Abstract
Advances in allograft processing have opened new horizons for clinical adaptation of flexor tendon allografts as delivery scaffolds for antifibrotic therapeutics. Recombinant adeno-associated-virus (rAAV) gene delivery of the growth and differentiation factor 5 (GDF-5) has been previously associated with antifibrotic effects in a mouse model of flexor tendoplasty. In this study, we compared the effects of loading freeze-dried allografts with different doses of GDF-5 protein or rAAV-Gdf5 on flexor tendon healing and adhesions. We first optimized the protein and viral loading parameters using reverse transcription polymerase chain reaction (RT-PCR), enzyme-linked immunosorbent assay (ELISA), and in vivo bioluminescent imaging. We then reconstructed flexor digitorum longus (FDL) tendons of the mouse hindlimb with allografts loaded with low and high doses of recombinant GDF-5 protein and rAAV-Gdf5 and evaluated joint flexion and biomechanical properties of the reconstructed tendon. In vitro optimization studies determined that both the loading time and concentration of the growth factor and viral vector had dose-dependent effects on their retention on the freeze-dried allograft. In vivo data suggest that protein and gene delivery of GDF-5 had equivalent effects on improving joint flexion function, in the range of doses used. Within the doses tested, the lower doses of GDF-5 had more potent effects on suppressing adhesions without adversely affecting the strength of the repair. These findings indicate equivalent antifibrotic effects of Gdf5 gene and protein delivery, but suggest that localized delivery of this potent factor should also carefully consider the dosage used to eliminate untoward effects, regardless of the delivery mode.
Keywords: Flexor tendon, allograft, adhesions, growth and differentiation factor 5, tissue engineering
Introduction
Fibrosis and adhesions are frequent complications of flexor tendon injury in the hand.1 Despite decades of research, an excellent outcome after flexor tendon surgery is still dependent on a skilled and experienced surgeon, a qualified team of occupational therapists, and a very motivated patient. One of the most effective advances in flexor tendon repair is the implementation of early post-operative mobilization, which has become feasible in part due to the development of stronger and more refined suturing techniques.2 Because of these advances, primary repair outcomes in Zone II injuries are now more successful, and grafts are less frequently used in flexor tendon reconstruction. However, tendon allografts can be the only option in cases of revision surgery and multi-tendon injuries in mutilating scenarios such as combat injuries,1,3 especially with limitations associated with autografts availability4,5 and the lack of clinically proven tissue-engineered biomaterial scaffolds.6
While clinical use of allografts has not been favored in flexor tendoplasty, recent advances in graft processing have enabled novel regenerative applications on the bench and in preclinical models. For example, intrasynovial flexor tendon allografts have been successfully decellularized without affecting the mechanical properties or chemical composition of the tissue7 and then revitalized by seeding different types of cells (tendon and sheath fibroblasts and stem cells).8 These studies demonstrate the conceptual feasibility of engineering intrasynovial flexor tendon grafts with epitenon cell layer seeding. However, cell-based tissue engineering approaches still face significant regulatory hurdles before they can become a clinical option.
Alternatively, tendon allografts can be decellularized to minimize the recipient’s immune response and can be modified with growth factors to enable their remodeling and incorporation into the host. The growth and differentiation factors (GDFs) 5, 6, and 7 are members of the bone morphogenetic protein (BMP) family and have been implicated in tendon development and repair.9–12 It has been previously demonstrated that freeze-dried tendon allografts loaded with recombinant adeno-associated virus (rAAV) for local and transient Gdf5 gene delivery significantly reduced tendon adhesions and restored the metatarsophalangeal (MTP) joint flexion in mice.13 Given that these growth factors are morphogens with varied and dose-dependent effects throughout the body that are not limited to tendon biology,14 we therefore sought to investigate doses that might enhance the repair strength while abating any fibrotic scarring. Considering the differences in kinetics of action of protein (immediate signaling effects) and viral gene delivery (delayed effects that involve transfection, gene expression, protein translation, and signaling), we hypothesized that rAAV-Gdf5 delivery via freeze-dried tendon allografts will provide a prolonged window of sustained therapeutic effects to improve the tendon biomechanical properties and abolish the fibrotic adhesions. To test this hypothesis, we set out first to optimize the retention of the rAAV particles or the recombinant GDF-5 protein on freeze-dried tendon allograft. We then compared the dose-dependent effects of rAAV-Gdf5 or GDF-5 protein on the MTP joint flexion and biomechanics of reconstructed mouse flexor digitorum longus (FDL) tendons.
Materials and methods
Preparation of FDL tendon allografts
FDL tendon grafts were aseptically dissected from donors (C57Bl/6 mice) and lyophilized as previously described.15 The grafts were then digitally imaged to determine their surface area (Image J software, http://rsb.info.nih.gov/ij). The lyophilized tendon grafts were placed in 100µL phosphate buffer solution (PBS) on ice containing rAAV2.5/CMV-LacZ (Virus Vector Core Facility, University of North Carolina, Chapel Hill, NC), rAAV2.5/CMV-Gdf5 (custom clone previously published13), or recombinant murine GDF-5 protein (R&D Systems, Minneapolis, MN). After the tendon grafts have been dipped in the rAAV or protein solution for a designated time (as described later), they were lyophilized and stored frozen at −80°C for 1–7 days until analyzed or used for tendon surgeries.
Assessment of rAAV loading and retention
To optimize the viral particle loading conditions, several experiments were performed. In the first experiment, the lyophilized grafts were rehydrated in a solution containing rAAV-LacZ (5 × 109 particles/100 µL) for 5, 15, 30, and 120 min as well as 24 h. In the second experiment, different concentrations of rAAV-LacZ (5 × 107 to 5 × 1010 particles/100 µL) were used to rehydrate the allografts for 120 min. To assess the retention of rAAV particles, the processed rAAV-LacZ-loaded FDL tendon grafts were digested in proteinase K (10 µg/mL). Real-time reverse transcription polymerase chain reaction (RT-PCR) was used to calculate the rAAV content in the tendon samples based on a standard curve in the range of 104–1010 particles/100 µL. Gene expression was measured in 9–12 grafts.
Assessment of rmGDF-5 protein loading and retention
To optimize therapeutic protein–loading conditions, FDL tendon grafts were processed aseptically by freeze-drying and then dipped in PBS solutions containing rmGDF-5 (10 or 50 ng/µL with 3% bovine serum albumin (BSA) as a carrier protein) for 2 or 24 h. To assess the retention of the protein, the processed rmGDF-5-loaded FDL tendon grafts were eluted in 120 µL blocking buffer (PBS with 3% BSA and 0.05% Tween-20) for 2 h on ice, and the eluate was analyzed by enzyme-linked immunosorbent assay (ELISA).16 The optical density (OD) for each well was read with a plate reader (Synergy Mx Multi-Mode Reader, BioTek, Winooski, VT) at 450 nm wavelength, and calibrated for GDF-5 concentration against a standard curve (10–1000 ng/mL), which was included in each ELISA plate. The limit of detection of the assay was 5 ng/mL, and the coefficient of variance for the assay <<1%. GDF-5 protein retention was assayed in 9–12 grafts.
Surgical procedure—FDL tendon defect reconstruction (tendoplasty)
All animal studies utilized C57Bl/6 mice, were performed in compliance with institutionally approved animal use and care protocols, and followed the latest version of the Declaration of Helsinki. A total of 24 h before the tendon reconstruction surgery, the flexor (calf) muscles of the left hind limb of the mouse was injected with BOTOX® (Allergan Pharmaceuticals, Irvine, CA) to induce transient paralysis of the flexor muscles in order to protect the reconstructed tendon from rupture upon recovery, while allowing controlled, incremental recovery of muscle forces (see Supplementary Material). The next day, aseptic FDL tendoplasty surgeries were preformed as previously described.13,15 Preoperatively, the animals were treated with one subcutaneous injection of buprenorphine (0.05 mg/kg). Next, the distal FDL tendon of the left hind paw was exposed and transected to create a 3 mm defect at the metatarsal level. A lyophilized allograft loaded with rAAV or recombinant protein (using the dip time of 2 h) was used to reconstruct the severed tendon using modified horizontal mattress suturing (8–0 nylon suture). Following recovery, the animals were allowed to ambulate freely, and post-operative subcutaneous injections of banamine (0.5 mg/kg) were given every 24 h for up to 3 days.
Bioluminescent imaging
To investigate the dose effects on gene delivery kinetics and biodistribution up to 14 days, which corresponds to peak adhesion formation in previous studies,13 rAAV-Luc-loaded allografts were implanted in the FDL tendons. Prior to bioluminescent imaging (BLI), an intraperitoneal injection of D-luciferin potassium salt (PerkinElmer, Waltham, MA) was administered to each animal. The rAAV-induced bioluminescence was then imaged using a 3-min exposure on the IVIS Spectrum Imaging System (PerkinElmer), and the signal intensity was quantified over a consistent region of interest encompassing the operated foot (n = 4 per treatment), as previously described.13
Assessment of MTP joint flexion
After 14 days of healing, the mice (n = 8 per treatment) were euthanized, and the hind limbs were dissected below the knee and stored frozen (−20°C) until tested. On the day of testing, the proximal FDL tendon was severed from the muscle at the tibia without compromising the healing tissue in the foot. The free tendon end was reinforced using tape and cyanoacrylate. The limb was then inversely suspended in a custom jig where the tibia was secured to prevent sliding and rotation. The flexion angle of the MTP joint under incremental loading was then measured as previously described.15 The flexion data were used to derive functional parameters, including the flexion range of motion (ROM) as previously described.13,15
Biomechanical tensile testing
Immediately following the assessment of MTP joint flexion, the tendon was released at the tarsal tunnel, and then tested in tension at a rate of 30 mm/min to failure on the Instron 8841 DynaMight™ axial servohydraulic testing system (Instron Corporation, Norwood, MA) as described.13,15 The maximum tensile force and stiffness were derived from force–displacement plots.
Statistical analysis
Data analysis included t-tests, analysis of variance (ANOVA) with Fisher’s least significant difference (LSD) post hoc multiple comparisons (α = 0.05), and non-linear regression to derive the MTP flexion parameters.
Results
Assessment of retention of rAAV particles and protein in tendon grafts in vitro
To optimize the performance of rAAV and rmGDF-5-loaded tendon grafts in vivo, we first sought to determine the effects of the concentration and dipping time on graft retention in vitro. Not surprisingly, we found significant incremental effects on the retention of rAAV-LacZ due to increasing the dipping time (Figure 1(a)). While there were no differences in retention between 5 and 60 min, increasing dip-coating time to 120 min significantly increased the retention of rAAV particles on the freeze-dried tendon graft compared to 5 and 15 min. Increasing the dip-coating time to 24 h significantly increased the retention of rAAV particles on the freeze-dried tendon graft compared to all other loading times. We also investigated the effects of rAAV concentration in the dipping solution, and observed a dose-dependent improvement in the retention of rAAV particles on the graft (Figure 1(b)) with increased dipping solution concentration.
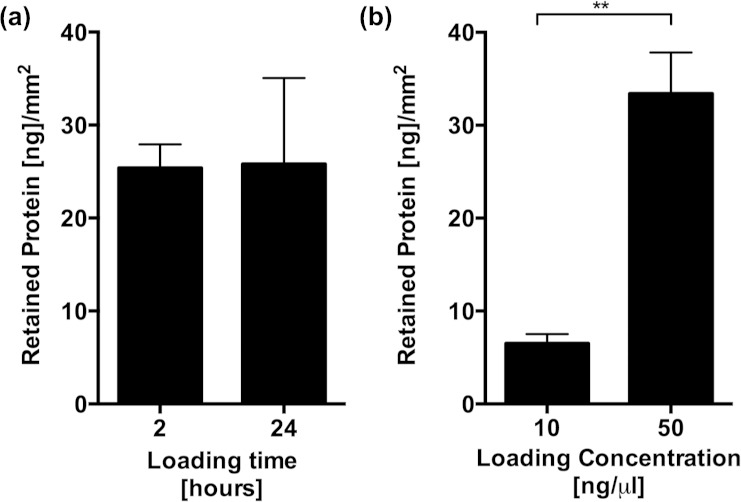
Figure 1.
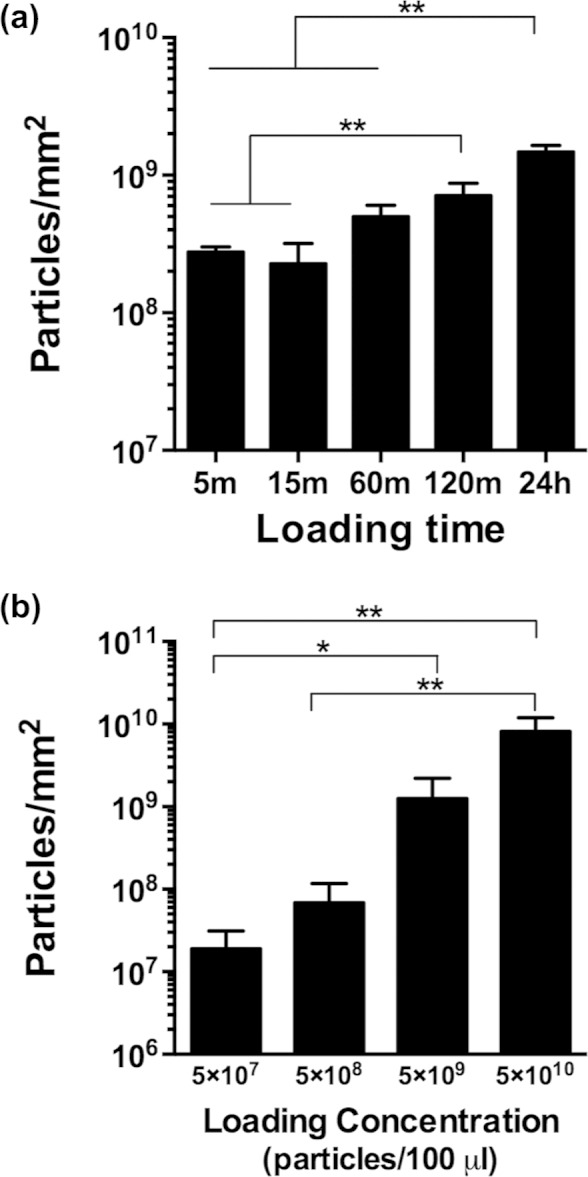
Retention of rAAV particles on tendon grafts, determined using a quantitative real time PCR assay and primers specific for LacZ. Data are presented as mean ± SEM, normalized to the surface area of the graft. Asterisks represent significant differences, *p < 0.05; **p < 0.01.
rAAV: recombinant adeno-associated-virus; PCR: polymerase chain reaction; SEM: standard error of the mean.
There were no differences in retention of rmGDF-5 between 2 and 24 h dipping times (Figure 2(a)). However, there were significant concentration-dependent effects on the retention of rmGDF-5 on the graft (Figure 2(b)).
Figure 2.
Retention of recombinant GDF-5 protein in tendon grafts determined using an ELISA assay. Data are presented as mean ± SEM, normalized to the surface area of the graft. Asterisks represent significant differences, **p < 0.01.
GDF-5: growth and differentiation factor 5; ELISA: enzyme-linked immunosorbent assay; SEM: standard error of the mean.
Given that we determined from the in vitro loading and retention studies that 2 h is an ideal time for loading that would in theory enable point-of-care reconstitution of the grafts, all subsequent in vivo experiments involved grafts that have been reconstituted with either rAAV or protein suspensions for 2 h.
Longitudinal assessment of rAAV-mediated gene delivery in vivo
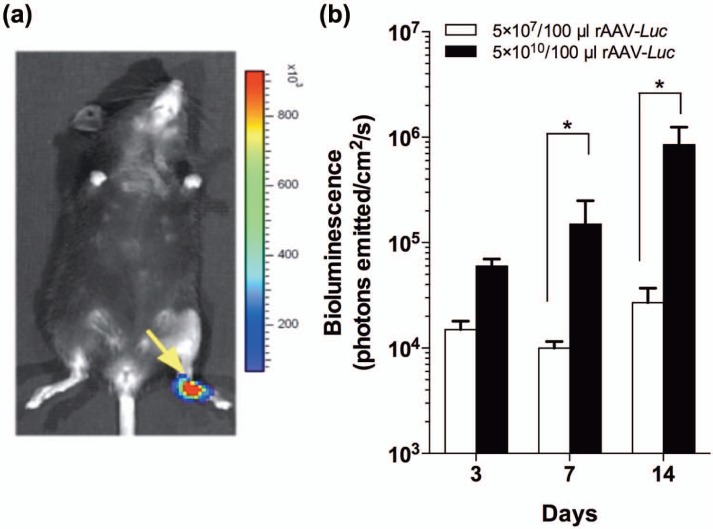
To determine the dose effects on the biodistribution and kinetics of reporter gene delivery and transduction in vivo, allografts were loaded with 5 × 107 or 5 × 1010 particles of rAAV-Luc, and implanted in FDL tendon defects. BLI was performed on days 3, 7, and 14 after grafting. As previously reported,13 bioluminescence was restricted to the grafted foot (Figure 3(a)). Furthermore, gene transduction, measured by BLI signal intensity, was dose-dependent, with the lower dose (5 × 107 particles/100 µL) inducing significantly less-intense bioluminescence at days 7 and 14 compared to the higher dose (p < 0.05; Figure 3(b)).
Figure 3.
Kinetics and biodistribution of rAAV and allograft-mediated gene expression. (a) Representative BLI of a mouse grafted with a freeze-dried FDL allograft loaded with 5 × 1010 particles of rAAV-Luc. (b) In vivo kinetics of Luc gene expression, based on bioluminescence intensity in a ROI encompassing the foot (mean value ± SEM).
BLI: bioluminescent imaging; ROI: region of interest; rAAV: recombinant adeno-associated-virus; SEM: standard error of the mean; FDL: flexor digitorum longus.
Effects of rAAV-Gdf5 and rmGDF-5 on allograft healing after FDL tendoplasty
To assess functional effects of GDF-5 gene and protein delivery, MTP flexion and biomechanical tensile testing were performed successively, MTP flexion tests (Figure 4) demonstrated that the lower dose (5 × 107 particles/100 µL) rAAV-Gdf5-loaded allografts had significantly improved MTP joint ROM (p < 0.05; Figure 4) at 14 days post grafting, while the higher dose (5 × 1010 particles/100 µL) allografts were not significantly different from rAAV-LacZ-loaded controls. Similarly, the lower dose of rmGDF-5-loaded allografts (10 ng/µL) significantly improved MTP ROM compared to controls (p < 0.05; Figure 4) at 14 days post reconstruction, while the higher doses (50 ng/µL) were not significantly different from controls.
Figure 4.
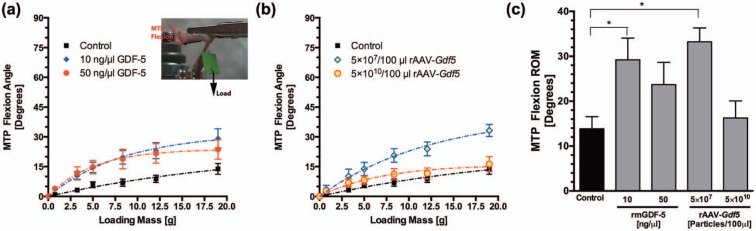
Assessment of MTP joint flexion (inset) following reconstruction with rmGDF-5 or rAAV-Gdf5-loaded allografts at 14 days post surgery. (a and b) Average MTP joint flexion curves and (c) maximum MTP flexion ROM over the range of applied weights of the control (rAAV-LacZ loaded) allografts, rmGDF-5-loaded allografts, and rAAV-Gdf5-loaded allografts. Data are presented as mean ± SEM. Asterisks represent significant differences from control repairs, *p < 0.05.
MTP: metatarsophalangeal; GDF-5: growth and differentiation factor 5; ROM: range of motion; rAAV: recombinant adeno-associated-virus; SEM: standard error of the mean.
The tensile strength and elasticity (maximum force and stiffness, respectively) tended to increase with both doses of rmGDF-5-loaded allografts but not the rAAV-Gdf5-loaded allografts (Figure 5), but these differences were not statistically different from the untreated controls.
Figure 5.
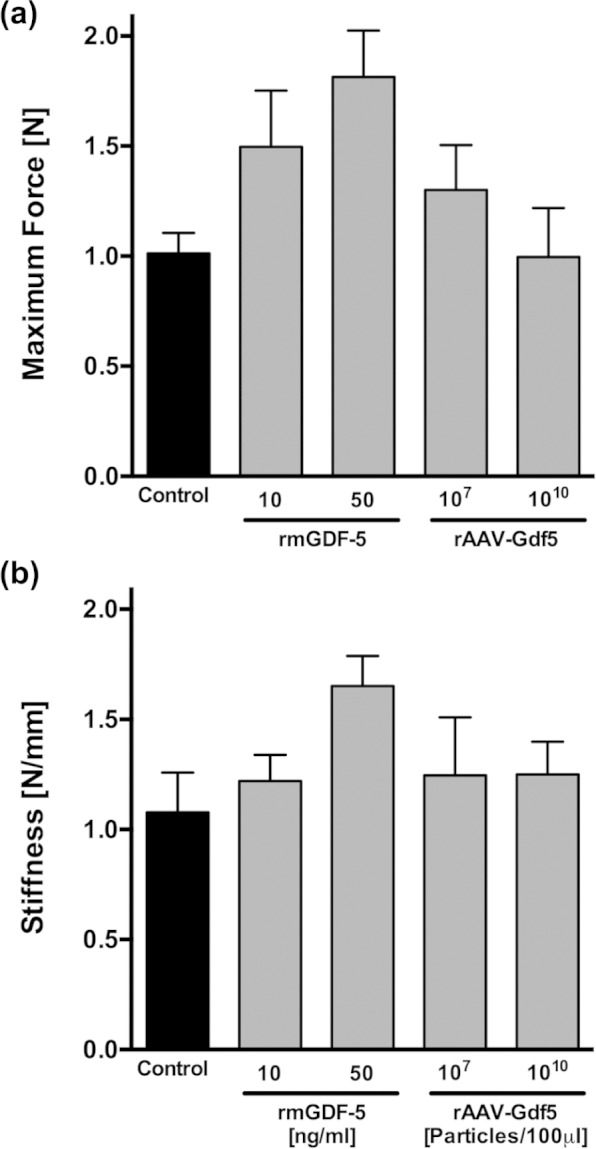
Assessment of tensile biomechanical properties of the FDL tendon following reconstruction with rmGDF-5 or rAAV-Gdf5-loaded allografts at 14 days post surgery. (a) Maximum tensile force (strength) and (b) tensile stiffness of the FDL tendons reconstructed with rAAV-LacZ-loaded (control) allografts, rmGDF-5-loaded allografts, and rAAV-Gdf5-loaded allografts. Biomechanical properties were measured at 14 days post surgery. Data are presented as mean ± SEM.
GDF-5: growth and differentiation factor 5; rAAV: recombinant adeno-associated-virus; SEM: standard error of the mean; FDL: flexor digitorum longus.
Discussion
Localized and sustained delivery systems of growth factors to sites of skeletal injury remain a substantial barrier in tissue engineering. A common component of growth factor delivery systems is a biomaterial carrier to provide localization and spatiotemporal regulation of their bioavailabilty after implantation. Biomaterial carriers can be classified in general terms into extracellular matrix (ECM)-mimicking polymer scaffolds or naturally derived ECM scaffolds.17 ECM scaffolds such as freeze-dried allografts have a number of desirable characteristics over synthetic polymers in tendon tissue engineering. Tendon allografts have been shown to maintain their biomechanical properties when freeze-dried.15 Their lack of cells and immunologically mismatched cell surface antigens minimize the foreign body response of the recipient’s immune system. They can also be infiltrated by host cells, including fibroblasts, allowing for their incorporation and remodeling in vivo.15,18 Previous studies have demonstrated the feasibility of creating tendon/ligament scaffolds from freeze-dried allografts,19 and others have demonstrated that decellularized allograft tendon can potentially be combined with donor cells to repair the anterior cruciate ligament (ACL)20 or flexor tendon.8 In addition, tendon allografts remain hydrophilic, which enables robust hydration and loading of therapeutics by simply dipping the grafts in an aqueous pharmaceutical solution.13 In this study, we optimized techniques to use freeze-dried flexor tendon allografts as growth factor and viral gene delivery systems. Both the concentration of the growth factor and titer of the viral vector had a dose-dependent effect on the retention of the therapeutics on the freeze-dried allograft. Maximum retention of GDF-5 protein was achieved within 2 h of reconstituting the graft in the therapeutic solution. More importantly, we found no significant differences between the therapeutic effects of the recombinant protein of rAAV-mediated gene delivery of GDF-5 in the range of doses used. This latter observation is consistent with some previous results that demonstrated that low doses of rmGDF-5 and rAAV-Gdf5 have significant effects on scratch closure rate of monolayer fibroblasts in vitro.13 The allograft-mediated delivery approach is a clinically compatible procedure. Conceptually, a Food and Drug Administration (FDA)-approved drug or factor can be combined with the allograft at the point-of-care (e.g. the operating room). However, while rAAV represents a class of gene delivery viral vectors with an acceptable safety profile and is being clinically tested in numerous of FDA-approved protocols (http://www.clinicaltrials.gov), there are currently no approved viral vectors for wide orthopedic use. Given that the effects of the protein and the rAAV gene delivery vector were equivalent, it is more likely that recombinant forms of the protein GDF-5 will have a faster route to the clinic, especially since recombinant BMPs are currently in clinical use in orthopedic surgery.
The effects of various growth factors on tendon healing have been extensively studied.21 Members of the BMP family, known as GDFs, have been of particular interest in this area because of their demonstrable induction of tendon phenotype in vitro and in vivo and their acceleration of tendon healing in preclinical models.9–12,16,22–24 GDF-5 (also called BMP-14) is one of the GDF isoforms whose genetic knockout in mice deregulates tail and Achilles tendon (collagen) ultrastructure leading to inferior biomechanics.10,22 It is for these observations that a number of therapeutic and tissue engineering strategies in tendon repair have focused on GDF-5. For example, Rickert et al.11 demonstrated that coating of surgical suture with GDF-5 accelerates Achilles tendon healing in a rodent model. Yet, the antifibrotic effects of GDF-5 on flexor tendon adhesions have only been recently reported,13 and confirmed in this study.
While growth factors often exert potent therapeutic effects, they can also trigger ectopic or untoward responses from targeted or untargeted tissues and cells. For example, factors such as GDFs are capable of driving ectopic differentiation of stem cells to tendon, cartilage, and bone at varying dosages.12,25 Indeed, within the doses tested, our findings suggest that the lower doses of GDF-5 (delivered either as protein or via rAAV) have more potent effects on suppressing the fibrotic response in tendon healing that leads to adhesions. Interestingly, in vivo investigations of tendon repair have previously raised concerns regarding dosage of GDF. For example, ectopic cartilage formation has been reported in preclinical animal models investigating GDF-5, especially at higher doses.26 A time course histological analysis of the stages of the repair response was beyond the scope of this study, but will be pursued in future studies to delineate the dose-dependent differences in the biology of tendon healing and the emergences of aberrant tissue differentiation, if any. Nevertheless, our data suggest that localized delivery of GDF-5 delivery must employ a minimal dosage of the growth factor to suppress fibrotic adhesions in our murine model. A more formal investigation of the dose-response effects of GDF-5 should be pursued in future studies.
The antifibrotic mechanism of action of GDF-5 is not understood. A common denominator in the abnormal fibrosis in a number of tissues is transforming growth factor β (TGF-β) presumably through inactivation of matrix metalloproteinases (MMPs).27–31 We have previously demonstrated that TGF-β/Smad3 loss-of-function in Smad3−/− mice leads to improved FDL tendon gliding and MTP joint flexion following surgical repair.32 Others have shown that specific blockade of TGF-β1/Smad3 signaling is a potent therapeutic intervention against fibrosis.31 In mesangial cells, TGF-β1 increases cell-associated ECM, including collagen IV and fibronectin and decreases the level and activity of MMP-2, thereby causing renal fibrosis.28 It has been demonstrated that BMP-7 antagonizes TGF-β1 effects by rescuing MMP-2 activity.28 Given that GDF-5 shares similar attributes with BMP-7 in terms of its receptor binding affinity and signaling,33 it is plausible that GDF-5 could exert similar effects on rescuing MMP-mediated remodeling, albeit this has yet to be demonstrated experimentally.
In summary, this study demonstrates that flexor tendon allografts can be manipulated effectively for localized therapeutic delivery, which opens new horizons for clinical utility of flexor tendon allografts, and suggests that localized delivery of potent growth factors, such as GDF-5, should carefully consider the dosage used to obtain the desired efficacy and eliminate untoward effects.
Supplementary Material
Footnotes
Declaration of conflicting interests: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or NIH.
Funding: This research was funded by Grants R01AR056696 and S10RR026542 from NIH. Additional funding was received from the Danish Rheumatism Association.
References
- 1. Taras JS, Lamb MJ. Treatment of flexor tendon injuries: surgeons’ perspective. J Hand Ther 1999; 12(2): 141–148. [DOI] [PubMed] [Google Scholar]
- 2. Strickland JW. The scientific basis for advances in flexor tendon surgery. J Hand Ther 2005; 18(2): 94–110. [DOI] [PubMed] [Google Scholar]
- 3. Coyle MP, Jr, Leddy TP, Leddy JP. Staged flexor tendon reconstruction fingertip to palm. J Hand Surg Am 2002; 27(4): 581–585. [DOI] [PubMed] [Google Scholar]
- 4. LaSalle WB, Strickland JW. An evaluation of the two-stage flexor tendon reconstruction technique. J Hand Surg Am 1983; 8(3): 263–267. [DOI] [PubMed] [Google Scholar]
- 5. Gelberman RH, Seiler JG, 3rd, Rosenberg AE, et al. Intercalary flexor tendon grafts. A morphological study of intrasynovial and extrasynovial donor tendons. Scand J Plast Reconstr Surg Hand Surg 1992; 26(3): 257–264. [DOI] [PubMed] [Google Scholar]
- 6. Chang J. Studies in flexor tendon reconstruction: biomolecular modulation of tendon repair and tissue engineering. J Hand Surg Am 2012; 37(3): 552–561. [DOI] [PubMed] [Google Scholar]
- 7. Zhang AY, Bates SJ, Morrow E, et al. Tissue-engineered intrasynovial tendons: optimization of acellularization and seeding. J Rehabil Res Dev 2009; 46(4): 489–498. [DOI] [PubMed] [Google Scholar]
- 8. Thorfinn J, Saber S, Angelidis IK, et al. Flexor tendon tissue engineering: temporal distribution of donor tenocytes versus recipient cells. Plast Reconstr Surg 2009; 124(6): 2019–2026. [DOI] [PubMed] [Google Scholar]
- 9. Aspenberg P, Forslund C. Enhanced tendon healing with GDF 5 and 6. Acta Orthop Scand 1999; 70(1): 51–54. [DOI] [PubMed] [Google Scholar]
- 10. Chhabra A, Tsou D, Clark RT, et al. GDF-5 deficiency in mice delays Achilles tendon healing. J Orthop Res 2003; 21(5): 826–835. [DOI] [PubMed] [Google Scholar]
- 11. Rickert M, Jung M, Adiyaman M, et al. A growth and differentiation factor-5 (GDF-5)-coated suture stimulates tendon healing in an Achilles tendon model in rats. Growth Factors 2001; 19(2): 115–126. [DOI] [PubMed] [Google Scholar]
- 12. Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest 1997; 100(2): 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Basile P, Dadali T, Jacobson J, et al. Freeze-dried tendon allografts as tissue-engineering scaffolds for Gdf5 gene delivery. Mol Ther 2008; 16(3): 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hsu C, Chang J. Clinical implications of growth factors in flexor tendon wound healing. J Hand Surg Am 2004; 29(4): 551–563. [DOI] [PubMed] [Google Scholar]
- 15. Hasslund S, Jacobson JA, Dadali T, et al. Adhesions in a murine flexor tendon graft model: autograft versus allograft reconstruction. J Orthop Res 2008; 26(6): 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rickert M, Wang H, Wieloch P, et al. Adenovirus-mediated gene transfer of growth and differentiation factor-5 into tenocytes and the healing rat Achilles tendon. Connect Tissue Res 2005; 46(4–5): 175–183. [DOI] [PubMed] [Google Scholar]
- 17. Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface 2011; 8(55): 153–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Potenza AD, Melone C. Evaluation of freeze-dried flexor tendon grafts in the dog. J Hand Surg Am 1978; 3(2): 157–162. [DOI] [PubMed] [Google Scholar]
- 19. Whitlock PW, Smith TL, Poehling GG, et al. A naturally derived, cytocompatible, and architecturally optimized scaffold for tendon and ligament regeneration. Biomaterials 2007; 28(29): 4321–4329. [DOI] [PubMed] [Google Scholar]
- 20. Tischer T, Vogt S, Aryee S, et al. Tissue engineering of the anterior cruciate ligament: a new method using acellularized tendon allografts and autologous fibroblasts. Arch Orthop Trauma Surg 2007; 127(9): 735–741. [DOI] [PubMed] [Google Scholar]
- 21. Goh JC, Ouyang HW, Teoh SH, et al. Tissue-engineering approach to the repair and regeneration of tendons and ligaments. Tissue Eng 2003; 9(Suppl. 1): S31–S44. [DOI] [PubMed] [Google Scholar]
- 22. Clark RT, Johnson TL, Schalet BJ, et al. GDF-5 deficiency in mice leads to disruption of tail tendon form and function. Connect Tissue Res 2001; 42(3): 175–186. [DOI] [PubMed] [Google Scholar]
- 23. Mikic B. Multiple effects of GDF-5 deficiency on skeletal tissues: implications for therapeutic bioengineering. Ann Biomed Eng 2004; 32(3): 466–476. [DOI] [PubMed] [Google Scholar]
- 24. Mikic B, Schalet BJ, Clark RT, et al. GDF-5 deficiency in mice alters the ultrastructure, mechanical properties and composition of the Achilles tendon. J Orthop Res 2001; 19(3): 365–371. [DOI] [PubMed] [Google Scholar]
- 25. Shimaoka H, Dohi Y, Ohgushi H, et al. Recombinant growth/differentiation factor-5 (GDF-5) stimulates osteogenic differentiation of marrow mesenchymal stem cells in porous hydroxyapatite ceramic. J Biomed Mater Res A 2004; 68(1): 168–176. [DOI] [PubMed] [Google Scholar]
- 26. Dines JS, Weber L, Razzano P, et al. The effect of growth differentiation factor-5-coated sutures on tendon repair in a rat model. J Shoulder Elbow Surg 2007; 16(Suppl. 5): S215–S221. [DOI] [PubMed] [Google Scholar]
- 27. Izumi N, Mizuguchi S, Inagaki Y, et al. BMP-7 opposes TGF-beta1-mediated collagen induction in mouse pulmonary myofibroblasts through Id2. Am J Physiol Lung Cell Mol Physiol 2006; 290(1): L120–L126. [DOI] [PubMed] [Google Scholar]
- 28. Wang S, Hirschberg R. BMP7 antagonizes TGF-beta-dependent fibrogenesis in mesangial cells. Am J Physiol Renal Physiol 2003; 284(5): F1006–F1013. [DOI] [PubMed] [Google Scholar]
- 29. Zeisberg M, Hanai J, Sugimoto H, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 2003; 9(7): 964–968. [DOI] [PubMed] [Google Scholar]
- 30. Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, et al. TGF-beta signaling in vascular fibrosis. Cardiovasc Res 2007; 74(2): 196–206. [DOI] [PubMed] [Google Scholar]
- 31. Liu X, Hu H, Yin JQ. Therapeutic strategies against TGF-beta signaling pathway in hepatic fibrosis. Liver Int 2006; 26(1): 8–22. [DOI] [PubMed] [Google Scholar]
- 32. Katzel EB, Wolenski M, Loiselle AE, et al. Impact of Smad3 loss of function on scarring and adhesion formation during tendon healing. J Orthop Res 2011; 29(5): 684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kirkbride KC, Townsend TA, Bruinsma MW, et al. Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. J Biol Chem 2008; 283(12): 7628–7637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.