Abstract
People believe that women are more emotionally intense than men, but the scientific evidence is equivocal. In this study, we tested the novel hypothesis that men and women differ in the neural correlates of affective experience, rather than in the intensity of neural activity, with women being more internally (interoceptively) focused and men being more externally (visually) focused. Adult men (n = 17) and women (n = 17) completed a functional magnetic resonance imaging study while viewing affectively potent images and rating their moment-to-moment feelings of subjective arousal. We found that men and women do not differ overall in their intensity of moment-to-moment affective experiences when viewing evocative images, but instead, as predicted, women showed a greater association between the momentary arousal ratings and neural responses in the anterior insula cortex, which represents bodily sensations, whereas men showed stronger correlations between their momentary arousal ratings and neural responses in the visual cortex. Men also showed enhanced functional connectivity between the dorsal anterior insula cortex and the dorsal anterior cingulate cortex, which constitutes the circuitry involved with regulating shifts of attention to the world. These results demonstrate that the same affective experience is realized differently in different people, such that women’s feelings are relatively more self-focused, whereas men’s feelings are relatively more world-focused.
Keywords: anterior insula, anterior cingulate, interoceptive, extraceptive, fMRI
Western culture is preoccupied with the question of whether women are more emotional than men. People assume that women are more emotionally intense, complex and expressive, whereas men are more stoic and reserved (Lutz, 1990; Pease and Pease, 1999; Baron-Cohen, 2003; Nagourney, 2006). There is such a profound belief in this sex difference that it influences political attitudes about who can be president of the United States (Nagourney, 2006) and is used to justify why women continue to be underrepresented in positions of economic and political power that require a level head and a steady hand (Lutz, 1990). Despite the prevailing belief that women are the more emotional sex, however, objective measures of emotion do not consistently show sex differences. For example, some studies report that women show larger physiological changes in evocative situations (Bradley et al., 2001), whereas others do not (Quigley and Barrett, 1999; Kelly et al., 2006), and some studies report the opposite pattern of results (Greenwald et al., 1989). Sometimes women smile more than men (LaFrance et al., 2003) and sometimes less (Ansfield, 2007). Men and women do not differ in the magnitude of physical reactivity like cortisol level and autonomic responding when exposed to an experimental social stress (e.g. preparation for speech), although they differed in reporting irritability and fear after the task (Kelly et al., 2008). Men and women also do not differ in their subjective reports of moment-to-moment emotional experiences in response to specific events as they occur in everyday life when measured using an experience-sampling procedure, although women describe themselves as more emotional when using memory-based self-report measures (Barrett et al., 1998), this is most likely because such measures tap stereotypes and other beliefs that are strongly gendered (Robinson and Clore, 2002).
One of the reasons for the inconsistent scientific results on sex differences is that studies mainly measure the ‘intensity’ of experience from a quantitative standpoint. One intriguing possibility is that men and women differ not in the extent to which they experience feelings, but rather in the ingredients involved in constructing those experiences (Barrett, 2006). More specifically, both philosophical (Lambie and Marcel, 2002) and experimental (Lindquist and Barrett, 2008) evidence indicate that affective experiences are constructed from both interoceptive (i.e. self-focused) and exteroceptive (i.e. world-focused) information (Barrett, 2009a). We hypothesized that perhaps men and women differ in the relative contributions of these aspects, such that women’s affective experience includes relatively more interoceptive information, whereas men’s includes relatively more exteroceptive information.
This hypothesis setting is derived from published empirical evidence in the psychological literature, although this idea has not yet been tested directly. Previous studies frequently suggest that women ‘somatize’ more than men, i.e. women report more functional somatic symptoms (Tibblin et al., 1990; Wool and Barsky, 1994), and the researchers proposed the idea that this is because women are more attentive to their internal state (Pennebaker, 1982; Tibblin et al., 1990). On the other hand, people suppose that ‘men are visual’ creatures (Smith, 2000) and express their emotions in ways consistent with visual perception. Men also have the advantage in visuospatial abilities to process and interpret visual information about external objects (Voyer et al., 1995). In addition, men have a more ‘externally oriented thinking’ style, measured by a questionnaire (Moriguchi et al., 2007), which might translate into their more externally focused experiences of emotion when compared with women who have a more ‘internal’ style. Moreover, human perceivers tend to make more ‘internal’ attributions about the emotional behaviors that they observe in women (i.e. people understand smiles, scowls and frowns to reveal something about a woman’s internal state), whereas they make more ‘external’ attributions about the emotional behaviors that they observe in men (i.e. expressions indicate something about a man’s situational demands; Brescoll and Uhlmann, 2008; Barrett and Bliss-Moreau, 2009).
In this study, we tested the hypothesis that men and women would not differ in the intensity of their momentary subjective affective experiences but that they would differ relatively in the neural correlates of those experiences. To this end, using functional neuroimaging, we investigated whether there are differences in the regional neural activations most robustly engaged as men and women rated their affective experiences while viewing evocative images. We predicted that women’s affective experience would be more strongly correlated with changes in activation in brain regions involved in representing bodily sensations, such as the anterior insula (AI), when compared with men. Based on its anatomical connections, the AI is considered to be a critical node in representing sensations from within the body and incorporating them into mental life, providing a critical substrate for the interoceptive awareness of affective experience (Mesulam and Mufson, 1982; Augustine, 1996; Craig, 2009). Importantly, the processing of the interoceptive information—information from one’s own body—should be always involved in constructing all sorts of perceptual, cognitive and emotional mental events (Barrett, 2009a, b), which is why neuroimaging studies routinely report increased AI activation in thousands of experiments across a range of psychological phenomena (Craig, 2002, 2009; Herbert and Pollatos, 2012). Thus, if we find an activation in the AI in response to a mental task, it is highly suggestive that interoceptive processing is relevant to the task.
In contrast, we predicted that men would be relatively more exteroceptively focused, deriving their subjective affective experiences more from representations of visual sensations, so that such experiences would be more correlated with activation in the visual cortex, such as the primary visual cortex (V1), because this activation reflects the motivational and attentional relevance of the outer visual world (Vuilleumier and Driver, 2007; Damaraju et al., 2009). Thirty-four normal healthy adults (17 male and 17 female) rated their subjective experiences of arousal (1 = low, 2 = mid and 3 = high) while viewing 132 full-color positive, negative and neutral images while undergoing functional magnetic resonance imaging (fMRI). Positive and negative images were matched on their intensity according to published norms (see Materials and Methods).
Furthermore, we explored sex differences in the functional connectivity between the AI and the anterior cingulate cortex (ACC) while men and women rated their affective experiences in response to evocative images. The dorsal portion of the ACC is intrinsically connected to the more dorsal aspect of the AI as part of a ‘ventral attention network’ (Corbetta et al., 2008) that is involved in the signaling of the importance of and the regulation of attention to events and objects in the world (Dosenbach et al., 2007). Moreover, it has been proposed that spindle (‘Von Economo’) neurons localized within the AI and ACC (Nimchinsky et al., 1999; Allman et al., 2002; Craig, 2009) subserve a fast signaling mechanism underlying the switch between the engagement of large-scale networks processing internal information vs those at least partly devoted to processing external cues (Sridharan et al., 2008; Menon and Uddin, 2010). We expected that if men’s subjective experience is more infused with exteroceptive information when compared with women, then this might occur because men show increased functional connectivity between the AI and the ACC when viewing images that evoked stronger subjective feelings.
MATERIAL AND METHODS
Participants
Participants were 34 right-handed healthy volunteers (17 male: age range 19–78 years, M = 42.9, s.d. = 22.9; 17 female: age range 22–83 years, M = 43.2, s.d. = 22.9). These participants were originally recruited to study the affective significance of novelty across the lifespan (Moriguchi et al., 2011). In this article, however, we focused on completely different hypotheses, and the analyses reported here do not overlap with those that have been previously published. We already confirmed that there were no age effects on the present results (data not shown; available on request), and therefore, the age effect was not considered in this study. The original sample contained more women than men (Moriguchi et al., 2011), thus the numbers of men and women were not balanced to test the sex difference hypothesis. Therefore, we randomly sampled to have an equal number of participants of men and women. (Note that both the behavioral and imaging results are essentially identical if the full sample is used.) There was no difference in age between the male and female groups [T(32) = 0.037, P = 0.97].
Affective picture stimuli
For the fMRI task, 132 full-color images were selected from the International Affective Picture System (IAPS; Lang et al., 1997) for each valence (positive, negative and neutral). Positive and negative pictures were equated for intensity [based on the norms (Lang et al., 2008)]. The task was run using E-Prime experimental software (Psychology Software Tools, Pittsburgh, PA) on a PC, from which images were projected onto a screen in the magnet bore. Participants viewed images through a mirror mounted on the head coil.
Scanning procedure for task-related fMRI
The imaging paradigm consisted of four event-related fMRI runs. During scanning, participants rated their subjective experiences of affective arousal as they viewed each image using a three-point scale (1 = low, 2 = mid and 3 = high) and answered with a three-button response box. These individual arousal ratings were used as a measure of momentary evaluation of their own affective states induced by the IAPS images with different valences in the scanner. Each run was 340 s in length and each image was presented for 3.5 s, with a stimulus onset asynchrony that varied from 4 to 16 s.
Image data were acquired using a Siemens Magnetom Trio Tim 3T whole body high-speed imaging device equipped for echo planar imaging (EPI) (Siemens Medical Systems, Iselin, NJ) with a 12-channel gradient head coil. Expandable foam cushions restricted head movement. After an automated scout image was acquired and shimming procedures were performed to optimize field homogeneity, a T1-weighted 3D MPRAGE sequence (TR/TE/flip angle = 2.53 s/3.39 ms/7°) with an in-plane resolution of 1.3 × 1.0 mm and 1.3 mm slice thickness was collected. Blood-oxygenation-level-dependent fMRI images were acquired using a gradient echo T2*-weighted sequence (TR/TE/flip angle = 2.0 s/30 ms/90°). At the beginning of each scan run, four volumes were acquired and discarded to allow longitudinal magnetization to reach equilibrium.
Processes and Analyses of imaging data for task-related fMRI
Image processing was carried out using Statistical Parametric Mapping software (SPM5, Wellcome Department of Imaging Neuroscience, London, UK). The EPI images were realigned to the first image of the time series, and co-registered to the participants’ T1-weighted images. Then the T1 images were transformed to a template brain in MNI (Montreal Neurological Institute) stereotactic space using high-dimensional warping implemented in SPM5. The parameters for the transformation were applied to the co-registered EPI images. The normalized images were smoothed by a 6-mm FWHM Gaussian kernel.
To test regionally specific effects, a first-level analysis was computed using the general linear model (GLM) with the canonical hemodynamic response function in SPM package, which models typical event-related hemodynamic response. Neural responses associated with the magnitude of momentary emotional experiences (i.e. subjective arousal ratings in the scanner) were assessed on event-by-event basis by parametric modulation analysis, which allows us to estimate the degree to which the hemodynamic response is modulated by the arousal rating in the design matrix. That is, the subjective rating scores were first mean-centered to zero across a run, and each hypothesized event-related hemodynamic response was modulated (multiplied) by the subjective rating score relevant to that event. The modulated hemodynamic time-course models were then concatenated to the nonmodulated conventional hemodynamic GLM model. The beta values estimated for this modulated term—which represent the correlational effect of the subjective rating with the hemodynamic responses—were calculated with this GLM for each individual subject across whole brain, and were then entered into a second-level random-effect analysis (Friston et al., 1999) to allow for population inferences and group analysis to test hypotheses related to sex differences.
Theoretically, we could have done this parametric modulation analysis in each negative, positive and neutral picture condition separately, but valence and subjective arousal are strongly associated, as negative and positive pictures were scored higher on arousal than neutral pictures. Within a single valence, there was a restricted range in arousal (e.g. participants often scored all the neutral pictures in a run ‘low’). Therefore, we did not segregate the data into separate valence conditions but analyzed the data including all the three conditions together to maximize the range of subjective arousal ratings. We also ran conventional analyses of sex difference of neural activity in response to affective pictures. The method and results are provided in Figure S1, S2, S3, S4, and Table S1 in Supplemental Information.
Region of interest definition
The primary hypotheses tested focused on two nodes of the salience network (bilateral AI and dACC) and the visual cortex (V1–V5). We used a functional-anatomic approach to identify specific regions within each of these cortical areas in the following manner. First, the anatomic areas were identified as bilateral AI, dACC and V1–V5 using the Automated Anatomical Labeling atlas (Tzourio-Mazoyer et al., 2002) from the WFU PickAtlas software (Maldjian et al., 2003). Next, within each of these anatomic areas, we identified the significantly activated clusters from the aforementioned parametric modulation analysis including all participants in the present study (height and extent threshold at P < 0.05, FDR-corrected). Each of these functional-anatomically defined clusters was set as region of interest (ROI). We then extracted, for each subject, the mean parameter estimates (beta values) from the parametric modulation analysis from each ROI using MarsBar software (http://marsbar.sourceforge.net/index.html; Brett et al., 2002). These values were then compared statistically between male and female groups.
In addition to this hypothesis-driven ROI approach, we also performed a whole brain analysis, and verified whether the clusters with significant sex difference from this whole brain analysis were included in our ROIs (the bilateral AI, dACC and V1–V5). For this analysis, we set alpha at P < 0.001 (uncorrected) for peak voxels, and specified a lower limit of 10 contiguous active voxels to constitute a cluster.
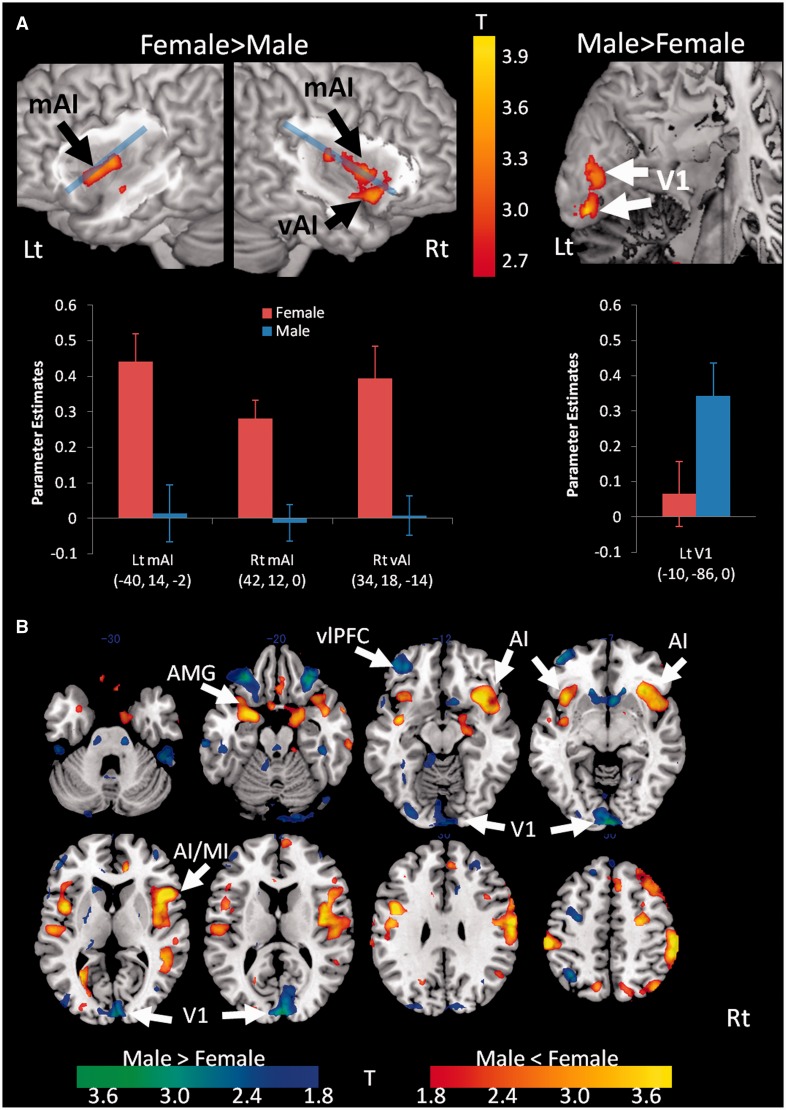
We found that, using the method just described for localizing the ROIs, the AI ROI was too large to determine the specific localization of activation within the bilateral AI (i.e. differentiation between ventral or dorsal AI [dAI]). Thus, we also confirmed the detailed localization (i.e. clusters) of sex difference in the parametric modulation analysis by visualizing the activation map [the significant clusters within the AI (P < 0.001, uncorrected, k = 10)] rendered on the surface of the insula (Figure 2A). These clusters were then used to extract parameter estimates as previously stated using MarsBar software, and the estimates in each sex group were illustrated graphically.
Fig. 2.
Sex differences in the association between neural responses and subjective arousal ratings. (A) The two upper left brain maps show the clusters within the insula that have significantly stronger association in women than in men between neural responses and subjective arousal ratings (P < 0.005 for the purpose of illustration). Blue line divides insula into dorsal and ventral sectors. Note that these regions were mostly located in more ventral or mid part of AI, not dorsal AI. The upper right brain map shows the clusters within the left primary visual area (V1) that have significantly stronger association in men than in women between neural responses and subjective arousal ratings (P < 0.005 for the purpose of illustration). The bar graphs below the brain map show parameter estimates of modulation effects of subjective ratings, with error bars indicating one standard error of the mean. Rt, right hemisphere; Lt, left hemisphere; vAI, ventral anterior insula (AI); mAI, mid AI; red bar (F), female; blue bar (M), male. Each coordinate below the graphs indicates the peak MNI coordinate (x, y, z mm) of each cluster. (B) Whole brain analysis of correlation between neural response to all IAPS pictures and subjective arousal rating (parametric modulation analysis) in men compared with women. Stronger correlation in women than men (t range 1.8–4.0 for illustration) is colored in red-yellow, and stronger correlation in men than women is colored in blue-green. Greater anterior insula (AI) extending to middle insula (MI) activation was confirmed in women than in men, whereas men show greater V1 association than women. Additional exploratory results demonstrate that women had greater association with arousal in other affective areas including the amygdala (AMG) extending to parahippocampal cortex than men, whereas men had greater association with arousal in ventrolateral prefrontal cortex (vlPFC) (see Table 1 for complete cluster table).
Functional connectivity analysis during task engagement
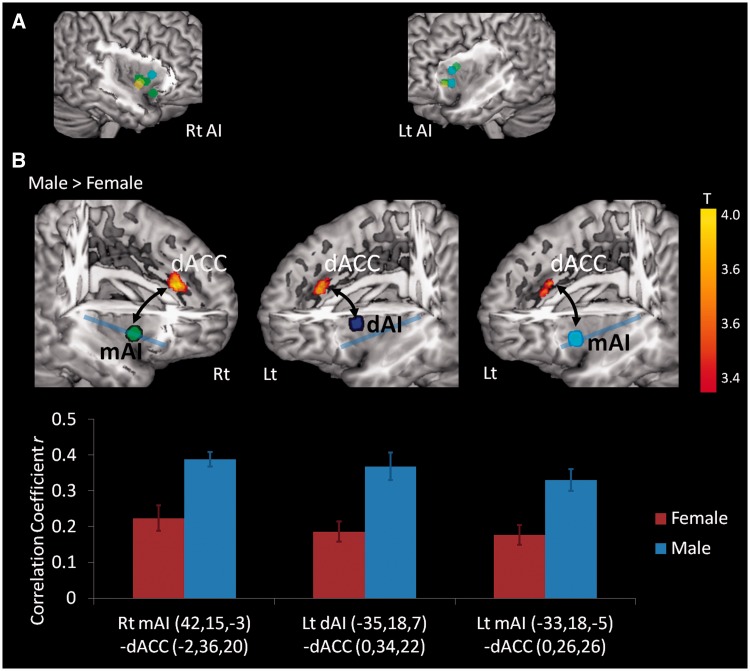
Next, we conducted a functional connectivity analysis between dACC and ROIs in the right and left AI. We used the functional connectivity toolbox version 12 (http://web.mit.edu/swg/software.htm). To define seed ROIs, we used previous meta-analyses (Wager and Barrett, 2004; Wager et al., 2008; Mutschler et al., 2009; Kurth et al., 2010; Deen et al., 2011). The coordinates reported in all these studies overlap with each other, so we selected one study (Kurth et al., 2010) that classified the insular coordinates into several functionally different categories. We selected eight insular MNI coordinates from the category ‘emotion’ [as affective regions; (−31, 24, −4), (−30, 12, 10), (42, 15, −3), (39, 7, 0), (28, 17, −15), (−31, 24, −6), (42, 8, −6) and (44, 10, −4)] and three from ‘attention’ [as cognitive/regulatory regions; (−35, 18, 7), (−33, 18, −5) and (36, 19, 3)] to cover a maximally broad area of AI (including both ventral and dorsal portions). Then we created spherical ROIs (4 mm radius) centered at each coordinate using MarsBar software (see Figure 3A). As our goal was to examine sex differences in connectivity between AI and ACC, we performed the functional connectivity analysis using a mask to restrict the search space to voxels in the right and left ACC ROIs (defined by Automated Anatomical Labeling system). The time course of the blood-oxygenation-level-dependent signal in the ROIs in each participant was extracted using the functional connectivity toolbox software, and the data were band-pass filtered with a frequency band from 0.008 to 0.16 Hz, which was confirmed by a spectral analysis as an effective frequency range of the theoretical task-related hemodynamic design in the present study. The correlations of time courses between the insula seed ROIs and the voxels within ACC ROIs were estimated by regression analyses co-varied with the main event-related task effect (i.e. the modeled hemodynamic response to observation of IAPS images during arousal ratings) and other nuisance variables (including white matter, cerebrospinal fluid signal and head motion parameters). Thus, the resulting maps represent voxels within the ACC in which the hemodynamic response during task-related activity is correlated with that of the AI. Next, for group comparison, we identified the statistically significant clusters in which there was a sex difference (height threshold P < 0.001 uncorrected, extent threshold k = 10).
Fig. 3.
Sex differences during task performance in the functional connectivity between insula ROIs and the dACC. (A) Seed ROI locations in the AI for functional connectivity analyses. The circles on the AI surface show 11 spherical ROIs. The coordinates at the center of each sphere are the ones reported in a meta-analysis (Kurth et al., 2010). The green and yellow ROIs are associated with ‘emotion’, and blue ROIs are associated with ‘attention’. In ‘emotion’ coordinates, green ROIs include multimodal integrated regions that might be also activated by the tasks for other different categories (i.e. not ‘specific’ to emotional tasks). On the other hand, yellow ROIs were the regions specific to emotional tasks (Kurth et al., 2010). (B) The upper figures show the ROIs rendered on brain surface maps (to illustrate insula, a part of temporal/frontal/parietal regions is cropped) and the clusters in ACC with a statistically significant sex difference (men vs women) of functional connectivity (during task performance) to the respective anterior insula (AI) ROI labeled with a colored dot. A blue line divides the insula into dorsal and ventral parts. Functional connectivity from each seed ROI [see (A)] to ACC was calculated. The three ROIs in the AI shown on the map were those that showed statistically significant stronger functional connectivity to the dACC in men than in women. Note that these seed regions in the AI were mostly located in the mid or dorsal AI (not ventral AI). The graphs below the brain map show mean functional connectivity (correlation coefficient r) in each sex group, with error bars indicating one standard error of the mean. Rt, right side; Lt, left side; dAI, dorsal AI; mAI, mid AI; red bar (F), female; blue bar (M), male. Each coordinate described below the graph represents center MNI coordinate (x, y, z, mm) at each ROI.
RESULTS
Sex differences in subjective arousal ratings of affective images
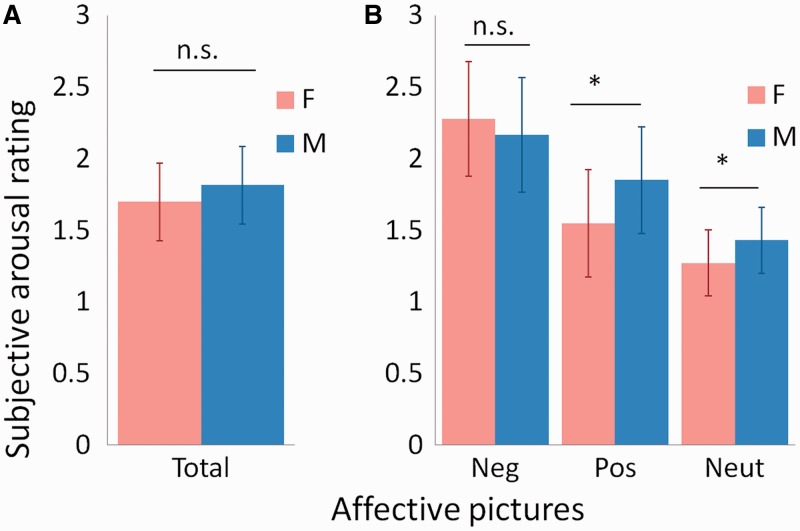
As expected, men and women did not differ in the intensity of their subjective experiences of arousal in response to evocative images overall [men: M (s.d.) = 1.70 (0.27); women: M (s.d.) = 1.81 (0.27); F(1, 32) = 1.56, P < 0.220, ηp2 = 0.047; Figure 1A]. On closer inspection, they did not differ in the intensity of their subjective experiences of arousal in response to negative images, although a significant valence (negative, positive and neutral) × sex (male, female) interaction [F(1.7, 53.6) = 5.72, P < .008, ηp2 = 0.152] indicated that men reported relatively stronger subjective experiences of arousal in response to positive and neutral images than women (Figure 1B). This effect held when the six sexual pictures were removed [F(1.7, 55.5) = 5.26, P < .011, ηp2 = 0.141]. The results indicate that women’s ratings do not fit the stereotype of stronger arousal levels on moment-to-moment experiences when compared with men.
Fig. 1.
Momentary emotional experience (ratings of subjective arousal level) measures in men and women. (A) The bar graph shows mean ratings of subjective arousal level when men and women observed all IAPS images, with error bars indicating 1 s.d. Men and women did not differ in the intensity of their subjective experiences of arousal in response to evocative images overall [F(1, 32) = 1.56, P < 0.220, ηp2 = 0.047]. Red bar (F), female; blue bar (M), male. (B) The bar graphs show mean ratings of subjective arousal level when men and women observed, each, negative, positive and neutral IAPS images, with error bars indicating 1 s.d. Men and women did not differ in the intensity of their subjective experiences of arousal in response to negative images [T(32) = 0.81, P < 0.0421], although a significant valence (negative, positive and neutral) × sex (male, female) interaction [F(1.7, 53.6) = 5.72, P < 0.008, ηp2 = 0.152] indicated that men reported relatively stronger subjective experiences of arousal than women in response to positive [T(32) = 2.34, P < 0.025] and neutral [T(32) = 2.03, P < 0.050] images. Red bar (F), female; blue bar (M), male.
Correlation of neural activation with momentary subjective ratings of affective images
As predicted, when compared with men, women’s subjective experiences of affect when viewing evocative images were relatively more strongly correlated with activation in the AI. Specifically, a parametric modulation analysis (using the subjective arousal ratings as the modulation parameter of each event-related response, see Materials and Methods) with a focused ROI showed that women’s affective experiences were more strongly correlated than men’s, with increased activation in the ventral and mid portion of AI (mAI) {the right ventral AI [peak MNI coordinate of the ROI (x, y, z) = (34, 18, 14) mm, T(32) = 3.63, P < 0.0002], mAI [(42, 12, 0), T(32) = 4.08, P < 0.00004] and left mAI [(−40, 14, −2), T(32) = 3.73, P < 0.0001]; see the magnitude of the correlations (parameter estimates) in Figure 2A}. The finding is consistent with the idea that women are relatively more focused on internal sensations from the body during the subjective experience of arousal when compared with men. On the other hand, as expected, men showed a relatively stronger association between their subjective arousal ratings and increased activation in primary visual cortex [left V1 (−10, −86, 0); see Figure 2A] and in the fusiform gyrus, which is a part of the ventral visual stream (−50, −30, −26). These findings were confirmed with a whole brain analysis (reported in the Figure 2B, and Table 1) and are consistent with the idea that men are relatively more externally focused during subjective affective experience compared with women.
Table 1.
Sex differences in parametric modulation analysis of neural response regressed against event-by-event subjective arousal rating
| Area | BA | MNI coordinates (mm) |
T | Z | Cluster size, k | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Female > male | |||||||
| Right inferior parietal lobule | 40 | 52 | −38 | 58 | 4.16 | 4.02 | 178 |
| Right inferior parietal lobule | 40 | 62 | −38 | 46 | 3.57 | 3.48 | |
| Right postcentral gyrus | 2 | 60 | −26 | 50 | 3.48 | 3.4 | |
| Right precentral gyrus | 4 | 62 | −10 | 26 | 4.12 | 3.99 | 82 |
| Right superior frontal gyrus | 6 | 16 | 30 | 62 | 4.1 | 3.97 | 97 |
| Right superior frontal gyrus | 8 | 28 | 28 | 58 | 3.98 | 3.86 | |
| Right precentral gyrus | 44 | 50 | 16 | 6 | 4.09 | 3.96 | 104 |
| Right insula | 13 | 42 | 12 | 0 | 3.47 | 3.39 | |
| Right medial frontal cortex | 8 | 8 | 28 | 44 | 3.85 | 3.74 | 25 |
| Left postcentral gyrus | 2 | −58 | −28 | 50 | 3.82 | 3.71 | 33 |
| Right inferior frontal gyrus | 47 | 34 | 18 | −14 | 3.6 | 3.5 | 39 |
| Left parahippocampal gyrus/amygdala | 18 | −28 | −60 | 4 | 3.56 | 3.47 | 21 |
| Left insula | 13 | −44 | 4 | 4 | 3.53 | 3.44 | 40 |
| Left insula | 13 | −40 | 14 | −2 | 3.45 | 3.37 | |
| Left inferior frontal gyrus | 9 | −44 | 4 | 30 | 3.48 | 3.4 | 20 |
| Male > female | |||||||
| Left fusiform gyrus | 20 | −50 | −30 | −26 | 4.16 | 4.02 | 27 |
| Left superior frontal gyrus | 9 | −10 | 46 | 38 | 3.95 | 3.83 | 27 |
| Right cuneus | 17 | 2 | −98 | −4 | 3.93 | 3.81 | 39 |
| Left middle frontal gyrus | 10 | −44 | 54 | −10 | 3.84 | 3.72 | 11 |
| Left anterior cingulate | 24 | −8 | 36 | 2 | 3.73 | 3.62 | 10 |
| Left caudate | −14 | 24 | 0 | 3.6 | 3.5 | 27 | |
| Right middle frontal gyrus | 11 | 30 | 34 | −22 | 3.56 | 3.47 | 14 |
| Left cuneus | 18 | −2 | −94 | 14 | 3.45 | 3.37 | 32 |
| Right cuneus | 18 | 6 | −94 | 10 | 3.44 | 3.36 | |
AI–ACC functional connectivity during task engagement
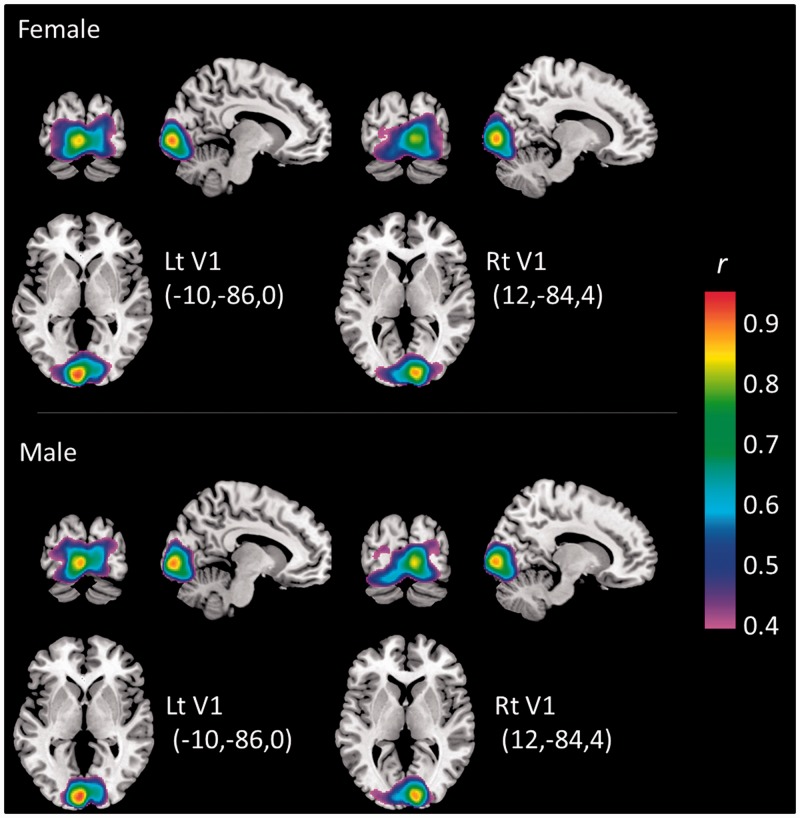
We also explored sex differences in the functional connectivity between the AI and the ACC while men and women rated their affective experiences. As noted before, this network is involved in the switch between the engagement of large-scale networks processing internal information vs those devoted to processing external cues (Menon and Uddin, 2010; Sridharan et al., 2008). We expected that this switching system would be enhanced in men if men’s subjective experience is more infused with exteroceptive information when compared with women. In fact, men did show stronger functional connectivity than women between AI and dorsal ACC (dACC) when viewing evocative images. Activations in the AI were positively correlated with activation in the dACC in both men and women during the task, but these correlations were stronger for men than for women [left dAI (−35, 18, 7) - dACC (3, 31 23), T(32) = 3.82, P < 0.001; right mAI (−33, 18, −5) - dACC (1, 25, 27), T(32) = 3.68, P < 0.001; left mAI (42, 15, −3) - dACC (−3, 29, 21), T(32) = 4.10, P < 0.001; all three P values were lower than the threshold of Bonferroni correction across 11 ROIs (α = 0.0045); see Figure 3B for correlation coefficients in each sex group, and Table 2 for all AI–ACC connectivity analyses]. Two of them were derived from the mid-dorsal AI seeds categorized as ‘attention’ [(−35, 18, 7) and (−33, 18, −5)]. In contrast, V1 had strong functional connectivity to other visual areas (V2–V5) in both men and women but there were no sex differences (Figure 4), supporting the specificity of the dAI–dACC effect.
Table 2.
Sex difference of AI–ACC functional connectivity during task engagement
| Area | AI seed ROI center x, y, z (mm) | ACC region connected to AI seed ROI Male vs Female |
||||
|---|---|---|---|---|---|---|
| Peak x, y, z (mm) | Correlation r |
T | k | |||
| Male | Female | |||||
| All images | ||||||
| Right | ||||||
| dAI | (36, 19, 3) | (−15, −49, 31) | −0.013 | −0.088 | 2.39 | – |
| (39, 7, 0) | (16, 44, 1) | 0.061 | −0.022 | 2.60 | – | |
| mAI | (42, 15, −3) | (−3, 29, 21) | 0.396 | 0.230 | 4.10** | 72 |
| (44, 10, −4) | (2, 19, −8) | 0.111 | −0.058 | 3.31* | – | |
| vAI | (42, 8, −6) | (0, 20, −8) | 0.106 | −0.043 | 3.57* | – |
| (28, 17, −15) | (18, −21, 43) | 0.011 | −0.049 | 2.33 | – | |
| Left | ||||||
| dAI | (−30, 12, 10) | (−1, −17, 29) | 0.164 | 0.067 | 2.75 | – |
| (−35, 18, 7) | (3, 31, 23) | 0.382 | 0.190 | 3.82** | 48 | |
| mAI | (−31, 24, −4) | (1, 31, 23) | 0.387 | 0.221 | 2.89* | – |
| (−33, 18, −5) | (1, 25, 27) | 0.340 | 0.182 | 3.68** | 22 | |
| vAI | (−31, 24, −6) | (1, 31, 23) | 0.408 | 0.245 | 2.98* | – |
| Negative | ||||||
| Right | ||||||
| dAI | (36, 19, 3) | (16, −49, 35) | 0.082 | −0.021 | 2.65 | – |
| (39, 7, 0) | (−8, 28, −11) | 0.092 | 0.002 | 2.98* | – | |
| mAI | (42, 15, −3) | (−1, 29, 23) | 0.437 | 0.272 | 3.63** | 12 |
| (44, 10, −4) | (18, 41, 1) | −0.007 | −0.145 | 3.55* | – | |
| vAI | (42, 8, −6) | (0, 19, −8) | 0.126 | −0.037 | 3.61* | – |
| (28, 17, −15) | (−12, −25, 49) | 0.071 | −0.028 | 2.20 | – | |
| Left | ||||||
| dAI | (−30, 12, 10) | (12, 38, −3) | 0.051 | −0.053 | 3.06* | – |
| (−35, 18, 7) | (5, 29, 25) | 0.394 | 0.220 | 3.66** | 19 | |
| mAI | (−31, 24, −4) | (1, 33, 23) | 0.420 | 0.231 | 3.43* | |
| (−33, 18, −5) | (1, 25, 27) | 0.361 | 0.200 | 3.55** | 10 | |
| vAI | (−31, 24, −6) | (1, 31, 21) | 0.425 | 0.226 | 3.62* | – |
| Positive | ||||||
| Right | ||||||
| dAI | (36, 19, 3) | (14, −9, 45) | 0.119 | 0.024 | 2.59 | – |
| (39, 7, 0) | (14, −11, 45) | 0.140 | −0.011 | 3.42* | – | |
| mAI | (42, 15, −3) | (−3, 29, 21) | 0.383 | 0.201 | 4.00** | 64 |
| (44, 10, −4) | (0, 19, −8) | 0.117 | −0.074 | 2.95* | – | |
| vAI | (42, 8, −6) | (0, 19, −8) | 0.105 | −0.068 | 2.98* | – |
| (28, 17, −15) | (−11, −19, 47) | 0.071 | −0.007 | 2.52 | – | |
| Left | ||||||
| dAI | (−30, 12, 10) | (1, −19, 29) | 0.151 | 0.050 | 2.66 | – |
| (−35, 18, 7) | (3, 31, 23) | 0.391 | 0.158 | 4.46** | 135 | |
| mAI | (−31, 24, −4) | (−5, 33, 29) | 0.431 | 0.287 | 2.91* | – |
| (−33, 18, −5) | (−1, 25, 27) | 0.335 | 0.187 | 3.27* | – | |
| vAI | (−31, 24, −6) | (8, −29, 51) | 0.119 | 0.027 | 2.41 | – |
| Neutral | ||||||
| Right | ||||||
| dAI | (36, 19, 3) | (3, 23, 31) | 0.420 | 0.301 | 2.48 | – |
| (39, 7, 0) | (7, −27, 35) | 0.232 | 0.093 | 2.93* | – | |
| mAI | (42, 15, −3) | (5, 21, 27) | 0.472 | 0.288 | 4.04** | 87 |
| (44, 10, −4) | (16, 44, 4) | 0.106 | −0.015 | 3.29* | – | |
| vAI | (42, 8, −6) | (2, 17, −11) | 0.144 | −0.035 | 3.24* | – |
| (28, 17, −15) | (4, 36, 6) | 0.260 | 0.150 | 2.82* | – | |
| Left | ||||||
| dAI | (−30, 12, 10) | (12, −35, 29) | 0.090 | −0.004 | 3.22* | – |
| (−35, 18, 7) | (1, 31, 23) | 0.375 | 0.200 | 2.94* | – | |
| mAI | (−31, 24, −4) | (16, −19, 41) | 0.066 | −0.026 | 2.78* | – |
| (−33, 18, −5) | (0, −41, 53) | 0.110 | −0.049 | 4.27** | 15 | |
| vAI | (−31, 24, −6) | (16, −17, 43) | 0.072 | −0.050 | 3.94* | – |
*P < 0.0045 (FWE correction across 11 AI ROIS), **P < 0.001, k = 10.
The table shows the coordinate of each seed in AI and the complete results of functional connectivity analysis, including the peak coordinates within ACC, correlation coefficients in men and women and t statistics from the analysis. As described in the text, among 11 seed ROIs in AI, men showed stronger functional connectivity from three AI ROIs to dACC than women in response to all the stimuli as well as each kind of stimuli (i.e. negative, positive and neutral images). There was no stronger functional connectivity between AI and dACC in women than in men.
Fig. 4.
Functional connectivity from V1 ROIs to other visual areas in each sex group. The figures show maps of functional connectivity (correlation coefficient r, range 0.4–1.0) based on a seed in the left V1 [left figures; MNI coordinate (x, y, z) = (10, 86, 0) mm] and right V1 [right figures; (x, y, z) = (12, 84, 4) mm] in women (upper figures) and men (lower figures). The two seed ROIs were obtained from the parametric modulation analysis from all participants (height and extent threshold at P < 0.05, FDR-corrected), and further restricted to V1 anatomically. Each coordinate in the figure is the center in each right and left V1 ROI. The figures indicate a similar localization of correlation in surrounding visual areas (V2–V5) in men and women. There was no significant difference in V1 functional connectivity between men and women.
DISCUSSION
These findings are provocative because they suggest that a different approach should be taken when considering the puzzle of sex differences in emotion. Men and women differed not in the overall intensity of their affective experience, but in the neural correlates of those experiences. Specifically, women’s subjective affective experience appears to be relatively more rooted in sensations from within the body, whereas men’s subjective affective experience is relatively more rooted in sensations from the world.
Note that women’s affective experiences were more strongly correlated than men’s with increased activation in the more ventral portion of AI. There are a number of recent studies reporting that the AI—in particular, the right ventral AI (Craig, 2009)—is associated with subjective affective experience (Wager and Barrett, 2004; Wager et al., 2008; Kurth et al., 2010). Our results extend previous findings showing that women (compared with men) have relatively greater activity in insula during negative mood induction (Hofer et al., 2006) and in the ventral AI extending from ventrolateral prefrontal regions in response to negative affective images (Caseras et al., 2007). On the other hand, our results that men place relatively more emphasis (than do women) on visual processing also builds on meta-analytic evidence showing that men produce more frequent activations in occipital cortex, including V1, during affective processing when compared with women (Wager et al., 2003; Stevens and Hamann, 2012), as well as findings that the affectively evocative aspects of the visual world modulate activity in the primary visual cortex (Posner and Gilbert, 1999; Vuilleumier and Driver, 2007). Recent reviews or meta-analysis about sex differences of neural correlates of emotion (Whittle et al., 2011; Stevens and Hamann, 2012) focused mainly on whether men and women respond differently to positive vs negative stimuli, and brain regions typically targeted when a neuroscience research aims at emotional reactions (e.g. the amygdala), although one meta-analysis of them indeed showed increased activation in visual cortex during emotional tasks for men compared with women (Stevens and Hamann, 2012). The previous neuroimaging studies only show sex differences from a quantitative standpoint of comparing intensities. Our results provide clearer explanation, however, such that women might experience more ‘self-focused’ emotion (i.e. they experience affect as the body’s response to the world around them), whereas men might experience more ‘world-focused’ emotion (i.e. they experience affect as a property of the world, much as people experience color).
A more speculative interpretation of our findings is that men are more effective at automatic affect regulation when compared with women. This is because the AI regions where men showed stronger functional connectivity with dACC were located in its more dorsal sector, which is part of the ventral attention network involved in the regulation of attention (Kurth et al., 2010). Such evidence inspires us to speculate that during evocative episodes, men might be more capable of shifting attention to the processing of exteroceptive cues (through the switching mechanism between the processing internal information vs external cues, see Sridharan et al., 2008; Menon and Uddin, 2010), whereas women remain more interoceptively focused on bodily sensations. Neuroimaging studies also provide support for the idea that men engage in more efficient automatic emotion regulatory processes than women (Thomas et al., 2001; Williams et al., 2005; Koch et al., 2007; Kempton et al., 2009; see the discussion in a recent review Whittle et al., 2011). Extensive evidence suggests that the dACC is a core brain region involved in emotional regulation (Ochsner and Gross, 2008). In light of these previous findings, our result of stronger dAI–dACC connectivity in men also suggests that men have more efficient neural systems for regulation of emotion (Koch et al., 2007; McRae et al., 2008), perhaps, in part, because they are more externally focused. Also we found stronger association between ventrolateral prefrontal cortex activity and subjective ratings in men, which is also suggestive of more regulative process in men.
We found additional results such as more activation related to emotional experiences in the amygdala in women. In fact, however, the previous studies have not shown consistent results regarding sex differences in amygdala response; for example, men exhibited greater amygdala response to sexual images (Hamann et al., 2004), but the same author summarized the pattern of the amygdala response in men and women to emotional stimuli in previously reported studies and suggested a great variety of patterns by different experimental paradigms (see the review in Hamann, 2005). The results from a couple of recent studies also vary in the link between sex and amygdala response to emotional material. For example, individual differences in trait anxiety scores were correlated with amygdala reactivity to angry facial expressions only among men (Carre et al., 2012). On the one hand, women showed greater left amygdala activation in contrast to men, who in turn showed greater right amygdala activation during viewing IAPS pictures (Aikins et al., 2010). Similarly, this sex-related lateralization (female-left/male-right) of amygdala activation was found in other studies (Cahill, 2003a, b), although other studies have failed to find this effect (Kensinger and Schacter, 2006, 2008; Talmi et al., 2008). The sex difference of amygdala seems still inconclusive, thus it is not easy to converge our present finding to the previous evidences. The only difference between our analysis and others is that we correlated amygdala activation with the ratings that represent moment-to-moment subjective emotional experiences. Therefore, our results might reflect neural association with emotional experiences better and the sex difference was enhanced. These are mostly speculations, however, and need further confirmation in the future.
Although we are focusing on the sex difference of emotional experiences especially in interoceptively or exteroceptively oriented neural process in the insula and visual cortex, the findings in this study could be viewed from other perspectives. For example, whereas women exhibited more activation during perception of ‘suffering’ or ‘compassion’ experiences in areas involved in basic emotional, empathic and moral phenomena, such as basal regions and cingulate and frontal cortices, activation in men was restricted mainly to the occipital cortex and parahippocampal gyrus (Mercadillo et al., 2011). This is partly consistent with our findings in terms of the dominance of the occipital cortex in men. According to the empathizing-systemizing theory (Baron-Cohen, 2005), women are stronger empathizers than men. Our ROIs—the insula and ACC—are key areas of the ‘social brain’ (Happe et al., 1996; Baron-Cohen et al., 1999; Castelli et al., 2002; Frith and Frith, 2003; Baron-Cohen, 2009), where sex differences have been previously found (Han et al., 2008; Rueckert and Naybar, 2008; Schulte-Ruther et al., 2008; Proverbio et al., 2009). In particular, AI is consistently involved in other complex mental phenomena like empathy, compassion, fairness and cooperation, such that AI plays an important role in ‘social emotions’ (affective states that arise when interacting with other people; see Lamm and Singer, 2010). In this regard, another explanation for our findings might be that women are more empathetic and have enriched social cognition relative to men, such that they show greater activation in social brain—the insula in particular—whereas men are better at systemizing reflected in greater activation in the occipital visual cortex. Given that the task used in this experiment was not a social task, and that the brain regions within the ‘social brain’ are largely overlapping with those in the ‘emotional brain’ (Barrett and Satpute, 2013), this explanation seems highly speculative.
If we were to speculate on the biological basis of this sex difference, a hormonal effect is the most interpretable and what we can most easily think of. In the brain—both human and nonhuman animals (e.g. rhesus monkeys, rats)—there are high concentrations of male sex hormone (androgen) receptors throughout cerebral cortex, especially in the visual cortex (Clark et al., 1988; Nunez et al., 2003; DonCarlos et al., 2006). Androgens, not estrogen, are also responsible for controlling the development of neurons in the visual cortex during embryogenesis, meaning that males have >20% more of these neurons than females (Nunez et al., 2000, 2001). These evidences are highly suggestive of hormonal effect on the men’s tendency to rely on exteroceptive visual input. On the other hand, it is still unclear whether female sex hormones influence directly interoceptive processes in insula. But some evidence implies this possibility; the insula was differentially activated in response to affective words between the follicular and luteal phases of the menstrual cycle (Protopopescu et al., 2005). The insula was also differently activated between different kinds of estrogen therapy for postmenopausal women (specifically, whether progestin was added or not) in response to emotional picture rating task (Shafir et al., 2012). The two studies show that insula activation in response to affective stimuli is considerably influenced by hormonal changes related to menstrual cycle in women. Further, neural response in insula to affective conditioned stimuli (simple geometric figures) was reduced in men but enhanced in women after treatment of cortisol, which like estrogen, is a steroid (Merz et al., 2010). These findings suggest the possibility that the estrogen may influence emotional experiences through insula activation representing interoceptive processing in women
Finally, our findings suggest that the same content (in this case, subjective experiences of affective arousal), although predictably engaging particular brain circuits, can engage nodes in these circuits to different degrees in different individuals. Even if the participants in the present study reported feeling the same experience, this report could contain subjective content that varied in its exteroceptive vs interoceptive focus. In this regard, our findings are consistent with a large body of behavioral evidence suggesting that even though people use the same words to represent and communicate their subjective affective experiences, they use those words to communicate particular properties of those experiences (e.g. ‘valence’ and ‘arousal’ properties; see Barrett, 2004). For example, some people use the word ‘angry’ to communicate a highly aroused, unpleasant feeling that involves the perception of offense, whereas others use the word to communicate just an ‘unpleasant’ feeling. Future studies should consider the possibility that it is the focus on the interoceptive vs exteroceptive aspects of affective experience, rather than its intensity per se, that might best reveal individual differences in subjective experience, whether due to sex, age, personality differences or psychopathology.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN Online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health Director’s Pioneer Award (DP1OD003312) and a National Institute on Aging grant (R01 AG030311) to L.F.B, as well as the following grants: R01-AG029480 to B.C.D. and shared resource/instrumentation grants NCRR P41-RR14075, 1S10RR023401, 1S10RR019307 and 1S10RR023043.
The authors thank Mary Foley and Larry White for their technical assistance.
REFERENCES
- Aikins DE, Anticevic A, Kiehl KA, Krystal JH. Sex-related differences in amygdala activity influences immediate memory. Neuroreport. 2010;21(4):273–76. doi: 10.1097/WNR.0b013e328335b3f9. doi:10.1097/WNR.0b013e328335b3f9. [DOI] [PubMed] [Google Scholar]
- Allman J, Hakeem A, Watson K. Two phylogenetic specializations in the human brain. Neuroscientist. 2002;8(4):335–46. doi: 10.1177/107385840200800409. [DOI] [PubMed] [Google Scholar]
- Ansfield ME. Smiling when distressed: when a smile is a frown turned upside down. Personality and Social Psychology Bulletin. 2007;33(6):763–75. doi: 10.1177/0146167206297398. doi:10.1177/0146167206297398. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research Brain Research Reviews. 1996;22(3):229–44. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. The Essential Difference: The Truth About the Male and Female Brain. New York: Basic Books; 2003. [Google Scholar]
- Baron-Cohen S. Testing the extreme male brain (EMB) theory of autism: let the data speak for themselves. Cognitive Neuropsychiatry. 2005;10(1):77–81. doi: 10.1080/13546800344000336. doi:10.1080/13546800344000336. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Autism: the empathizing-systemizing (E-S) theory. Annals of the New York Academy of Sciences. 2009;1156:68–80. doi: 10.1111/j.1749-6632.2009.04467.x. doi:10.1111/j.1749-6632.2009.04467.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, O'Riordan M, Stone V, Jones R, Plaisted K. Recognition of faux pas by normally developing children and children with Asperger syndrome or high-functioning autism. Journal of Autism and Developmental Disorders. 1999;29(5):407–18. doi: 10.1023/a:1023035012436. [DOI] [PubMed] [Google Scholar]
- Barrett LF. Feelings or words? Understanding the content in self-report ratings of experienced emotion. Journal of Personality and Social Psychology. 2004;87(2):266–81. doi: 10.1037/0022-3514.87.2.266. doi:10.1037/0022-3514.87.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF. Solving the emotion paradox: categorization and the experience of emotion. Personality and Social Psychology Review. 2006;10(1):20–46. doi: 10.1207/s15327957pspr1001_2. doi:10.1207/s15327957pspr1001_2. [DOI] [PubMed] [Google Scholar]
- Barrett LF. The future of psychology: connecting mind to brain. Perspectives on Psychological Science. 2009a;4(4):326–39. doi: 10.1111/j.1745-6924.2009.01134.x. doi:10.1111/j.1745-6924.2009.01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF. Variety is the spice of life: a psychological construction approach to understanding variability in emotion. Cognition and Emotion. 2009b;23(7):1284–306. doi: 10.1080/02699930902985894. doi:10.1080/02699930902985894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Bliss-Moreau E. She's emotional. He's having a bad day: attributional explanations for emotion stereotypes. Emotion. 2009;9(5):649–58. doi: 10.1037/a0016821. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Satpute A. Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Current Opinion in Neurobiology. 2013 doi: 10.1016/j.conb.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Robin L, Pietromonaco PR, Eyssell KM. Are women the “More Emotional'' sex? Evidence from emotional experiences in social context. Cognition and Emotion. 1998;12(4):555–78. [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: sex differences in picture processing. Emotion. 2001;1(3):300–19. [PubMed] [Google Scholar]
- Brescoll VL, Uhlmann EL. Can an angry woman get ahead? Status conferral, gender, and expression of emotion in the workplace. Psychological Science. 2008;19(3):268–75. doi: 10.1111/j.1467-9280.2008.02079.x. doi:10.1111/j.1467-9280.2008.02079.x. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract] presented at the 8th International Conference on Functional Mapping of the Human Brain. Neuroimage. 2002;16(2) abstract 497. Available on CD-ROM. [Google Scholar]
- Cahill L. Sex- and hemisphere-related influences on the neurobiology of emotionally influenced memory. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003a;27(8):1235–41. doi: 10.1016/j.pnpbp.2003.09.019. doi:10.1016/j.pnpbp.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Cahill L. Sex-related influences on the neurobiology of emotionally influenced memory. Annals of the New York Academy of Sciences. 2003b;985:163–73. doi: 10.1111/j.1749-6632.2003.tb07080.x. [DOI] [PubMed] [Google Scholar]
- Carre JM, Fisher PM, Manuck SB, Hariri AR. Interaction between trait anxiety and trait anger predict amygdala reactivity to angry facial expressions in men but not women. Social Cognitive and Affective Neuroscience. 2012;7(2):213–21. doi: 10.1093/scan/nsq101. doi:10.1093/scan/nsq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseras X, Mataix-Cols D, An SK, et al. Sex differences in neural responses to disgusting visual stimuli: implications for disgust-related psychiatric disorders. Biological Psychiatry. 2007;62(5):464–71. doi: 10.1016/j.biopsych.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125(Pt 8):1839–49. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Clark AS, MacLusky NJ, Goldman-Rakic PS. Androgen binding and metabolism in the cerebral cortex of the developing rhesus monkey. Endocrinology. 1988;123(2):932–40. doi: 10.1210/endo-123-2-932. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–24. doi: 10.1016/j.neuron.2008.04.017. doi:10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–66. doi: 10.1038/nrn894. doi:10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Damaraju E, Huang YM, Barrett LF, Pessoa L. Affective learning enhances activity and functional connectivity in early visual cortex. Neuropsychologia. 2009;47(12):2480–87. doi: 10.1016/j.neuropsychologia.2009.04.023. doi:10.1016/j.neuropsychologia.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cerebral Cortex. 2011;21(7):1498–506. doi: 10.1093/cercor/bhq186. doi:10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DonCarlos LL, Sarkey S, Lorenz B, et al. Novel cellular phenotypes and subcellular sites for androgen action in the forebrain. Neuroscience. 2006;138(3):801–7. doi: 10.1016/j.neuroscience.2005.06.020. doi:10.1016/j.neuroscience.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(26):11073–8. doi: 10.1073/pnas.0704320104. doi:10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10(1):1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2003;358(1431):459–73. doi: 10.1098/rstb.2002.1218. doi:10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Cook EW, Lang PJ. Affective judgment and psychophysiological response: dimensional covariation in the evaluation of pictorial stimuli. Journal of Psychophysiology. 1989;3(1):51–64. [Google Scholar]
- Hamann S. Sex differences in the responses of the human amygdala. Neuroscientist. 2005;11(4):288–93. doi: 10.1177/1073858404271981. [DOI] [PubMed] [Google Scholar]
- Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nature Neuroscience. 2004;7(4):411–6. doi: 10.1038/nn1208. doi:10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- Han S, Fan Y, Mao L. Gender difference in empathy for pain: an electrophysiological investigation. Brain Research. 2008;1196:85–93. doi: 10.1016/j.brainres.2007.12.062. doi:10.1016/j.brainres.2007.12.062. [DOI] [PubMed] [Google Scholar]
- Happe F, Ehlers S, Fletcher P, et al. ‘Theory of mind' in the brain. Evidence from a PET scan study of Asperger syndrome. Neuroreport. 1996;8(1):197–201. doi: 10.1097/00001756-199612200-00040. [DOI] [PubMed] [Google Scholar]
- Herbert BM, Pollatos O. The body in the mind: on the relationship between interoception and embodiment. Topics in Cognitive Science. 2012;4(4):692–704. doi: 10.1111/j.1756-8765.2012.01189.x. doi:10.1111/j.1756-8765.2012.01189.x. [DOI] [PubMed] [Google Scholar]
- Hofer A, Siedentopf CM, Ischebeck A, et al. Gender differences in regional cerebral activity during the perception of emotion: a functional MRI study. Neuroimage. 2006;32(2):854–62. doi: 10.1016/j.neuroimage.2006.03.053. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Forsyth JP, Karekla M. Sex differences in response to a panicogenic challenge procedure: an experimental evaluation of panic vulnerability in a non-clinical sample. Behaviour Research and Therapy. 2006;44(10):1421–30. doi: 10.1016/j.brat.2005.10.012. doi:10.1016/j.brat.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Tyrka AR, Anderson GM, Price LH, Carpenter LL. Sex differences in emotional and physiological responses to the Trier Social Stress Test. Journal of Behavior Therapy and Experimental Psychiatry. 2008;39(1):87–98. doi: 10.1016/j.jbtep.2007.02.003. doi:10.1016/j.jbtep.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton MJ, Haldane M, Jogia J, et al. The effects of gender and COMT Val158Met polymorphism on fearful facial affect recognition: a fMRI study. International Journal of Neuropsychopharmacology. 2009;12(3):371–81. doi: 10.1017/S1461145708009395. doi:10.1017/S1461145708009395. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. Journal of Neuroscience. 2006;26(9):2564–70. doi: 10.1523/JNEUROSCI.5241-05.2006. doi:10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Neural processes supporting young and older adults' emotional memories. Journal of Cognitive Neuroscience. 2008;20(7):1161–73. doi: 10.1162/jocn.2008.20080. [DOI] [PubMed] [Google Scholar]
- Koch K, Pauly K, Kellermann T, et al. Gender differences in the cognitive control of emotion: an fMRI study. Neuropsychologia. 2007;45(12):2744–54. doi: 10.1016/j.neuropsychologia.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure and Function. 2010;214(5-6):519–34. doi: 10.1007/s00429-010-0255-z. doi:10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFrance M, Hecht MA, Paluck EL. The contingent smile: a meta-analysis of sex differences in smiling. Psychological Bulletin. 2003;129(2):305–34. doi: 10.1037/0033-2909.129.2.305. [DOI] [PubMed] [Google Scholar]
- Lambie JA, Marcel AJ. Consciousness and the varieties of emotion experience: a theoretical framework. Psychological Review. 2002;109(2):219–59. doi: 10.1037/0033-295x.109.2.219. [DOI] [PubMed] [Google Scholar]
- Lamm C, Singer T. The role of anterior insular cortex in social emotions. Brain Structure and Function. 2010;214(5-6):579–91. doi: 10.1007/s00429-010-0251-3. doi:10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: affect, activation and action. In: Lang PJ, Balaban RF, editors. Attention and Orienting: Sensory and Motivational Processes. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1997. pp. 97–134. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. 2008. Technical Report A-8. Gainesville, FL: University of Florida. [Google Scholar]
- Lindquist KA, Barrett LF. Constructing emotion: the experience of fear as a conceptual act. Psychological Science. 2008;19(9):898–903. doi: 10.1111/j.1467-9280.2008.02174.x. doi:10.1111/j.1467-9280.2008.02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz CA. Engendered emotion: gender, power and the rhetoric of emotional control in American discourse. In: Lutz CA, Abu-Lughod L, editors. Language and the Politics of Emotion: Studies in Emotion and Social Interaction. Cambridge: Cambridge University Press; 1990. pp. 69–91. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gabrieli JJD, Gross JJ. Gender differences in emotion regulation: an fMRI Study of Cognitive Reappraisal. Group Processes Intergroup Relations. 2008;11(2):143–62. doi: 10.1177/1368430207088035. doi:10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214(5-6):655–67. doi: 10.1007/s00429-010-0262-0. doi:10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercadillo RE, Diaz JL, Pasaye EH, Barrios FA. Perception of suffering and compassion experience: brain gender disparities. Brain and Cognition. 2011;76(1):5–14. doi: 10.1016/j.bandc.2011.03.019. doi:10.1016/j.bandc.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, et al. Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology. 2010;35(1):33–46. doi: 10.1016/j.psyneuen.2009.07.009. doi:10.1016/j.psyneuen.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: efferent cortical output and comments on function. Journal of Comparative Neurology. 1982;212(1):38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Maeda M, Igarashi T, et al. Age and gender effect on alexithymia in large, Japanese community and clinical samples: a cross-validation study of the Toronto Alexithymia Scale (TAS-20) BioPsychoSocial Medicine. 2007;1:7. doi: 10.1186/1751-0759-1-7. doi:10.1186/1751-0759-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Negreira A, Weierich M, et al. Differential hemodynamic response in affective circuitry with aging: an fMRI study of novelty, valence, and arousal. Journal of Cognitive Neuroscience. 2011;23(5):1027–41. doi: 10.1162/jocn.2010.21527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler I, Wieckhorst B, Kowalevski S, et al. Functional organization of the human anterior insular cortex. Neuroscience Letters. 2009;457:66–70. doi: 10.1016/j.neulet.2009.03.101. doi:10.1016/j.neulet.2009.03.101. [DOI] [PubMed] [Google Scholar]
- Nagourney A. Calling Clinton ‘Angry,’ G.O.P. Chairman Goes on the Attack. New York Times. February 6, 2006:A16. [Google Scholar]
- Nimchinsky EA, Gilissen E, Allman JM, Perl DP, Erwin JM, Hof PR. A neuronal morphologic type unique to humans and great apes. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(9):5268–73. doi: 10.1073/pnas.96.9.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, Huppenbauer CB, McAbee MD, Juraska JM, DonCarlos LL. Androgen receptor expression in the developing male and female rat visual and prefrontal cortex. Journal of Neurobiology. 2003;56(3):293–302. doi: 10.1002/neu.10236. doi:10.1002/neu.10236. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Jurgens HA, Juraska JM. Androgens reduce cell death in the developing rat visual cortex. Brain Research Developmental Brain Research. 2000;125(1-2):83–8. doi: 10.1016/s0165-3806(00)00126-7. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Lauschke DM, Juraska JM. Cell death in the development of the posterior cortex in male and female rats. Journal of Comparative Neurology. 2001;436(1):32–41. [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17(2):153–8. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease A, Pease B. Why Men Don't Listen and Women Can't Read Maps: How We're Different and What to Do About it. London: Orion Publishing Group; 1999. [Google Scholar]
- Pennebaker JW. The Psychology of Physical Symptoms. New York: Springer-Verlag; 1982. [Google Scholar]
- Posner MI, Gilbert CD. Attention and primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(6):2585–7. doi: 10.1073/pnas.96.6.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Altemus M, et al. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(44):16060–5. doi: 10.1073/pnas.0502818102. doi:10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proverbio AM, Adorni R, Zani A, Trestianu L. Sex differences in the brain response to affective scenes with or without humans. Neuropsychologia. 2009;47(12):2374–88. doi: 10.1016/j.neuropsychologia.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Quigley KS, Barrett LF. Emotional learning and mechanisms of intentional psychological change. In: Brandtstadter J, Lerner RM, editors. Action and Self-Development: Theory and Research Through the LifeSpan. Thousand Oaks, CA: Sage; 1999. [Google Scholar]
- Robinson MD, Clore GL. Belief and feeling: evidence for an accessibility model of emotional self-report. Psychological Bulletin. 2002;128(6):934–60. doi: 10.1037/0033-2909.128.6.934. [DOI] [PubMed] [Google Scholar]
- Rueckert L, Naybar N. Gender differences in empathy: the role of the right hemisphere. Brain and Cognition. 2008;67(2):162–7. doi: 10.1016/j.bandc.2008.01.002. doi:10.1016/j.bandc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Schulte-Ruther M, Markowitsch HJ, Shah NJ, Fink GR, Piefke M. Gender differences in brain networks supporting empathy. NeuroImage. 2008;42(1):393–403. doi: 10.1016/j.neuroimage.2008.04.180. doi:10.1016/j.neuroimage.2008.04.180. [DOI] [PubMed] [Google Scholar]
- Shafir T, Love T, Berent-Spillson A, et al. Postmenopausal hormone use impact on emotion processing circuitry. Behavioural Brain Research. 2012;226(1):147–53. doi: 10.1016/j.bbr.2011.09.012. doi:10.1016/j.bbr.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FJ. Men are Visual; Women are Verbal. New York: Rivercross Pub; 2000. [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(34):12569–74. doi: 10.1073/pnas.0800005105. doi:10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50(7):1578–93. doi: 10.1016/j.neuropsychologia.2012.03.011. doi:10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Talmi D, Anderson AK, Riggs L, Caplan JB, Moscovitch M. Immediate memory consequences of the effect of emotion on attention to pictures. Learning and Memory. 2008;15(3):172–82. doi: 10.1101/lm.722908. doi:10.1101/lm.722908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, et al. Amygdala response to facial expressions in children and adults. Biological Psychiatry. 2001;49(4):309–16. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Tibblin G, Bengtsson C, Furunes B, Lapidus L. Symptoms by age and sex. The population studies of men and women in Gothenburg, Sweden. Scandinavian Journal of Primary Health Care. 1990;8(1):9–17. doi: 10.3109/02813439008994923. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychological Bulletin. 1995;117(2):250–70. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362(1481):837–55. doi: 10.1098/rstb.2007.2092. doi:10.1098/rstb.2007.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Barrett LF. From affect to control: functional specialization of the insula in motivation and regulation. 2004. Available at: http://www.affective-science.org/pubs/2004/Wager_Edfest_submitted_copy.pdf.
- Wager TD, Barrett LF, Bliss-Moreau E, et al. The neuroimaging of emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. The Handbook of Emotion. 3rd edn. New York: Guilford; 2008. pp. 249–71. [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19(3):513–31. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Whittle S, Yucel M, Yap MB, Allen NB. Sex differences in the neural correlates of emotion: evidence from neuroimaging. Biological Psychology. 2011;87(3):319–33. doi: 10.1016/j.biopsycho.2011.05.003. doi:10.1016/j.biopsycho.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Williams LM, Barton MJ, Kemp AH, et al. Distinct amygdala-autonomic arousal profiles in response to fear signals in healthy males and females. NeuroImage. 2005;28(3):618–26. doi: 10.1016/j.neuroimage.2005.06.035. doi:10.1016/j.neuroimage.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Wool CA, Barsky AJ. Do women somatize more than men? Gender differences in somatization. Psychosomatics. 1994;35(5):445–52. doi: 10.1016/S0033-3182(94)71738-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.