Abstract
Depressive cognitive schemas play an important role in the emergence and persistence of major depressive disorder (MDD). The current study adapted emotion regulation techniques to reflect elements of cognitive behavioural therapy (CBT) and related psychotherapies to delineate neurocognitive abnormalities associated with modulating the negative cognitive style in MDD. Nineteen non-medicated patients with MDD and 19 matched controls reduced negative or enhanced positive feelings elicited by emotional scenes while undergoing functional magnetic resonance imaging. Although both groups showed significant emotion regulation success as measured by subjective ratings of affect, the controls were significantly better at modulating both negative and positive emotion. Both groups recruited regions of dorsolateral prefrontal cortex and ventrolateral prefrontal cortex (VLPFC) when regulating negative emotions. Only in controls was this accompanied by reduced activity in sensory cortices and amygdala. Similarly, both groups showed enhanced activity in VLPFC and ventral striatum when enhancing positive affect; however, only in controls was ventral striatum activity correlated with regulation efficacy. The results suggest that depression is associated with both a reduced capacity to achieve relief from negative affect despite recruitment of ventral and dorsal prefrontal cortical regions implicated in emotion regulation, coupled with a disconnect between activity in reward-related regions and subjective positive affect.
Keywords: depression, emotion regulation, attention, amygdala, cognitive control, prefrontal cortex
INTRODUCTION
Major depressive disorder (MDD) is among the most prevalent, costly and debilitating psychiatric disorders (World Health Organization, 2003). A ‘negative cognitive triad’ is thought to play a key role in the initiation and maintenance of depressed mood, consisting of a persistent negative idiosyncratic appraisal of the self, the future and the world (Beck et al., 1979). It has also been argued that one of the key abnormalities behind depressive illnesses is dysfunction in neural systems supporting adaptive emotion regulation (Davidson et al., 2002). The goal of a number of psychotherapies therefore is to target negative biases and to increase the efficacy of emotion regulation. Consequently, elucidating the role of neural regions involved in resolving negative biases and fostering adaptive emotional responding in depression is vitally important for improving the efficiency, efficacy and durability of the therapeutic response (Linden, 2006; Clark and Beck, 2010).
The effortful regulation of emotion is thought to involve dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC) and dorsomedial prefrontal cortex (DMPFC), which modulate, either directly or indirectly, emotion encoding in regions such as the amygdala and ventral striatum (Ochsner et al., 2002, 2004; Urry et al., 2006; Wager et al., 2008; Han et al., 2011). However, the efficacy associated with emotion regulation varies depending on the strategy adopted as indicated by neural, physiological and behavioural markers of affect (e.g. Gross, 1998; Goldin et al., 2008; Kross et al., 2009). For example, some strategies may even exacerbate emotional dysfunction (Gross, 1998; Campbell-Sills and Barlow, 2007). Consideration of the specific regulation strategy adopted is therefore critical when interpreting the clinical significance of results from emotion regulation studies.
Considerable research has demonstrated the importance of the ventromedial prefrontal cortex, including the subgenual region (Mayberg et al., 2005), in the etiology of depression (Drevets et al., 1992; Koenigs et al., 2008). Notably, much of this evidence comes from studies using positron emission tomography, which is often more robust than functional magnetic resonance imaging (fMRI) to the signal loss caused by susceptibility artefact (Devlin et al., 2000; Veltman et al., 2000). However, it is also important to note the evidence that lateral prefrontal regions involved in emotion regulation have also been implicated in current models of MDD (Phillips et al., 2003; Price and Drevets, 2010). Past research has demonstrated that MDD is associated with reduced resting metabolism in DLPFC (Mayberg, 2002) and hyper-metabolism in VLPFC (Drevets et al., 1992). In addition, histological evidence from post-mortem studies indicates that neuronal and glial density within the DLPFC and VLPFC of depressed patients is reduced (Rajkowska et al., 1999, 2001). While some suggest that recovery from MDD following medication is related to enhanced activity in DLPFC (Kennedy et al., 2001; Fales et al., 2009), others have found that successful treatment with cognitive behavioural therapy (CBT) was associated with reduced activity within DLPFC and VLPFC (Goldapple et al., 2004; Ritchey et al., 2011). When healthy, these regions are thought to modulate stimulus encoding in a manner that ultimately influences activity in emotion-related brain regions including the amygdala and ventral stratum, which have both been implicated in the pathophysiology of depression (Drevets et al., 1992; Phillips et al., 2003). One way to better understand the role of DLPFC and VLPFC in emotion regulation and CBT is to examine these two processes in the context of active emotion regulation.
To date, strategies used in emotion regulation studies of depression have differed from those adopted in typical cognitive-based therapies. For example, patients have been asked to take the perspective of a detached observer (Beauregard et al., 2006; Erk et al., 2010), or imagine that the situation is fake or unreal (Johnstone et al., 2007; Heller et al., 2009; Light et al., 2011). This is crucial because various forms of regulation differentially recruit regions of the prefrontal cortex (Ochsner et al., 2004; Goldin et al., 2008; Kross et al., 2009). Furthermore, only one study to date (Heller et al., 2009) has examined patients’ capacity to explicitly up-regulate positive affect. This knowledge gap is particularly critical given that reduced behavioural responsiveness to positive emotions predicts poorer prognosis (Rottenberg et al., 2002), and a number of studies have found that amygdala reactivity to positive but not negative cues is correlated with depression severity (Suslow et al., 2010; Victor et al., 2010). Thus, collectively, the evidence suggests that distinct emotion regulation strategies can have different effects at a behavioural and physiological level, and emotional reactivity to positive stimuli predicts therapeutic response. Consequently, further research using emotion regulation techniques that mirror strategies used in cognitive therapies and target positive as well as negative affect is essential.
Here we used functional magnetic resonance imaging (fMRI) in conjunction with an emotion regulation task we adapted to incorporate elements of CBT and cognitive-based therapies. Patients with MDD and matched controls attempted to reduce their emotional response to sad stimuli and enhance their response to positive ones. We tested the hypothesis that depression would be associated with abnormal recruitment of prefrontal regions implicated in emotion regulation. Specifically, we predicted that patients with depression would show reduced regulation efficacy, coupled with functional abnormalities in DLPFC and VLPFC. In addition, we predicted that this would be accompanied by dysfunctional modulation of amygdala and ventral striatum by negative and positive regulation trials, respectively.
METHODS
Participants
Nineteen medication-free outpatients with a primary diagnosis of MDD were recruited for study participation from London Health Sciences Centres and via community advertisements in London, Ontario (Mage = 26.79, s.d. = 11.4, range = 16–59; 13 females, 6 males). Ten patients were anti-depressant naïve at the time of scan, and the remainder were medication-free for at least three months (Mmonths = 30.8, range = 3–60 months). All participants were experiencing a major depressive episode at the time of scanning, as determined by a clinical research assistant or the primary investigator using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition Text Revision, DSM-IV-TR, (SCID; First et al., 2002, both of whom underwent the required training as per the SCID manual. Patients with a history of head injury, neurologic illness, or depression resulting from a general medical condition or substance as determined by the SCID, were excluded. Patients with a comorbid diagnosis other than anxiety or past alcohol abuse were also excluded. Seven participants were experiencing their first major depressive episode at the time of the scan, while the remainder were experiencing at least their second major depressive episode. All patients reached diagnostic criteria for MDD, which was not attributed to any other comorbid diagnosis. Seven patients had comorbid anxiety disorders: two with social anxiety disorder (SAD) without agoraphobia, four with post-traumatic stress disorder (PTSD), one with PTSD and SAD without agoraphobia and two had a history of alcohol abuse. One patient had last abused alcohol a year prior to the time of scan, and the other patient last abused one month from the time of scan. Notably, the between-group fMRI analyses described below were repeated after excluding patients with PTSD or patients with alcohol abuse. These additional analyses did not produce substantively different results, and so are not presented. Patients who presented as euthymic at the time of contact with the research program, who reported claustrophobia or who had any contraindications for participation in the MRI scanner were not enrolled in the study. Additionally, data from one subject was excluded because treatment with anti-depressants was commenced between the date of the SCID interview and the scan session. A control group (CTL) of 19 healthy volunteers matched for age, sex and handedness were recruited from the community for the study. Participants in the CTL had no history of psychiatric illness as determined by the SCID, and reported having no first-degree relative with a known DSM-IV axis-1 or axis-2 disorder (Mage = 27.63, s.d. = 11.0, range = 18–54; 13 females, 6 males). There was no significant difference in age between groups [t(36) = 0.231; P > 0.8] nor were there significant differences in intelligence quotient (IQ) on the Wechsler Abbreviated Scale of Intelligence [WASImean(s.d): MDD = 108.65 (12.3), CTL = 113.17 (8.9); t(33) = 1.251, P > 0.2; WASI scores were missing from 3 participants (2 in the MDD group) due to attrition]. Immediately before scanning, participants completed the Beck Depression Inventory (BDI; Beck et al., 1996). As expected, participants with MDD had significantly higher BDI scores than controls [BDImean(s.d): MDD = 25.53 (10.4), CTL = 1.6 (2.3); t(36) = 9.768, P < 0.001]; the mean BDI score for the MDD group was indicative of moderate depression, although severity ranged from mild to severe. The mean estimated length of the current depressive episode at the time of scan based on subject report was 7.8 weeks (ranging from 2 to 54 weeks), although the median length was 4 weeks. All subjects granted informed written consent, and the study was approved by the Health Science Research Ethics Board at the University of Western Ontario, Canada.
Task design
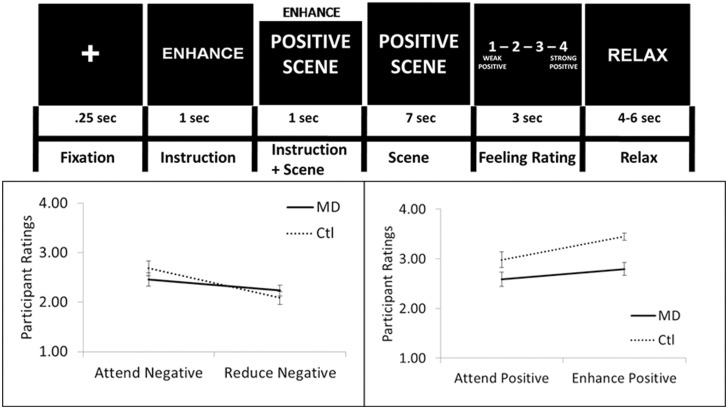
The emotion regulation task was designed to have participants actively engage in a strategy to alter the feelings elicited by sad (negative) and positive emotional scenes (for task details see Figure 1), similar to previous studies in healthy controls (Ochsner et al., 2002, 2004). However, the current method differed from previous studies in that the regulation techniques were developed to reflect a strategy, similar to those used in cognitive behavioural and other cognitive-based therapies (CBT), to address the cognitive triad of dysfunctional schematic thinking associated with MDD (Beck et al., 1979). This strategy targeted the tendency of depressed patients to have negative thoughts about the self (e.g. feelings of worthlessness), the world or environment (e.g. the world is unfair) and the future (e.g. the future is hopeless). In the enhance condition, participants were instructed to ‘Acknowledge that the scene is positive. Further, that it does affect you, things can and do get even better and the scene does reflect the real world’. During the reduce condition, participants were instructed to ‘Acknowledge that the scene is negative. However, it does not affect you, things do not stay this bad, and the scene does not reflect the whole world’. It was further emphasized that participants should, using internal dialogue, elaborate on any aspect of the script using self-relevant examples they believed would be most effective. There were 20 trials in each of the four experimental conditions (i.e. attend positive, attend negative, reduce negative, enhance positive), for a total of 80 trials across four runs. Additionally, the trial order in each run was randomized, and the four runs were counterbalanced across subjects. In a separate session, before being scanned, participants were trained to use the regulation strategies and underwent a practice session of the task.
Fig. 1.
The emotion regulation task and behavioural results. Top—Sample of an enhance-positive trial. Each trial of the emotion regulation task was composed of five events: (i) a fixation cross; (ii) an instruction about the type of strategy to use while viewing the scene (i.e. attend positive or negative, reduce negative or enhance positive); (iii) a scene depicting either positive or negative emotional significance (i.e. a standardized image shown to elicit contentment/amusement or sadness); (iv) a rating screen with a 4-point Likert scale during which participants rated the feelings evoked by the picture; (v) a screen with the word ‘relax’, during which time the participants could clear their minds before the next trial. The three instructional words of ‘attend’, ‘enhance’ and ‘reduce’ each corresponded to an emotion regulation strategy that was taught to the participants before beginning the experiment. During the attend conditions, participants were instructed to identify the feeling associated with the scene and experience whatever feelings come naturally without changing them. The positive and negative scenes were taken from the IAPS database (IAPS image not shown, see Supplementary Table 1). Bottom-Left—Mean emotional rating for negative trials [y-axis: strength of emotional response (ranging from 1 = weak to 4 = strong)] reveals a main effect of instruction and a significant group × instruction interaction showing enhanced regulation efficacy in CTL relative to MDD group. Error bars depict standard error of the mean. Bottom-right—mean emotional rating for positive trials (y-axis: 1 = weak positive to 4 = strong positive emotional response) reveals a main effect of group, a main effect of instruction and a significant group by instruction interaction characterized by enhanced regulation efficacy in CTL relative to MDD group (p). Error bars depict standard error of the mean. All effects were significant at P < 0.05.
Stimuli
A total of 20 sad and 20 positive scenes were used in the task, each one appearing twice (never in the same run), once in an attend condition and once in a regulate condition, with the order counterbalanced across participants. The emotional scenes were taken from the International Affective Picture System (IAPS; Lang et al., 2008), and were not significantly different in terms of normative ratings of arousal [Mpositive (s.d.) = 5.03 (0.55), Msad (s.d.) = 5.08 (0.62); P > 0.8]. To increase the relationship between the stimuli and the emotions central to depression, images were chosen on the basis of refined normative ratings developed by Mikels et al. (2005). These refined ratings allowed us to identify a subset of scenes that elicited one discrete emotion more than others. Specifically, our sad scenes were those that reliably elicit sadness, and the positive scenes were images that reliably elicit contentment/amusement (Mikels et al., 2005). A list of the IAPS images used can be found in Supplementary Table 1.
fMRI data acquisition
The experimental task was completed at the Centre for Metabolic Mapping, in the Robarts Research Institute’s 3T Siemens scanner equipped with a 32 channel head coil. Participants completed six functional MRI runs during which blood-oxygenation-level-dependent (BOLD) changes were measured using a T2*-gradient echo-planar sequence (EPI; time to repetition = 3000 ms, time to echo = 30 ms; 120 × 120 mm matrix; field of view = 24 cm). Seventy-nine volumes were collected per run, resulting in run durations of 3.95 min. Complete brain coverage was obtained with 45 interleaved slices of 2 mm by 2 mm in plane and a slice thickness of 2.5 mm (forming voxels of 2 × 2 × 2.5 mm). Our current parameters involved whole-brain coverage and were not specifically optimized for signal detection in the ventral prefrontal cortex (PFC). There was notable susceptibility-artefact in regions of the ventral PFC (see Supplementary Figure 1), which may account for a lack of effects in these regions (Devlin et al., 2000; Veltman et al., 2000). The session ended with a high-resolution anatomical scan that covered the whole brain (time to repetition = 2300 ms, time to echo = 4.25 ms; Field of View = 25.6 cm; 192 axial slices; voxel dimensions = 1 mm isovoxels; 256 × 256 mm matrix).
Behavioural analysis
Participants’ mean emotional ratings were calculated for each of the four conditions from the trial-by-trial emotional rating screen (the 4-point Likert scale). The individual means were entered into two independent 2 (Group: CTL, MDD) × 2 (Condition: Regulation, Attend) ANOVAs (analyses of variance, one for trials with sad scenes and the other for positive scene trials). We also computed both negative (sad trials) and positive (positive trials) regulation efficacy scores at the individual subject level. These scores reflected the mean absolute difference between emotional ratings during regulate-minus-attend trials. Thus, in the context of the current task a higher value for either negative or positive regulation efficacy was indicative of greater regulation success. This was used to examine correlations between regulation efficacy and ratings of depression severity (BDI), as well as regulation efficacy and functional activity.
fMRI analysis
Individual and group analyses were conducted using Analysis of Functional NeuroImages software (Cox, 1996). The first four volumes of each of the six runs were discarded to insure that magnetization equilibrium was reached. Motion correction was completed by registering all (BOLD) data in each run of the task to the first volume of the last experimental run. Next, the functional data were aligned to the anatomical data and both were transformed into the standard space of Talairach and Tournoux. The dataset for each subject was spatially smoothed with a 4 mm isotropic Gaussian kernel, and the time series data of each voxel was scaled such that the coefficients produced by the regression analysis represented the percent signal change from the mean voxel activity. A first-level general linear model regression analysis was performed including a regressor for each of the four conditions of interest (attend positive, attend negative, enhance positive, reduce negative), which began at emotional scene onset and ended with emotional scene offset (a duration of 8 s). Regressors of no-interest were modelled for trials in which no response was detected, for the instruction epoch, and for the emotional rating and relax epochs. Participants were instructed to only respond during the rating epoch so as to ensure that BOLD activity related to motor responses did not confound the events of interest. All regressors were produced by convolving the train of stimuli with the gamma-variate haemodynamic response function. To account for voxel-wise correlated drifting, baseline plus linear drift and quadratic trend were also modelled. This produced beta coefficients and t-values for each of our experimental conditions at each voxel, which were then used in the group analyses described below.
To test our primary hypotheses concerning the neural correlates of emotion regulation in MDD, we performed analyses examining the effects of group (MDD vs CTL) on regulating positive and negative affect. This between-group analysis was performed using the mixed-effects multilevel analysis function in the AFNI software package (Chen et al., 2012). A two-sample mixed effects analysis was then possible for each of the experimental conditions. Whole-brain analysis of the BOLD data identified significant clusters that survived a family-wise error rate (FWE) correction to P < 0.05 (k > 47 contiguous voxel; P < 0.005 uncorrected threshold, two-tailed). For the amygdala, an a priori region of interest (ROI), we used a more liberal threshold of P < 0.01 (uncorrected, k ≥ 10 contiguous voxels) consistent with thresholds adopted in previous studies of emotion in depression (Fales et al., 2008; Victor et al., 2010).
RESULTS
Behavioural results
To assess emotion regulation ability, affect ratings were obtained from each participant on each trial. For sad trials, the 2 (Group) × 2 (Instruction) ANOVA revealed no main effect of group [F(1, 36) = 0.042, P > 0.8]. However, a main effect of instruction [F(1, 36) = 47.10, P < 0.001] emerged; negative affect ratings were significantly reduced in the regulate relative to attend condition. Importantly, a significant group × instruction interaction was also revealed [F(1, 36) = 9.59, P < 0.005; Figure 1A] characterized by enhanced regulation of negative emotional reactivity in the CTL group. Follow-up within-group contrasts of the reduce vs attend sad conditions revealed that both the CTL group [t(18) = −7.11, P < 0.001, two-tailed] and MDD group [t(18) = −2.64, P < 0.05] reported significantly less negative reactivity during the regulate condition. A follow-up test of regulation efficacy scores confirmed that the CTL group were significantly more effective than those with MDD at regulating sad affect [t(36) = 2.05, P < 0.05).
For positive trials, the 2 (Group) × 2 (Instruction: attend positive, enhance positive) ANOVA revealed a main effect of group such that the CTL group rated the scenes as more positive overall [F(1, 36) = 9.08, P < 0.005], and a main effect of instruction such that both groups reported significantly greater positive reactivity when enhancing positive affect [F(1, 36) = 27.91, P < 0.001]. There was also a significant two-way interaction [F(1, 36) = 4.186, P < 0.05, Figure 1B], which indicated that the CTL group showed enhanced up-regulation of positive affect. Follow-up within-group contrasts of enhance positive vs attend positive conditions revealed that whereas the CTL group reported a significant increase in positive emotional reactivity [t(18) = 6.46, P < 0.001], the MDD group’s rating of positivity in the enhance condition reflected only a trend [t(18) = 1.97, P = 0.065, two-tailed]. Lastly, the regulation efficacy score for positive stimuli was significantly greater in the CTL relative to MDD group [t(36) = 3.01, P < 0.005].
To determine whether a relationship existed between regulation efficacy for both the sad and positive emotional contexts, a correlation analysis within each group was performed. This analysis revealed a significant positive correlation between negative and positive regulation efficacy in both the CTL (r = 0.705, P < 0.001) and MDD groups (r = 0.631, P < 0.005). Thus, the capacity to regulate negative affect was also associated with more effective regulation of positive affect. Finally, there was no significant relationship between either positive or negative regulation efficacy and depression severity (BDI score; P > 0.2) within the MDD group.
fMRI results
BOLD response to trials with sad scenes
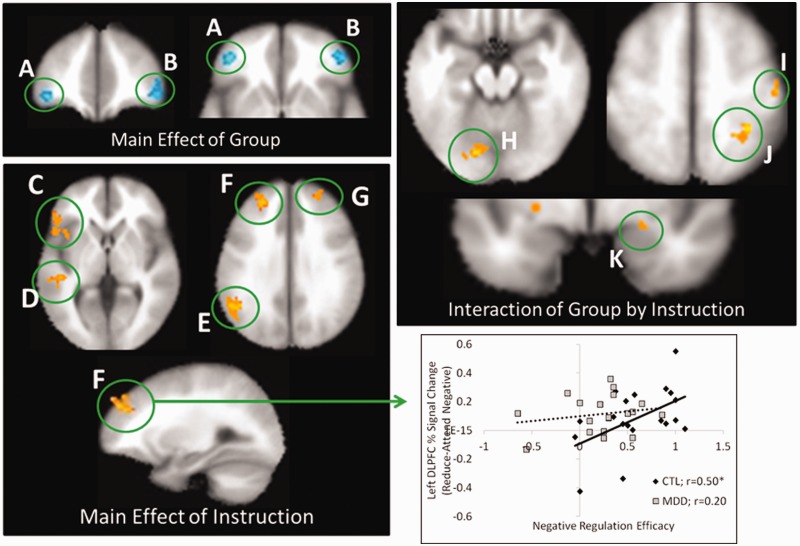
We first investigated the BOLD response for trials with sad scenes between groups (P < 0.005; P < 0.05 FWE corrected; see Table 1 for full summary). Irrespective of instruction, sad scenes produced greater activity in an anterior region of VLPFC [Brodmann location (BA) 10/47] in the CTL relative to MDD group (main effect of group; Figure 2A and B). Next, collapsing across groups, we examined the neural response to down-regulate negative affect (main effect of instruction: reduce negative–attend negative; Figure 3C–G). Consistent with previous studies of emotion regulation in both healthy and depressed individuals, we found significantly greater activity for the reduce negative condition in left DLPFC (BA 8/9/10) and right DLPFC (BA 10/9), left VLPFC (BA 47/45), left temporoparietal junction (BA 39) and left middle temporal gyrus (BA 21). For the interaction term comparing the two groups in negative affect regulation capacity [MDD (reduce-attend negative) vs CTL (reduce-attend; Figure 2H–K)], significant activity was observed in left lingual gyrus (BA 18), right postcentral gyrus (BA 3/4) and right inferior parietal lobe (BA 40/7). The nature of this interaction in all regions was similar. Whereas for the CTL group, attempts to reduce sadness were accompanied by reductions in activity in these regions (P < 0.05 in each case), similar attempts were associated with enhanced activity in all these areas for the MDD group (P < 0.05 in each case, except the middle occipital gyrus, P = 0.057). Notably, for the a priori amygdala ROI, an interaction was also observed (Figure 2K) whereby CTLs showed greater activity in the attend relative to reduce condition, and patients with MDD displayed the opposite effect. Bar plots of percent signal change for each group and condition can be found in Supplementary Figures 2 and 3.
Table 1.
Significantly active clusters from the group by instruction analysis of sad conditions
| Location | R/L | BA | X, Y, Z | Cluster size | T-value |
|---|---|---|---|---|---|
| Main effect of group: CTL (reduce + attend negative) > MDD (reduce + attend negative) | |||||
| VLPFC | R | 10/47 | 45, 45, −1 | 81 | 3.36 |
| VLPFC | L | 10/47 | −41, 41, −4 | 55 | 3.65 |
| Main effect of instruction: MDD and CTL (reduce negative > attend negative) | |||||
| VLPFC | L | 47/45 | −48, 21, 1 | 152 | 3.42 |
| DLPFC | L | 9/8/10 | −28, 46, 37 | 166 | 3.53 |
| DLPFC/superior frontal gyrus | R | 10/9 | 26, 48, 31 | 62 | 3.49 |
| DLPFC/middle frontal gyrus | L | 6/9 | −40, 8, 50 | 57 | 3.25 |
| Middle frontal gyrus | L | 6/8 | −44, 12, 59 | 93 | 3.59 |
| Superior frontal gyrus | R | 6/8 | 16, 24, 62 | 84 | 3.40 |
| Supplemental motor area | R/L | 6 | −4, 5, 68 | 410 | 3.67 |
| Temporoparietal junction | L | 39 | −51, −57, 23 | 552 | 3.73 |
| Temporal pole/middle temporal gyrus | L | 38/21 | −51, 14, −33 | 82 | 3.61 |
| Middle temporal gyrus | L | 21 | −55, −31, −7 | 140 | 3.49 |
| Culmen/cerebellum | R | 36, −50, −34 | 260 | 3.82 | |
| Interaction: MDD (reduce − attend negative) > CTL (reduce − attend negative) | |||||
| Precentral gyrus/middle frontal gyrus | L | 6/9 | −47, −1, 43 | 72 | 3.33 |
| Postcentral gyrus | R | 3/4 | 45, −21, 50 | 70 | 3.42 |
| Inferior parietal lobe | R | 40/7 | 29, −51, 53 | 74 | 3.51 |
| Precuneus | L | 19 | −20, −84, 32 | 117 | 3.38 |
| Cuneus | R | 19 | 30, −82, 30 | 56 | 3.39 |
| Middle occipital gyrus | L | 19/18 | −36, −86, 7 | 83 | 3.36 |
| Lingual gyrus | L | 18 | −16, −77, 30 | 62 | 3.35 |
| Amygdala* | R | 23, 1, −11 | 14 | 2.99 | |
The Brodmann location (BA) is provided, along with coordinates for the centre of mass in Montreal Neurological Institute (MNI) space (X, Y, Z).
Cluster size represents the number of contiguous voxels sharing a face, and the T-value is the mean T-value for all voxels in the cluster.
All clusters were FWE corrected to P < 0.05 (uncorrected threshold of P < 0.005), with the exception of the amygdala(*), which was thresholded at P < 0.01 (uncorrected).
Fig. 2.
BOLD response for the whole brain analysis of negative trials and the relation to regulation efficacy. Top-left—main effect of group demonstrates greater activity in the left (A) and right (B) VLPFC of the CTL relative to the MDD group. Bottom-left—Main effect of instruction shows enhanced activity for reduce negative relative to attend negative trials in the (C) left VLPFC, (D) left middle temporal gyrus, (E) left temporoparietal junction, (F) left DLPFC and (G) right DLPFC. Top-right—Group × instruction interaction revealed that whereas brain activity was attenuated in the CTL group on reduce relative to attend negative trials, activity was enhanced in the MDD group within the (H) left lingual gyrus, (I) right postcentral gyrus, (J) right inferior parietal lobe and (K) right amygdala* (displayed at a thresholded of P < 0.01, two-tailed, uncorrected). Bottom-right—Negative regulation efficacy was positively correlated with percent signal change in the reduce vs attend conditions in the DLPFC (F) of the CTL group (P < 0.05), but not the MDD group (P > 0.4). All regions were thresholded at P < 0.005, two-tailed and corrected to P < 0.05 (FWE, k > 47), except where noted (*). Active clusters are displayed on the averaged T1-weighted Talairach–Tournoux template (TT_avg152) in AFNI. Refer to Supplementary Figure 2 for bar plots of percent signal change.
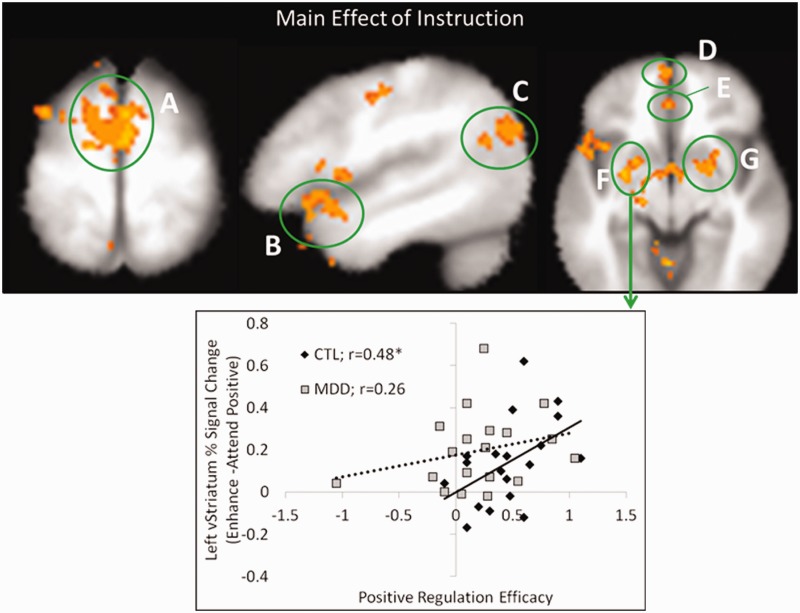
Fig. 3.
BOLD response for the whole brain analysis of positive trials and the relation to regulation efficacy. Top—Main effect of instruction revealed increased activity for enhance positive relative to attend positive trials in the (A) bilateral DMPFC/supplemental motor area, (B) left VLPFC, (C) left temporoparietal junction, (D) bilateral anterior VMPFC, (E) bilateral pgACC, (F) left ventral striatum and (G) right ventral striatum. Bottom—Positive regulation efficacy was positively correlated with the difference in percent signal change between the enhance vs attend positive conditions in the left ventral striatum (F) of the CTL group, but not the MDD group. All regions were thresholded at P < 0.005, two-tailed and corrected to P < 0.05 (FWE, k > 47). Active clusters are displayed on the averaged T1-weighted Talairach–Tournoux template (TT_avg152) in AFNI. Refer to Supplementary Figure 3 for bar plots of percent signal change.
BOLD response to trials with positive scenes
We analyzed the BOLD response to trials with scenes that elicit positive affect (P < 0.005; P < 0.05 FWE corrected; see Table 2 for full results). A main effect of group emerged in bilateral DMPFC/supplemental motor area (BA 6), characterized by greater activity in the MDD compared with CTL group. Collapsing across groups, there was significantly greater activity in positive trials during the enhance relative to attend condition (main effect of instruction; Figure 3A–G) in bilateral DMPFC/supplemental motor area (BA 6), bilateral anterior VMPFC (BA 10), bilateral perigenual anterior cingulate cortex (pgACC: BA 32/24), left VLPFC (BA 47, extending into superior temporal gyrus), left VLPFC (BA 47, extending into lateral orbital frontal cortex), left temporoparietal junction (BA 39) and right and left ventral striatum. Conversely, there was greater activity in the right DLPFC (BA 9) in the attend relative to enhance condition irrespective of group. Finally, the interaction of MDD (enhance-attend positive) vs CTL (enhance-attend positive) revealed no significant clusters of activity.
Table 2.
Significantly active clusters from the group by instruction analysis of positive conditions
| Location | R/L | BA | X, Y, Z | Cluster size | T-value |
|---|---|---|---|---|---|
| Main effect of group: MDD (enhance + attend positive) > CTL (reduce + attend positive) | |||||
| DMPFC/supplemental motor area | R/L | 6 | 0, 6, 56 | 52 | 3.46 |
| Main effect of instruction: MDD and CTL (enhance positive > attend positive) | |||||
| Superior frontal gyrus/middle frontal gyrus | L | 8 | −19, 33, 57 | 111 | 3.44 |
| Middle frontal gyrus/precentral gyrus | L | 6 | −36, 1, 59 | 74 | 3.62 |
| Middle frontal gyrus | L | 6 | −38, 10, 55 | 58 | 3.29 |
| VLPFC/anterior superior temporal gyrus | L | 47 | −48, 15, −11 | 285 | 3.48 |
| VLPFC/lateral orbital frontal cortex | L | 47 | −34, 32, −7 | 95 | 3.42 |
| VLPFC/insula | R | 44/13 | 45, 4, 7 | 134 | 3.47 |
| DMPFC/supplemental motor area | L/R | 6 | −4, −3, 65 | 1027 | 3.67 |
| Perigenual anterior cingulate cortex | L/R | 32/24 | −4, 39, −2 | 77 | 3.56 |
| Anterior VMPFC | L/R | 10 | −2, 60, −4 | 52 | 3.43 |
| Insula/caudate | L | 13 | −30, 8, 13 | 409 | 3.50 |
| Precentral/postcentral gyrus | L | 6 | −44, −12, 41 | 73 | 3.31 |
| Superior temporal gyrus | R | 38 | 41, 21, −29 | 245 | 3.53 |
| Temporal parietal junction/middle temporal gyrus | L | 39 | −46, −67, 23 | 369 | 3.52 |
| Anterior middle temporal gyrus | L | 38 | −44, 12, −33 | 53 | 3.47 |
| Precuneus | L | 7 | −10, −54, 49 | 95 | 3.50 |
| Parahippocampal gyrus | L | 35 | −18, −18, −15 | 80 | 3.58 |
| Parahippocampal gyrus | L | 36 | −24, −28, −25 | 50 | 3.49 |
| Striatum | L | −26, 11, 10 | 132 | 3.53 | |
| Ventral striatum | L | −26, 0, −10 | 128 | 3.70 | |
| Ventral striatum | R | 22, 3, −12 | 108 | 3.51 | |
| Caudate | R | 20, 11, 20 | 101 | 3.54 | |
| Uncus/amygdala | R | 12, −5, −31 | 49 | 3.67 | |
| Cerebellum/lingual gyrus | R/L | −6, −51, −8 | 943 | 3.52 | |
| Cerebellum | R | 34, −50, −34 | 291 | 3.67 | |
| Cerebellum | R | 22, −27, −33 | 106 | 3.61 | |
| Main effect of instruction: MDD and CTL (attend positive > enhance positive) | |||||
| DLPFC/middle frontal gyrus | R | 9 | 48, 19, 34 | 54 | 3.41 |
| Interaction: MDD(enhance − attend positive) > CTL (enhance − attend positive) | |||||
| No significant clusters | |||||
All clusters were FWE corrected to P < 0.05 (uncorrected threshold of P < 0.005).
The Brodmann location (BA) is provided, along with coordinates for the centre of mass in MNI space (X, Y, Z).
Cluster size represents the number of contiguous voxels sharing a face, and the T-value is the mean T-value for all voxels in the cluster.
Comparison of positive and negative regulation
As an exploratory analysis, we compared the regulation of positive (enhance-attend) with the regulation of negative (reduce-attend) directly. This revealed a significant main effect of regulation condition irrespective of group. Specifically, there was greater activity in the MPFC and left ventral striatum during the enhancement of positive scenes, while there was greater activity in the DLPFC and inferior parietal lobe during the regulation of sad scenes. There was no significant group by regulation condition interaction (see Supplementary Figure 4 and Supplementary Table 2 for full results).
Relationship between brain activity and regulation efficacy
Given that the main effect of instruction for both sad and positive trials revealed recruitment of DLPFC and VLPFC for both groups, we next examined whether there were group differences in the relationship between activity in these regions and regulation efficacy. There was a significant positive correlation between negative regulation efficacy and percent signal change for reduce minus attend negative conditions within DLPFC (Figure 2F and bottom right panel) of the CTL (r = 0.50, P < 0.05, two-tailed] but not the MDD group (r = 0.198, P > 0.4, two-tailed], and a similar trend within the VLPFC (Figure 2C; CTL: r = 0.45, P = 0.056, two-tailed; MDD: r = −0.01, P > 0.9, two-tailed). For positive trials, we found no significant within-group correlations in either DLPFC or VLPFC regions. However, given the role of the ventral striatum in reward processing and that dysfunction in this region is thought to contribute to depression (Pizzagalli et al., 2009), we performed two additional correlations in the ventral striatum clusters identified by the main effect of instruction. A significant positive correlation between positive regulation efficacy and percent signal change for enhance minus attend positive trials emerged in the left ventral striatum of the CTL but not the MDD group (Figure 3F and bottom panel; CTL: r = 0.48, P < 0.05, two-tailed; MDD: r = 0.26, P > 0.2, two-tailed). There was a similar trend for the right ventral striatum (Figure 3G; CTL: r = 0.40, P = 0.09, two-tailed; MDD: r = 0.25, P > 0.3). One possibility is that the null correlation result in depressed patients could be attributed to restricted variance. To examine this possibility, an F-test of equality of variance was performed. This test indicated that this null finding could not be explained by unequal variance between groups for either negative regulation efficacy scores (P > 0.5) or positive regulation efficacy scores (P > 0.1). It is important to note, however, between group comparisons of the r-values (Fisher r-to-z) and regression slopes identified in these analyses reveal no significant between-group differences (P > 0.1, all two-tailed).
Relationship between brain activity and depression severity
Two whole-brain analyses were performed within the MDD group to determine whether depression severity, as indexed by the BDI, correlated with the magnitude of BOLD change from regulate-minus-attend conditions (one for sad trials, the other for positive trials). This revealed no significant clusters of activity after correcting for multiple comparisons.
DISCUSSION
The current study adapted experimental emotion regulation techniques to reflect elements of cognitive theory and associated psychotherapies to delineate neurocognitive abnormalities associated with modulating the negative cognitive triad in MDD. Although the CTL group was significantly better than depressed patients at regulating both positive and negative affect, evidence was also observed for the successful regulation of sad affect, and to a lesser extent, positive affect in participants with MDD. The capacity to regulate negative affect was highly correlated with the capacity to regulate positive affect. At the neural level, significantly greater recruitment of DLPFC and VLPFC during the regulate vs attend negative stimulus condition was observed in both groups; however, a significant correlation between brain activity and subjective indices of regulation success existed only in CTLs. Additionally, a group by instruction interaction revealed that only CTLs exhibited reduced activity in regions implicated in the representation of sensory information and emotional encoding. For example the amygdala, and visual areas known to respond more robustly to emotionally significant stimuli (Vuilleumier et al., 2001; Pessoa et al., 2002; Ishai et al., 2004; Padmala and Pessoa, 2008, 2011; Lindquist et al., 2012). Similarly, during positive affect enhancement, we observed recruitment of VLPFC and DMPFC in both groups. We also observed increased activity in neural regions associated with reward and emotionally salient stimulus encoding, particularly bilateral regions of ventral striatum; however, only in controls was activity in this region significantly correlated with ratings of positive affect. The results provide partial evidence that a dissociation exists in depressed patients between activity in neural regions associated with emotional control and encoding, and indices of regulation success.
Neural regions for the control of emotion
In the current task, both groups exhibited a similar increase in activity of dorsal PFC regions and VLPFC while regulating both sad and positive affect. These neural regions are widely implicated in healthy emotion regulation (Ochsner et al., 2004; Ochsner and Gross, 2005; Mitchell, 2011; Mitchell and Greening, 2012). One means by which DLPFC is thought to exert emotional control is via an attention-related amplification of goal-specific or alternate representations in occipito-temporal cortices, which compete in an inhibitory fashion with emotional representations (Blair and Mitchell, 2009; Mitchell, 2011). It has also been suggested that dorsal regions of prefrontal cortex are involved in explicit reasoning about how emotional associations can be changed (Ochsner and Gross, 2005), or are involved in the neural representation of social and emotional processes (Wood and Grafman, 2003; Moll et al., 2005). In addition, dorsal regions might reduce activity in emotion-related brain areas such as the amygdala via second-order connections with MPFC (Delgado et al., 2008). It has been suggested that VLPFC is involved in updating the representation of optimal motor responses (Mitchell et al., 2009; Greening et al., 2011), in the active regulation of an emotional response (Phan et al., 2005; Wager et al., 2008) or in updating the representation of both optimal motor and emotional responses (Kringelbach and Rolls, 2003; Mitchell, 2011). In line with these ideas, lesions to lateral areas of DLPFC (relative to medial PFC) are associated with increased vulnerability to depression (Koenigs et al., 2008), and activity in both DLPFC and VLPFC has been associated with regulation success in healthy controls (Phan et al., 2005; Wager et al., 2008). The current study demonstrated, however, that despite both groups showing similar patterns of activation in DLPFC during negative affect regulation, only in the CTL group was this activity significantly correlated with regulation efficacy. This finding should be interpreted with caution, however, as the follow-up comparisons of the two correlations revealed no significant between-group differences. Notably, this type of between-group comparison of correlations can suffer from low power (Yarkoni, 2009), and so larger sample sizes are required to address this question. Furthermore, relative to individuals with MDD, negative affect regulation in the CTL group was associated with significantly reduced activity in neural regions associated with encoding emotional sensory information, including visual areas, and the amygdala (Padmala and Pessoa, 2008, 2011). Together with the behavioural results, the current findings suggest that appropriate levels of prefrontal cortex activity may exist in patients with MDD without commensurate relief of negative affect or reductions in sensory encoding of negative stimuli. The results of the current study are consistent with each of the models of DLPFC and VLPFC discussed above, and suggest that these regions are involved in the modulation of emotional stimuli and not simply response suppression. Further work is required to determine whether the reduced efficacy is associated with dysfunction in outputs of emotion-related brain areas targeted by PFC, or due to abnormalities in the nature of the computations performed by PFC during emotion regulation. For example, PFC activity in patients may be disproportionately devoted to representing task-demands or conflict rather than being allocated to performing executive control over emotion. Alternatively, the modified context formed during emotion regulation may be represented in PFC, but varies in emotional content between groups (Moll et al., 2005).
The current study is only the second to examine the online enhancement of positive affect in patients with MDD, and the first to relate activation to indices of regulation success. Significantly enhanced activity in left VLPFC , DMPFC, and regions of DLPFC was observed during attempts to up-regulate positive affect. Similar increases in activation during this condition were also observed in both groups in the ventral striatum. However, only in the CTL group was this enhancement significantly correlated with regulation success. Interestingly, this region is believed to be a target of PFC regions like VLPFC during emotion regulation (Wager et al., 2008; Peters et al., 2009). The current results are consistent with suggestions that emotion-related abnormalities within the striatum are implicated in depression (Epstein et al., 2006; Bluhm et al., 2009; Heller et al., 2009; Osuch et al., 2009; Robinson et al., 2012). They are also consistent with previous research showing that greater DLPFC and VLPFC activation to emotional cues before a course of CBT was positively correlated with treatment success (Ritchey et al., 2011). When enhancing positive emotion, irrespective of group, we also observed a cluster in VLPFC that included a small part of anterior superior temporal lobe. In depression, generalized self-blame has been found to be associated with functional connectivity disruption between the anterior superior temporal lobe and regions of the subgenual anterior cingulate cortex (Green et al., 2012). This raises the possibility that reappraisal involves modifications to the representation of self-concepts, a process that is ineffective in depression (Beck et al., 1979).
Limitations
This study uses a standard explicit emotion regulation paradigm in depressed patients that incorporated strategies from cognitive theories of depression and associated psychotherapies. It is important to acknowledge the limitations that any laboratory-based task has as it is an approximation of the therapeutic context. CBT involves training over many sessions. Furthermore, the stimuli used in the present study to trigger an emotional response were standardized images rather than autobiographical or idiosyncratic cues (Eddington et al., 2009; Lemogne et al., 2009), which is an important consideration given evidence that different stimuli can yield distinct effects (Siegle et al., 2007; Kross et al., 2009). Additionally, we have no direct means of assessing task compliance in the current task. It is possible that rumination by the depressed group might have impaired their ability to use the regulation strategy throughout the entire trial (Levens et al., 2009). Nevertheless, it is notable that the current emotion regulation strategy modulated neural areas previously shown to be affected in patients following a formal treatment course with CBT (e.g. Goldapple et al., 2004; Jensen et al., 2012). Moreover, patients also reported significant down-regulation of negative affect, and a near significant up-regulation of positive affect.
CONCLUSION
The current study raises the possibility that depression is not associated with a failure to recruit neural regions implicated in the regulation of emotion, but rather, that the recruitment of such regions is less effective in modulating subjective emotional states and activity in emotional and sensory brain areas. Because activity in regions associated with cognitive control was appropriate in depressed patients, these results are also consistent with suggestions that depression is associated with significant regulatory efforts over negative affect without commensurate relief (Segal et al., 2006; Farb et al., 2010). This may explain why depression can persist in the presence of normal (e.g. Beauregard et al., 2006), excessive (e.g. Johnstone et al., 2007) or reduced (e.g. Erk et al., 2010) recruitment of areas associated with emotion regulation. Further work is required to determine whether the reduced efficacy is due to abnormalities in the nature of the computations performed by PFC (e.g. impaired cognitive control, Siegle et al., 2007) or due to dysfunction in the output of emotion-related regions targeted by PFC. Nevertheless, the current study highlights the potential use of neuroimaging to test the efficacy of existing or novel therapies (Linden, 2006; De Raedt et al., 2010), and also the need to delineate potential synergistic interactions between different pharmacological interventions and psychotherapies.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN Online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This work was funded by an Ontario Mental Health Foundation Young Investigator’s Fellowship to D.G.V.M. The authors would like to thank the First Episode Mood and Anxiety Program, London Health Sciences Centre and Dr Jeffrey Reiss for assistance with participant recruitment. We would also like to thank Betsy Schaefer for help with participant screening, and P. Stables for fruitful discussion concerning this work.
REFERENCES
- Beauregard M, Paquette V, Levesque J. Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport. 2006;17:843–6. doi: 10.1097/01.wnr.0000220132.32091.9f. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York: The Guilford Press; 1979. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Blair RJ, Mitchell DG. Psychopathy, attention and emotion. Psychological Medicine. 2009;39:543–55. doi: 10.1017/S0033291708003991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm R, Williamson P, Lanius R, et al. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry and Clinical Neurosciences. 2009;63:754–61. doi: 10.1111/j.1440-1819.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Barlow DH. Incorporating emotion regulation into comceptualizations of treatments of anxiety and mood disorders. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: The Guilford Press; 2007. [Google Scholar]
- Chen G, Saad ZS, Nath AR, Beauchamp MS, Cox RW. FMRI group analysis combining effect estimates and their variances. Neuroimage. 2012;60:747–65. doi: 10.1016/j.neuroimage.2011.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DA, Beck AT. Cognitive theory and therapy of anxiety and depression: convergence with neurobiological findings. Trends in Cognitive Sciences. 2010;14:418–24. doi: 10.1016/j.tics.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–74. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- de Raedt R, Koster EH, Joormann J. Attentional control in depression: a translational affective neuroscience approach. Cognitive, Affective, and Behavioral Neuroscience. 2010;10:1–7. doi: 10.3758/CABN.10.1.1. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–38. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Russell RP, Davis MH, et al. Susceptibility-induced loss of signal: comparing PET and fMRI on a semantic task. Neuroimage. 2000;11:589–600. doi: 10.1006/nimg.2000.0595. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. Journal of Neuroscience. 1992;12:3628–41. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddington KM, Dolcos F, Mclean AN, Krishnan KR, Cabeza R, Strauman TJ. Neural correlates of idiographic goal priming in depression: goal-specific dysfunctions in the orbitofrontal cortex. Social Cognitive and Affective Neuroscience. 2009;4:238–46. doi: 10.1093/scan/nsp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. American Journal of Psychiatry. 2006;163:1784–90. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Erk S, Mikschl A, Stier S, et al. Acute and sustained effects of cognitive emotion regulation in major depression. Journal of Neuroscience. 2010;30:15726–34. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biological Psychiatry. 2008;63:377–84. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, et al. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. Journal of Affective Disorders. 2009;112:206–11. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Mayberg H, et al. Minding one's emotions: mindfulness training alters the neural expression of sadness. Emotion. 2010;10:25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I disorders, research version, Patient Edition. (SCID-I/P). 2002 New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Goldapple K, Segal Z, Garson C, et al. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Archives of General Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Mcrae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S, Lambon Ralph MA, Moll J, Deakin JF, Zahn R. Guilt-selective functional disconnection of anterior temporal and subgenual cortices in major depressive disorder. Archives of General Psychiatry. 2012:1–8. doi: 10.1001/archgenpsychiatry.2012.135. [DOI] [PubMed] [Google Scholar]
- Greening SG, Finger EC, Mitchell DG. Parsing decision making processes in prefrontal cortex: response inhibition, overcoming learned avoidance, and reversal learning. Neuroimage. 2011;54:1432–41. doi: 10.1016/j.neuroimage.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–37. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Han T, Alders GL, Greening SG, Neufeld RW, Mitchell DG. Do fearful eyes activate empathy-related brain regions in individuals with callous traits? Social Cognitive and Affective Neuroscience. 2011;7:958–68. doi: 10.1093/scan/nsr068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:22445–50. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A, Pessoa L, Bikle PC, Ungerleider LG. Repetition suppression of faces is modulated by emotion. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9827–32. doi: 10.1073/pnas.0403559101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Berna C, Loggia ML, Wasan AD, Edwards RR, Gollub RL. The use of functional neuroimaging to evaluate psychological and other non-pharmacological treatments for clinical pain. Neuroscience Letters. 2012;520:156–64. doi: 10.1016/j.neulet.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27:8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. American Journal of Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Calamia M, Raymont V, Tranel D, Grafman J. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. Journal of Neuroscience. 2008;28:12341–8. doi: 10.1523/JNEUROSCI.2324-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage. 2003;20:1371–83. doi: 10.1016/S1053-8119(03)00393-8. [DOI] [PubMed] [Google Scholar]
- Kross E, Davidson M, Weber J, Ochsner K. Coping with emotions past: the neural bases of regulating affect associated with negative autobiographical memories. Biological Psychiatry. 2009;65:361–6. doi: 10.1016/j.biopsych.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. 2008. Technical report A-8. Gainesville, FL: University of Florida. [Google Scholar]
- Lemogne C, le Bastard G, Mayberg H, et al. In search of the depressive self: extended medial prefrontal network during self-referential processing in major depression. Social Cognitive and Affective Neuroscience. 2009;4:305–12. doi: 10.1093/scan/nsp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens SM, Muhtadie L, Gotlib IH. Rumination and impaired resource allocation in depression. Journal of Abnormal Psychology. 2009;118:757–66. doi: 10.1037/a0017206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light SN, Heller AS, Johnstone T, et al. Reduced right ventrolateral prefrontal cortex activity while inhibiting positive affect is associated with improvement in hedonic capacity after 8 weeks of antidepressant treatment in major depressive disorder. Biological Psychiatry. 2011;70:962–8. doi: 10.1016/j.biopsych.2011.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DE. How psychotherapy changes the brain–the contribution of functional neuroimaging. Molecolar Psychiatry. 2006;11:528–38. doi: 10.1038/sj.mp.4001816. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behavioral and Brain Science. 2012;35:121–43. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg H. Depression, II: localization of pathophysiology. American Journal of Psychiatry. 2002;159:1979. doi: 10.1176/appi.ajp.159.12.1979. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Mikels JA, Fredrickson BL, Larkin GR, Lindberg CM, Maglio SJ, Reuter-Lorenz PA. Emotional category data on images from the international affective picture system. Behaviour Research Methods. 2005;37:626–30. doi: 10.3758/bf03192732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DG. The nexus between decision making and emotion regulation: a review of convergent neurocognitive substrates. Behavioral Brain Research. 2011;217:215–31. doi: 10.1016/j.bbr.2010.10.030. [DOI] [PubMed] [Google Scholar]
- Mitchell DG, Greening SG. Conscious perception of emotional stimuli: brain mechanisms. Neuroscientist. 2012;18:386–98. doi: 10.1177/1073858411416515. [DOI] [PubMed] [Google Scholar]
- Mitchell DG, Luo Q, Avny SB, et al. Adapting to dynamic stimulus-response values: differential contributions of inferior frontal, dorsomedial, and dorsolateral regions of prefrontal cortex to decision making. Journal of Neuroscience. 2009;29:10827–34. doi: 10.1523/JNEUROSCI.0963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. Opinion: the neural basis of human moral cognition. Nature Reviews Neuroscience. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Osuch EA, Bluhm RL, Williamson PC, Theberge J, Densmore M, Neufeld RW. Brain activation to favorite music in healthy controls and depressed patients. Neuroreport. 2009;20:1204–8. doi: 10.1097/WNR.0b013e32832f4da3. [DOI] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Affective learning enhances visual detection and responses in primary visual cortex. Journal of Neuroscience. 2008;28:6202–10. doi: 10.1523/JNEUROSCI.1233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Reward reduces conflict by enhancing attentional control and biasing visual cortical processing. Journal of Cognitive Neuroscience. 2011;23:3419–32. doi: 10.1162/jocn_a_00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Mckenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11458–63. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learning and Memory. 2009;16:279–88. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry. 2003;54:515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. American Journal of Psychiatry. 2009;166:702–10. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biological Psychiatry. 2001;49:741–52. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biological Psychiatry. 1999;45:1085–98. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R. Neural correlates of emotional processing in depression: changes with cognitive behavioral therapy and predictors of treatment response. Journal of Psychiatric Research. 2011;45:577–87. doi: 10.1016/j.jpsychires.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Cools R, Carlisi CO, Sahakian BJ, Drevets WC. Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. American Journal of Psychiatry. 2012;169:152–9. doi: 10.1176/appi.ajp.2011.11010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J, Kasch KL, Gross JJ, Gotlib IH. Sadness and amusement reactivity differentially predict concurrent and prospective functioning in major depressive disorder. Emotion. 2002;2:135–46. doi: 10.1037/1528-3542.2.2.135. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Kennedy S, Gemar M, Hood K, Pedersen R, Buis T. Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Archives of General Psychiatry. 2006;63:749–55. doi: 10.1001/archpsyc.63.7.749. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Suslow T, Konrad C, Kugel H, et al. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biological Psychiatry. 2010;67:155–60. doi: 10.1016/j.biopsych.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltman DJ, Friston KJ, Sanders G, Price CJ. Regionally specific sensitivity differences in fMRI and PET: where do they come from? Neuroimage. 2000;11:575–88. doi: 10.1006/nimg.2000.0581. [DOI] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Archives of General Psychiatry. 2010;67:1128–38. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–41. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nature Reviews Neuroscience. 2003;4:139–47. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Investing in Mental Health. Geneva, Switzerland: Department of Mental Health and Substance Dependence; 2003. [Google Scholar]
- Yarkoni T. Big correlations in little studies: inflated fMRI correlations refelct low statistical power. Perspectives on Psychological Science. 2009;4:294–8. doi: 10.1111/j.1745-6924.2009.01127.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.