Abstract
Mesenchymal stem cells (MSC) are multipotential cells with utility in tissue engineering and regenerative medicine. However, the immunological properties and immunogenicity of allogeneic MSC remain poorly defined. Recent studies investigating their immunogenicity remain inconclusive and this has hampered their clinical application. This study investigated the (1) immunogenicity and (2) immunomodulatory properties of bone marrow-derived MSC using an allogeneic mouse model involving Balb/c (responder) and C3H (stimulator) mice. Dermal fibroblasts (DF) were used as controls for cells of mesenchymal origin. Adaptations of the lymphocyte transformation assay (LTA) and mixed lymphocyte reaction (MLR) were used to investigate the immunogenicity and immunomodulatory properties of allogeneic undifferentiated and chondrogenic-differentiated MSC and DF. Both MSC and DF displayed a similar phenotypic profile with the exception of lower expression of CD44 and CD105 in DF. Tri-lineage differentiation of MSC and DF into adipocytes, chondrocytes and osteocytes confirmed their multipotency. In LTA, both undifferentiated and chondrogenic-differentiated allogeneic MSC stimulated lymphocyte proliferation. Allogeneic DF were non-stimulatory but chondrogenic-differentiated DF triggered responder lymphocyte proliferation. In one-way MLR, both allogeneic MSC and DF significantly suppressed Balb/c lymphocyte proliferation. The current challenges in distinguishing between MSC and fibroblasts were apparent throughout the work. These findings support the notion that although MSC possess immunosuppressive properties, they may not be immunoprivileged. Thus, clinical application of allogeneic MSC should be taken with due consideration of their potential immunogenicity.
Keywords: Mesenchymal stem cells, dermal fibroblasts, immunogenicity, immunosuppression, mixed lymphocyte reaction, lymphocyte transformation assay
Introduction
Bone marrow-derived mesenchymal stem cells (MSC) belong to a wider group of mammalian adult stem cells broadly defined as fibroblastic-like, plastic-adherent, non-haematopoietic cells possessing self-renewal properties and the capacity to differentiate in vitro into adipocytes, chondrocytes and osteoblasts.1–3 MSC have been widely reported to have immunomodulatory capacity and an immunoprivileged status.4–7 The lack of major histocompatibility class II (MHC II) and co-stimulatory molecules CD40, CD80 and CD86 and low MHC I expression by MSC is thought to be in part responsible for their immunoprivileged status.8 Immunosuppressive properties offer potential utility of MSC in cell-based therapies such as in the prevention of graft versus host disease (GvHD) following allogeneic bone marrow transplantation9–11 and in the treatment of autoimmune diseases.12 Immunoprivileged status would mean that allogeneic MSC could be used without the risk of immune rejection, a scenario that is attractive for commercial companies to develop ‘off-the-shelf’ cell therapies and tissue-engineered products. To date, although the published data regarding their immunosuppressive properties are compelling, the evidence for the immunoprivileged status of allogeneic MSC is controversial.13–16
Most of the evidence for MSC immunoprivilege and immunosuppression has been obtained using adaptations of the lymphocyte transformation assays (LTA) and mixed lymphocyte reaction (MLR), respectively. These assays have been widely used for nearly five decades as in vitro correlates for alloreactivity, for the determination of histo-incompatibility in tissue matching and as tests for immunological tolerance.17–19 The reliability and sensitivity of these assays is dependent upon the optimisation of the assay conditions, and different conditions have yielded conflicting outcomes in some instances.
An important consideration for clinical application of allogeneic MSC is their potential for differentiation. Applications, which may involve tissue or organ regeneration, require MSC differentiated into tissue-specific cells. However, differentiation has been reported to lead to the loss of both immunosuppressive properties and immunoprivileged status,20–22 and this would be a major concern for clinical applications.
In light of the reported immunoprivilege of MSC, we hypothesised that depending on assay conditions, allogeneic MSC would be capable of stimulating a proliferative response in allogeneic lymphocytes. Hence, the aims of this study were to investigate the (1) immunogenicity and (2) immunomodulatory properties of both undifferentiated and differentiated allogeneic bone marrow-derived MSC using adaptations of the LTA and MLR, respectively. We employed an allogeneic mouse model in which Balb/c (H2-d) and C3H (H2-k) mice were used as responder (recipient) and stimulator (donor), respectively. Dermal fibroblasts (DF) were used as controls for cells of mesenchymal origin and similarly tested. Unlike in standard MLR and LTA, which are commonly carried out over a 2–5-day period, assays were carried out over a 15-day period accompanied with medium replacement at 3-day intervals. We report that undifferentiated allogeneic MSC are immunogenic in LTA. Following chondrogenic differentiation, both allogeneic MSC and DF significantly stimulated lymphocyte proliferation. With regard to immunomodulatory properties, both undifferentiated and chondrogenic-differentiated allogeneic and syngeneic MSC and DF suppressed lymphocyte proliferation in one- and two-way MLR.
Materials and methods
Animals
Female Balb/c (H2-d) and C3H (H2-k) mice were obtained from (Harlan Laboratories, UK) and maintained in the University of Leeds Central Biomedical Services with food and water administered ad libitum. MSC and DF were isolated from 4–8-week-old mice, while mononuclear cells (MNC) were isolated from 6- to 12-week-old mice. Animals were humanely sacrificed using a Schedule 1 procedure in accordance with institutional and national guidelines.
Isolation and expansion of MSC and DF
MSC
MSC were isolated from the femurs of Balb/c and C3H mice using an adaptation of the plastic adherence method described by Phinney et al.23 Briefly, following femoral aspiration, the resulting cell suspensions were plated at a density of 105 cells cm−2 in T25 flasks (Nunc, Denmark) in culture medium (Dulbecco’s modified Eagle’s medium-low glucose (DMEM-LG; Invitrogen), 10% (v/v) foetal calf serum (FCS; Lonza, UK), 2 mM l-glutamine and 100 U mL−1 penicillin/streptomycin (Invitrogen, UK)). The cells were incubated in a humidified incubator at 37°C in an atmosphere of 5% (v/v) CO2 in air for 24 h before the first medium change. Culture medium was changed every 3–4 days and cells were passaged using Hy-Q-Tase™ (Thermo-Fisher Scientific, UK). Cultures were maintained up to passage 15 (P15).
DF
DF were isolated using an adaptation of the method described by Kitano and Okada.24 Briefly, abdominal skin pieces (~2 cm2) were incubated in 0.5 mg mL−1 dispase II (Sigma) overnight at 4°C. The dermis was separated from the epidermis and digested in 0.5 mg mL−1 collagenase 1A (Sigma) at 37°C for 4 h. The cell suspensions were washed and cultured in Dulbecco’s modified Eagle’s medium-high glucose (DMEM-HG; Lonza) plus 10 (v/v) FCS, 2 mM l-glutamine and 100 U mL−1 penicillin/streptomycin. Culture medium was changed every 3–4 days and subsequent passages carried out 0.05% (v/v) trypsin–ethylenediaminetetraacetic acid (EDTA; Lonza).
Characterisation of Balb/c and C3H MSC and DF
Flow cytometry
The following mouse-specific antibodies were used and, unless indicated, were obtained from eBioscience, UK; fluorescein isothyocyanate (FITC)-conjugated anti-Sca-1 (clone D7), CD90.2 (clone 5a-8; Invitrogen), CD80 (clone 16-10A1), CD11b (clone M1/70), MHC I (clone 34-1-25), MHC II (clone M5/114.15.2), CD44 (clone IM7), phycoerythrin (PE)-conjugated anti-CD29 (clone HMb1-1), CD86 (clone PO3.1) and CD45 (clone 30-F11; Invitrogen). The following isotype controls were used: FITC-conjugated 1gG2a (clone 6H3) (MBL), IgG2b (clone 3D12) (MBL), IgG (clone 2E12), PE-conjugated IgG2b (clone 3D12) (Invitrogen) and IgG (clone 2E12). Briefly, cells were detached using Hy-Q-Tase™ (MSC) or 0.05% (v/v) trypsin-EDTA (DF), washed and resuspended at 106 cells mL−1 in phosphate-buffered saline (PBS; Lonza). Equal volumes (200 µL) of cell suspension and blocking buffer (PBS and 20% (v/v) FCS) were mixed and incubated for 20 min. Specific conjugated antibody solutions were added to the cell suspensions followed by further incubation for 1 h at 4°C in the dark. The labelled cells were then washed thrice, resuspended in cold PBS and analysed on a FACSCalibur cytometer. Data were collected at 105 events and analysed using CellQuest software (BD Biosciences, UK) and FlowJo (Tree Star, USA).
Tri-lineage differentiation
MSC and DF multipotency was tested by differentiation into adipocytes, chondrocytes and osteocytes using adaptations of methods described in Tropel et al.2 Culture medium supplements and chemicals were obtained from Sigma, UK, unless stated otherwise.
For adipogenic differentiation, MSC (P10) and DF (P6) were seeded into 6-well plates at 105 cells per well and cultured until 90% confluent at which point they were incubated in adipogenic differentiation medium (ADM) comprising culture medium with 1 µM dexamethasone, 0.1 µM indomethacin, 0.5 mM 3-isobutyl-1-methylxanthine and 10 µg mL−1 insulin. ADM was interchanged with maintenance medium (culture medium with 10 µg mL−1 insulin) every 2 days and the cycle repeated for 14 days. The presence of intracellular lipids was detected by oil red-O staining as described in Vidal et al.25 For osteogenic differentiation, cells were similarly seeded as previously described. On confluence, the cells were incubated in osteogenic differentiation medium (culture medium with 0.1 µM dexamethasone, 0.1 mM ascorbic-2-phosphate and 10 mM β-glycerophosphate). Calcium deposition was analysed using alizarin red-S staining as described in Vidal et al.25 Chondrogenic differentiation was assessed using modifications of the micromass culture described in Denker et al.26 Briefly, 10 µL of MSC or DF (107 cells mL−1) was plated as micromass cultures in 24-well plates in chondrogenic differentiation medium (culture medium with 0.15 mM ascorbic 2 phosphate, 0.1 µM dexamethasone, 1 mM pyruvic acid, 6.25 µg mL−1 selenous acid, transferrin and insulin and 0.01 µg mL−1 transforming growth factor-β3 (TGFβ3). The cells were incubated for 21 days with medium changed every 3 days. Chondrogenesis was assessed using alcian blue (Biostain Ready Reagents, UK) as described in Denker et al.26 All controls (non-differentiation conditions) for the tri-lineage assay were cultured in the DMEM-LG (MSC) or DMEM-HG (DF) culture medium.
Immunological assays
Isolation of Balb/c and C3H MNC
MNC were isolated from mouse spleens and lymph nodes (inguinal and axillary) as described in Bøyum.27 Briefly, tissues were gently teased and passed through a cell sieve using transport medium (Roswell Park Memorial Institute (RPMI)-1640 medium, 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (Sigma, UK) and 100 µg mL−1 penicillin/streptomycin) followed by density gradient separation using Lymphoprep™ (Axis-Shield, UK). The resultant cell suspension was washed, counted using the Trypan blue dye-exclusion method and resuspended in lymphocyte culture medium [LCM] (RPMI-1640, 10% v/v FCS, 20 mM HEPES buffer, 2 mM l-glutamine, 50 mM β-mercaptoethanol and 100 U mL−1 penicillin/streptomycin).
Mitotic inactivation
C3H MNC, MSC and DF and Balb/c MSC and DF used in LTA and MLR were mitotically inactivated by treatment with 10 µg mL−1 mitomycin-C for 30 min followed by five washes with transport medium and final re-suspension in lymphocyte culture medium.
Medium changes for MLR and LTA cultures
Medium changes were performed every 3-day period for all LTA and MLR cultures. Briefly, half the culture medium volume (100 µL) was aseptically removed from each well and an equal volume of fresh LCM was replaced.
Measurement of lymphocyte proliferation
After incubation, LTA and MLR cultures were pulsed with 10 µL per well of 0.025 µCi (2 Ci mmol−1) 3H-thymidine (Perkin-Elmer, UK) for the last 16 h of each time point. Upon completion of incubation, the cells were harvested and 3H-thymidine incorporated into cellular DNA was measured with raw data obtained as counts per minute (CPM). Lymphocyte stimulation for LTA and MLR as represented by stimulation index (SI) was calculated using the formula
LTA
Responder Balb/c MNC (5 × 104 cells per well) were co-cultured with mitomycin-C-treated and untreated C3H MSC or DF (allogeneic LTA) and Balb/c MSC or DF (syngeneic LTA) at a 1:1 cell ratio (n = 6) in uncoated 96-well plates (Sterilin, UK). Positive controls (n = 6) comprised Balb/c MNC stimulated with 10 µg mL−1 concanavalin-A (Con-A). Negative controls (n = 6) comprised Balb/c MNC alone. The cultures were incubated for 3, 6, 9, 12 and 15 days accompanied by medium changes every 3 days. Lymphocyte proliferation was measured by 3H-thymidine uptake.
MLR
Mixed lymphocyte cultures comprising responder Balb/c MNC (5 × 104 cells) and mitomycin-C-treated (one-way MLR) or untreated (two-way MLR) stimulator C3H MNC were co-cultured in the presence of Balb/c and C3H MSC or DF at a 1:1:1 ratio in 200 µL of lymphocyte culture medium (n = 6). Positive controls for allogeneic stimulation comprised co-cultures of Balb/c MNC and C3H MNC only. Balb/c MNC were used as controls for non-proliferating cells. To test for mitotic inactivation, monocultures of untreated and mitomycin-C-treated C3H MNC were either left unstimulated or stimulated with 10 µg mL−1 Con-A. Similarly, individual cultures of unstimulated and Con-A-stimulated Balb/c and C3H MSC and DF were used as controls. The cultures were incubated for 3, 6, 9, 12 and 15 days accompanied by medium changes every 3 days. Lymphocyte proliferation was measured by 3H-thymidine uptake.
LTA and one-way MLR for chondrogenic-differentiated MSC and DF
Balb/c and C3H MSC (P5) and DF (P5) were differentiated in pellet culture as described by Bosnakovski et al.28 Briefly, 1 × 106 cells were pelleted at 500g for 10 min and incubated for 21 days in chondrogenic differentiation medium with medium changes after 3 days. On completion, the cell pellets were disaggregated using 100 µg mL−1 collagenase 1A for 30 min at 37°C, washed and resuspended in fresh lymphocyte culture medium. The cells were seeded at 5 × 104 cells in 20 µL medium in uncoated V-bottom 96-well plates (Sterilin, UK) and incubated overnight. The next day, Balb/c MNC (LTA) or Balb/c MNC + mitomycin-C-treated C3H MNC (one-way MLR) were added to each well and the cultures were treated as described above.
Statistical analysis
All statistical analyses were carried out using Microsoft® Excel 2010 and GraphPad Prism® (GraphPad, USA). All replicate CPM data from LTA and MLR at each time point were first transformed to Log10 prior to analysis by one-way analysis of variance (ANOVA). Differences between means at each time point were calculated using the minimum significant difference (MSD) at 95% confidence limit (CL) by the T-method for equal samples sizes and the T′-method for unequal sample sizes. The means, upper and lower 95% CL obtained from Log10 transformed data were then back-transformed and the data were plotted as mean values ± 95% CL.
Results
MSC and DF share morphological and phenotypical similarities
Balb/c and C3H MSC were harvested by femoral aspiration and isolated using their propensity to adhere to tissue culture plastic. Non-adherent cells were lost during ensuing medium changes. Initial cultures were composed of sparse cells with several distinct morphologies. After the first passage, it took an average of 3–4 weeks for the cells to reach confluence. At P3, the proliferative capacity of the cells increased dramatically and homogeneous cultures of bipolar, spindle-shaped fibroblastic-like cells ensued with an average cell doubling time (CDT) of 8–12 h (Figure 1(a)). These cultures retained their morphological homogeneity up to the P15. DF cultures were homogeneously fibroblastic throughout culture (Figure 1(b)) and morphologically similar to P3-P15 MSC.
Figure 1.
Isolation and characterisation of mouse MSC and DF. P3) Mouse MSC (a) and DF (b) showed typical bipolar spindle-shaped morphology (×100). Analysis of selected markers showed that MSC (c) and DF (d) differed in their expression of CD44 and CD105 (results (c) and (d) representative for P3 and P10 Balb/c and C3H MSC and DF, respectively). Tri-lineage differentiation of P10 MSC (e) and P6 DF (f) was tested with oil red-O for adipogenesis, alcian blue for chondrogenesis and alizarin red-S for osteogenesis.
MSC: mesenchymal stem cells; DF: dermal fibroblasts.
The phenotype of both Balb/c and C3H MSC was determined by flow cytometry using a panel of markers commonly used in stem cell analysis. P3 (early) and P12 (late) cultures were analysed to determine whether prolonged culture led to any phenotypic changes. Both Balb/c and C3H P3 (Figure 1(c)) and P12 MSC exhibited a similar phenotype consistent with that attributed to MSC. Sca-1 and CD44 were highly expressed (>95%) by MSC at both passages (Table 1). CD29 and CD90.2 expression was greater than 95% in all but P3 C3H MSC (89% and 87%, respectively). MHC I expression was partial in both P3 and P12 C3H (77 and 57%) and Balb/c MSC (62% and 57%). CD105 expression was higher in P12 (>95%) than P3 MSC (<60%). MHC II, CD11b, CD34, CD45, CD80 and CD86 were negative (<5%) in all MSC samples.
Table 1.
Phenotypic characterisation of Balb/c and C3H MSC and DF by flow cytometry.
| Marker | C3H MSC |
Balb/c MSC |
C3H DF |
Balb/c DF |
||
|---|---|---|---|---|---|---|
| P3 | P12 | P3 | P12 | P6 | P6 | |
| Sca-1 | 97% | 98.40% | 95.30% | 98% | 98.40% | 100% |
| MHC I | 76.80% | 57.30% | 62.30% | 56.50% | 82% | 90.90% |
| MHC II | 0.10% | 1.17% | 2.80% | 1.50% | 1.20% | 1.38% |
| CD11b | 0% | 1.30% | 1.20% | 0.80% | 1.66% | 1.57% |
| CD29 | 88.50% | 97.40% | 100% | 100% | 80.14% | 90.30% |
| CD34 | 0.20% | 0.21% | 0.85% | 0.14% | – | – |
| CD44 | 99% | 97% | 99.60% | 100% | 53.20% | 42.10% |
| CD45 | 0.01% | 0.74% | 1.21% | 1.24% | 1.34% | 0.10% |
| CD80 | 0.03% | 3.02% | 1.40% | 1.20% | 2.01% | 1.14% |
| CD86 | 0.02% | 1.03% | 1.20% | 1.04% | 1.40% | 2.85% |
| CD90.2 | 87% | 98.80% | 99.80% | 100% | 78% | 54.20% |
| CD105 | 59% | 99% | 57% | 99.20% | 1.30% | 1.49% |
MHC I: major histocompatibility class I; MHC II: major histocompatibility class II; MSC: mesenchymal stem cells; DF: dermal fibroblasts.
Cultured early passage (P3) and late passage (P6) MSC and intermediate passage (P6) DF were stained with conjugated antibodies as described in section ‘Materials and Methods’. Events (10,000) were collected using a FACSCalibur cytometer and results were analysed using CellQuest and FlowJo software. Results are expressed as % of positively stained cells relative to isotype controls.
DF were also analysed using the same markers for MSC (except CD34). Due to their fast growth, only intermediate passage (P6) cells were analysed. Both Balb/c and C3H DF were positive for Sca-1, MHC I and CD29 but were negative for MHC II, CD11b, CD45, CD80, CD86, CD105 and expressed intermediate levels of CD44 and CD90.2 (Figure 1(d)). Of the positive markers, only Sca-1 had greater than 95% expression. CD90.2, MHC I and CD29 expression was 54%, 91% and 90%, respectively, for Balb/c and 78%, 82% and 80%, respectively, for C3H (Table 1). All the negative markers had expression less than 5% in both types of DF. In the case of CD44, expression was 42% and 53%, respectively, in Balb/c and C3H DF. Collectively, the phenotype displayed by DF and MSC showed differences in the expression of CD105 (negative in DF and positive in MSC) and CD44 (intermediate in DF and positive in MSC). There were also some variations in marker expression between the two mouse species and different cell passages.
MSC and DF exhibit similar differentiation capacity
P10 Balb/c and C3H MSC were cultured in ADM, chondrogenic and osteogenic differentiation media. After incubation, both Balb/c and C3H MSC tested positive for adipogenesis with oil red-O, chondrogenesis with alcian blue and osteogenesis with alizarin red-S (Figure 1(e)). Interestingly, both Balb/c and C3H DF underwent adipogenic, chondrogenic and osteogenic differentiation when cultured and tested under the same conditions used for MSC (Figure 1(f)).
Allogeneic MSC stimulate lymphocyte proliferation
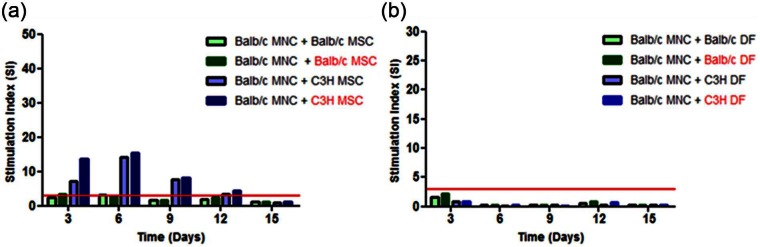
To test the immunoprivilege of allogeneic MSC, LTA incorporating 1:1 co-cultures of Balb/c MNC with either mitomycin-C-treated C3H (allogeneic) or Balb/c (syngeneic) MSC were cultured with half the medium changed and replaced at 3-day intervals during incubation. The cultures were then analysed for lymphocyte proliferation after 3, 6, 9, 12 and 15 days. 3H-thymidine uptake counts between the syngeneic and allogeneic LTA were compared based on the premise that counts significantly greater than those in syngeneic LTA were indicative of a stimulatory response. At days 6 and 9, allogeneic LTA produced significantly (p < 0.05) higher counts compared to syngeneic LTA with highest counts at day 6 (Figure 2(a)). This trend was also observed in LTA comprising mitotically active (mitomycin-C untreated) C3H and Balb/c MSC as stimulators and Balb/c MNC. The counts obtained for syngeneic LTA were comparable to those from control Balb/c MNC monocultures (Figure 2(b)). Interestingly, LTA involving Balb/c MNC and either mitomycin-C-treated or untreated syngeneic and allogeneic DF failed to stimulate lymphocyte proliferation at all the time points (Figure 2(c)). To demonstrate that the Balb/c MNC were viable, co-culture of these cells with Con-A resulted in significant stimulation (Figure 2(d)). Overall, only the SI of LTA involving mitotically active and inactivated allogeneic MSC were greater than 3 at all the time points except for day 15 (Supplementary Figure 1). The mitomycin-C concentration (10 µg mL−1) used to mitotically inactivate the cells without impacting on cell viability was determined using carboxyfluorescein succinimidyl ester (CFSE) dilution (Supplementary Figure 2).
Figure 2.
Immunogenicity of allogeneic mouse MSC and DF. For the LTA test for immunogenicity, responder Balb/c MNC (5 × 104 cells per well) were co-cultured with stimulator C3H (allogeneic) and Balb/c (syngeneic) MSC (a) or DF (c) at a 1:1 ratio. Balb/c and C3H MNC and MSC (b) or DF (d) cultured alone or stimulated with Con-A were controls for non-proliferating and proliferating cells. Mitotically inactivated cells are highlighted in red. Culture medium was changed every 3 days by replacing 100 µL with an equal volume of fresh medium and the cells were cultured for 3, 6, 9, 12 and 15 days and lymphocyte proliferation measured by the incorporation of 3H-thymidine. CPM data (n = 6) were Log10 transformed to compute upper and lower 95% CL. Data were then back-transformed for presentation. Data are presented as mean CPM ± 95% CL. The symbol ‘*’ indicates significant difference between Balb/c MNC + C3H MSC and Balb/c MNC + Balb/c MSC (a); (e) at a given time point.
MSC: mesenchymal stem cells; DF: dermal fibroblasts; LTA: lymphocyte transformation assay; MNC: mononuclear cells; CPM: counts per minute; CL: confidence limit.
Allogeneic and syngeneic MSC and DF possess immunosuppressive properties
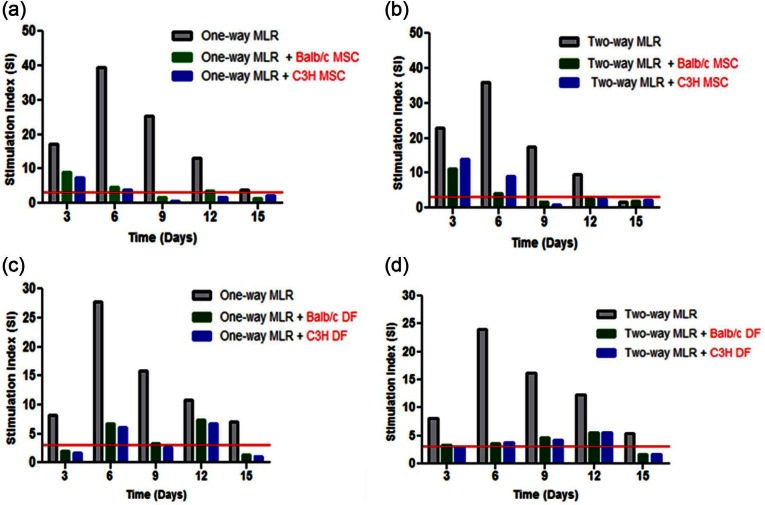
The immunosuppressive properties of MSC were tested in one-way MLR. Responder Balb/c MNC and mitotically inactivated stimulator C3H MNC were co-cultured with either C3H (allogeneic) or Balb/c (syngeneic) mitotically inactivated MSC at a 1:1:1 ratio for 3, 6, 9, 12 and 15 days with half the medium changed and replaced at 3-day intervals. The positive control (Balb/c MNC + mitotically inactivated C3H MNC) showed that Balb/c lymphocytes were significantly stimulated (p < 0.05) with 3H-thymidine uptake peaking at day 6 followed by a gradual decline in stimulation with time. When both syngeneic and allogeneic mitotically inactivated MSC were co-cultured in the one-way MLR, 3H-thymidine uptake was significantly suppressed at days 6, 9 and 12 with up to 90% suppression of the Balb/c response observed at day 6 with both allogeneic and syngeneic MSC (Figure 3(a)). In order to determine the immunosuppressive potency of MSC from each mouse strain, mitotically inactivated Balb/c and C3H MSC were cultured in two-way MLR. Both Balb/c and C3H MSC significantly suppressed the two-way MLR at days 3, 6, 9 and 12 (Figure 3(b)). Interestingly, the counts for the two-way MLR control (Balb/c MNC + C3H MNC) were not significantly different from those of the one-way MLR. Overall, mitotically inactivated Balb/c and C3H MSC exhibited comparable potency in suppressing both the one-way and two-way MLR. In similar experiments to test the immunosuppressive potential of DF, 3H-thymidine uptake in one-way MLR cultures containing syngeneic or allogeneic DF was suppressed significantly (p < 0.05) at days 3, 6 and 9 (Figure 3(c)). Up to 75% of the one-way MLR was suppressed at day 6 by both allogeneic and syngeneic DF. A similar trend was observed in the two-way MLR in which approximately 80% of the response was suppressed by both C3H and Balb/c DF (Figure 3(d)). Collectively, these data showed that like MSC, DF were similarly immunosuppressive in both one- and two-way MLR. Significant suppression was however observed earlier in MLR with DF (days 3–9) than in MLR with MSC (days 6–12). Some of the SI for both syngeneic and allogeneic MSC and DF mediated immunosuppression at specific time points were greater than 3 (Supplementary Figure 3).
Figure 3.
Immunomodulatory capacity of MSC and DF. To test for the immunosuppressive potency of allogeneic (C3H) and syngeneic (Balb/c) MSC and DF, one-way MLR comprising responder Balb/c MNC (5 × 104 cells per well) and mitotically inactivated stimulator C3H MNC were co-cultured with mitotically inactivated allogeneic or syngeneic (with respect to the responder) MSC (a) or DF (c) at a 1:1:1 cell ratio. Two-way MLR also comprising 1:1:1 co-cultures of Balb/c MNC with C3H MNC and mitotically inactivated Balb/c and C3H MSC (b) or DF (d). Mitotically inactivated cells are highlighted in red. Culture medium was changed every 3 days by replacing 100 µL with an equal volume of fresh medium and lymphocyte proliferation measured by 3H-thymidine uptake. CPM data (n = 6) were Log10 transformed to compute upper and lower 95% CL. Data were then back-transformed for presentation. Data (n = 6) are presented as mean CPM ± 95% CL. The symbol ‘*’ indicates significant difference between one-way MLR and one-way MLR + C3H/Balb/c MSC (a) or DF (c); two-way MLR and two-way MLR + C3H/Balb/c MSC (b) or DF (d) at a given time point.
MSC: mesenchymal stem cells; DF: dermal fibroblasts; LTA: lymphocyte transformation assay; MNC: mononuclear cells; MLR: mixed lymphocyte reaction; CPM: counts per minute; CL: confidence limit.
Allogeneic chondrogenic-differentiated MSC and DF are immunosuppressive but stimulate lymphocyte proliferation
Chondrogenic-differentiated Balb/c and C3H MSC and DF were tested for their immunogenicity and immunosuppressive capacity. The controls for these experiments showed appropriate responses for unstimulated cells (Figure 4(a)) and con-A-stimulated cells (Figure 4(b)). In LTA involving allogeneic MSC-Chon or DF-Chon, significant counts (p < 0.05) relative to the Balb/c MNC control were produced at days 3, 6 (peak) and 9 (Figure 4(c)) showing that both the allogeneic were immunogenic. In the one-way MLR, the counts for the positive control peaked at day 6 and followed a similar trend seen before. When syngeneic and allogeneic MSC-Chon were co-cultured in the one-way MLR, significant suppression (p < 0.05) of 3H-thymidine uptake was observed at days 3, 6 and 9 in comparison to one-way MLR positive control with approximately 50% suppression achieved at day 6 (Figure 4(d)). A similar trend was obtained with syngeneic or allogeneic DF-Chon. There were no significant differences (p < 0.05) in the suppression of the one-way MLR between allogeneic or syngeneic MSC-Chon and DF-Chon. On the whole, the SI of allogeneic but not syngeneic MSC-Chon and DF-Chon was equal to or greater than 3 at all the time points but day 15 for the LTA (Figure 4(e)). However, in the one-way MLR, SI equal to or higher than 3 was obtained in all cultures with either syngeneic or allogeneic differentiated MSC and DF at all the time points but day 15 (Figure 4(f)).
Figure 4.
Immunogenicity and immunosuppressive properties of chondrogenic-differentiated MSC and DF. Monocultures of unstimulated (a) and con-A-stimulated (b) Balb/c and C3H MNC and chondrogenic-differentiated MSC and DF were used as controls for non-proliferating and proliferating cells, respectively. LTA for stimulatory capacity comprised co-cultures of responder Balb/c MNC (5 × 104 cells per well) and stimulator C3H (allogeneic) and Balb/c (syngeneic) chondrogenic-differentiated MSC or DF at a 1:1 ratio (c). One-way MLR test for immunosuppression comprised 1:1:1 ratios of co-cultures of responder Balb/c MNC with stimulator C3H MNC and allogeneic or syngeneic (with respect to the responder MNC) MSC and DF (d). Red indicates cells that were mitotically inactivated by treatment with mitomycin-C. Culture medium was changed every 3 days by replacing 100 µL with an equal volume of fresh medium. Lymphocyte proliferation was measured by the incorporation of 3H-thymidine. CPM data (n = 6) were Log10 transformed to compute upper and lower 95% CL. Data were then back-transformed for presentation. Data (n = 6) are presented as mean CPM ± 95% CL (a, c, e, g). The symbol ‘*’ indicates significant differences between Balb/c MNC only and Balb/c MNC + C3H differentiated MSC or DF (c) and between one-way MLR only and one-way MLR + C3H or Balb/c differentiated MSC and DF (d) at a given time point. Data are also presented as SI (b, d, f, h). The red line at SI = 3 denotes statistical biological positive whereby SI > 3 indicates significant stimulation of responder MNC.
MSC: mesenchymal stem cells; DF: dermal fibroblasts; LTA: lymphocyte transformation assay; MNC: mononuclear cells; MLR: mixed lymphocyte reaction; CPM: counts per minute; CL: confidence limit; SI: stimulation index.
Discussion
The aims of this study were to investigate the notions that allogeneic MSC are immunoprivileged7,29,30 and immunosuppressive5,31 even after differentiation8,32 in light of the immunoprivileged status of allogeneic MSC being brought into question.15,30,33 Here, our emphasis was on investigating both in vitro immunoprivilege and immunomodulation before and after chondrogenic differentiation using modifications of the LTA and MLR. DF were employed as controls, but showed striking similarities to MSC in some but not all cases.
The most important finding of this study was the demonstration that allogeneic C3H MSC significantly stimulated Balb/c MNC in LTA. Stimulation was measured by statistical comparison of 3H-thymidine uptake in LTA to Balb/c MNC only cultures, as well as computation of the SI, which according to the International Union of Immunological Societies (IUIS) in 1976, values equal to or above 3 are deemed “statistical biological positive” or significant stimulation of responder lymphocytes by stimulating agent.34,35 These findings were in contrast with most previous in vitro findings.30,31,33,36–38 Interestingly, although some in vitro studies have shown that allogeneic MSC were immunoprivileged, in vivo tests have suggested otherwise.14–16,30,33
These discrepancies raise the important question: whether in vitro assays are a reliable correlate for in vivo immunogenicity given the variations in methodologies across studies. For instance, in this study, the culture medium was changed during the course of incubation, an approach that has not been adopted in any of the previous studies. Another factor was the ratio of allogeneic MSC to responder lymphocytes. We used a 1:1 ratio after preliminary experiments showed that increasing the responder-to-stimulator cell ratio from 1:1 to 1:5 resulted in a decrease in 3H-thymidine uptake and SI in cultures. This implied that increasing the MSC ratio would lead to inhibition of lymphocyte proliferation, which could be construed as immunoprivilege. Le Blanc et al.4 had previously demonstrated using human MSC that low MSC doses were stimulatory in LTA, while higher doses were inhibitory but this was not explored further. There is now overwhelming evidence that MSC inhibit lymphocyte proliferation in a dose-dependent manner and studies in which high MSC-to-lymphocyte ratios have been used resulted in no detectable lymphocyte stimulation.33,39 This suggests that the results of in vitro assays of immunogenicity will depend on the MSC-to-lymphocyte ratio.
Klyushnenkova et al.29 reported that MSC failed to stimulate allogeneic separated lymphocytes but when MSC were cultured with allogeneic unseparated peripheral blood mononuclear cells (PBMNC), the 3H-thymidine counts were significantly higher at day 8 of culture than for PBMNCs cultured alone.Although not explored further by the authors, these findings suggested the role of accessory cells in PBMNCs, particularly antigen-presenting cell (APC) in the response of responder lymphocytes to stimulator MSC, a possible role for the indirect or semi-direct response. Another study which used porcine MSC failed to detect a proliferative response in an allogeneic LTA, although the opposite was found in in vivo tests using the same MSC.30 The allogeneic LTA was only tested for 3 days and given that MSC are known to be poorly immunogenic, the in vitro findings could have simply been due to the limited time for an adequate response via the indirect or semi-direct pathway to be detected. In fact, there is a growing hypothesis that MSC employ an ‘evasive’ tactic rather than immunoprivilege, as recently argued by Ankrum et al.40 We suggest that in an in vitro setting, such ‘evasive’ approaches in addition to limited incubation time can be further exaggerated by the declining nutrient reserves and accumulation of metabolic by-products thus hampering the detection of and initiation of an allogeneic response. Thus, our approaches to increase the incubation period to 15 days and introduce medium changes were aimed at remedying some of the shortcomings of the traditional LTA and MLR approach.
Interestingly, allogeneic C3H DF failed to stimulate Balb/c lymphocyte proliferation in LTA in contrast to C3H MSC at the 1:1 ratio. However, it must be noted that DF were tested only at this ratio, and thus, this result cannot be interpreted as demonstrative of DF immunoprivilege. Following chondrogenic differentiation, both allogeneic, but not syngeneic, MSC and DF stimulated lymphocyte proliferation. Collectively, the LTA findings suggested that both allogeneic MSC and DF may be immunologically rejected following implantation. Although undifferentiated allogeneic DF were non-stimulatory in LTA, their chondrogenic-differentiated progeny were stimulatory. Chondrogenic differentiation of MSC has been reported to render them immunogenic.21,22 Chen et al.22 reported that expression of CD80 and CD86 coupled with elevated levels of MHC I were major contributors to the immunogenicity of chondrogenic-differentiated MSC.
Although DF have been widely used as control cells in some MSC studies,39 the evidence with regard to their immunogenicity is equally controversial. Human DF have previously been reported to be non-immunogenic, whereas smooth muscle, endothelial and epidermal cells were immunogenic.41 Our findings were similar to those reported in studies conducted nearly four decades ago;42 yet the immunological properties of DF have only gained prominence in the last decade.
The unique approach taken in this study is that LTA and MLR experiments were carried out over a 15-day period instead of the traditional 3–5 days. This modification was borne out of the hypothesis that the traditional 3–5-day approach favours the direct pathway of allorecognition but not necessarily the indirect and semi-direct pathways, which are thought to require more time to be initiated both in vitro and in vivo.43,44 To mitigate for this extended culture period, medium changes (half the culture medium was replaced with an equal amount of fresh medium) at 3-day intervals were carried out. This was done to ensure consistent nutrient supply to proliferating cells and avoiding drastic pH changes. Lymphocyte stimulation is a highly energy-dependent process requiring increased glucose uptake and reduced oxygen intake, which lowers the pH and affects cell division and viability.45 Even when oxygen supply is maintained, stimulated lymphocytes undergo aerobic glycolysis, which, without nutrient replenishment, could lead to increased acidity and metabolic arrest.46 We tested Balb/c lymphocyte proliferation after stimulation by Con-A with and without medium changes. Significant lymphocyte stimulation lasted for 15 days in the former and 5 days in the later. This enabled us to conduct our experiments for longer periods than usual (Figure 5).
Figure 5.
Effect of medium replacement on Balb/c MNC proliferation. Cells (1 × 105 cells per well) were stimulated with Con-A (5–25 µg mL−1) and cultured for 3, 5, 10, 15 and 20 days with and without medium changes. The medium changes were carried out every 3 days by replacing 100 µL of medium per well. Unstimulated cells (no Con-A) were used as controls for non-proliferating cells. CPM data (n = 6) were Log10 transformed to compute upper and lower 95% CL. Data were then back-transformed for presentation and is presented as mean CPM ± 95% CL for cultures (a) with medium changes and (b) without medium changes. Lymphocyte stimulation is also presented as SI for cultures (c) with and (d) without medium changes. The red line at SI = 3 denotes statistical biological positive; only SI above 3 were considered significant responses against Con-A.
MNC: mononuclear cells; CPM: counts per minute; CL: confidence limit; SI: stimulation index.
The one-way and two-way MLR were used to determine the immunomodulatory capacity of both allogeneic and syngeneic MSC. Significant suppression of responder lymphocyte proliferation was obtained in all cases. These findings supported a growing body of evidence, which used mouse,33 human8,29 and primate31 models to demonstrate the in vitro immunosuppressive potency of MSC. Also, both syngeneic and allogeneic DF suppressed one-way and two-way MLR in a similar manner to that displayed by MSC. The overall suppression of one-way MLR by both syngeneic and allogeneic MSC and DF was between 60% and 95%, which was consistent with previous studies. Chondrogenic-differentiated allogeneic MSC and DF significantly suppressed one-way MLR, although the suppression at day 6 (50%) appeared lower to that obtained with undifferentiated MSC and DF. Previous studies have shown that MSC lost their immunosuppressive potency following chondrogenic21,22 and osteogenic32 differentiation as well as in in vivo studies.20 The fact that both allogeneic and syngeneic MSC and DF suppressed one-way MLR suggested that the mechanisms involved were independent of the MHC as has previously been reported with human MSC.4 The mechanisms by which MSC suppress lymphocyte proliferation in vitro are not fully understood. It is thought that suppression is mediated, at least in part by factors such as prostaglandin E2 (PGE2), indoleamine 2,3-dioxygenase (IDO), transforming growth factor-β1 (TGFβ1) and nitrous oxide (NO) secreted by MSC upon activation by allogeneic lymphocytes.5,7 However, other studies have suggested that neither MSC production of these factors was responsible for T-lymphocyte suppression.37 Cell-to-cell contact has been reported to be critical for MSC-mediated immunosuppression through induction of T-cell anergy,36 although earlier studies showed that the responsiveness of T-cells that have been previously suppressed by MSC could be restored.47,48 It has been argued that fibroblast-mediated immunosuppression is independent of PGE2, IL-10 and IDO48,49 despite being similar to that exhibited by MSC.50 This suggests that the immunosuppressive mechanisms employed by MSC and DF may be different but this is yet to be explored.
The phenotype of MSC has also been thought to contribute to their perceived immunoprivilege. Their lack of MHC II, low to intermediate expression of MHC I and lack of co-stimulatory markers CD80, CD86 and CD40 was demonstrated in this study in both low (P3) and high passage (P12) MSC. This phenotype renders MSC incapable of directly stimulating allogeneic CD4+ helper lymphocytes. However, even in the absence of the direct pathway, MSC could potentially stimulate allogeneic CD4+ lymphocytes via the indirect or semi-direct pathways in the presence of accessory cells. For this reason, we used unseparated MNC in all the experiments. However, it will be important for future studies of the mechanisms of MSC- and DF-mediated immunomodulation to investigate the roles of these allorecognition pathways.
To our knowledge, this study is the first to demonstrate the tri-lineage differentiation potential of both mouse MSC and DF into adipocytes, chondrocytes and osteocytes. This test remains the only functional assay for defining MSC. Although it has been widely reported that DF are capable of differentiation into cells of other types, their tri-lineage capacity remains controversial. Previous studies have demonstrated their inability to differentiate or their capacity to differentiate into either one or two39,51,52 but not all the three cell lineages. A growing school of thought suggests that MSC and DF are phenotypically and functionally indistinguishable.53,54 However, we found differences in the expression of CD44 and CD105, which were strongly expressed in MSC but weakly (CD44) or not (CD105) expressed in DF. Wagner et al.55 reported that human fibroblasts expressed CD44 but not CD105 and were incapable of differentiation into fat and bone. These differences in marker expression, although providing a measure of distinction, are unreliable since MSC from different species and studies have been found to have different marker expression profiles.
Conclusion
Our findings and the available literature demonstrated the complexity of studying the immunological properties of MSC in vitro as well as the difficulty of distinguishing between MSC and DF. The results have shown that undifferentiated and differentiated allogeneic MSC, although potent immunosuppressors, were immunogenic.
Supplementary Information
Supplementary Figure 1.
Stimulation of Balb/c responder MNC by allogeneic MSC and DF. LTA to determine the immunogenicity of C3H (allogeneic) and syngeneic (Balb/c) MSC (A) and DF (B) were set up as described in Figure 2. CPM data were used to compute the SI, which is a ratio of 3H-thymidine uptake between responder MNC alone and co-cultures of responder MNC + MSC or DF. The red line at SI = 3 denotes statistical biological positive whereby SI > 3 indicates significant stimulation of responder MNC.
Supplementary Figure 2.
CFSE dilution assay for the determination of mitotic inactivation of stimulator C3H MHC. Representative histograms plotted to show CFSE fluorescence intensities of unstimulated cells (A) and Con-A stimulated cells (B) treated with 5 and 10 µg.ml−1 mitomycin C. Positive (Con-A stimulated) and negative (unstimulated) control cells were not treated with mitomycin C. A shift to the left of peak fluorescence intensities denotes CFSE dilution which in turn represents separate cell fractions of daughter cells. The peaks of the Con-A stimulated cells were further resolved using the cell proliferation platform on FlowJo™ to expose groups of cells with the different CFSE fluorescence intensities which corresponded to specific generations of cell division (C). The data are representative of three experiments.
Supplementary Figure 3.
Suppression of the one-way and two-way MLR by mouse MSC and DF. One-way MLR to determine the immunosuppressive properties of C3H (allogeneic) and syngeneic (Balb/c) MSC (A) and DF (C) as well as two-way MLR to determine the suppressive potency of each mouse strain MSC (B) and DF (D) were set up as described in Figure 3. CPM data were used to compute the SI, which is a ratio of 3H-thymidine uptake between the one-way or two-way MLR and co-cultures of the one-way or two-way MLR + MSC or DF. The red line at SI = 3 denotes statistical biological positive whereby SI > 3 indicates significant stimulation of responder MNC.
Acknowledgments
The authors would like to acknowledge the assistance provided by Dr Gareth Howell for assistance with flow cytometry; Blessing Mukonoweshuro for conception and design, data analysis and interpretation and article writing; Christopher JF Brown for conception and design, data analysis and interpretation; John Fisher for conception and final approval of article; and Eileen Ingham for conception and design, data analysis and interpretation and final approval of article.
Footnotes
Declaration of conflicting interests: The authors indicate no potential conflict of interest.
Funding: This work was funded by the University of Leeds’ Faculty of Biological Sciences and The Beit Trust Charity (Charity Number 232478) as part of B.M.’s PhD studentship and also through WELMEC, a Centre of Excellence in Medical Engineering funded by the Wellcome Trust and EPSRC, under grant number WT 088908/Z/09/Z. J.F. and E.I. also receive funding from the NIHR funded Leeds Musculoskeletal Biomedical Research Centre (LMBRU).
References
- 1. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284(5411): 143–147. [DOI] [PubMed] [Google Scholar]
- 2. Tropel P, Noel D, Platet N, et al. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res 2004; 295(2): 395–406. [DOI] [PubMed] [Google Scholar]
- 3. Meirelles LS, Nardi NB. Murine marrow derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br J Haematol 2003; 123(4): 702–711. [DOI] [PubMed] [Google Scholar]
- 4. Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 2003; 57(1): 11–20. [DOI] [PubMed] [Google Scholar]
- 5. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105(4): 1815–1822. [DOI] [PubMed] [Google Scholar]
- 6. Rasmusson I, Ringdén O, Sundberg B, et al. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res 2005; 305(1): 33–41. [DOI] [PubMed] [Google Scholar]
- 7. Ryan JM, Barry FP, Murphy JM, et al. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm 2005; 2(1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 2003; 31(10): 890–896. [DOI] [PubMed] [Google Scholar]
- 9. Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008; 371(9624): 1579–1586. [DOI] [PubMed] [Google Scholar]
- 10. Ringdén O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation 2006; 81(10): 1390–1397. [DOI] [PubMed] [Google Scholar]
- 11. Polchert D, Sobinsky J, Douglas G, et al. IFNγ activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol 2008; 38(6): 1745–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Laar J, Tyndall A. Adult stem cells in the treatment of autoimmune diseases. Rheumatology 2006; 45(10): 1187–1193. [DOI] [PubMed] [Google Scholar]
- 13. Schu S, Nosov M, O’Flynn L, et al. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med 2012; 16(9): 2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prigozhina TB, Khitrin S, Elkin G, et al. Mesenchymal stromal cells lose their immunosuppressive potential after allotransplantation. Exp Hematol 2008; 36(10): 1370–1376. [DOI] [PubMed] [Google Scholar]
- 15. Nauta AJ, Westerhuis G, Kruisselbrink AB, et al. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 2006; 108(6): 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eliopoulos N, Stagg J, Lejeune L, et al. Allogeneic marrow stromal cells are immune rejected by MHC class I-and class II-mismatched recipient mice. Blood 2005; 106(13): 4057–4065. [DOI] [PubMed] [Google Scholar]
- 17. Mangi RJ, Mardiney MR., Jr. The mixed lymphocyte reaction. Transplantation 1971; 11(4): 369. [PubMed] [Google Scholar]
- 18. Schwarz MR. The mixed lymphocyte reaction: an in vitro test for tolerance. J Exp Med 1968; 127(5): 879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hughes D, Caspary E. Lymphocyte transformation in vitro measured by tritiated thymidine uptake. Int Arch Allergy Appl Immunol 1970; 37: 506–531. [DOI] [PubMed] [Google Scholar]
- 20. Huang X-P, Sun Z, Miyagi Y, et al. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation 2010; 122(23): 2419–2429. [DOI] [PubMed] [Google Scholar]
- 21. Technau A, Froelich K, Hagen R, et al. Adipose tissue-derived stem cells show both immunogenic and immunosuppressive properties after chondrogenic differentiation. Cytotherapy 2011; 13(3): 310–317. [DOI] [PubMed] [Google Scholar]
- 22. Chen X, McClurg A, Zhou GQ, et al. Chondrogenic differentiation alters the immunosuppressive property of bone marrow-derived mesenchymal stem cells, and the effect is partially due to the upregulated expression of B7 molecules. Stem Cells 2007; 25(2): 364–370. [DOI] [PubMed] [Google Scholar]
- 23. Phinney DG, Kopen G, Isaacson RL, et al. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem 1999; 72(4): 570–585. [PubMed] [Google Scholar]
- 24. Kitano Y, Okada N. Separation of the epidermal sheet by dispase. Br J Dermatol 2006; 108(5): 555–560. [DOI] [PubMed] [Google Scholar]
- 25. Vidal MA, Kilroy GE, Johnson JR, et al. Cell growth characteristics and differentiation frequency of adherent equine bone marrow-derived mesenchymal stromal cells: adipogenic and osteogenic capacity. Vet Surg 2006; 35(7): 601–610. [DOI] [PubMed] [Google Scholar]
- 26. Denker AE, Nicoll SB, Tuan RS. Formation of cartilage-like spheroids by micromass cultures of murine C3H10T1/2 cells upon treatment with transforming growth factor-β1. Differentiation 1995; 59(1): 25–34. [DOI] [PubMed] [Google Scholar]
- 27. Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol 1976; 5: 9–15. [PubMed] [Google Scholar]
- 28. Bosnakovski D, Mizuno M, Kim G, et al. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells in pellet cultural system. Exp Hematol 2004; 32(5): 502–509. [DOI] [PubMed] [Google Scholar]
- 29. Klyushnenkova E, Mosca JD, Zernetkina V, et al. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci 2005; 12(1): 47–57. [DOI] [PubMed] [Google Scholar]
- 30. Poncelet AJ, Vercruysse J, Saliez A, et al. Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation 2007; 83(6): 783–790. [DOI] [PubMed] [Google Scholar]
- 31. Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002; 30(1): 42–48. [DOI] [PubMed] [Google Scholar]
- 32. Liu H, Kemeny DM, Heng BC, et al. The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells. J Immunol 2006; 176(5): 2864–2871. [DOI] [PubMed] [Google Scholar]
- 33. Sudres M, Norol F, Trenado A, et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol 2006; 176(12): 7761–7767. [DOI] [PubMed] [Google Scholar]
- 34. Klein R, Schwenk M, Heinrich-Ramm R, et al. Diagnostic relevance of the lymphocyte transformation test for sensitization to beryllium and other metals (IUPAC Technical Report). Pure Appl Chem 2004; 76(6): 1269–1281. [Google Scholar]
- 35. Frome EL, Newman LS, Cragle DL, et al. Identification of an abnormal beryllium lymphocyte proliferation test. Toxicology 2003; 183(1): 39–56. [DOI] [PubMed] [Google Scholar]
- 36. Glennie S, Soeiro I, Dyson PJ, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005; 105(7): 2821–2827. [DOI] [PubMed] [Google Scholar]
- 37. Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 2003; 75(3): 389. [DOI] [PubMed] [Google Scholar]
- 38. Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003; 101(9): 3722. [DOI] [PubMed] [Google Scholar]
- 39. Jones S, Horwood N, Cope A, et al. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol 2007; 179(5): 2824–2831. [DOI] [PubMed] [Google Scholar]
- 40. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotech (Research) 2014; 32(3): 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Theobald VA, Lauer JD, Kaplan FA, et al. ‘Neutral allografts’ – lack of allogeneic stimulation by cultured human cells expressing MHC class I and class II antigens. Transplantation 1993; 55(1): 128–132. [DOI] [PubMed] [Google Scholar]
- 42. Wagner H, Wyss C. Cell-mediated immune responsesin vitro V. A comparative study of in vitro immunogenicity of splenic lymphocytes, neoplastic lymphoid cells and fibroblasts. Eur J Immunol 1973; 3(9): 549–555. [DOI] [PubMed] [Google Scholar]
- 43. Chitilian HV, Laufer TM, Stenger K, et al. The strength of cell-mediated xenograft rejection in the mouse is due to the CD4+ indirect response. Xenotransplantation 1998; 5(1): 93–98. [DOI] [PubMed] [Google Scholar]
- 44. Costa C, Pizzolato MC, Shen Y, et al. CD86 blockade in genetically modified porcine cells delays xenograft rejection by inhibiting T-cell and NK-cell activation. Cell Transplant 2004; 13(1): 75–87. [DOI] [PubMed] [Google Scholar]
- 45. Roos D, Loos J. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes: II. Relative importance of glycolysis and oxidative phosphorylation on phytohaemagglutinin stimulation. Exp Cell Res 1973; 77(1): 127–135. [DOI] [PubMed] [Google Scholar]
- 46. Frauwirth KA, Thompson CB. Regulation of T lymphocyte metabolism. J Immunol 2004; 172(8): 4661–4665. [DOI] [PubMed] [Google Scholar]
- 47. Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002; 99(10): 3838–3843. [DOI] [PubMed] [Google Scholar]
- 48. Cappellesso-Fleury S, Puissant-Lubrano B, Apoil P-A, et al. Human fibroblasts share immunosuppressive properties with bone marrow mesenchymal stem cells. J Clin Immunol 2010; 30(4): 607–619. [DOI] [PubMed] [Google Scholar]
- 49. Sato T, Kirimura Y, Mori Y. The co-culture of dermal fibroblasts with human epidermal keratinocytes induces increased prostaglandin E2 production and cyclooxygenase 2 activity in fibroblasts. J Invest Dermatol 1997; 109(3): 334–339. [DOI] [PubMed] [Google Scholar]
- 50. Haniffa MA, Wang X-N, Holtick U, et al. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol 2007; 179(3): 1595–1604. [DOI] [PubMed] [Google Scholar]
- 51. Blasi A, Martino C, Balducci L, et al. Dermal fibroblasts display similar phenotypic and differentiation capacity to fat-derived mesenchymal stem cells, but differ in anti-inflammatory and angiogenic potential. Vasc Cell 2011; 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. French M, Rose S, Canseco J, et al. Chondrogenic differentiation of adult dermal fibroblasts. Ann Biomed Eng 2004; 32(1): 50–56. [DOI] [PubMed] [Google Scholar]
- 53. Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Cytotherapy 2012; 14(5): 516–521. [DOI] [PubMed] [Google Scholar]
- 54. Haniffa MA, Collin MP, Buckley CD, et al. Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica 2009; 94(2): 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 2005; 33(11): 1402–1416. [DOI] [PubMed] [Google Scholar]