Abstract
Beauty is in the eye of the beholder. How attractive someone is perceived to be depends on the individual or cultural standards to which this person is compared. But although comparisons play a central role in the way people judge the appearance of others, the brain processes underlying attractiveness comparisons remain unknown. In the present experiment, we tested the hypothesis that attractiveness comparisons rely on the same cognitive and neural mechanisms as comparisons of simple nonsocial magnitudes such as size. We recorded brain activity with functional magnetic resonance imaging (fMRI) while participants compared the beauty or height of two women or two dogs. Our data support the hypothesis of a common process underlying these different types of comparisons. First, we demonstrate that the distance effect characteristic of nonsocial comparisons also holds for attractiveness comparisons. Behavioral results indicated, for all our comparisons, longer response times for near than far distances. Second, the neural correlates of these distance effects overlapped in a frontoparietal network known for its involvement in processing simple nonsocial quantities. These results provide evidence for overlapping processes in the comparison of physical attractiveness and nonsocial magnitudes.
Keywords: attractiveness, beauty, comparison, fMRI, social cognition
INTRODUCTION
Beauty is as relative as light and dark. Thus, there exists no beautiful woman, none at all, because you are never certain that a still far more beautiful woman will not appear and completely shame the supposed beauty of the first.
—Paul Klee, Swiss artist (Klee and Klee, 1957–64, p. 243)
Few if any characteristics have as far ranging implications as physical attractiveness. In everyday life interactions, physical appearance is the most obvious and accessible personal characteristic, having a major impact on impression formation. This influence is all the more important as people rely on physical attractiveness to make assumptions about others. Attractive persons are perceived as more intelligent, honest, kind, sociable, dominant, talented and mentally healthy than less attractive ones (Thornike, 1920; Dion et al., 1972; Kaplan, 1978; Feingold, 1992). This Beautiful is good stereotype serves as the basis for discriminatory behaviors. Attractive people—whether children or adults—are consistently treated more favorably (Langlois et al., 2000). They are not only paid higher incomes but also receive more help in emergency situations and benefit from milder judicial condemnation and sentences (Moss and Page, 1972; Efran, 1974; Piliavin et al., 1975; Sigall and Ostrove, 1975; Hamermesh and Biddle, 1994). Psychology has proved the maxim wrong: People do judge the book by its cover.
In light of the potency of physical attractiveness, it is essential to ask how people judge the appearance of others. Psychological research has, to date, helped answer this question by focusing on both the contents and processes associated with attractiveness judgments. On the content-side, attractiveness judgments rely on the assessment of a number of physical characteristics, such as symmetry, averageness, youthfulness, or waist-to-hip ratio (Langlois and Roggman, 1990; Singh, 1993; Perrett et al., 1999; for review see Rubenstein et al., 2002). On the process-side, the relative evaluation of these characteristics seems particularly important: Attractiveness judgments are made in relation to external or internalized standards (Brown et al., 1992), such as the norms prescribed by the canon of beauty in a given society and era (Pettijohn and Tesser, 1999; Dion, 2002). Physical attractiveness evaluations are therefore not absolute. Rather, they are comparative in nature.
How does the brain weigh someone’s attractiveness in relation to a given standard and perform such subjective comparisons? Previous research has shown that the more attractive a face the greater the activity in reward related areas, such as the nucleus accumbens (NAcc) and the orbitofrontal cortex (OFC), suggesting that these regions encompass a representation of attractiveness value (Bray and O’Doherty, 2007; Kim et al., 2007; Cloutier et al., 2008; Kawabata and Zeki, 2008; Bzdok et al., 2011). However, the process underlying the comparison of these magnitudes to perform an attractiveness judgment has not been investigated so far.
The assignment of values to stimuli and the comparison of these values in the service of a behavioral choice may very well engage different brain processes. Abundant literature indeed suggests that the main comparator in the brain for numbers, simple nonsocial magnitudes and rewards is located in a frontoparietal network encompassing the intraparietal sulcus (IPS) and medial prefrontal areas rather than in regions associated with primary reward (Pinel et al., 2001, 2004; Dehaene et al., 2003; Fias et al., 2003; Cohen Kadosh et al., 2008; Wunderlich et al., 2009; Hare et al., 2011). The activity of this network depends on the distance between the two compared magnitudes. The closer two magnitudes (e.g. two numbers), the more difficult the comparison, and the greater the activity of this frontoparietal network (Cohen Kadosh et al., 2005; Nieder and Dehaene, 2009). This network is recruited by comparisons of numbers, size, line lengths, time, beverage taste and monetary rewards (Rao et al., 2001; Pinel et al., 2004; Cohen Kadosh et al., 2005; Wunderlich et al., 2009; Hare et al,. 2011) but its role in comparisons of personal subjective characteristics, such as physical attractiveness, has not been demonstrated so far.
The objective of the present study was to investigate the extent to which attractiveness comparisons rely on the same cognitive and neural mechanisms as comparisons of size and other nonsocial magnitudes. Specifically, we tested whether attractiveness comparisons obey a distance effect and whether this distance effect involves the frontoparietal network identified by research on nonsocial comparisons. Finally, we tested whether this comparative process is similarly engaged by comparisons of persons and nonsocial targets.
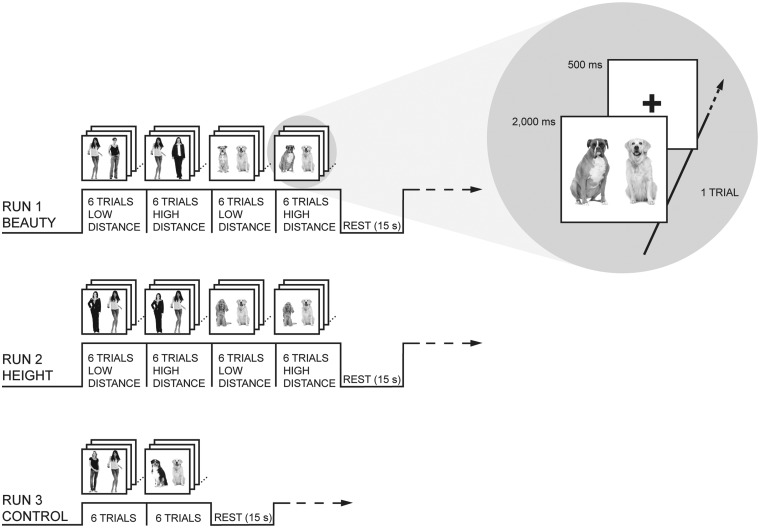
For this purpose, we examined a sample of female participants who compared the beauty and height of two women or two dogs whose pictures were displayed on the MRI scanner screen. We used dogs as nonsocial targets because they present the advantage of being similar to humans in several respects—they are living creatures, they can elicit emotions, people spontaneously judge their beauty or their height—but at the same time they are not as socially relevant as other people. People identify themselves with other humans, which is a core social cognitive process that does not characterize interactions with animals (Festinger, 1954). For half of our comparisons, the targets were close to each other on the compared dimension (low distance conditions). For the other half, one target was markedly more beautiful or taller than the other one (high distance conditions). These comparative conditions were contrasted to noncomparative control conditions, in which participants had to indicate whether both targets, i.e. both women or both dogs, had their mouth open (Figure 1).
Fig. 1.
Experimental design. Stimuli were black and white full-length pictures of women or dogs displayed in pairs. Participants had to compare the height (Which woman—or dog—is taller?) or beauty (Which woman—or dog—is more beautiful?) of these targets. In the noncomparative control conditions, participants had to indicate whether both targets had their mouth open or not. Stimuli were presented in blocks of six trials organized in a counterbalanced order. A block of rest (15 s) consisting only of a fixation cross was presented every four active blocks in the beauty and height comparison runs and every two active blocks in the control run. A trial consisted of a pair of women or dogs presented for 2 s followed by a 0.5 s fixation cross.
METHODS
Participants
Behavioral and neuroimaging studies have shown that men and women judge and react differently to female faces of various levels of attractiveness (Kaplan, 1978; Jankowiak et al., 1992; Kenrick et al., 1993; Kranz and Ishai, 2006). Therefore, to recruit a homogeneous sample and avoid effects of romantic interest, we focused our investigation to a sample of female participants. We recruited 25 right-handed British women with normal or corrected-to-normal vision (M = 23.46 years, s.d. = 4.23). The study was approved by the ethics committee of Bangor University School of Psychology. Prior to scanning, all participants gave their written informed consent according to the Declaration of Helsinki. They received monetary compensation for their participation in the study. None of them owned a dog at the moment of the experiment and none of them reported experiencing intense fear of dogs.
Stimuli
Stimuli were black and white full-length photographs of 21 women and 21 dogs collected from a commercial online image data base (http://en.fotolia.com/). These pictures were selected from a set of 152 photographs of women and 161 photographs of dogs, whose beauty had been assessed by a separate sample of female participants (Supplementary Material). The photographs used in the functional magnetic resonance imaging (fMRI) experiment encompassed targets who were high (7 women, 7 dogs), middle (7 women, 7 dogs), and low (7 women, 7 dogs) in beauty.
Procedure
A computer display consisted of two women or two dogs appearing at the center of a white screen. The center-to-center distance between the two targets subtended a horizontal visual angle of 5.8°.
In the beauty conditions, the two targets had the same height—the vertical visual angle was of 9.3° for the pictures of women and 6.6° for the pictures of dogs—and only differed in their beauty. In the low distance beauty condition, participants had to compare targets high and middle in beauty (half of the trials) and targets middle and low in beauty (the other half of the trials). In the high distance beauty condition, all trials consisted of comparing targets high and low in beauty.
In the height conditions, the targets differed in their height but were matched for their beauty (presentation of two targets high, middle, or low in beauty in one-third of the trials each). The vertical visual angle subtended by the pictures of women was of 9.3° and 9.4° in the low distance height comparison and of 9° and 9.5° in the high distance comparison. The vertical visual angle subtended by the pictures of dogs was of 6.6° and 6.7° in the low distance comparison condition and of 6.5° and 6.8° in the high distance comparison condition.
In the control conditions, the two targets were matched for their beauty and their height (same vertical visual angles as for the beauty conditions). For half of the trials, the two targets had the mouth open; for the other half only one or no target had the mouth open.
There were 576 trials in total: 96 in the low distance beauty comparison condition, 96 in the high distance beauty comparison condition, 96 in the low distance height comparison condition, 96 in the high distance height comparison condition, and 192 in the control condition. For each condition, half of the trials depicted pairs of women and the other half pairs of dogs. The same photos were used in the beauty, height, and control conditions. The size of the dogs relative to the size of the women was calculated so that both kinds of target covered the same area on the screen.
During fMRI scanning, participants viewed stimuli via a 45° angled mirror positioned above the head coil reflecting the projection of a computer screen. A trial consisted of a pair of women or dogs presented for 2 s followed by a 0.5 s fixation cross. Participants’ task was to decide which of the two targets was more beautiful or taller by pressing a button with the corresponding hand. In the control condition, they had to press a button with their left hand if both targets had the mouth open and with their right hand otherwise. The experiment was divided into 3 runs of 192 trials each (1 beauty comparison run, 1 height comparison run, and 1 control condition run). The order of the runs was counterbalanced across participants. Within 1 run, stimuli were organized in blocks of 6 trials, and thus each block lasted 15 s. Blocks were presented in a counterbalanced order. A block of rest (15 s) consisting only of a fixation cross was presented every four active blocks in the beauty and height comparison runs and every two active blocks in the control run.
Before the experiment, participants performed outside of the scanner a 39-trial training session with different stimuli as those used for the fMRI session. During the scanning session, the instruction (‘compare beauty,’ ‘compare height,’ and ‘mouth open’) was given before the respective run.
Following the fMRI session, participants were asked to rate the beauty of the 21 women and 21 dogs they had to compare in the scanner on a 7-point scale (ranging from 1: very ugly to 7: very beautiful; results of the post-hoc validation of the stimuli are presented in the Supplementary Material).
fMRI acquisition, preprocessing and analysis
We acquired the data on a Phillips 3T Achieva MR scanner equipped with an eight channel head volume coil. We acquired the functional (T2*-weighted) images using blood oxygenation level dependency contrast (repetition time TR = 2500 ms; echo time TE = 35 ms; Field of View (FoV) = 224 mm; flip angle = 65°; matrix = 88 × 84 reconstructed to 112 × 112; 1 volume = 40 axial slices; slice thickness = 3 mm; no gap; voxel size = 2.6 × 2.6 × 3 mm3). We discarded the first five scans of each run to allow for scanner equilibration. A total of 723 volumes remained corresponding to the three runs of 241 images. In addition, for each subject, we acquired a T1-weighted anatomical MRI (TE = 3.8 ms; FoV = 288 × 232 × 175 mm3; flip angle = 8°; matrix = 288 × 232 × 175; 175 slices; slice thickness = 1 mm; no gap; voxel size = 1 × 1 × 1 mm3).
We analyzed the data with SPM8 (http://www.fil.ion.ucl.ac.uk/spm) implemented in Matlab R2008a. We realigned the images using the first scan of each run as reference and we coregistered functional and anatomical data. We applied gray matter segmentation on the coregistered images and we then normalized them into standard stereotaxic space (Talairach and Tournoux, 1988). During normalization, we resampled the images at a voxel size of 3 × 3 × 3 mm3 and afterward smoothed them with a FWHM 8 × 8 × 8 Gaussian kernel. We analyzed individual subject data with standard neuroimaging methods based on the general linear model, providing contrasts for group effects analyzed at the second level.
At the first level, we modeled each experimental condition with a boxcar reference vector of 15 s convolved with the canonical hemodynamic response function implemented in SPM8. We filtered low-frequency signal drifts using a cutoff period of 128 s. To remove variations in signal due to movement artifacts, we included the movement parameters calculated during the realignment in the model as parameters of no interest. To estimate the model parameters, we used a Restricted Maximum Likelihood method. We created contrasts between each experimental condition and the baseline and then entered the contrast of parameter estimate images into a second-level group analysis using an analysis of variance (ANOVA) employing a random-effect model.
Whole-brain analyses
At the second level, we investigated with whole-brain analyses the main effect of distance in the beauty and height conditions separately and performed a conjunction analysis of these two contrasts. We also investigated the main effect of distance in the women and dog conditions separately and performed a conjunction analysis of these two contrasts. In addition, we examined the interactions between the factor distance on the one hand and the factors target and judgment on the other hand.
Moreover, to test whether the differences in brain activity revealed by the distance effects were caused by differences in task difficulty, we ran the same conjunctions as described earlier but, in addition, we modeled the response times at the first level (for each trial of each participant) as parameters of no interest.
Finally, we computed the main effects for the factors judgment and target and compared the task-related activations with the control task.
We report neural changes below a voxelwise statistical threshold of P < 0.001 uncorrected for multiple comparisons and a spatial extent threshold of P < 0.05 Familywise error (FWE) corrected for multiple comparisons. Statistical maps were labeled using the MRIcro atlas (http://www.mricro.com) and the Talairach and Tournoux atlas (Talairach and Tournoux, 1988).
Region of interest analyses
To bring the present results into line with previous research on both number comparison and attractiveness judgment, we performed a series of region of interest (ROI) analyses. These analyses aimed at (i) checking that the observed activations in the IPS anatomically matched those found for number comparisons and (ii) investigating whether the distance effects extended to the NAcc and the OFC, two regions involved in the representation of attractiveness value (Cloutier et al., 2008; Kawabata and Zeki, 2008).
For the ROI over the IPS, we defined two 10-mm spheres centered on the mean coordinates of maxima reported by Cohen Kadosh et al. (2008) in their meta-analysis on number processing (left IPS: x, y, z = −31, −50, 45; right IPS: x, y, z = 37, −46, 42).
In addition, we defined two separate ROIs over the nucleus accumbens and the OFC by applying the WFU PickAtlas Tool version 3.03 standardized mask (Maldjian et al., 2003, 2004) of the bilateral nucleus accumbens and the orbital gyrus, respectively. For all ROIs, we report neural changes below a voxelwise statistical threshold of P < 0.05 FWE corrected for multiple comparisons and a spatial extent threshold of 10 voxels.
RESULTS
Behavioral data
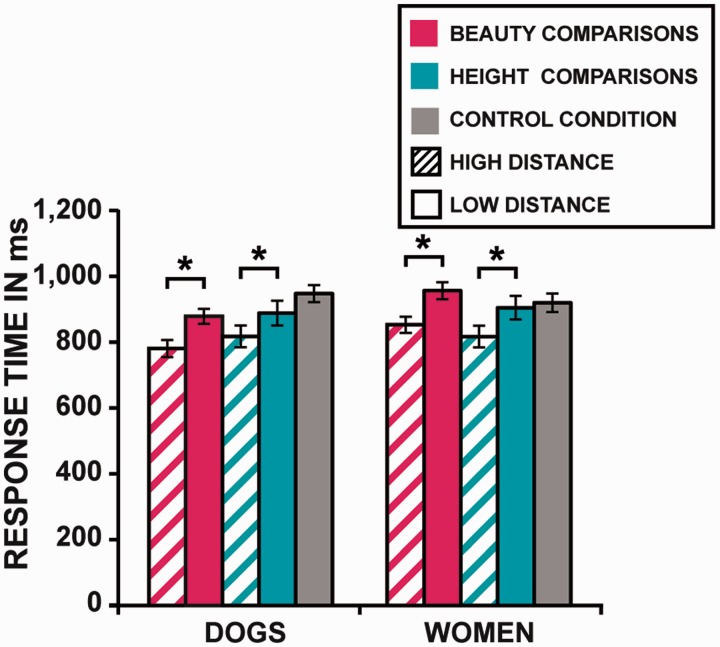
We calculated mean reaction times (RTs) for every subject in each condition. These means were submitted to a 2 (distance) × 2 (judgment) × 2 (target) repeated measure ANOVA (Figure 2). The main effect of distance was significant: Participants responded faster to high distance than low distance comparisons [F(1, 24) = 130.20, P < 0.001; η2 = 0.84]. Post-hoc two-tailed t-tests applied with a Bonferroni correction testing the effect of distance for each dimension and target category indicated that participants were faster for high distance than low distance beauty and height comparisons of women [for beauty: t(25) = 8.92, P < 0.001; for height: t(25) = 6.26, P < 0.001] and dogs [for beauty: t(25) = 6.70, P < 0.001; for height: t(25) = 6.01, P < 0.001]. There was no significant interaction between the factor distance and the two other factors [all F(1, 24) < 2.23, all P > 0.15; see Supplementary Material for the other main effects and interactions].
Fig. 2.
Response times in the different experimental conditions. Error bars represent ± s.e.m. *P < 0.001.
fMRI data: whole-brain analyses
Distance effects
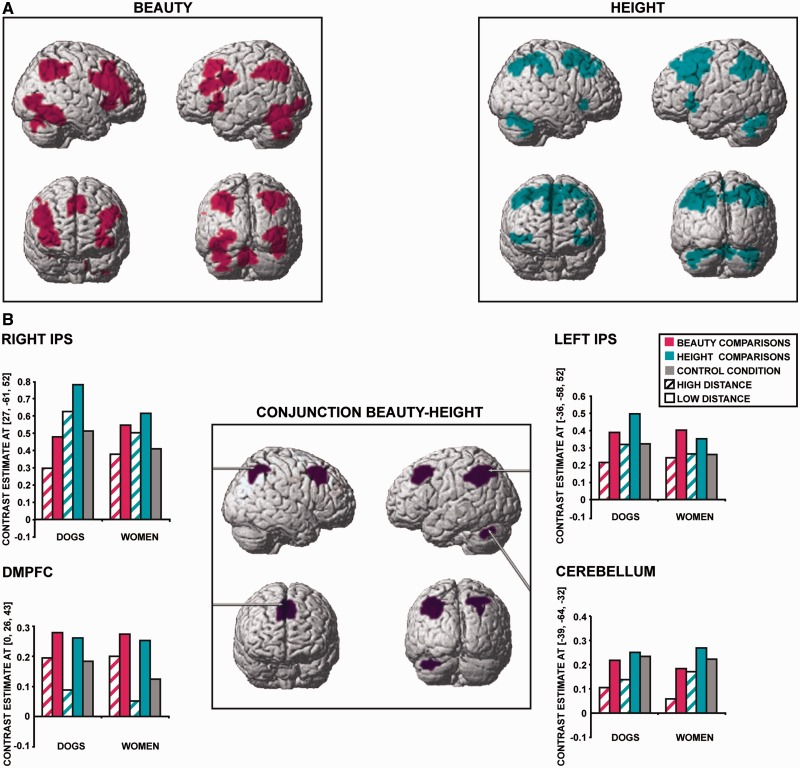
Distance effects in the beauty and height conditions analyzed separately revealed two identical networks composed of the inferior frontal gyrus (IFG), the dorsomedial prefrontal cortex (DMPFC), the left insula, two parietal clusters peaking in the left and right IPS, respectively, and the bilateral cerebellum (Table 1 and Figure 3A). In addition, low distance beauty comparisons elicited greater activity than high distance beauty comparisons in the left precentral gyrus and in the right fusiform gyrus; conversely low distance height comparisons elicited greater activity than high distance height comparisons in the inferior orbitofrontal gyrus (OFG; Table 1 and Figure 3A).
Table 1.
Distance effects: anatomical locations and coordinates of activation
| Regions | Side | Cluster size (voxels) | MNI coordinates |
z scores | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Distance effect in the beauty conditions | ||||||
| IFG/MFG | R | 914 | 45 | 38 | 28 | 6.03 |
| DMPFC | L/R | 287 | 3 | 23 | 49 | 5.75 |
| Insula | L | 164 | −33 | 17 | −5 | 5.06 |
| Precentral gyrus | L | 392 | −39 | 5 | 31 | 5.48 |
| IPS | L | 455 | −33 | −58 | 43 | 6.43 |
| R | 534 | 27 | −58 | 43 | 6.12 | |
| Fusiform gyrus | R | 520 | 39 | −58 | −14 | 5.08 |
| Cerebellum | L | 665 | −27 | −64 | −32 | 4.85 |
| L/R | 133 | −6 | −79 | −35 | 4.94 | |
| Distance effect in the height conditions | ||||||
| DMPFC | L/R | 787 | −3 | 29 | 37 | 5.33 |
| IFG | L | 164 | −39 | 23 | 28 | 4.76 |
| Inferior OFG | R | 72 | 51 | 20 | −5 | 3.88 |
| Insula | L | 82 | −51 | 14 | −8 | 3.92 |
| IPS | L | 545 | −39 | −55 | 52 | 4.80 |
| R | 401 | 27 | −61 | 52 | 4.39 | |
| Cerebellum | L | 170 | −42 | −61 | −47 | 4.57 |
| R | 146 | 36 | −64 | −32 | 4.68 | |
| L/R | 115 | 6 | −67 | −26 | 4.27 | |
| Distance effect in the women conditions | ||||||
| IFG | L | 98 | −36 | 23 | 25 | 4.73 |
| DMPFC | L/R | 1133 | 0 | 23 | 49 | 5.75 |
| Insula | L | 94 | −30 | 20 | −5 | 5.50 |
| IPS/IPL | L | 405 | −33 | −52 | 37 | 4.75 |
| IPS | R | 313 | 24 | −58 | 52 | 4.63 |
| Cerebellum/IOG | R | 240 | 45 | −58 | −14 | 4.35 |
| L | 127 | −27 | −64 | −32 | 4.51 | |
| L | 74 | −36 | −79 | −11 | 4.32 | |
| Distance effect in the dog conditions | ||||||
| IFG/MFG | R | 549 | 45 | 41 | 19 | 5.41 |
| DMPFC | L/R | 327 | 3 | 26 | 46 | 5.08 |
| Insula | L | 165 | −48 | 14 | −8 | 4.64 |
| R | 76 | 30 | 23 | −8 | 4.20 | |
| Precentral gyrus | L | 460 | −39 | 5 | 31 | 4.98 |
| IPS | L | 557 | −36 | −58 | 52 | 5.51 |
| R | 673 | 30 | −64 | 52 | 5.49 | |
| Cerebellum | R | 238 | 30 | −67 | −50 | 4.99 |
| L/R | 877 | −6 | −79 | −29 | 4.97 | |
All values, P < 0.001 uncorrected for multiple comparisons; cluster extent threshold of P < 0.05 FWE corrected for multiple comparisons. IOG, inferior occipital gyrus; MNI coordinates, Montreal Neurological Institute coordinates.
Fig. 3.
Distance effects. Statistical parametric maps overlaid onto the canonical MNI brain (whole-brain random-effect analysis, voxel level P < 0.001 uncorrected, cluster level P < 0.05 FWE corrected). (A) Distance effects in the beauty and height conditions separately. (B) Conjunction between the beauty and height distance effects. Histograms display the parameter estimates in the right (MNI coordinates: x, y, z = 27, −61, 52) and left IPS (x, y, z = −36, −58, 52), the DMPFC (x, y, z = 0, 26, 43), and the cerebellum (x, y, z = −39, −64, −32).
In accordance with our hypothesis, we found that the distance effects for beauty and height significantly overlapped in the DMPFC, in left and right parietal clusters peaking in each IPS, and in the left cerebellum (Table 2 and Figure 3B). To exclude that this effect was driven by comparisons of dogs, we investigated the distance effect in the women and dog conditions separately (Table 1). Results indicated that both distance effects activate a network composed of the IFG, the DMPFC, the left insula, two parietal clusters peaking in the left and right IPS, respectively, and of the bilateral cerebellum. In addition, low distance comparisons of dogs elicited greater activity than high distance comparisons of dogs in the left precentral gyrus and in the right insula. The conjunction analysis of the distance effects for women and dogs revealed significant overlap in the right IFG, the DMPFC, the left angular gyrus/IPS area, the right IPS, and in the left cerebellum (Table 2).
Table 2.
Conjunction analyses: anatomical locations and coordinates of activation for the conjunction analyses at the whole-brain level, and at the whole-brain level while controlling for response times (RTs)
| Regions | Side | Whole-brain analysis |
Whole-brain analysis with RTs as covariates |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CS | MNI coordinates |
Z-score | CS | MNI coordinates |
z-score | ||||||
| X | y | z | x | y | z | ||||||
| Conjunction analyses of the beauty and height distance effects | |||||||||||
| DMPFC | L/R | 236 | 0 | 26 | 43 | 5.10 | 229 | 0 | 26 | 43 | 4.97 |
| Angular gyrus/IPS | L | 272 | −36 | −58 | 52 | 4.48 | 277 | −36 | −58 | 52 | 4.54 |
| IPS | R | 127 | 27 | −61 | 52 | 4.39 | 169 | 27 | −61 | 52 | 4.43 |
| Cerebellum | L | 74 | −39 | −64 | −32 | 3.83 | 74 | −39 | −64 | −32 | 3.82 |
| Conjunction analyses of the women and dog distance effects | |||||||||||
| IFG | R | 259 | 51 | 26 | 31 | 4.37 | 264 | 51 | 26 | 31 | 4.44 |
| DMPFC | L/R | 254 | 3 | 26 | 46 | 5.08 | 251 | 3 | 26 | 43 | 5.01 |
| Angular gyrus/IPS | L | 342 | −36 | −55 | 49 | 4.75 | 341 | −36 | −55 | 49 | 4.76 |
| IPS | R | 257 | 27 | −61 | 49 | 4.60 | 273 | 27 | −61 | 49 | 4.70 |
| Cerebellum | L | 104 | −33 | −61 | −32 | 4.13 | 105 | −33 | −61 | −32 | 4.17 |
All values, P < 0.001 uncorrected for multiple comparisons; cluster extent threshold of P < 0.05 FWE corrected for multiple comparisons. CS, cluster size (voxels).
We found a significant interaction between the factors distance and judgment in the ventral anterior cingulate cortex (vACC; x, y, z = 3, 47, 4, cluster size = 176 voxels, z = 4.16), in the left medial temporal gyrus (MTG; x, y, z = −57, −58, 19, cluster size = 111 voxels, z = 4.84), and in the left precuneus (x, y, z = −18, −55, 19, cluster size = 71 voxels, z = 3.79). These interactions correspond, however, to deactivations, i.e. areas which activity was decreased rather than increased by the task (Supplementary Figure S1). As a consequence, they only provide information about the networks that are inhibited during the task and not about those that actively contribute to its execution. The other two-way and three-way interactions between the factor distance and the other two factors did not reveal any significant cluster of activated voxels.
Distance effects with RTs as covariates
We replicated all distance effect results when controlling for response times (Table 2). These analyses led to identical clusters of activated voxels suggesting that task difficulty, as measured by response times, does not account for the results we observe.
Main effects of judgment and target
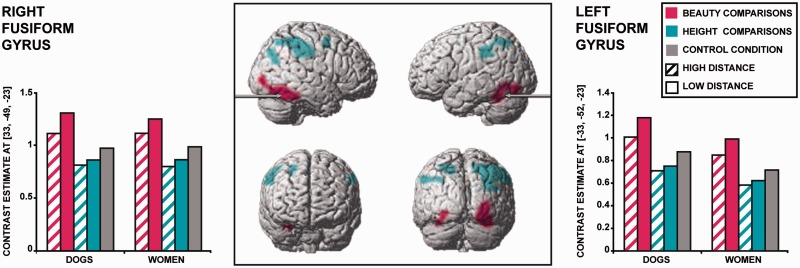
We found that beauty comparisons trigger more activity than height comparisons in right and left clusters both encompassing part of the cerebellum and of the fusiform gyrus (Table 3 and Figure 4). The coordinates of both cluster peaks are close to those previously described for the fusiform face area (FFA; Grill-Spector et al., 2004) and the fusiform body area (FBA; Peelen et al., 2007). The opposite contrast—Height > Beauty comparisons—revealed greater activation in the right middle frontal gyrus (MFG), in the left supramarginal gyrus, in the bilateral superior parietal lobe (SPL) including a peak in the right IPS, and in the right inferior parietal lobe (IPL).
Table 3.
Main effects of judgment and target: anatomical locations and coordinates for the main effects of judgment and target at the whole-brain level
| Regions | Side | Cluster size (voxels) | MNI coordinates |
z scores | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Main effect of judgment | ||||||
| Beauty > height comparisons | ||||||
| Cerebellum/fusiform gyrus | R | 343 | 33 | −49 | −23 | 4.37 |
| L | 133 | −33 | −52 | −23 | 4.13 | |
| Height > beauty comparisons | ||||||
| MFG | R | 78 | 24 | 2 | 52 | 5.24 |
| Supramarginal gyrus | L | 119 | −57 | −34 | 40 | 4.65 |
| IPS | R | 496 | 21 | −58 | 58 | 5.90 |
| SPL | L | 110 | −15 | −64 | 55 | 5.30 |
| IPL | R | 71 | 33 | −76 | 43 | 3.94 |
| Height > control conditiona | ||||||
| MFG | R | 85 | 24 | 2 | 52 | 6.08 |
| IPS | R | 409 | 21 | −58 | 58 | 6.97 |
| SPL | L | 188 | −18 | −61 | 55 | 6.47 |
| Main effect of target | ||||||
| Women > dogs | ||||||
| IFG | R | 107 | 48 | 20 | 25 | 3.92 |
| Fusiform gyrus | R | 93 | 42 | −40 | −20 | 7.17 |
| MTG | R | 607 | 48 | −61 | 10 | >8 |
| L | 99 | −45 | −70 | 13 | 5.06 | |
| Cuneus | L/R | 498 | 9 | −88 | 22 | >8 |
| Dogs > women | ||||||
| Supramarginal gyrus | L | 288 | −54 | −34 | 34 | 5.23 |
| Fusiform gyrus | L | 1008 | −27 | −82 | −11 | >8 |
| IOG/fusiform gyrus | R | 548 | 27 | −82 | −5 | >8 |
All values, P < 0.001 uncorrected for multiple comparisons; cluster extent threshold of P < 0.05 FWE corrected for multiple comparisons. IOG, inferior occipital gyrus.
aBeauty comparisons did not elicit significantly more activity than the control condition.
Fig. 4.
Beauty vs height comparisons. Statistical parametric maps overlaid onto the canonical MNI brain (whole-brain random-effect analysis, voxel level P < 0.001 uncorrected, cluster level P < 0.05 FWE corrected). Blue clusters indicate regions more activated by height than beauty comparisons. Pink clusters correspond to regions more activated by beauty than height comparisons. Histograms display parameter estimates of the local maxima in the right (x, y, z = 33, −49, −23) and left fusiform clusters (x, y, z = −33, −52, −23).
Height comparisons triggered greater activity than the control condition in the right MFG as well as in the right IPS and in the left SPL (Table 3). Beauty comparisons did not elicit more activity than the control condition. No area showed higher activity in the control condition than in either of the other conditions.
Concerning the main effect of target (Table 3), comparisons of women elicited greater activity than comparisons of dogs in the right IFG, in the right fusiform gyrus—the coordinates are close to those previously described for the FFA and the FBA (Grill-Spector et al., 2004; Peelen et al., 2007)—, in the bilateral MTG and in the medial cuneus. Conversely, comparisons of dogs elicited greater activity in the left supramarginal gyrus and in the bilateral fusiform gyrus / inferior occipital gyrus—in a more posterior area of the fusiform gyrus than that more highly activated for women.
fMRI data: ROI analyses
We found a significant distance effect within the mask over the bilateral IPS for both beauty (left IPS: x, y, z = −33, −58, 43, cluster size = 128 voxels, z = 6.43; right IPS: x, y, z = 30, −55, 40, cluster size = 126 voxels, z = 6.14) and height comparisons (left IPS: x, y, z = −39, −55, 52, cluster size = 75 voxels, z = 4.80). But we did not observe any significant distance effect—neither for beauty nor for height—within the ROIs defined around the NAcc and the OFC.
DISCUSSION
This study confirms the hypothesis that physical attractiveness comparisons engage the same mechanism as comparisons of simple nonsocial magnitudes, such as size. First, we found that the distance effect that is characteristic of nonsocial comparisons also holds for beauty comparisons. Behavioral results indicated—for all our comparisons—longer response times for near than far distances. Second, these distance effects overlapped in a frontoparietal network known for its involvement in nonsocial comparisons. These results provide evidence for overlapping processes in the comparison of physical attractiveness and nonsocial magnitudes.
Previous research suggests that this network—in particular the IPS—encompasses a system in which quantities are represented according to a mental line. The number line is, for example in Western cultures, a horizontal axis going from left (lower numbers) to right (higher numbers), which may account for the distance effect (Zorzi et al., 2002). Numerically close numbers (e.g. 2 and 3) are spatially closer on the number line than numerically more distant numbers (e.g. 2 and 8), and as a consequence more difficult to discriminate and compare (Nieder, 2005; Dehaene, 2011). In the present experiment, we find that beauty and height comparisons also obey a distance effect and that this distance effect relies on the activity of the IPS. One can thus tentatively assume that comparisons on these two dimensions follow a similar process. To compare the attractiveness of two persons, people would thus ‘extract’ or compute a certain quantity of beauty and then project it along a mental line to perform the actual comparison.
An alternative explanation is that the IPS is more engaged in difficult comparisons because of its function in eye-movements and response selection demands. If these processes were responsible for our IPS activations we would, however, expect activity in regions that are also typically recruited by these functions, such as the frontal eye field (Corbetta and Shulman, 2002), which was not activated in this study. In addition, if low distance trials trigger more IPS activity because they are more difficult, we would expect IPS activity to correlate with RTs. This was not the case here. Our height condition triggered more activity in the IPS than the control condition although RTs were longer in the control condition. Moreover, to formally exclude this possibility, we have run the same analyses modeling RTs as a covariate and found exactly the same brain activations. Therefore, nonspecific effects of response selection demands seem unlikely to account for our results.
In addition to these frontoparietal commonalities, our results indicate differences in the way the brain processes the two dimensions. Beauty judgments preferentially recruit the lateral fusiform gyrus—peak coordinates are close to those previously described for the FFA and FBA—whereas height processing more intensively involves the parietal cortex. The activations observed in the fusiform gyrus are in line with previous studies showing that the FFA is especially recruited by attractiveness judgments because of its role in face perception and identification (Nakamura et al., 1998; Senior, 2003; Kranz and Ishai, 2006; Iara et al., 2008). The present findings confirm, therefore, the involvement of the fusiform gyrus in beauty judgment and, in addition, suggest the existence of a new important cerebral network for attractiveness evaluations.
Research on the neural correlates of attractiveness judgments has indeed so far focused on perceptual and emotional systems. Besides face responsive areas, attractiveness judgments have also been associated with reward related brain regions (Bzdok et al., 2011). Attractive faces, as compared with unattractive faces, typically elicit activity in the OFC and in the ventral striatum (Kranz and Ishai, 2006; Bray and O’Doherty, 2007; Kim et al., 2007; Winston et al., 2007; Cloutier et al., 2008).
Yet, our results suggest that the comparative process takes place outside of the reward system. We investigated activity in the NAcc and in the OFC with whole-brain and ROI analyses: Neither region exhibited any significant distance effect. Conversely, our study suggests that beauty comparisons primarily engage a frontoparietal comparison system. This result is in fact remarkably consistent with research on other rewarding stimuli. Several studies, using, for example money or beverages, have shown that reward comparisons recruit the bilateral IPS and the DMPFC rather than the reward system (Wunderlich et al., 2009; Hare et al., 2011). These comparative regions seem thus to play a central role in the processing of a wide range of magnitudes and values, including attractiveness judgments.
Future research can now probe whether this frontoparietal network is involved in the assessment of other meaningful personal characteristics than beauty, such as trustworthiness or intelligence. This assumption is supported by evidence demonstrating that comparing simple social characteristics that are readily rank-ordered also trigger activity in the IPS. Chiao et al. (2009) have shown that comparisons of the ranks of US navy officers activate the IPS. It is important to keep in mind, however, that military ranks are organized in a clear and objective order that is explicitly taught. This stands in marked contrast to the highly subjective nature of most characteristics in everyday social judgments. Thus, in this respect, comparisons of military ranks seem closer to comparisons of numbers than to comparisons of social characteristics.
Previous fMRI experiments designed to investigate subjective judgments have failed to show IPS activity for comparisons of intelligence (Lindner et al., 2008) or animal ferocity (Thioux et al., 2005). These studies used, however, paradigms involving noncomparative control conditions rather than a distance effect. Control conditions may be less suitable to study comparisons than the distance effect because they often trigger processes that rely on the activity of the parietal cortex as well. In this study, for example the absence of a difference in brain activity between the beauty and control conditions is probably due to the fact that our control task—identifying whether both targets have the mouth open—involved spatial components that have activated the IPS. The high levels of IPS activity in our control conditions speak in favor of this hypothesis (Figure 3B). This phenomenon has also been observed with numbers. Numerical comparison experiments that used spatial control tasks also failed to find greater IPS activation (Göbel et al., 2004). The present experiment, in line with those performed in the field of numerical cognition, suggests that the distance effect is a more appropriate tool to examine the neural correlates of comparisons than contrasts with control conditions.
CONCLUSION
In this experiment, we investigated the neural correlates of attractiveness comparisons using functional MRI and a paradigm derived from the cognitive theories of magnitude comparisons. We have found that beauty comparisons greatly resemble comparisons of nonsocial quantities, such as sizes. Not only do beauty comparisons obey the same distance effect, but this behavioral effect also comes with activity in a frontoparietal network known for its involvement in nonsocial comparisons. These results, therefore, suggest that attractiveness comparisons rely on the same comparative process as nonsocial comparisons. We propose that this finding paves the way for investigations into other important subjective social judgments, such as intelligence or trustworthiness comparisons.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
The authors thank Jason Mitchell for his helpful comments. This work was supported by the bilateral program between the Economic and Social Research Council of the UK (ESRC) and the German Research Foundation (DFG) [grant number: RES-062-23-0946: The Neural Substrates of Social Comparison].
REFERENCES
- Bray S, O’Doherty J. Neural coding of reward-prediction error signals during classical conditioning with attractive faces. Journal of Neurophysiology. 2007;97:3036–45. doi: 10.1152/jn.01211.2006. [DOI] [PubMed] [Google Scholar]
- Brown JD, Novick NJ, Lord KA, Richards JM. When Gulliver travels: social context, psychological closeness, and self-appraisals. Journal of Personality and Social Psychology. 1992;62:717–27. [Google Scholar]
- Bzdok D, Langner R, Caspers S, et al. ALE meta-analysis on facial judgments of trustworthiness and attractiveness. Brain Structure and Function. 2011;215:209–23. doi: 10.1007/s00429-010-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Oby ER, Li Z, Parrish T, Bridge DJ. Neural representations of social status hierarchy in human inferior parietal cortex. Neuropsychologia. 2009;47:354–63. doi: 10.1016/j.neuropsychologia.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Cloutier J, Heatherton TF, Whalen PJ, Kelley WM. Are attractive people rewarding? Sex differences in the neural substrates of facial attractiveness. Journal of Cognitive Neuroscience. 2008;20:941–51. doi: 10.1162/jocn.2008.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh R, Henik A, Rubinsten O, et al. Are numbers special? The comparison systems of the human brain investigated by fMRI. Neuropsychologia. 2005;43:1238–48. doi: 10.1016/j.neuropsychologia.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R, Lammertyn J, Izard V. Are numbers special? An overview of chronometric, neuroimaging, developmental and comparative studies of magnitude representation. Progress in Neurobiology. 2008;84:132–47. doi: 10.1016/j.pneurobio.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Review Neuroscience. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dehaene S. The Number Sense: How the Mind Creates Mathematics. New York: Oxford University Press; 2011. [Google Scholar]
- Dehaene S, Piazza M, Pinel P, Cohen L. Three parietal circuits for number processing. Cognitive Neuropsychology. 2003;20:487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- Dion KK. Cultural perspectives on facial attractiveness. In: Rhodes G, Zebrowitz LA, editors. Facial Attractiveness: Evolutionary, Cognitive and Social Perspectives. Westport, CT: Ablex Publishing; 2002. pp. 239–59. [Google Scholar]
- Dion KK, Berscheid E, Walster E. What is beautiful is good. Journal of Personality and Social Psychology. 1972;24:285–90. doi: 10.1037/h0033731. [DOI] [PubMed] [Google Scholar]
- Efran MG. The effect of physical appearance on the judgment of guilt, interpersonal attraction, and severity of recommended punishment in a simulated jury task. Journal of Research in Personality. 1974;8:45–54. [Google Scholar]
- Feingold A. Good-looking people are not what we think. Psychological Bulletin. 1992;111:304–41. [Google Scholar]
- Festinger L. A theory of social comparison processes. Human Relations. 1954;7:117–40. [Google Scholar]
- Fias W, Lammertyn J, Reynvoet B, Dupont P, Orban GA. Parietal representation of symbolic and nonsymbolic magnitude. Journal of Cognitive Neuroscience. 2003;15:47–56. doi: 10.1162/089892903321107819. [DOI] [PubMed] [Google Scholar]
- Göbel SM, Johansen-Berg H, Behrens T, Rushworth MFS. Response-selection-related parietal activation during number comparison. Journal of Cognitive Neuroscience. 2004;16:1536–51. doi: 10.1162/0898929042568442. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nature Neuroscience. 2004;7:555–62. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- Hamermesh DS, Biddle JE. Beauty and the labor market. American Economic Review. 1994;84:1174–94. [Google Scholar]
- Hare TA, Schultz W, Camerer CF, O’Doherty JP, Rangel A. Transformation of stimulus value signals into motor commands during simple choice. Proceedings of the National Academy of Sciences USA. 2011;108:18120–5. doi: 10.1073/pnas.1109322108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaria G, Fox CJ, Waite CT, Aharon I, Barton JJS. The contribution of the fusiform gyrus and superior temporal sulcus in processing facial attractiveness: neuropsychological and neuroimaging evidence. Neuroscience. 2008;155:409–22. doi: 10.1016/j.neuroscience.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowiak WR, Hill EM, Donovan JM. The effects of sex and sexual orientation on attractiveness judgments: an evolutionary interpretation. Ethology and Sociobiology. 1992;13:73–85. [Google Scholar]
- Kaplan RM. Is beauty talent? Sex interaction in the attractiveness halo effect. Sex Roles. 1978;4:195–204. [Google Scholar]
- Kawabata H, Zeki S. The neural correlates of desire. PLoS One. 2008;3(8):e3027. doi: 10.1371/journal.pone.0003027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenrick DT, Montello DR, Gutierres SE, Trost MR. Effects of physical attractiveness on affect and perceptual judgments: when social comparison overrides social reinforcement. Personality and Social Psychology Bulletin. 1993;19:195–9. [Google Scholar]
- Kim H, Adolphs R, O’Doherty JP, Shimojo S. Temporal isolation of neural processes underlying face preference decisions. Proceedings of the National Academy of Science U S A. 2007;104:18253–8. doi: 10.1073/pnas.0703101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee P, Klee F. The Diaries of Paul Klee 1898-1918. Berkeley (CA): University of California Press; 1957, trans. 1964. [Google Scholar]
- Kranz F, Ishai A. Face perception is modulated by sexual preference. Current Biology. 2006;16:63–8. doi: 10.1016/j.cub.2005.10.070. [DOI] [PubMed] [Google Scholar]
- Langlois JH, Roggman LA. Attractive faces are only average. Psychological Science. 1990;1:115–21. [Google Scholar]
- Langlois JH, Kalakanis L, Rubenstein AJ, Larson A, Hallam M, Smoot M. Maxims or myths of beauty? A meta-analytic and theoretical review. Psychological Bulletin. 2000;126:390–423. doi: 10.1037/0033-2909.126.3.390. [DOI] [PubMed] [Google Scholar]
- Lindner M, Hundhammer T, Ciaramidaro A, Linden DEJ, Mussweiler T. The neural substrates of person comparison—an fMRI study. NeuroImage. 2008;40:963–71. doi: 10.1016/j.neuroimage.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage. 2004;21:450–5. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Moss MK, Page RA. Reinforcement and helping behavior. Journal of Applied Social Psychology. 1972;2:360–71. [Google Scholar]
- Nakamura K, Kawashima R, Nagumo S, et al. Neuroanatomical correlates of the assessment of facial attractiveness. Neuroreport. 1998;9:753–7. doi: 10.1097/00001756-199803090-00035. [DOI] [PubMed] [Google Scholar]
- Nieder A. Counting on neurons: the neurobiology of numerical competence. Nature Review Neuroscience. 2005;6:177–90. doi: 10.1038/nrn1626. [DOI] [PubMed] [Google Scholar]
- Nieder A, Dehaene S. Representation of number in the brain. Annual Review of Neuroscience. 2009;32:185–208. doi: 10.1146/annurev.neuro.051508.135550. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Burt DM, Penton-Voak IS, Lee KJ, Rowland DA, Edwards R. Symmetry and human facial attractiveness. Evolution and Human Behavior. 1999;20:295–307. [Google Scholar]
- Peelen MV, Atkinson AP, Andersson F, Vuilleumier P. Emotional modulation of body-selective visual areas. Social Cognitive and Affective Neuroscience. 2007;2:274–83. doi: 10.1093/scan/nsm023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettijohn TF, II, Tesser A. Popularity in environmental context: facial feature assessment of American movie actresses. Media Psychology. 1999;1:229–47. [Google Scholar]
- Piliavin IM, Piliavin JA, Rodin J. Costs, diffusion, and the stigmatized victim. Journal of Personality and Social Psychology. 1975;32:429–38. [Google Scholar]
- Pinel P, Dehaene S, Rivière D, LeBihan D. Modulation of parietal activation by semantic distance in a number comparison task. NeuroImage. 2001;14:1013–26. doi: 10.1006/nimg.2001.0913. [DOI] [PubMed] [Google Scholar]
- Pinel P, Piazza M, Le Bihan D, Dehaene S. Distributed and overlapping cerebral representations of number, size, and luminance during comparative judgments. Neuron. 2004;41:983–93. doi: 10.1016/s0896-6273(04)00107-2. [DOI] [PubMed] [Google Scholar]
- Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Nat Neurosci. 2001;4:317–23. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- Rubenstein AJ, Langlois JH, Roggman LA. What makes a face attractive and why: the role of averageness in defining facial beauty. In: Rhodes G, Zebrowitz LA, editors. Facial Attractiveness: Evolutionary, Cognitive and Social Perspectives. Westport, CT: Ablex Publishing; 2002. pp. 1–33. [Google Scholar]
- Senior C. Beauty in the brain of the beholder. Neuron. 2003;38:525–8. doi: 10.1016/s0896-6273(03)00293-9. [DOI] [PubMed] [Google Scholar]
- Sigall H, Ostrove N. Beautiful but dangerous: effects of offender attractiveness and nature of the crime on juridic judgment. Journal of Personality and Social Psychology. 1975;31:410–4. [Google Scholar]
- Singh D. Adaptive significance of female physical attractiveness: role of waist-to-hip ratio. Journal of Personality and Social Psychology. 1993;65:293–307. doi: 10.1037//0022-3514.65.2.293. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Thioux M, Pesenti M, Costes N, De Volder A, Seron X. Task-independent semantic activation for numbers and animals. Cognitive Brain Research. 2005;24:284–90. doi: 10.1016/j.cogbrainres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Thorndike E. The constant error in psychological ratings. Journal of Applied Psychology. 1920;4:25–9. [Google Scholar]
- Winston JS, O’Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45:195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Wunderlich K, Rangel A, O’Doherty JP. Neural computations underlying action-based decision making in the human brain. Proceedings of the National Academy of Sciences U S A. 2009;106:17199–204. doi: 10.1073/pnas.0901077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzi M, Priftis K, Umilta C. Brain damage: neglect disrupts the mental number line. Nature. 2002;417:138–9. doi: 10.1038/417138a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.