Abstract
Eudaimonic well-being reflects traits concerned with personal growth, self-acceptance, purpose in life and autonomy (among others) and is a substantial predictor of life events, including health. Although interest in the aetiology of eudaimonic well-being has blossomed in recent years, little is known of the underlying neural substrates of this construct. To address this gap in our knowledge, here we examined whether regional gray matter (GM) volume was associated with eudaimonic well-being. Structural magnetic resonance images from 70 young, healthy adults who also completed Ryff’s 42-item measure of the six core facets of eudaimonia, were analysed with voxel-based morphometry techniques. We found that eudaimonic well-being was positively associated with right insular cortex GM volume. This association was also reflected in three of the sub-scales of eudaimonia: personal growth, positive relations and purpose in life. Positive relations also showed a significant association with left insula volume. No other significant associations were observed, although personal growth was marginally associated with left insula, and purpose in life exhibited a marginally significant negative association with middle temporal gyrus GM volume. These findings are the first to our knowledge linking eudaimonic well-being with regional brain structure.
Keywords: gray matter volume, insula, psychological well-being, eudaimonia, personal growth, purpose in life, positive relations
INTRODUCTION
Recent years have seen a blossoming interest in the origins of human well-being and happiness (Seligman and Csikszentmihalyi, 2000). Within the broader positive psychology literature, a distinction is commonly made between subjective (or hedonic) well-being and eudaimonic (or psychological) well-being (Ryan and Deci, 2001). Subjective well-being reflects positive emotional functioning (Diener, 1984). In contrast, eudaimonic well-being is more closely linked to human potentials: aspects of life relating to flourishing and character development (Ryff, 1989; Waterman, 1993). Although substantial interest has been directed at delineating the consequences of eudaimonia (Ryff, 1989), to date, limited work has examined this construct within a neuroscientific framework (Kringelbach and Berridge, 2009). This is unfortunate as this approach offers the possibility of important insights into the underlying biological bases of this trait. Here, we sought to address this gap in the literature by examining whether regional gray matter (GM) volume was associated with eudaimonia in a large sample of young, healthy adults.
Eudaimonic well-being: a brief outline
Aristotle (1925/1998) distinguished between the experience of pleasure (i.e. hedonia) and the notion of a ‘virtuous activity of [the] soul’ (p. 18), or eudaimonia. In this model, eudaimonia was thought of as the conscious and life-long active exercise of intellect and character virtues. Contemporary models of eudaimonia have developed these Aristotelian foundations, incorporating insights from research on human development detailed in the work of scholars such as Erikson, Allport and Maslow (Ryff, 1989). This research led to the most widely used measure of eudaimonia: the six-factor Ryff Scales of Psychological Well-being. These correlated scales assess autonomy, personal growth, self-acceptance, purpose in life, environmental mastery and positive relations with others, forming a superordinate eudaimonia factor (Ryff and Singer, 2006). This six-factor model captures the qualities of belonging and benefiting others, flourishing, thriving and exercising excellence, although notably it omits some intellect qualities, character traits and values that Aristotle (1925/1998) would have emphasized, including wisdom, bravery, generosity and justice. Several studies have supported the psychometric bases of this six-factor model (cf. Ryff and Singer, 2006; but also see, Springer and Hauser, 2006).
Eudaimonic well-being is increasingly recognized as an important domain of individual differences (Ryff, 1989, 1995). For instance, both eudaimonic well-being and its facets are heritable (Archontaki et al., 2013), and low eudaimonia acts a risk factor for depression (Wood and Joseph, 2010). Low eudaimonic well-being has also been linked to poor physical health and with biomarkers linked to health (Ryff et al., 2004, 2006). In a large sample of elderly US women, eudaimonic well-being was predictive of lower levels of cardiovascular risk, pro-inflammatory cytokines and daily salivary cortisol levels, and with longer duration rapid eye movement (REM) sleep (Ryff et al., 2004). Of interest, these associations are largely independent to those observed for subjective well-being suggesting a distinct pattern of biomarkers reflects eudaimonia.
Neural correlates of eudaimonia
Although much attention has been directed towards the origins of human well-being (both subjective and eudaimonic, Seligman and Csikszentmihalyi, 2000), little work to date has directly addressed the neural bases of eudaimonia. The first and only study directly examining neural correlates of eudaimonic well-being found evidence for an association with frontal cortex activity: greater left than right superior frontal cortex activation (measured using electroencephalography) is associated with eudaimonic (and hedonic) well-being (Urry et al., 2004). No study (to our best knowledge) has utilized magnetic resonance imaging (MRI) techniques to characterize structural or functional correlates of eudaimonia; however, several studies have identified structural correlates (GM volume) of depressive symptoms (in turn, negatively associated with eudaimonic well-being, Wood and Joseph, 2010), including insular cortex (Hwang et al., 2010; Sprengelmeyer et al., 2011; Bechdolf et al., 2012), amygdala (Sheline et al., 1998; von Gunten et al., 2000; but also see, Frodl et al., 2002) and hippocampus (Campbell et al., 2004). There is some basis, then, for speculating that these regions may reflect variation in eudaimonic well-being.
In addition to these empirical findings, Kringelbach and Berridge (2009) have suggested that neural regions associated with the ‘default network’ (Gusnard et al., 2001; Buckner et al., 2008)—specifically, the anterior cingulate cortex, orbitofrontal cortex and medial prefrontal cortex—may be related to eudaimonic well-being, on account of links from the default network to representations of self (Lou et al., 1999) and internal modes of cognition (Buckner et al., 2008), these cognitive processes are closely analogous to the characteristics ascribed to eudaimonia. Finally, Archontaki et al. (2013) recently noted that the mechanism underlying eudaimonia must be capable of exerting control across all facets of eudaimonia, implying a top-down control mechanism over multiple systems processing emotional, reward incentive and motivational information related to eudaimonic well-being. Although several cortical regions are active during self-control activity, right ventrolateral prefrontal cortex activation is present regardless of domain (Cohen and Lieberman, 2010), leading Archontaki et al. (2013) to hypothesize links from this region to eudaimonic well-being.
The current study
Little work to date has examined the neural bases of eudaimonic well-being. To address this gap in the literature, here we examined whether regional GM volume was associated with the general factor of eudaimonia and the six eudaimonia sub-scales. Such neuroanatomical studies, as noted earlier have yielded novel links between GM volume and a broad range of psychological traits (Kanai and Rees 2011; Lewis et al., 2012)—including traits with clear relevance for eudaimonic well-being such as depressive symptoms (Hwang et al., 2010; Sprengelmeyer et al., 2011; Bechdolf et al., 2012) and mindfulness (Hölzel et al., 2008)—and so present as a plausible approach for delineating the neural architecture of eudaimonic well-being. We analysed structural MRI volumes collected from a sample of healthy, young adults (n = 70) using voxel-based morphometry (VBM) methods to assess whether differences in regional brain structure correlated with individual differences in eudaimonic well-being. In line with limited prior work in this literature, and several brain regions linked to traits of relevance to eudaimonic well-being, we elected to conduct whole-brain analyses so as to provide a more conservative test of neuroanatomical associations with eudaimonia (see ‘Methods’ section for full details).
METHODS
Participants
Seventy healthy participants (42 females; mean age = 24.6 ± 3.76 years) were recruited from the local community of University College London. The study was approved by the local ethical committee, and written informed consent was obtained from all participants.
Eudaimonic well-being measures
Eudaimonic well-being was assessed using the 42-item Ryff Scales of Psychological Well-being (Ryff et al., 1989). The six scales (and an example item for each scales) are as follows: autonomy: ‘I tend to be influenced by people with strong opinions’; environmental mastery: ‘I am quite good at managing the many responsibilities of my daily life’; personal growth: ‘I think it is important to have new experiences that challenge how you think about yourself and the world’; positive relations with others: ‘People would describe me as a giving person, willing to share my time with others’; purpose in life: ‘Some people wander aimlessly through life, but I am not one of them’ and self-acceptance: ‘In many ways I feel disappointed about my achievements in life’ (reverse scored). All responses were made on seven-point scales (from 1: strongly agree to 7: strongly disagree). Scale scores were computed as the sum of relevant items, reversing items where appropriate. Most of the sub-scales exhibited adequate reliability [αs = 0.64 (autonomy), 0.67 (mastery), 0.52 (personal growth), 0.76 (positive relations), 0.76 (purpose in life) and 0.89 (self-acceptance)]. A latent factor of ‘eudaimonia’ was derived as the first principle component extracted from the six sub-scales. This latent factor explained 58.34% of total variance and showed moderate-to-high positive loadings on each of the sub-scales (all >0.55).
MRI data acquisition
MR images were acquired on a 1.5-T Siemens Sonata MRI scanner (Siemens Medical, Erlangen, Germany). High-resolution anatomical images were acquired using a T1-weighted 3-D Modified Driven Equilibrium Fourier Transform sequence (repetition time = 12.24 ms; echo time = 3.56 ms; field of view = 256 × 256 mm and voxel size = 1 × 1 × 1 mm).
Voxel-based morphometry
The MR images were first segmented for GM and white matter using the segmentation tools in SPM8 (http://www.fil.ion.ucl.ac.uk/spm). Subsequently, we performed Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra in SPM8 for inter-subject registration of the GM images. To ensure that local GM volumes were conserved after spatial transformation, the image intensity of each voxel was modulated by the Jacobian determinants of the deformation fields. Registered images were then smoothed with a Gaussian kernel (full with half maximum = 8 mm) and transformed to Montreal Neurological Institute (MNI) stereotactic space using affine and non-linear spatial normalization implemented in SPM8 for multiple regression analysis.
The gender and age of the participants as well as whole-brain GM volume were included as covariates in the design matrix, thus regressing out effects correlated with these variables. As noted earlier, because little prior work facilitated targeted hypotheses we remained largely agnostic with regard to specific regions, instead focusing our analyses on the whole brain. Therefore, we analysed the data using a mask volume that comprised the entire brain with the exception of the cerebellum, which was thus excluded from analysis. The mask volume was constructed from a probabilistic MNI structural atlas (Mazziotta et al., 2001). Within this mask volume, clusters were initially identified as contiguous groups of voxels that exceeded an uncorrected threshold of voxel-wise P < 0.001. We employed a threshold of P(corr) < 0.05 corrected for multiple comparisons across the entire mask volume at a cluster level using non-stationary correction (Hayasaka et al., 2004) to identify regions in which GM volume correlated significantly with eudaimonia scores.
RESULTS
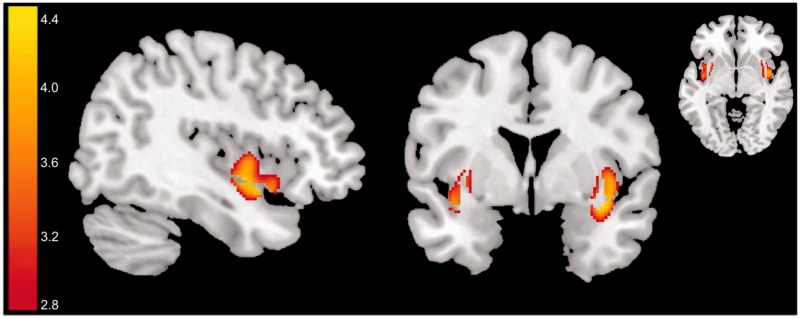
Eudaimonia was positively and significantly correlated with the GM volume of right insular cortex [P(corr) = 0.03, r = 0.47, t(65) = 4.33; Table 1 and Figure 1]. No clusters exhibited significant (corrected) negative correlations with eudaimonia.
Table 1.
Summary of GM volume associations with eudaimonia and eudaimonia sub-scales
| Trait | Area | H | MNI coordinates of peak voxel |
Correlation (Pearson’s r) | t(65) | Cluster size (voxels) | P(corr) | ||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||||
| Eudaimonia | Insula | R | 39 | 5 | −14 | 0.47 | 4.33 | 419 | 0.03* |
| Personal growth | Insula | R | 42 | 15 | −11 | 0.48 | 4.38 | 277 | 0.05* |
| Insula | L | −33 | 6 | −2 | 0.45 | 4.09 | 554 | 0.07ns | |
| Positive relations | Insula | R | −39 | −1 | −8 | 0.51 | 4.76 | 720 | 0.04* |
| Insula | L | 39 | 3 | −14 | 0.50 | 4.63 | 543 | 0.04* | |
| Purpose in life | MTG | R | 63 | 3 | −23 | −0.44 | 4.00 | 566 | 0.08ns |
| Insula | R | 42 | 2 | 3 | 0.46 | 4.23 | 416 | 0.02* | |
MTG, middle temporal gyrus; H, hemisphere; L, left; R, right.
P(corr) denotes the P-values corrected for multiple comparison at the cluster level across the volume of interest (see ‘Methods’ section for details). Significant results are denoted by *P ≤ 0.05, corrected; ns indicates non-significant results.
Fig. 1.
Regions where GM volume was associated with eudaimonia are overlaid on a T1-weighted standard MNI template; t-values are overlaid for regions that showed significant positive correlation in the VBM analyses (see the main text and Table 1). The colour bar illustrates the corresponding t-values.
Within the sub-scales of the eudaimonia measures, the following associations were found. Purpose in life, positive relations and personal growth scores all showed positive correlations with right insular cortex volumes [P(corr) = 0.02, r = 0.46, t(65) = 4.23; P(corr) = 0.04, r = 0.51, t(65) = 4.76 and P(corr) = 0.05, r = 0.48, t(65) = 4.38, respectively].
In addition to these links with right insula volume, positive relations and personal growth scores were also positively correlated with left insular cortex volume [P(corr) = 0.04, r = 0.50, t(65) = 4.63 and P(corr) = 0.07, r = 0.45, t(65) = 4.09, respectively]. Finally, purpose in life showed a marginal negative correlation with middle temporal gyrus [P(corr) = 0.08, r = −0.44, t(65) = 4.00]. No other associations between eudaimonia sub-scales and regional GM volume exceeded the corrected threshold used here.
DISCUSSION
This study found evidence for a positive association between GM volume in the right insular cortex and eudaimonic well-being in a large sample of young, healthy adults. These links with right insular cortex volume were also reflected in positive associations with several facets of eudaimonic well-being such as personal growth, positive relations and purpose in life. Positive relations and personal growth also exhibited positive associations with left insular cortex at nominal and marginally significant levels, respectively.
Although insular cortex has not been explicitly linked to differences in eudaimonic well-being in previous theorizing, these results are broadly consistent with prior research showing a negative association between insula volume and depression (Hwang et al., 2010; Sprengelmeyer et al., 2011; Bechdolf et al., 2012). The finding that low levels of eudaimonia are associated with increased risk of depressive symptoms (Wood and Joseph, 2010) suggests that eudaimonia and depression may share important biological links. In addition, eudaimonia is fundamentally linked to notions of agency (Waterman, 1993), and recent work has identified insular cortex as a source of agentic control (Lee and Reeve, 2013). The insula has also been linked to facilitation of self-awareness (Craig, 2009), as well as to the regulation of bodily states and modulation of decision making based on interoceptive information about these bodily states (Singer et al., 2009). Additional linkages to the guidance of behaviour have also been postulated, based on the activation of insular cortex during the segregation of internal and external stimuli for their relevance and subsequent incorporation in the active guidance of behaviour (Menon and Uddin, 2010). Finally, mindfulness meditation, a trait with prima facie links to eudaimonia, has been associated with right insular cortex GM volume (Hölzel et al., 2008).
This set of higher-order functions attributed to the insula are not mutually exclusive. Indeed, jointly, they closely reflect the multi-faceted psychological description of eudaimonia as reflected in high levels of self-awareness, self-reflection and cognitive and emotional control in the service of dynamic goal selection and goal pursuit, and self governance and reflection (Ryff, 1995). In sum, then, insula cortex may facilitate eudaimonic well-being by generating a set of capacities which jointly act to integrate interoceptive states with external circumstances, and successfully manage this emotional milieu. Nonetheless, it should be recalled that the insula is implicated in many functions (cf. Craig, 2009; Singer et al., 2009; Menon and Uddin, 2010) and, thus, further work is required to confirm the mechanism by which insular cortex and eudaimonia are linked.
Specific limitations and recommendations for future work require mention. First, this study did not control for subjective (hedonic) well-being, which is moderately associated with eudaimonic well-being (Ryff and Keyes, 1995). As such, we cannot conclude that insula cortex is a unique correlate of eudaimonia, over and above neural bases of subjective well-being, and future work should address this shortcoming. Second, although these results provide insight into the neural bases of eudaimonic well-being, the data cannot speak to causal directions; that is, the present data are unable to establish whether greater insular cortex facilitates eudaimonia, or if greater eudaimonic well-being causes increased insula GM volume. Longitudinal studies addressing development and change in cortical structure (Hedman et al., 2012) will be helpful to address this question. Some work to date has addressed this issue, examining the influence of mindfulness meditation on structural changes in insula and hippocampus (Hölzel et al., 2011); however, this work failed to find changes following such interventions in insular cortex (although such changes were observed in hippocampus).
In summary, we provide evidence that GM volume in the right insular cortex, a brain region with links to depression as well as to interoception and control over bodily states (among other behaviours), is positively associated with eudaimonic well-being. These findings provide initial evidence of individual differences in brain structure showing links to an important psychosocial trait with links in turn to mental and physical health.
Acknowledgments
This work was funded by the Wellcome Trust (G.R.).
REFERENCES
- Archontaki D, Lewis GJ, Bates TC. Genetic influences on psychological well-being: a nationally representative twin study. Journal of Personality. 2013;81(2):221–30. doi: 10.1111/j.1467-6494.2012.00787.x. [DOI] [PubMed] [Google Scholar]
- Aristotle (1925/1998). The Nicomachean Ethics. Ross, W.D., trans. Oxford: Oxford University Press.
- Bechdolf A, Wood SJ, Nelson B, et al. Amygdala and insula volumes prior to illness onset in bipolar disorder: a magnetic resonance imaging study. Psychiatry Research. 2012;201:34–9. doi: 10.1016/j.pscychresns.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Buckner R, Andrews-Hanna J, Schacter D. The brain’s default network: anatomy, function and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. American Journal of Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Cohen JR, Lieberman MD. The common neural basis of exerting self-control in multiple domains. In: Hassin R, Ochsner K, Trope Y, editors. Self Control in Society, Mind, and Brain. Oxford: Oxford Scholarship Online Monographs; 2010. pp. 141–62. [Google Scholar]
- Craig AD. How do you feel — now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Diener E. Subjective well-being. Psychological Bulletin. 1984;95:542–75. [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzche T, et al. Enlargement of the amygdala in patients with a first episode of major depression. Biological Psychiatry. 2002;51:708–14. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. NeuroImage. 2004;22:676–87. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Hedman AM, van Haren NEM, Schnack HD, Kahn RS, Hulshoff Pol HE. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Human Brain Mapping. 2012;33:1987–2002. doi: 10.1002/hbm.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Carmody J, Vangel M, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Research: Neuroimaging. 2011;191:36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Ott U, Gard T, et al. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Social Cognitive and Affective Neuroscience. 2008;3:55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JP, Lee TW, Tsai SJ, et al. Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. Journal of Geriatric Psychiatry and Neurology. 2010;23:171–84. doi: 10.1177/0891988710363713. [DOI] [PubMed] [Google Scholar]
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nature Reviews Neuroscience. 2011;12:231–42. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Berridge KC. Towards a functional neuroanatomy of pleasure and happiness. Trends in Cognitive Sciences. 2009;13:479–87. doi: 10.1016/j.tics.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Reeve J. Self-determined, but not non-self-determined, motivation predicts activations in the anterior insular cortex: an fMRI study of personal agency. Social, Cognitive, and Affective Neuroscience. 2013;8(5):538–45. doi: 10.1093/scan/nss029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GJ, Kanai R, Bates TC, Rees G. Moral values are associated with individual differences in regional brain volume. Journal of Cognitive Neuroscience. 2012;24:1657–63. doi: 10.1162/jocn_a_00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou HC, Kjaer TW, Friberg L, Wildschiodtz G, Holm S, Nowak M. A 15O-H2O PET study of meditation and the resting state of normal consciousness. Human Brain Mapping. 1999;7:98–105. doi: 10.1002/(SICI)1097-0193(1999)7:2<98::AID-HBM3>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, et al. A probabilistic atlas and reference system for the human brain: international consortium for brain mapping (ICBM) Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2001;356:1293–322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RM, Deci EL. On happiness and human potentials: a review of research on hedonic and eudaimonic well-being. Annual Review of Psychology. 2001;52:141–66. doi: 10.1146/annurev.psych.52.1.141. [DOI] [PubMed] [Google Scholar]
- Ryff CD. Happiness is everything, or is it—explorations on the meaning of psychological well-being. Journal of Personality and Social Psychology. 1989;57:1069–81. [Google Scholar]
- Ryff CD. Psychological well-being in adult life. Current Directions in Psychological Science. 1995;4:99–104. [Google Scholar]
- Ryff CD, Keyes CLM. The structure of psychological well-being revisited. Journal of Personality and Social Psychology. 1995;69:719–27. doi: 10.1037//0022-3514.69.4.719. [DOI] [PubMed] [Google Scholar]
- Ryff CD, Love GD, Urry HL, et al. Psychological well-being and ill-being: do they have distinct or mirrored biological correlates? Psychotherapy and Psychosomatics. 2006;75:85–95. doi: 10.1159/000090892. [DOI] [PubMed] [Google Scholar]
- Ryff CD, Singer BH. Best news yet on the six-factor model of wellbeing. Social Science Research. 2006;35:1103–19. [Google Scholar]
- Ryff CD, Singer BH, Dienberg Love G. Positive health: connecting well-being with biology. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2004;359:1383–94. doi: 10.1098/rstb.2004.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman MEP, Csikszentmihalyi M. Positive psychology: an introduction. American Psychologist. 2000;55:5–14. doi: 10.1037//0003-066x.55.1.5. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–8. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences. 2009;13:334–40. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Steele JD, Mwangi B, et al. The insular cortex and the neuroanatomy of major depression. Journal of Affective Disorders. 2011;133:120–7. doi: 10.1016/j.jad.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Springer KW, Hauser RM. An assessment of the construct validity of Ryff's Scales of Psychological Well-Being: method, mode, and measurement effects. Social Science Research. 2006;35:1080–102. [Google Scholar]
- Urry HL, Nitschke JB, Dolski I, et al. Making a life worth living: neural correlates of well-being. Psychological Science. 2004;15:367–72. doi: 10.1111/j.0956-7976.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- von Gunten A, Fox NC, Cipolotti L, Ron MA. A volumetric study of hippocampus and amygdala in depressed patients with subjective memory problems. Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12:493–8. doi: 10.1176/jnp.12.4.493. [DOI] [PubMed] [Google Scholar]
- Waterman AS. Two conceptions of happiness—contrasts of personal expressiveness (eudaimonia) and hedonic enjoyment. Journal of Personality and Social Psychology. 1993;64:678–91. [Google Scholar]
- Wood AM, Joseph S. The absence of positive psychological (eudemonic) well-being as a risk factor for depression: a ten year cohort study. Journal of Affective Disorders. 2010;122:213–7. doi: 10.1016/j.jad.2009.06.032. [DOI] [PubMed] [Google Scholar]