programmed according to the mastermix manufacturer’s instructions. Primers were custom designed by a commercial supplier (Primer Design Ltd). The aim of the experiments was to quantify expression of skeletal muscle genes by the human MSCs in the presence of differentiating mouse myoblasts which would be expected to concurrently express equivalent mouse skeletal muscle genes. Given that the relative copy number of human target sequences was often low in comparison to the equivalent mouse sequences in the same samples, and given the relatively high degree of homology between many of the mouse and human genes, some optimisation of annealing temperature was required in order to ensure strict species specificity. Given such strict requirements for species specificity, it was also not possible to design exon spanning primers for every gene, hence the additional DNAse treatment prior to reverse transcription. Primers were validated by amplification of cDNA prepared from monocultures of differentiating C2C12 myoblasts and primary human skeletal muscle myoblasts for mouse and human targets, respectively. The primer pair sequences and annealing temperature for each pair along with the melt temperature of the amplification products as measured by post-amplification melt curve analysis are listed in Table 1.
Table 1.
Primer sequences and annealing temperatures (Ta) for gene expression analysis by quantitative RT-PCR. Values of amplification product melting temperature (Tm) were measured experimentally by post-amplification melt curve analysis.
| Gene | Species | Sense | Antisense | Ta (°C) | Tm (°C) |
|---|---|---|---|---|---|
| Myod1 | Mouse | AGGACACGACTGCTTTCTTC | CGGAACCCCAACAGTACAA | 60.0 | 84.6 |
| MYOD1 | Human | CCACAGGGCAAGGACACAG | TAGCCCCTCAAGGTTCAGC | 70.0 | 87.5 |
| Myog | Mouse | GCCCAGTGAATGCAACTCCC | CAGCCGCGAGCAAATGATCT | 60.0 | 87.0 |
| MYOG | Human | GCCCTGATGCTAGGAAGCC | CTGAATGAGGGCGTCCAGTC | 70.0 | 85.0 |
| Myh2 | Mouse | GGACCTCATGCTGGATGTG | GCGTGGGTTTCCTCATACTT | 60.0 | 84.6 |
| MYH2 | Human | TAGTAATGTAGAAACGGTCTCCAAA | CCTTTGATTTCAGTTCACTCAGTT | 68.0 | 80.7 |
| Ttn | Mouse | TTTCTTGTGAGGTCAGCGGG | CTGGAGCGACTCACACTGAT | 60.0 | 79.9 |
| TTN | Human | TAGTAAAATGCTTAAAGCAGGCATAA | TACTGTTAGAACTTTGCCTTCATC | 68.0 | 78.8 |
| RUNX2 | Human | GAGCAATTAAAGTTACAGTAGATGGA | GAGGCGGTCAGAGAACAAAC | 68.0 | 81.0 |
| PPARG | Human | AACACTAAACCACAAATATACAACAAG | GGCATCTCTGTGTCAACCAT | 68.0 | 78.6 |
| ALCAM | Human | AAAAGTGCTACATCCCCTTGAA | TGTCAGCCTTGGTTGTCTTG | 68.0 | 81.3 |
RT-PCR: reverse transcriptase polymerase chain reaction.
Experimental threshold cycle times were determined from amplification curves using ABI StepOne Plus software. High-resolution melt curves were acquired after amplification in order to assess the validity and specificity of amplification in comparison to positive controls. Amplification of target sequence was determined as nil (below measurable limits) if the value of CT exceeded 32 cycles or the melt curve showed multiple or incorrect peaks. Data were deemed valid if the melt curve showed good specificity in comparison to positive controls.
A GeNorm reference gene selection kit was used to determine the most stable reference genes for the experimental conditions employed. Informed by the GeNorm test, values of CT were normalised to aggregate expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), cytochrome c-1 (CYC1) and beta-2-microglobulin (B2M). Relative gene expression was calculated relative to expression at the beginning of the experiments (day 0) and plotted as fold change plus or minus the standard error of the mean. Statistical differences with respect to expression at day 0 and between static and strained cultures were determined by application of Student’s t-test.
Proliferation assay
Six replicate samples were used for each condition. After seeding, the C2C12s were allowed to attach, spread and proliferate for 24 h after which the cultures were assumed to be in the log phase of growth. The Alamar Blue (resazurin) viability assay was used to determine the rate of proliferation using the manufacturer’s protocol. First, the growth medium was aspirated and replaced with exactly 2 mL of fresh differentiation medium per well. Alamar Blue working solution (150 µM, 200 µL) was added to each well, and the plates were incubated at 37°C for 2 h; 200-µL aliquots of medium were removed in triplicate to black 96-well plates, and the fluorescence was measured using a multiwell plate reader with excitation and emission wavelengths of 560 and 590 nm, respectively. The growth medium was then aspirated from the sample wells and replaced with fresh medium. A total of 48 h after seeding, the assay was repeated giving a total of two time points from which the population doubling rate (rd) was calculated for each well using equation (1), where f1 and f2 are the background-subtracted fluorescence values at time point 1 (t1) and time point 2 (t2), respectively. Mean values of rd were plotted plus or minus the standard error of the mean.
| (1) |
Results and discussion
Cyclic mechanical strain of C2C12 monocultures
C2C12 myoblasts in monoculture fused to form multinucleated myotubes expressing MHC (Figure 2). The relative degree of myotube bulk orientation and the apparent number of myotubes appeared qualitatively increased in cultures subjected to cyclic strain compared to static cultures. However, even when subjected to cyclic strain, some variability in the direction of bulk orientation was observed with the most common orientation being approximately 45° relative to the strain axis. This contrasts with the findings from other studies that showed parallel orientation of myotubes along the strain axis.19,24 It has been shown that fibroblasts (which share a mesenchymal morphology with myoblasts and MSCs) generally adopt orientations perpendicular to an applied uniaxial strain field in order to minimise the deformation of stress fibres.25 It may be that the observed orientation at 45° is a combination of this effect and a second effect particular to myotubes which acts to orient the myotubes parallel to the strain axis and which has yet to be fully characterised.
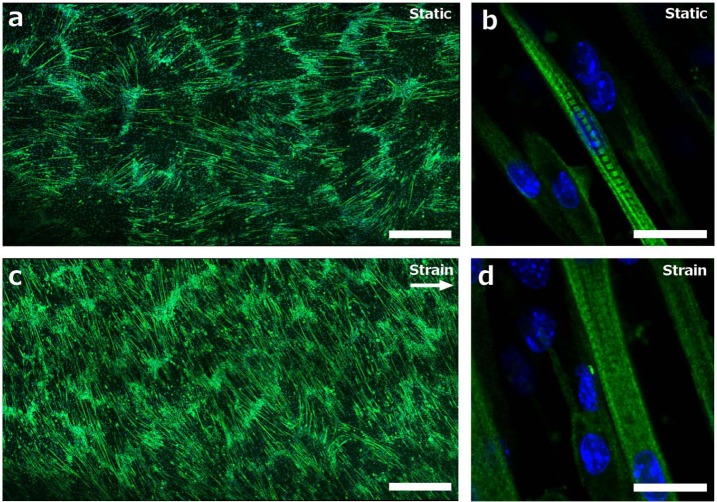
Figure 2.
Confocal micrographs of MHC expression. Green staining corresponds to MHC and blue to nuclei (DAPI staining); (a) large-scale tile scan of static culture: scale bar = 750 µm, (b) high magnification scan of static culture: scale bar = 25 µm, (c) large-scale tile scan of culture subjected to cyclic strain: scale bar = 750 µm and (d) high magnification scan of culture subjected to cyclic strain: scale bar = 25 µm.
DAPI: diamidinophenylindole; MHC: myosin heavy chain.
After a period of differentiation, the C2C12 myotubes displayed myosin in a striated arrangement, indicating sarcomeric assembly and maturation of the nascent myotubes (Figure 2(b) and (d)). However, the application of cyclic strain led to a qualitative reduction in striation, with both the number of striated myotubes (relative to non-striated) and the extent of striation clearly reduced. A similar finding has also been reported elsewhere,24 and may indicate that contrary to some other reports, cyclic mechanical strain of the amplitudes employed here may perturb sarcomeric assembly and maturation in vitro.
The application of cyclic strain also affected the rate of proliferation of C2C12s (Figure 3). After strain, a significant increase in proliferation rate was observed relative to before the application of strain, an effect also reported elsewhere and attributed to the modulation of extracellular signal-regulated kinase (ERK) phosphorylation as a direct result of mechanical stimulation.22 A small but statistically insignificant decrease in proliferation rate was observed for the cells that were not subjected to cyclic strain, as would be expected as the cells begin to terminally differentiate and withdraw from the cell cycle in response to serum withdrawal.
Figure 3.
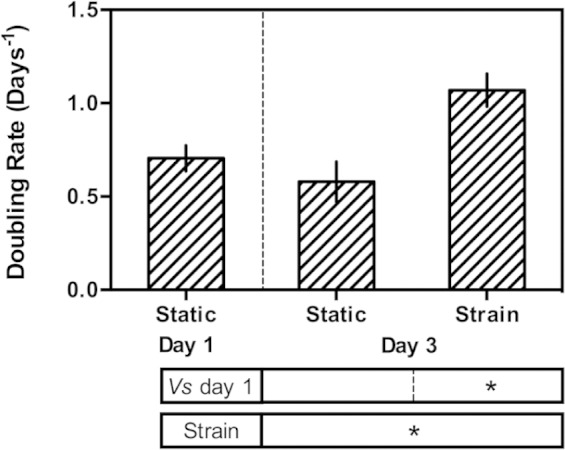
Proliferation of C2C12s expressed as doubling rate (number of doublings per day). Statistical differences arising from the application of cyclic strain and relative to day 1 (before application of strain) were determined using Student’s t-test. *denotes a statistically significant difference with p ≤ 0.05.
Whether cyclic mechanical strain is beneficial or detrimental for skeletal muscle myogenesis in vitro is unclear from the literature, with different studies drawing often very different conclusions. The choice of strain direction may be one aspect of experimental design that leads to variability between studies. The majority of the studies have been carried out using devices that deform a flexible-bottom culture plate using a vacuum (e.g. the FlexCell device used in this study). Such devices may be configured to exert uniaxial strain (as observed in the extension of a muscle) or equibiaxial (radial) strain which deforms cells in two orthogonal axes, and indeed, both geometries have been employed in studies on skeletal muscle myogenesis. It may be expected that uniaxial strain promotes myogenesis, and indeed, this has been reported,19 and yet, the opposite has also been demonstrated elsewhere.24 Likewise, equibiaxial strain may be expected to lead to decreased myogenesis as it has no physiological relevance for skeletal muscle, and yet a positive effect has been reported.20,21 In contrast, the detrimental effect of equibiaxial strain on myogenesis has also been reported in two independent studies, and both sets of researchers demonstrated a rational mechanism in the activation of cell cycle regulators via focal adhesion kinase (FAK) or ERK, respectively, leading to increased proliferation and a consequent decrease in myogenesis and terminal differentiation.22,23 The only study in which a direct comparison of uniaxial and equibiaxial cyclic strain was compared in skeletal muscle myogenesis found that uniaxial strain led to an increased number of myotubes formed from a larger number of myoblasts, whereas equibiaxial strain made little difference to the progression of myogenesis.19 One effect of strain which is clear, however, is in the imposition of orientation and long-range order in the nascent myotubes in vitro. Uniaxial strain has been reported to produce oriented cultures of parallel myotubes, whereas equibiaxial strain leads to no such long-range order.19,24
It seems likely from the results presented here and the seemingly confusing picture painted by the published literature that in fact the effect of cyclic mechanical strain on skeletal muscle myogenesis in vitro may be multifaceted. An increase in proliferation has clearly been demonstrated both in this study and elsewhere.22,23 As withdrawal from the cell cycle and cessation of proliferation is an integral process in terminal differentiation, it may be that in upregulating proliferation terminal differentiation is prevented or at least delayed. No such clear reduction in differentiation was observed here, however, possibly indicating a second process or group of processes by which cyclic strain may actually promote myogenesis or at least myotube fusion. Indeed, if two separate effects of cyclic strain act to prevent or delay onset of terminal differentiation while promoting other processes downstream of cell cycle withdrawal, this may go some way to explaining the apparent contradictions and variation in the published literature as subtle differences in experimental protocol may lead to different balances of the opposing effects. It should be noted, however, that the mechanism of a pro-myogenic effect of cyclic strain has yet to be elucidated.
Cyclic mechanical strain of MSCs in monoculture and coculture
In contrast to the C2C12s, after the period of culture under differentiation conditions, no evidence of myotube formation or MHC expression was observed by ICC in the MSC monocultures (Figure 4). In coculture, many small and some large MHC-positive myotubes formed and the two cell populations appeared homogeneously distributed on the collagen I–coated substrates (Figure 4(e) and (f)). A primary antibody against human specific lamin-A (a component of the nuclear envelope) was employed in order to distinguish the two cell populations by ICC. For most fields-of-view, the MHC-positive myotubes contained only mouse nuclei and had apparently formed only from C2C12s. However, a small number (approximately 1% or less) of MHC-positive myotubes clearly contained nuclei of both mouse and human or just human origin originating from both the C2C12s and MSCs or only from the MSC population (Figure 5). Great care was taken during confocal microscopy to ensure no crosstalk between channels by sequentially scanning with each excitation laser and detecting only narrow emission bands. The human nuclei were clearly located within the myotubes rather than above or below, as there was a clear reduction in intensity of MHC staining at the nuclear location as indicated by the white arrows in Figure 5(b).
Figure 4.
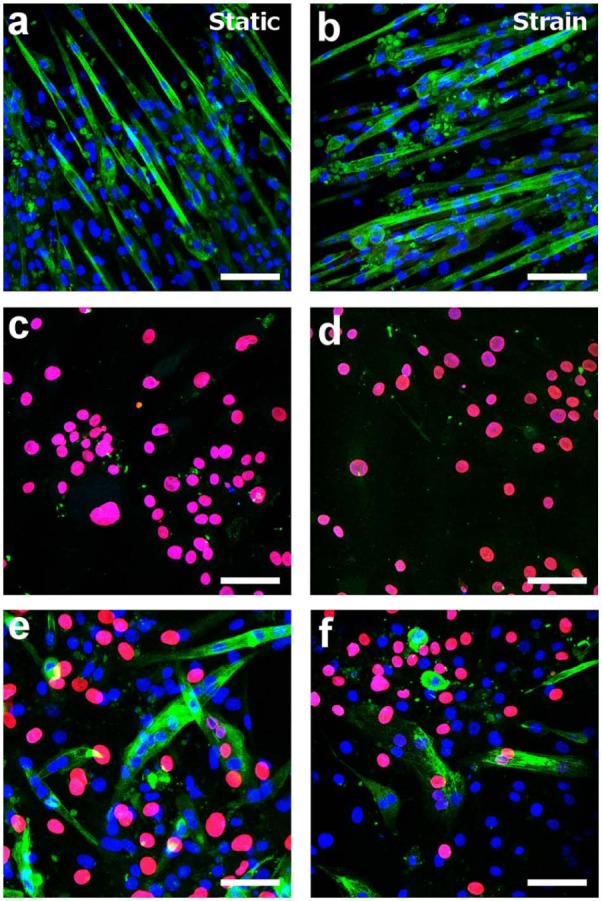
Confocal micrographs of monocultures and cocultures of C2C12s and MSCs under static and strained culture conditions. Green staining corresponds to MHC, red staining corresponds to human lamin-A (marker of human nuclei) and blue corresponds to nuclei (DAPI staining). Scale bars = 75 µm. (a) Static monoculture of C2C12s, (b) C2C12 monoculture subjected to cyclic strain, (c) static monoculture of MSCs, (d) MSC monoculture subjected to cyclic strain, (e) static coculture and (f) coculture subjected to cyclic strain.
MHC: myosin heavy chain; MSC: mesenchymal stem cell; DAPI: diamidinophenylindole.
Figure 5.
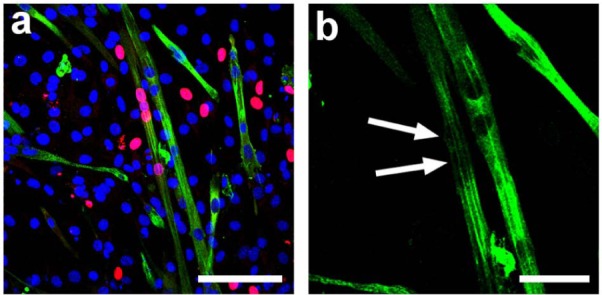
Confocal micrograph of a representative example of co-fusion of C2C12s and MSCs. Green staining corresponds to MHC, red staining corresponds to human lamin-A (marker of human nuclei) and blue corresponds to nuclei (DAPI staining). (a) Overlay of confocal channels. Scale bar = 100 µm. (b) high magnification scan of MHC channel. Arrows indicate location of human lamin-A positive nuclei. Scale bar = 50 µm.
MHC: myosin heavy chain; MSC: mesenchymal stem cell; DAPI: diamidinophenylindole.
Characteristically, the C2C12 myoblasts expressed the MRF MyoD1, both in monoculture (not shown) and in the coculture experiments (Figure 6). Some cytoplasmic localisation of MyoD1 was observed, although the majority of expression was localised to the nuclei suggesting functional activity in modulating downstream transcription. Some MyoD1 expression was observed in the MSCs in monoculture, although localisation to the nucleus was never observed. In monocultures of both the C2C12s and MSCs and in the coculture experiments, there was no qualitative difference in the expression and spatial distribution of MyoD1 in response to cyclic strain. In the small number of mixed mouse/human myotubes resulting from the coculture experiments, MyoD1 was localised to both the mouse and human nuclei, as indicated by colocalisation with human lamin-A (Figure 6). Although the antibody against MyoD1 was reactive against both murine and human forms, the observed colocalisation with human lamin-A indicated likely regulation of human skeletal muscle regulators and proteins downstream of MyoD1, assuming sufficient homology between murine and human forms of the transcription factor.
Figure 6.
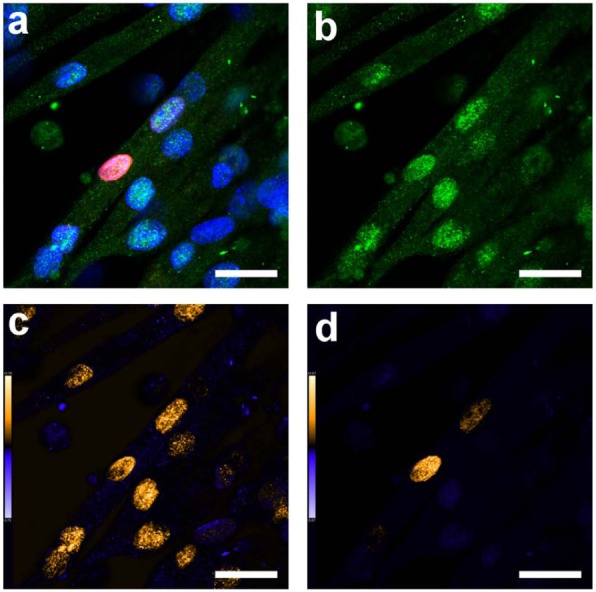
Representative confocal micrographs of MyoD1 expression in a myotube containing both mouse and human nuclei. Green staining corresponds to MyoD1 (reactive for both mouse and human), red staining corresponds to human lamin-A (marker of human nuclei) and blue corresponds to nuclei (DAPI staining). (a) Overlay of confocal channels; (b) MyoD1 channel; (c) colocalisation analysis of MyoD1 and DAPI (all nuclei) and (d) colocalisation analysis of MyoD1 and human lamin-A. The gold colour scale shows regions in which strong expression (greater than the mean for the image) was observed for both stains. The blue colour scale shows regions in which strong expression was only measured in one of the two stains. Scale bars = 25 µm.
DAPI: diamidinophenylindole.
Expression of skeletal muscle genes
Quantitative gene expression analysis of C2C12s in monoculture revealed a pattern of expression characteristic of terminally differentiating myoblasts (Table 2 and Figure 7). Relative to day 0 (proliferative myoblasts prior to terminal differentiation), Myod1 expression was slightly reduced and expression of Myog (myogenin) was significantly increased as expected when myogenic cells undergo terminal differentiation, withdraw from the cell cycle and begin fusion.26 Positive expression of Myh2 (encoding MHC) and Ttn (encoding the sarcomeric protein titin) was also observed, with significant increases in expression relative to day 0. In response to the application of cyclic strain, only Ttn expression was affected, with significantly reduced expression following the application of strain. While most published studies examining the effect of strain on myogenesis make no mention of Ttn expression, a recent study reported a decrease in observed striation of myotubes which was also observed here (Figure 2).24 It seems likely, therefore, that this strain regimen may perturb sarcomeric assembly as the myotubes mature, resulting in less striation and reduced expression of Ttn. However, it is not entirely clear by what mechanism this perturbation may occur. It may be that cyclic strain slows the differentiation and subsequent maturation, or at least delays onset of terminal differentiation, by the same mechanism as that responsible for the observed increase in proliferation (Figure 3). However, given that expression of Myog and Myh2, respectively, a regulator of terminal differentiation and a key sarcomeric protein, were unchanged in response to strain, it seems more likely that the process of maturation and sarcomeric assembly may be distinctly mechanosensitive via a mechanism not yet elucidated.
Table 2.
Expression of skeletal muscle markers in monoculture experiments.
MSC: mesenchymal stem cell; +: positive expression, −: no measurable expression under the conditions of the assay.
Figure 7.
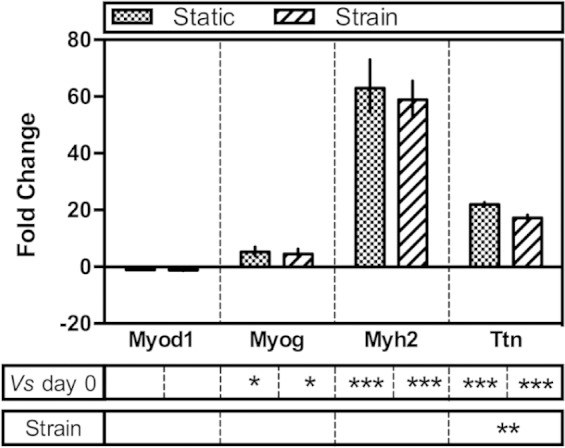
Expression of murine skeletal muscle genes in C2C12 monocultures by quantitative RT-PCR. Data are expressed as fold change relative to day 0 plus or minus the standard error of the mean. Statistical differences were measured by Student’s t-test and denoted with asterisks. ***statistical difference at p ≤ 0.001, **statistical difference at p ≤ 0.01 and *statistical difference at p ≤ 0.05.
RT-PCR: reverse transcriptase polymerase chain reaction.
In monoculture, no expression of skeletal muscle markers was observed in the MSCs, irrespective of the application of cyclic strain (Table 2). While there is some evidence in the literature that adipose-derived MSCs, such as those used in this study, are more likely to differentiate down the myogenic lineage than those from bone marrow, there remains little clarity on what conditions are required for such differentiation. It is perhaps unsurprising, however, that the adipose-derived MSCs failed to differentiate in response to simple serum withdrawal. Indeed, only recently have adipose-derived MSCs been shown to express myogenin and also undergo fusion in vitro under such conditions.13 Sarcomeric assembly was not shown, however, and the effect was only observed on matrices with carefully tuned mechanical properties.
In the coculture experiments, quantitative gene expression by PCR was carried out using strictly species-specific primers to interrogate expression of both murine and human skeletal muscle markers (i.e. expression originating from the C2C12s and MSCs respectively). Expression of murine markers revealed a very similar expression profile to the monoculture experiments and also showed the same response to cyclic strain, with significantly reduced expression of Ttn in the cultures subjected to strain (Table 3 and Figure 8(a)). Expression of human skeletal muscle markers in coculture revealed a strikingly different expression profile compared to the monoculture experiments however (Table 3 and Figure 8(b)). As in the monocultures, no expression of MYOD1 or MYOG was observed. Positive expression of MYH2 and TTN were observed, however, in contrast to the monoculture experiments although cyclic strain had no effect on levels of expression. The positive expression of MYH2 agrees with the ICC of the cocultures (Figure 5) as multinuclear myotubes containing human nuclei (from the MSCs) were observed.
Table 3.
Expression of skeletal muscle markers in coculture experiments.
: denotes positive expression; −: denotes no measurable expression under the conditions of the assay.
Figure 8.
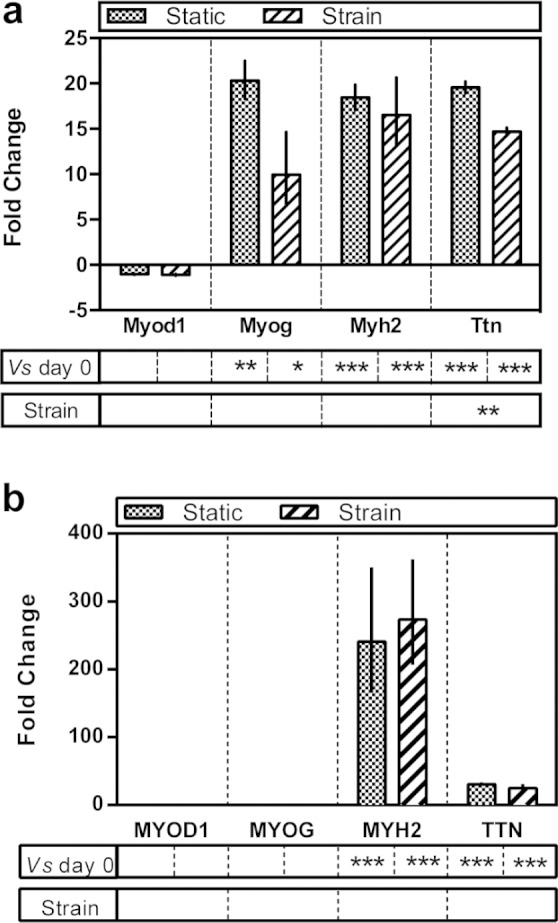
Expression of skeletal muscle genes in coculture experiments by quantitative RT-PCR (a) murine genes and (b) human genes. Data are expressed as fold change relative to day 0 plus or minus the standard error of the mean. Statistical differences were measured by Student’s t-test and denoted with asterisks. ***statistical difference at p ≤ 0.001, **statistical difference at p ≤ 0.01 and *statistical difference at p ≤ 0.05.
RT-PCR: reverse transcriptase polymerase chain reaction.
With the absence of expression of the key MRFs MyoD1 and myogenin, it seems highly unlikely that the MSCs had actually undergone myogenic differentiation in the true sense. Rather the observed phenomenon resonates more strongly with work published in the 1980s in which the existence of MRFs was first postulated.27 In this seminal study, primitive human cells collected from amniotic fluid were artificially fused with maturing murine myotubes. In much the same way as observed here, expression of human skeletal muscle–specific proteins soon followed which was taken as evidence that key MRFs must exist and that the cytoplasm-borne factors may activate transcription of skeletal muscle–specific genes. In their study, the different species of the two cell types was used as a tool in order to distinguish between expression arising from transcription from the two different nuclei within the same multinuclear cell. Whereas in their study, the co-fusion event was artificially induced using a chemical agent, the fusion demonstrated here is apparently spontaneous. Similar co-fusion of human MSCs with murine myoblasts has also been demonstrated more recently.14–17 In all such studies, however, the authors attribute the observed expression of human muscle markers to myogenic differentiation.
Expression of genes of other lineages
Given that no bulk myogenic differentiation of MSCs occurred and that only a small number of co-fusion events were observed, expression of key markers of the other major mesodermal lineages by the MSCs was also assessed (Figure 9). In monoculture, no significant changes in expression of runt related transcription factor 2 (RUNX2 a key regulator of osteogenic specification) or PPARG (peroxisome proliferator-activated receptor (PPAR)-gamma, a regulator of adipogenesis) were observed relative to day 0 or in response to cyclic strain. A small but statistically significant decrease in expression of activated leukocyte cell adhesion molecule (ALCAM), a marker of the multipotent MSC phenotype,28 was observed in static monocultures indicating a possible decrease in the multipotent fraction of the MSC population. However, given the small change (less than two-fold) and the lack of observed changes in RUNX2 and PPARG expression, it seems likely that this may indicate simply a small shift closer to senescence in the absence of other mechanical, biological or chemical factors.
Figure 9.
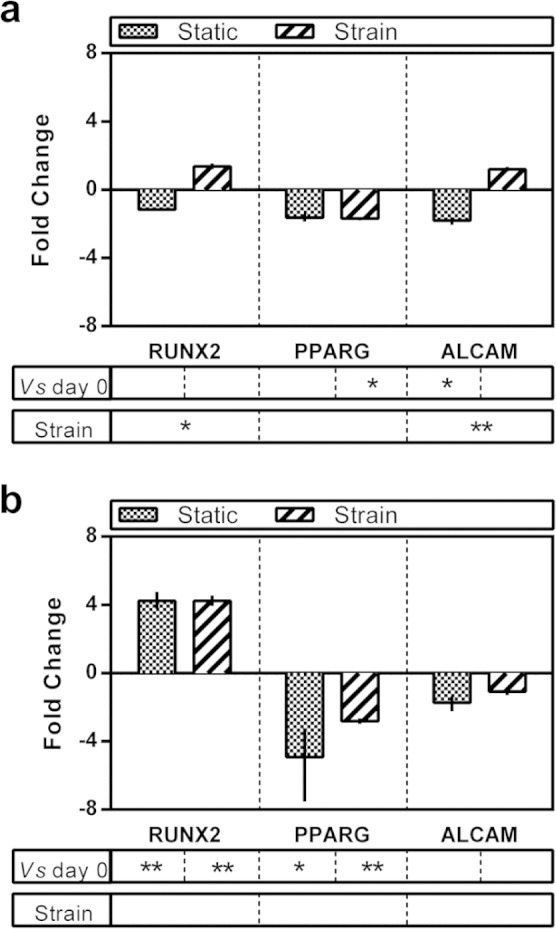
Expression of human mesodermal genes by quantitative RT-PCR. (a) MSC monoculture experiments. (b) Coculture experiments. Data are expressed as fold change relative to day 0 plus or minus the standard error of the mean. Statistical differences were measured by Student’s t-test and denoted with asterisks. ***statistical difference at p ≤ 0.001, **statistical difference at p ≤ 0.01 and *statistical difference at p ≤ 0.05.
RT-PCR: reverse transcriptase polymerase chain reaction; MSC: mesenchymal stem cell.
In coculture, a several-fold increase in RUNX2 expression and concurrent reduced expression of PPARG was observed although cyclic strain apparently made little difference to levels of expression. Some factor or combination of factors originating from the C2C12s in the coculture milieu clearly constitutes a pro-osteogenic environment for MSCs. Nonetheless the increase in RUNX2 and decrease in PPARG expression were only fourfold relative to day 0 and therefore indicate only a rather moderate differentiation event. Furthermore, expression of ALCAM was unchanged relative to day 0 irrespective of cyclic strain. It seems likely, therefore, that the majority of the MSCs in coculture remained in a multipotent state throughout the experiment.
Conclusion
Uniaxial cyclic mechanical strain was found to increase proliferation of C2C12 myoblasts. Although progression of terminal differentiation and fusion of myotubes was apparently unaffected by cyclic strain, maturation and sarcomeric assembly was perturbed. In coculture with human adipose–derived MSCs, a small number of MSCs fused with the C2C12 myotubes and expression of human sarcomeric proteins was observed irrespective of the application of cyclic strain. No expression of human MRFs was observed, however, indicating that true myogenic differentiation of MSCs did not occur, at least not via the usual sequence of signalling events. Nonetheless, the myogenic potential of adipose-derived MSCs was clearly evident, and the potential for such approaches in tissue engineering remains highly promising.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This study was funded by the Engineering and Physical Sciences Research Council through a doctoral prize fellowship.
References
- 1. Vindigni V, Mazzoleni F, Rossini K, et al. Reconstruction of ablated rat rectus abdominis by muscle regeneration. Plast Reconstr Surg 2004; 114: 1509–1515. [DOI] [PubMed] [Google Scholar]
- 2. Stern-Straeter J, Riedel F, Bran G, et al. Advances in skeletal muscle tissue engineering. In Vivo 2007; 21: 435–444. [PubMed] [Google Scholar]
- 3. Koning M, Harmsen MC, van Luyn MJA, et al. Current opportunities and challenges in skeletal muscle tissue engineering. J Tissue Eng Regen Med 2009; 3: 407–415. [DOI] [PubMed] [Google Scholar]
- 4. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–147. [DOI] [PubMed] [Google Scholar]
- 5. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002; 13: 4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone-marrow mesenchymal stem-cells exposed to 5-azacytidine. Muscle Nerve 1995; 18: 1417–1426. [DOI] [PubMed] [Google Scholar]
- 7. Gang EJ, Bosnakovski D, Simsek T, et al. Pax3 activation promotes the differentiation of mesenchymal stem cells toward the myogenic lineage. Exp Cell Res 2008; 314: 1721–1733. [DOI] [PubMed] [Google Scholar]
- 8. Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001; 7: 211–228. [DOI] [PubMed] [Google Scholar]
- 9. Mizuno H, Zuk PA, Zhu M, et al. Myogenic differentiation by human processed lipoaspirate cells. Plast Reconstr Surg 2002; 109: 199–209. [DOI] [PubMed] [Google Scholar]
- 10. Lin YF, Liu L, Li ZY, et al. Pluripotency potential of human adipose-derived stem cells marked with exogenous green fluorescent protein. Mol Cell Biochem 2006; 291: 1–10. [DOI] [PubMed] [Google Scholar]
- 11. Gang EJ, Jeong JA, Hong SH, et al. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells 2004; 22: 617–624. [DOI] [PubMed] [Google Scholar]
- 12. Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126: 677–689. [DOI] [PubMed] [Google Scholar]
- 13. Choi YS, Vincent LG, Lee AR, et al. Mechanical derivation of functional myotubes from adipose-derived stem cells. Biomaterials 2012; 33: 2482–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi DQ, Reinecke H, Murry CE, et al. Myogenic fusion of human bone marrow stromal cells, but not hematopoietic cells. Blood 2004; 104: 290–294. [DOI] [PubMed] [Google Scholar]
- 15. Lee JH, Kosinski PA, Kemp DM. Contribution of human bone marrow stem cells to individual skeletal myotubes followed by myogenic gene activation. Exp Cell Res 2005; 307: 174–182. [DOI] [PubMed] [Google Scholar]
- 16. Gentile A, Toietta G, Pazzano V, et al. Human epicardium-derived cells fuse with high efficiency with skeletal myotubes and differentiate toward the skeletal muscle phenotype: a comparison study with stromal and endothelial cells. Mol Biol Cell 2011; 22: 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee JH, Kemp DM. Human adipose-derived stem cells display myogenic potential and perturbed function in hypoxic conditions. Biochem Biophys Res Commun 2006; 341: 882–888. [DOI] [PubMed] [Google Scholar]
- 18. Behm DG, Chaouachi A. A review of the acute effects of static and dynamic stretching on performance. Eur J Appl Physiol 2011; 111: 2633–2651. [DOI] [PubMed] [Google Scholar]
- 19. Pennisi CP, Olesen CG, de Zee M, et al. Uniaxial cyclic strain drives assembly and differentiation of skeletal myocytes. Tissue Eng Part A 2011; 17: 2543–2550. [DOI] [PubMed] [Google Scholar]
- 20. Zhang SJ, Truskey GA, Kraus WE. Effect of cyclic stretch on beta1D-integrin expression and activation of FAK and RhoA. Am J Physiol Cell Physiol 2007; 292: C2057–C2069. [DOI] [PubMed] [Google Scholar]
- 21. Chandran R, Knobloch TJ, Anghelina M, et al. Biomechanical signals upregulate myogenic gene induction in the presence or absence of inflammation. Am J Physiol Cell Physiol 2007; 293: C267–C276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kook SH, Son YO, Choi KC, et al. Cyclic mechanical stress suppresses myogenic differentiation of adult bovine satellite cells through activation of extracellular signal-regulated kinase. Mol Cell Biochem 2008; 309: 133–141. [DOI] [PubMed] [Google Scholar]
- 23. Kumar A, Murphy R, Robinson P, et al. Cyclic mechanical strain inhibits skeletal myogenesis through activation of focal adhesion kinase, Rac-1 GTPase, and NF-kappaB transcription factor. FASEB J 2004; 18: 1524–1535. [DOI] [PubMed] [Google Scholar]
- 24. Boonen KJM, Langelaan MLP, Polak RB, et al. Effects of a combined mechanical stimulation protocol: value for skeletal muscle tissue engineering. J Biomech 2010; 43: 1514–1521. [DOI] [PubMed] [Google Scholar]
- 25. Greiner AM, Chen H, Spatz JP, et al. Cyclic tensile strain controls cell shape and directs actin stress fiber formation and focal adhesion alignment in spreading cells. PLoS One 2013; 8: e77328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wright WE, Sassoon DA, Lin VK. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell 1989; 56: 607–617. [DOI] [PubMed] [Google Scholar]
- 27. Blau HM, Chiu CP, Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell 1983; 32: 1171–1180. [DOI] [PubMed] [Google Scholar]
- 28. McMurray RJ, Gadegaard N, Tsimbouri PM, et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater 2011; 10: 637–644. [DOI] [PubMed] [Google Scholar]