Abstract
Data from developmental psychology suggests a link between the growth of socio-emotional competences and the infant's sensitivity to the salience of social stimuli. The aim of the present study was to find evidence for this relationship in healthy adults. Thirty-five participants were recruited based on their score above the 85th or below the 15th percentile of the empathy quotient questionnaire (EQ, Baron-Cohen and Wheelwright, 2004). Functional magnetic resonance imaging (fMRI) was used to compare neural responses to cues of social and non-social (monetary) reward. When compared to the high-EQ group, the low-EQ group showed reduced activity of the brain s reward system, specifically the right nucleus accumbens, in response to cues predictive of social reward (videos showing gestures of approval)—but increased activation in this area for monetary incentives. Our data provide evidence for a link between self-reported deficits in social proficiency and reduced sensitivity to the motivational salience of positive social stimuli.
Keywords: NAcc, social interest, empathy, social reward, autism
INTRODUCTION
Humans differ in their ability to negotiate social situations. Whereas some effortlessly pick up verbal and non-verbal social signals, others struggle with this task, which can affect how they are perceived by others. The identification of many social signals depends on the capacity to experience empathy. Although empathy is a multi-facet construct and multiple different definitions exist (see Davis, 1994; Wiseman, 1996; Decety and Jackson, 2004), many psychological theorists (e.g., Deutsch and Madle, 1975; Davis, 1983) divide empathy into a cognitive component (i.e. the ability to use social cues to identify the mental states of other persons) and an emotional (or affective) component (i.e. the tendency to react emotionally to the emotions of others). Both components are prerequisites to successful social interaction. Social communication typically includes subtle (often non-verbal) social signals, such as facial expressions. Cognitive empathy allows one to pick up these cues and interpret them according to one’s own theory of mind. Emotional empathy (or emotional reactivity; Lawrence et al., 2004) is not necessarily required to understand social situations, but it is believed to be helpful, and individuals failing to demonstrate (appropriate) emotional reactions during a conversation are likely to be perceived as cold or indifferent.
A growing literature associates the ability to experience empathy with depth of social interest, beginning as early as infancy. Social interest refers to the motivation to look at others, to be with others or to relate with others on a personal level (Grelotti et al., 2002; Chevallier et al., 2012). Developmental psychology suggests that early individual differences in social interest might be responsible for later differences in social proficiency (Dawson et al., 2005; Schultz, 2005; Chevallier et al., 2012). Specifically, it has been argued that children who show a greater interest in social information (e.g. caregiver’s face) are better able to recognize social signals (eye gaze, emotional expressions) as cues of future events, and that this mechanism helps them to develop distinct representations of self and others (Vaughan Van Hecke et al., 2007; Parlade et al., 2009). At the opposite extreme, diminished social interest is thought to play an important role in the social disconnection typical of children with autism spectrum disorders (ASD; Kohls et al., 2013; Schultz et al., 2000; Grelotti et al., 2002; Dawson et al., 2005; Schultz, 2005; Chevallier et al., 2012; Dawson et al., 2012). Recent data indicate that social reward processing deficits in individuals with ASD may be associated with abnormal patterns of brain activity, particularly in brain areas involved in processing rewards (Kohls et al., 2013; Scott-Van Zeeland et al., 2010; Dichter et al., 2011; Kohls et al., 2011; Dichter et al., 2012; Lin et al., 2012).
In contrast, little is still known about how social interest relates to social proficiency in typically developed individuals. Kohls et al. (2009) found preliminary evidence in children for a correlation between performance during a social reward task and levels of empathy (Kohls et al., 2009). Sims et al. (2012) demonstrated that associating reward with different faces in a conditioning paradigm amplified the extent to which participants automatically mimicked happy facial expressions with their own face (facial mimicry). Interestingly, this effect was restricted to individuals with high levels of social proficiency, suggesting a link between reward processing and empathic reaction that is modulated by individual differences in social proficiency (Sims et al., 2012). Here, we set out to explore if evidence for a relationship between sensitivity to social reward and social proficiency can be identified at brain level. Sensitivity to the rewarding component of social stimuli has been found to be reflected by activation of the mesolimbic reward system during viewing or anticipation of social stimuli (Aharon et al., 2001; Spreckelmeyer et al., 2009; Strathearn et al., 2009; Rademacher et al., 2010). Moreover, during perception of positive social stimuli (happy faces), activation of the reward system, i.e. the ventral striatum, has been shown to be correlated to individual trait empathy in adults (Chakrabarti et al., 2006). To our knowledge, no imaging study has yet tested for a link between activation of the reward system during anticipation of social stimuli and social proficiency. Here, we addressed this question by comparing neural responses in individuals rated high vs low in empathy during processing of cues that are predictive of social vs non-social (monetary) reward. Our primary index of empathy was the empathy quotient (EQ; Baron-Cohen and Wheelwright, 2004), a scale that attempts to quantify self-experienced social proficiency based on self-assessment with regard to both components of empathy (labeled cognitive empathy and emotional reactivity) as well as social skill.
The main target of our functional brain activation analysis was the nucleus accumbens (NAcc). The NAcc has been found to be recruited by cued anticipation of different types of reward, including social reward (Knutson et al., 2001a; O'Doherty et al., 2002; Kirsch et al., 2003; Knutson et al., 2003; Rademacher et al., 2010; Spreckelmeyer et al., 2012). Extent of NAcc activation during reward anticipation was found to increase with expected reward value (Knutson et al., 2001a; Spreckelmeyer et al., 2009) and to vary as a function of subjective preference (O'Doherty et al., 2006; Kim et al., 2007; Clithero et al., 2011). Therefore, NAcc activation in response to reward-predicting cues can be interpreted as an indicator of the reward’s motivational salience, possibly reflecting the individual’s positive affect at the prospect of gaining that particular reward (Knutson and Greer, 2008). With regard to our study, we hypothesized that individuals with high EQ are more likely to be positively aroused by the prospect of perceiving a social reward than individuals with low EQ level, and therefore show higher reward-related neural response in the NAcc. To maximize the reward value and the ecological validity of the social reward stimuli, we used video clips of individuals showing strong signs of social approval.
Previous imaging results from individuals with ASD suggest aberrant reward processing across a variety of incentive types (Kohls et al., 2013). However, for individuals without ASD, there is little evidence that limited social proficiency is associated with a diminished response to rewards. To test if EQ score selectively modulated reward processing of social cues, we included monetary reward as a comparison condition.
EXPERIMENTAL PROCEDURES
Participants
Thirty-five healthy men were included in the study, 15 of whom constituted the high-EQ group [mean age (±s.d.) = 23.47 ± 2.33 years] and 20 the low-EQ group [mean age (±s.d.) = 24.70 ± 5.96 years]. The study sample was constrained to men to control for sex effects that are evident in trait empathy (Baron-Cohen and Wheelwright, 2004). Additionally, it was requisite to self-identify as heterosexual, so that variance with regard to individual preference of the social stimuli was reduced (all social videos depicted females). All participants were selected based on their score on a shortened Internet version of the EQ questionnaire (German version of the EQ; (Baron-Cohen and Wheelwright, 2004). Participants were selected from a total sample of 464 men who responded to study advertisements (i.e. flyers, hand-outs and online ads). Participants scoring below the 20th and above the 80th percentile in the short version of the questionnaire were invited to the laboratory, where their EQ was validated with the full version of the EQ questionnaire. The cut-off values of EQ ≥50 for individuals in the high-EQ group and <30 for the low-EQ group, respectively, were selected to ascertain individuals ranking below the 15th or above the 85th percentile within the population (Baron-Cohen and Wheelwright, 2004).
The mean EQ (±s.d.) of the high-EQ group was 57.27 ± 6.49 (range 50–72); the mean EQ of the low-EQ group was 21.05 ± 6.70 (range 8–29). All participants were right-handed non-smokers who had no neurological or psychiatric history. None of the participants were taking psychotropic medication or had any neurological or psychiatric history [as assessed by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM IV) personality disorders]. Two participants in the low-EQ group scored above the clinical cut-off score for ASD of 32 on the autism quotient questionnaire (AQ) and had previously undergone clinical examination for ASD without fulfilling the diagnostic criteria (according to DSM IV). The study was approved by the Ethics Committee of the Medical Faculty of the RWTH Aachen University. Participants gave written informed consent and were compensated for their participation.
Personality questionnaires
The EQ questionnaire (Baron-Cohen and Wheelwright, 2004) consists of 60 questions (40 related to empathy and 20 filler items). Extensive research has revealed a continuous distribution of EQ in both Western (Lawrence et al., 2004; Wakabayashi et al., 2007; Berthoz et al., 2008; Von Horn et al., 2010) and Eastern societies (Wakabayashi et al., 2007; Kim and Lee, 2010), with men typically scoring lower than women (Baron-Cohen and Wheelwright, 2004; Wakabayashi et al., 2007; Berthoz et al., 2008; Von Horn et al., 2010), and a high likelihood for a diagnosis of ASD in individuals scoring extremely low (Baron-Cohen and Wheelwright, 2004; Wakabayashi et al., 2007). It may be subdivided into three subscales (i) ‘cognitive empathy’, covering the cognitive component of empathy (see Introduction); (ii) ‘emotional reactivity’, covering the emotional component of empathy; and (iii) ‘social skills’, giving an estimation of self-assessed social competence (Lawrence et al., 2004).
To get a more comprehensive picture of personality differences between the two groups, all participants also completed a questionnaire battery including the following instruments: the alexithymia questionnaire (Toronto Alexithymia Scale (TAS)-20; Bagby et al., 1994), the AQ (Baron-Cohen et al., 2001), the rejection sensitivity questionnaire (Downey and Feldman, 1996; German version by Staebler et al., 2010), the temperament and character inventory (TCI; Cloninger et al., 1994) and the Lubben social network scale (LSNS-R; Lubben and Gironda, 2004).
The LSNS-R was originally developed to screen for social isolation among older adults but has been found to be useful in a wide range of research and clinical settings for all age-groups. The LSNS-R includes six items concerning kinship and non-kin ties, asking for the quantity and quality of social contacts/attachments among relatives, friends and neighbors. LSNS-R includes questions like ‘How many [e.g. friends] do you see or hear from at least once a month?’, ‘How many [e.g. friends] do you feel at ease with that you can talk about private matters?’ or ‘If a [e.g. friend] needs to make an important decision, how often are you asked for advice?’. Separate scores were calculated for the categories ‘friends’ and ‘family’.
Films facial expression recognition test
Deficits in social processing, especially in ASD, have been associated with difficulties in facial expression recognition (Schneider et al., in press; Barton et al., 2007; Schneider et al., 2012; Tanaka et al., 2012; Weigelt et al., 2012). To account for this possibility, all participants completed the ‘Films facial expression recognition test’ (Banissy et al., 2011). In this task, participants are required to match one of three facial expressions to a previously presented emotional adjective (e.g. ‘disdainful’, ‘pleased’ or ‘uneasy’). Performance was assessed through accuracy and response time.
FMRI study design
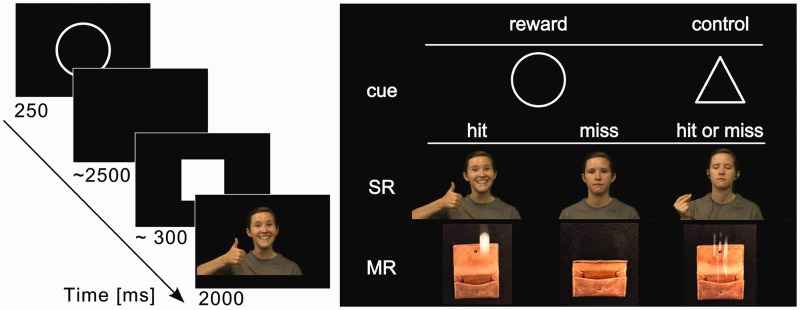
The functional magnetic resonance imaging (fMRI) experiment consisted of a monetary and social incentive delay task (Figure 1) modified from Spreckelmeyer et al. (2009). The main alteration to the original version was the utilization of dynamic stimuli, i.e. video clips, rather than static pictures, to create a more naturalistic and ecologically valid context for the task. The experiment was tailored to examine the neural correlates of reward anticipation based on the work by Knutson et al. (see Knutson and Greer, 2008, for a review). Immediately after seeing a visual cue, participants had to respond within a certain time window to gain reward. Cues informed the participant whether reward could be gained (reward trials, prompted by a circle) or not (control trials, prompted by a triangle). In the social reward condition (SR), a sufficiently fast response (hit) in a reward trial resulted in seeing a video of a woman smiling warmly, nodding her head affirmatively and gesturing social approval by making a ‘thumbs up’ gesture with her right hand (approval mode, see exemplary stimuli in Supplementary Material Supplement 1). If the participant reacted too slowly (miss), the video showed a woman with a neutral facial expression who just looked at the participant without showing any gestures (neutral-face mode). In social control trials, the outcome video showed a woman wearing headphones who had her eyes closed and snapped her fingers to an imaginary beat (listening-to-music mode). This scenario was chosen as control stimulus because the amount of body movement is comparable with that of the social reward clips. Yet, the reward value is diminished, because it is obvious to the participant that the person on the video is disengaged.
Fig. 1.
Experimental design. Cues (circle, triangle) determined the possible outcome of trials in the two outcome conditions: social reward (SR) or monetary reward (MR). If in a reward trial (circle), the response to the target (square) was fast enough (hit), the participant was presented with a video of a woman showing signs of social approval (SR condition) or of money falling into a wallet (MR condition). Misses in reward trials resulted in neutral outcome videos (SR: neutral face/ MR: empty wallet). In control trials (triangle), both hits and misses resulted in seeing a control video (SR: woman with eyes closed and listening to music/MR: confetti falling into a wallet).
In the monetary reward condition (MR), hits were followed by a video of two euro coins dropping into an opened wallet, whereas misses were followed by a video clip of a slowly closing empty wallet. In control trials, outcome consisted of a video showing confetti falling into an open wallet. Participants were informed that their performance had no (positive or negative) influence on the compensation they received for participation.
SR and MR condition were presented in two separate blocks, with block order pseudo-randomized and counter-balanced across participants. At the beginning of the experiment, the participants were informed about the order of conditions. In both outcome conditions (SR or MR), receiving a reward in the reward trials (n per condition = 40) depended on participants’ ability to respond quickly enough to a cued target symbol (white square). Performance was irrelevant in the control trials (n per condition = 40). However, participants were encouraged to respond as fast as possible to all cued targets.
To ensure an approximately equal number of positive outcome events (i.e. reward) across subjects, task performance level was standardized to approximate an equal number of hits in about two-thirds of the reward trials for all participants. To that end, the time interval to respond to the target was adjusted to individual reaction times dynamically, trial-by-trial. Starting from the individual’s mean reaction time during the practice session before scanning, target duration was shortened by 20 ms every time the participant gained three hits in a row, or extended by 20 ms when two consecutive trials resulted in a miss. Thus, performance level was kept comparable across individuals (at ∼62%, see Table 1) by increasing or lowering difficulty in a step-wise manner, depending on individual’s ongoing performance. Measures of reaction times provided an estimate of motivation for the different trial types.
Table 1.
Mean scores (+s.d.) of personality instruments for both groups and results of statistical comparison [unpaired t-test, Bonferroni-corrected: *P(corr.) < 0.05, **P (corr.) < 0.01] for the EQ with its subscales, AQ, alexithymia, rejection sensitivity, TCI subscores, social networks (using LSNS) and performance on the FFERT
| Personality instrument | High-EQ (n = 15) |
Low-EQ (n = 20) |
P (two-tailed) | η2 | ||
|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | |||
| EQ | 57.27 | 6.49 | 21.05 | 6.70 | <0.0001** | 0.884 |
| Cognitive empathy | 21.00 | 3.55 | 5.65 | 4.16 | <0.0001 ** | 0.801 |
| Emotional reactivity | 17.80 | 2.96 | 5.50 | 2.44 | <0.0001** | 0.844 |
| Social skills | 11.67 | 1.95 | 4.70 | 2.62 | <0.0001** | 0.703 |
| AQ | 14.47 | 4.45 | 24.8 | 7.85 | <0.0001** | 0.393 |
| Alexithymia | 31.47 | 10.68 | 49.15 | 8.89 | <0.0001** | 0.46 |
| Rejection sensitivity quotient | 7.45 | 2.98 | 9.36 | 3.37 | 0.09 | 0.079 |
| TCI | ||||||
| Reward dependence | 54.33 | 10.38 | 34.80 | 10.12 | <0.0001** | 0.527 |
| Novelty seeking | 43.6 | 10.39 | 42.55 | 12.28 | 0.79 | 0.005 |
| Harm avoidance | 24.27 | 13.1 | 39.95 | 16.19 | <0.01* | 0.211 |
| Persistence | 52.80 | 11.61 | 47.20 | 13.79 | 0.21 | 0.046 |
| Self-directedness | 61.93 | 12.12 | 50.85 | 16.90 | 0.04 | 0.12 |
| Cooperativeness | 69.67 | 10.08 | 50.40 | 9.85 | <0.0001** | 0.486 |
| LSNS | ||||||
| Friendships | 29.13 | 2.00 | 22.50 | 5.05 | <0.0001** | 0.378 |
| Family | 42.20 | 19.61 | 33.80 | 17.01 | 0.19 | 0.048 |
| FFERT (% hit) | 83.68 | 6.42 | 84.84 | 5.08 | 0.56 | 0.011 |
| FFERT reaction time hits (ms) | 459.95 | 155.57 | 432.97 | 184.10 | 0.65 | 0.005 |
FFERT, Films facial expression recognition test.
Stimulus presentation and recording of reaction times were performed using the software Presentation (Neurobehavioral Systems, Inc., San Francisco, CA). Participants indicated their response by pressing the button of a fiber-optic response box with the index finger of their right hand. Before entering the scanner, participants performed a practice session composed of six trials per condition (SR, MR). After scanning, participants rated the perceived reward value of the monetary and the social videos on a 7-point scale from ‘not rewarding at all’ to ‘very rewarding’.
Experimental design
Figure 1 depicts the details of the experimental setup. The monetary reward condition (MR) and the social reward condition (SR) were presented blockwise, with order of blocks counter-balanced across participants. For both reward types (SR and MR), each trial started with the presentation of one of the two cues for 250 ms, followed by a delayed anticipation period for a variable length of time (jittered between 2250 and 2750 ms), followed by the target (individually adjusted presentation time between 150 and 450 ms). Outcome videos were presented for 2000 ms, starting 500 ms after target onset. Cue types (n per condition = 40) were pseudo-randomly ordered within SR and MR blocks, each with inter-trial intervals jittered between 2500 and 5000 ms, resulting in a mean trial duration of ∼9400 ms, and also including a 12-s pause after 40 trials.
Outcome stimuli
In the SR condition, videos of 20 female actors in three different gesture modes (i.e. 60 videos) served as outcome stimuli (see exemplary stimuli in Supplementary Material Supplement 1). The order of photos from different actors was pseudo-randomized to avoid anticipation effects. The video stimuli were selected from a large set of validated videos recorded and evaluated at the Center for Autism Research, The Children’s Hospital of Philadelphia (G.K., R.T.S.). Video clips in the MR condition were generated by author A.G. and processed using Photoshop Professional and AviDemux 2.5 (http://www.avidemux.org/). Selection of video stimuli for the present study was based on two previous experiments where male subjects were asked to rate how authentic they thought the women on the social video clips behaved and how rewarding the SR and MR video clips were perceived, respectively (for detailed explanation, see Supplementary Material Supplement 2). Based on these ratings, SR and MR videos were matched for their reward value. This procedure resulted in a final set of 60 video clips [i.e. 20 different actors showing three different modes of action: approval (= hits), neutral face (= miss) and listening to music (= control) in the SR condition, and a set of three MR videos (hit, miss and control)].
Data acquisition and analysis
Behavioral data
Reaction times were analyzed using a repeated-measures analysis of variance (rm-ANOVA) with ‘condition’ (SR vs MR) and ‘cue type’ (reward cue vs control cue) as the within-subjects factor, and ‘group’ (high-EQ vs low-EQ) as the between-subjects factor.
Subjectively perceived reward value of the outcome stimuli was derived from post-scan ratings and analyzed in a 2 × 3 × 2 rm-ANOVA with the factors ‘condition’ (SR vs MR), ‘outcome’ (hit, miss or control) and ‘group’ (high-EQ vs low-EQ). The Huynh–Feldt epsilon correction was used to correct for non-sphericity. Paired t-tests were used for post hoc analyses. Effect sizes are reported using partial η2.
Group differences in self-report measures (TCI subscales, alexithymia, AQ and LSNS subscores family and friendship) were tested using unpaired Student t-tests.
fMRI data
Images were acquired on a 3 Tesla Siemens® Trio MR scanner at the RWTH Aachen University, equipped with echo planar imaging capabilities using a standard head coil for radio frequency transmission and signal reception. For each participant, two series of 360 echo planar imaging scans covering the whole brain were acquired, with each series lasting ∼12 min (36 3.5 mm isotropic slices; interslice gap = 0.35 mm; matrix size = 64 × 64; field of view = 224 mm; repetition time = 2.2 s; echo time = 30 ms; flip angle = 90°).
Images were preprocessed and analyzed using Statistical Parametric Mapping (SPM8; University College, London). The first three volumes were discarded to allow for T1 equilibration effects. Functional images of both tasks were realigned, normalized and resliced to a final voxel size of 2 × 2 × 2 mm, and smoothed with an isotropic 6-mm full-width half-maximum Gaussian kernel. Before model estimation, a high-pass temporal filter of 128 s was applied (Ashburner and Friston, 2005).
A mixed effects General Linear Model (Friston et al., 1995; Josephs et al., 1997) was used for a two-level statistical analysis. In the first stage, blood-oxygen-level-dependent responses were modeled by delta functions at stimulus onset, which were then convolved with a standard hemodynamic response function. For each subject, the SR and the MR condition were modeled individually with five regressors. Anticipation was modeled by convolving a delta function (at anticipation onset = presentation of the cue) for each of the two cue types, and outcome was modeled by convolving the delta function (outcome onset = start of the video) for each of three possible feedback conditions (hit, miss and control). Thus, 10 regressors were modeled for each subject: four for the anticipation phase (social reward, social control, monetary reward and monetary control) and six for the outcome phase (hit, miss or control video) in the SR or MR condition. In addition, six movement parameters derived from the realignment procedure were included as additional regressors, as well as one regressor of no interest, modeling the duration of the pause between the SR and the MR block.
To address the question if incentive salience processing can be linked to individual social proficiency, the second-level analysis was constrained to the anticipation phase. We defined the onset of the anticipation phase as the time point when the cue appears on the screen to inform the participant about the potential outcome of the trial. A 2 × 2 × 2 full-factorial design for voxel-wise comparisons across subjects was applied, including the factors ‘group’ (high-EQ vs low-EQ), ‘condition’ (SR vs MR) and ‘cue type’ (reward cue vs control cue). Results of the whole-brain random effects analyses were corrected for multiple comparisons [family-wise error corrected, P (FWE) <0.05] on cluster level by setting a minimal cluster size of 220 continuous voxel on P(unc.) <0.001 voxel level [cluster-size was determined using the CorrClusTh-program (http://www.sph.umich.edu/∼nichols/JohnsGems5.html)]. Coordinates of significant local maxima are reported in a standard stereotaxic reference space (Montreal Neurological Institute), and functional overlays are displayed on the Montreal Neurological Institute template image.
In addition, and in accordance with our hypothesis that group effects would modulate the fMRI blood-oxygen-level-dependent response in the NAcc, small volume corrections (FWE-corrected P < 0.05) were performed for a priori defined regions of interest (ROI) of the right and left NAcc. The combined ROI mask of the bilateral NAcc was defined based on anatomical masks of the right and left NAcc (derived from the Harvard-Oxford Atlas) and applied to the data using the ROI toolbox of the WFU pickatlas utility (Lancaster et al., 2000; Maldjian et al., 2003).
To understand the nature of potential interaction effects, statistical analyses of fMRI activations were conducted on parameter estimates extracted from peak voxels. A 2 × 2 × 2 rm-ANOVA with factors ‘condition’ (SR vs MR), ‘cue type’ (reward cue vs control cue) and ‘group’ (high-EQ vs low-EQ) was performed in the Statistical Package for the Social Sciences (SPSS 11), followed by post hoc t-tests.
Behavioral results
Personality scores
The group-specific means and standard deviations of the questionnaires are listed in Table 1. Groups differed significantly on all three subfactors of the EQ, with the low-EQ group scoring lower than the high-EQ group with regard to ‘cognitive empathy’, ‘emotional reactivity’ and ‘social skill’ (all P < 0.001). Furthermore, the low-EQ group reported more difficulties describing own and others’ feelings (i.e. high alexithymia scores), scored lower on TCI (social) reward dependence and cooperativeness, but scored higher on the AQ (all P < 0.001) and the TCI harm avoidance scale (P < 0.01). No significant group differences were found with regard to social rejection sensitivity, or on the TCI dimensions novelty-seeking, persistence and self-directedness. With regard to the social network scale, significantly lower values in the friendships category were reported by the low-EQ than the high-EQ group (P < 0.001), indicating that subjects with low EQ establish fewer and less intimate peer relations. No such difference was observed for family bonds.
Films facial expression recognition test
Groups did not differ in their accuracy or reaction time on the Films facial expression recognition test (P > 0.05, see Table 1).
Incentive delay task - behavioral performance
Subjects were successful in responding quickly enough to the target symbol, pressing the button too early or not at all in <4% of all trials in both SR and MR conditions in both groups (Table 2). The adaptive manipulation of the hit rate (i.e. individual adjustment of the target time window) resulted in similar hit rates (of ∼62%) in both the SR and MR condition in both groups (Table 2, all P > 0.05).
Table 2.
Behavioral data
| Measures | High-EQ (n = 15) |
Low-EQ (n = 20) |
||||||
|---|---|---|---|---|---|---|---|---|
| Social |
Monetary |
Social |
Monetary |
|||||
| Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | |
| Button press on target (%) | 97.0 | 2.7 | 96.5 | 3.2 | 97.6 | 2.6 | 96.9 | 4.3 |
| Hit rate reward (%) | 62.8 | 3.0 | 61.7 | 2.8 | 62.1 | 3.3 | 61.6 | 4.6 |
| Hit rate control (%) | 46.5 | 13.1 | 45.2 | 11.9 | 49.2 | 15.7 | 55.4 | 17.9 |
| RT reward (ms) | 259.4 | 21.0 | 232.4 | 24.9 | 264.6 | 18.7 | 244.9 | 24.8 |
| RT control (ms) | 285.0 | 31.2 | 244.1 | 17.8 | 286.1 | 23.1 | 249.7 | 24.8 |
| Rating hit | 6.1 | 0.9 | 5.3 | 1.3 | 5.1 | 1.0 | 5.2 | 1.1 |
| Rating miss | 1.5 | 0.5 | 1.1 | 0.3 | 1.9 | 0.7 | 1.4 | 0.6 |
| Rating control | 2.3 | 1.0 | 3.0 | 1.7 | 2.3 | 0.8 | 3.4 | 1.5 |
Mean values (+s.d.) of hit rates, reaction times (RT) and stimulus rating for both tested groups.
Reaction times for hits
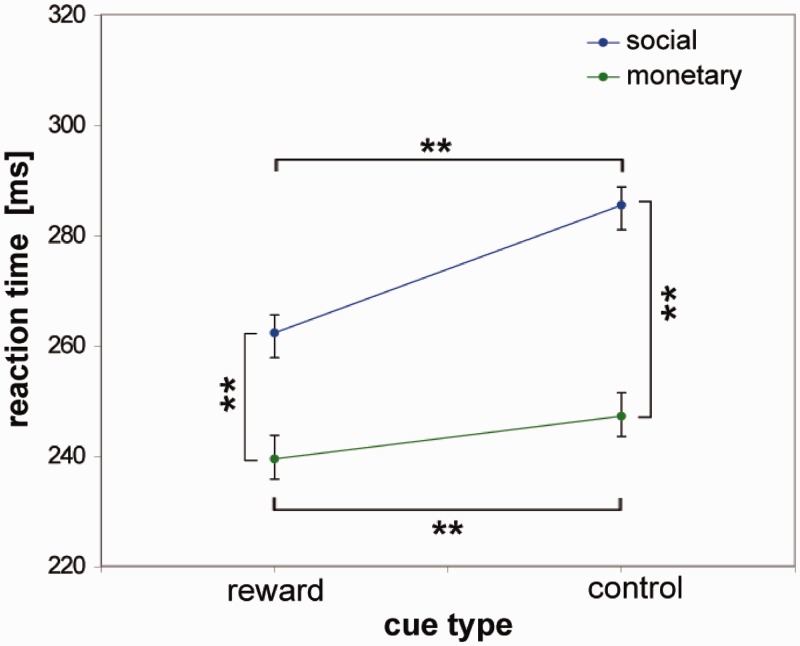
Statistical analysis of reaction times showed a main effect of ‘cue type’ [F(1, 33) = 43.73; P < 0.001; η2 = 0.57], reflecting faster reaction times for potential reward outcome as compared with control outcome (Figure 2, P < 0.001). Further, a main effect of ‘condition’ was found [F(1, 33) = 100.63; P < 0.001; η2 = 0.75], reflecting faster reaction times if a monetary outcome video was anticipated than if a social outcome video was expected (P < 0.001). The interaction of ‘condition × cue type’ [F(1, 33) = 15.50; P < 0.001, η2 = 0.32] indicated that although responses were faster for reward than control trials for both conditions (slower reactions to social control cues than to monetary control cues [286 (±26) vs 247 (±22) ms, P < 0.001] and to social reward cues than to monetary reward cues [262 (±20) vs 240 (±25) ms, P < 0.001]), the reaction time difference was more pronounced in the social than in the monetary condition. No main effect of ‘group’ [F(1, 33) = 0.82; P = 0.37] or interaction effects were found [‘group × condition’: F(1, 33) = 0.91; P = 0.35, ‘group × cue type’: F(1, 33) = 1.34; P = 0.25 and ‘group × condition × cue type’: F(1, 33) = 0.14; P = 0.71].
Fig. 2.
Reaction times. Responses in hit trials were faster in the monetary than the social condition, and in response to reward cues relative to control cues (**P < 0.01). An interaction of ‘condition × cue type’ indicated that reaction time differences of reward and control cued trials were more pronounced for the SR than the MR condition.
Rating of subjectively perceived value of outcome stimuli
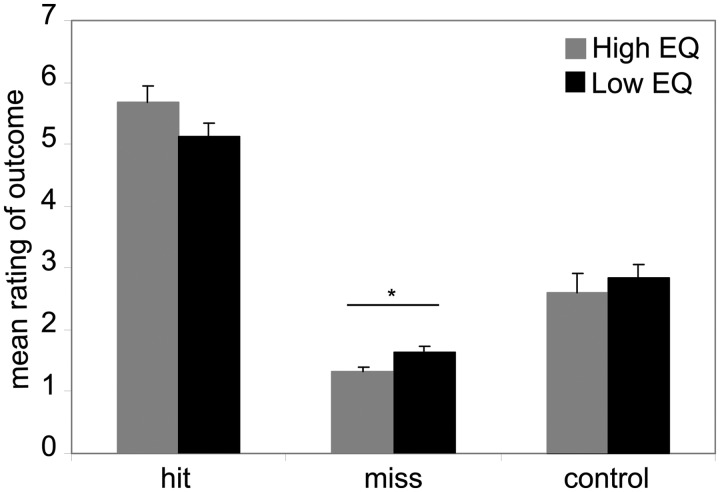
A three-way ANOVA on mean ratings of the individual video clips revealed a main effect of ‘outcome’ (hit, miss and control) [F(2, 66) = 247.97, P < 0.001; η2 = 0.88]. Hit clips (social approval video or money falling into wallet) were perceived as significantly more rewarding than miss clips (neutral face video or empty wallet; P < 0.001) or control stimuli (listening-to-music-video or confetti falling into wallet; P < 0.001), independent of condition (SR or MR). No main effects of the factors ‘group’ or ‘condition’ were found (both P > 0.05). Mean ratings for the social hit-outcome videos did not significantly differ from ratings of the monetary hit-outcome videos (P > 0.05). However, miss outcome in the MR condition was rated as less rewarding than in the SR condition (P < 0.001), and control outcome in the SR condition was rated less rewarding than in the MR condition (P < 0.001) [‘condition × outcome’ interaction: F(2, 66) = 21.82, P < 0.001; η2 = 0.40]. A ‘group × outcome’ interaction [F(2, 66) = 3.54, P = 0.035; η2 = 0.10] could be linked to group differences for rating the miss videos. High-EQ participants rated miss videos as less rewarding than low-EQ participants (P = 0.012, Figure 3). No three-way interaction of ‘group × outcome × condition’ [F(2, 66) = 2.28, P = 0.11] was detected.
Fig. 3.
Mean rating (+ SEM) of subjectively perceived reward value of the outcome options ‘hit’, ‘miss’ and ‘control’ for both conditions for each group (high EQ = gray, low EQ = black). The interaction effect of ‘group × outcome’ [F(2, 66) = 3.54, P = 0.035 < 0.05] originates from a significantly different rating score between groups only for the miss videos [*P (two-sided) < 0.05].
Imaging results
Main effects of cue type (anticipated reward vs control)
Whole-brain analyses of the main effect of cue type (i.e. reward vs control, collapsed across MR and SR conditions) revealed several brain areas that were recruited during reward anticipation, including bilateral NAcc, midbrain/ventral tegmental area, thalamus, left insula and left amygdala [P (FWE) < 0.05, see Table 3].
Table 3.
Brain activations during anticipation phase, whole-brain analysis, full-factorial ANOVA, P (FWE) < 0.05 cluster corrected for multiple comparisons
| Cluster P(FWE) | Cluster size | Peak Z | x | y | z | Region | BA | R/L |
|---|---|---|---|---|---|---|---|---|
| Main effect of reward value | ||||||||
| Reward > control | ||||||||
| <1.0e-020 | 4716 | 6.1 | 8 | 0 | 66 | Supplementary motor area | 6 | R |
| 5.8 | −8 | −2 | 66 | Supplementary motor area | 6 | L | ||
| 5.4 | 16 | −16 | 66 | Superior frontal lobule | 6 | R | ||
| 0.0014 | 482 | 5.6 | 16 | 6 | −10 | Nucleus accumbens/amygdala | R | |
| 0.0002 | 678 | 4.8 | −6 | −16 | 0 | Thalamus | L | |
| 4.5 | 6 | −16 | 0 | Thalamus | R | |||
| 4.4 | −8 | −16 | −18 | Midbrain | L | |||
| 0.0065 | 363 | 4.7 | −18 | 0 | −10 | Amygdala | L | |
| 4 | −14 | 8 | −4 | Pallidum/nucleus accumbens | L | |||
| 3.4 | −6 | −4 | −16 | Brain stem | L | |||
| 0.0069 | 359 | 4.6 | −30 | 26 | 0 | Insula | L | |
| 3.7 | −46 | 6 | 2 | Insula | 44 | L | ||
| 3.3 | −46 | 10 | -6 | Insula | L | |||
| 0.0302 | 253 | 4.2 | −8 | −58 | 62 | Precuneus | L | |
| 3.6 | −28 | −54 | 60 | Superior parietal lobule | L | |||
| 3.5 | −16 | −70 | 54 | Superior parietal lobule | L | |||
| 0.0335 | 246 | 4.2 | 52 | −4 | 52 | Middle frontal gyrus | 6 | R |
| 4.1 | 48 | 0 | 44 | Precentral gyrus | 6 | R | ||
| Social reward > social control (both groups) | ||||||||
| 2.89e-015 | 3968 | 5.4 | 8 | 0 | 66 | Supplementary motor area | 6 | R |
| 5.2 | 20 | −20 | 66 | Precentral gyrus | 6 | R | ||
| 5.1 | −8 | −2 | 66 | Supplementary motor area | L | |||
| 328 | 5 | 14 | 4 | −12 | Nucleus accumbens | R | ||
| 363 | 4.6 | −8 | −18 | −18 | Midbrain | L | ||
| 4.4 | −4 | −18 | 0 | Thalamus | L | |||
| 4 | 6 | −16 | 0 | Thalamus | R | |||
| 232 | 4 | −32 | 28 | 0 | Insula | L | ||
| 3.8 | −36 | 16 | 4 | Insula | L | |||
| 3.7 | −32 | 24 | 10 | Insula | L | |||
| Monetary reward > monetary control (both groups) | ||||||||
| 0.0016 | 475 | 4.1 | 4 | −4 | 64 | Supplementary motor area | 6 | R |
| 3.8 | −6 | −2 | 64 | Supplementary motor area | 6 | L | ||
| 3.5 | 12 | −2 | 74 | Supplementary motor area | 6 | R | ||
| Main effect of feedback type | ||||||||
| Social > money | ||||||||
| 1.16e-005 | 931 | 6.1 | 50 | −36 | 6 | Superior temporal gyrus | R | |
| 5.4 | 50 | −26 | −6 | Middle temporal gyrus | R | |||
| 3.7 | 48 | −16 | −16 | |||||
| 2.78e-006 | 1082 | 5.5 | −16 | −92 | −6 | Middle occipital gyrus | V3 | L |
| 5.4 | −28 | −90 | −8 | Inferior occipital gyrus | V4 | L | ||
| 4.9 | −24 | −96 | 2 | Inferior occipital gyrus | V3 | L | ||
| 3.31e-006 | 1063 | 5.2 | 20 | −88 | −8 | Lingual gyrus | 18 | R |
| 5 | 10 | −90 | 16 | Cuneus | 18 | R | ||
| 4.4 | 36 | −82 | −10 | Inferior occipital gyrus | 18 | R | ||
| 0.0001 | 681 | 5.2 | 48 | 2 | 46 | Precentral gyrus | 6 | R |
| 4.4 | 50 | 16 | 26 | Inferior frontal gyrus (P. opercularis) | 44 | R | ||
| 4.1 | 38 | 16 | 24 | Inferior frontal gyrus (P. triangularis) | R | |||
| Money > social | ||||||||
| 0.0003 | 615 | 6.7 | 10 | −78 | −2 | Lingual gyrus | 18 | R |
| 5 | −8 | −78 | −2 | Lingual gyrus | 18 | L | ||
| 3.4 | −12 | −90 | 4 | Superior occipital gyrus | 17 | L | ||
| Effects of group | ||||||||
| Low EQ > high EQ | ||||||||
| 0.0174 | 291 | 5 | −34 | −70 | −10 | Fusiform gyrus | R | |
| 4.3 | −24 | −88 | −6 | Inferior occipital gyrus | V4 | R | ||
| 4.3 | −32 | −78 | 0 | Middle occipital gyrus | V5 | R | ||
| Interactions | ||||||||
| ‘High > low EQ in social > monetary task’ | ||||||||
| 0.0069 | 359 | 4.5 | −32 | −94 | 12 | Middle occipital gyrus | 19 | L |
| 3.6 | −28 | −88 | −4 | Middle occipital gyrus | L | |||
| 3.5 | −46 | −70 | 8 | Middle temporal gyrus | 39 | L | ||
| ROI analyses | ||||||||
| Interaction in ‘High > low EQ in social > monetary task’ | ||||||||
| 0.0318 | 7 | 3.1 | 14 | 16 | −8 | Nucleus accumbens | R | |
| ROI analyses in single reward contrasts | ||||||||
| High EQ: ‘social reward vs control’ | ||||||||
| 0.0227 | 16 | 4 | 12 | 6 | −10 | Nucleus accumbens | R | |
| 3.4 | 14 | 14 | −8 | R | ||||
| 0.0281 | 10 | 3.6 | −12 | 6 | −8 | Nucleus accumbens | L | |
| High EQ: ‘monetary reward vs control’ | ||||||||
| n.s. | n.s. | |||||||
| Low EQ: ‘social reward vs control’ | ||||||||
| n.s. | n.s. | |||||||
| Low EQ: ‘monetary reward vs control’ | ||||||||
| 0.0366 | 4 | 3.2 | 12 | 8 | −6 | Nucleus accumbens | R | |
BA, Brodmann area; L, left hemisphere; R, right hemisphere.
Main effect of condition (anticipated monetary vs social outcome)
Whole-brain comparisons between anticipated social vs anticipated monetary outcome (i.e. main effect of ‘condition’) showed significantly greater activation of a region spanning the right temporoparietal junction and superior temporal sulcus [peak activation: x = 50, y = −36, z = −6, P (FWE) < 0.05] during anticipation of social compared with monetary outcome (‘social reward – social control > monetary reward – monetary control’, see Table 3). The temporoparietal junction has been implicated in the attribution of mental state information to other people (Van Overwalle and Baetens, 2009), and has previously been found to be activated during social reward anticipation (Spreckelmeyer et al., 2012). Additional clusters were located in the right inferior frontal cortex and in bilateral primary visual fields extending into the cuneus region of the right hemisphere. The reverse contrast, anticipation of monetary compared with social outcome (‘monetary reward – monetary control > social reward – social control’), yielded increased activation of higher-order visual processing areas, i.e. BA 18 and lingual gyrus.
Main effect of group
No main effect of ‘group’ was found in any reward-related brain regions. The only significant group difference was detected in the left visual ventral stream [peak activation: x = −34, y = −70, z = −10, P (FWE) < 0.05] when contrasting low-EQ > high-EQ (see Table 3).
Interaction of condition × group
The only significant interaction on whole-brain level was found in the left visual ventral stream for the contrast ‘high > low EQ in social > monetary condition’ [peak activation: x = −32, y = −94, z = 12, P (FWE) < 0.05].
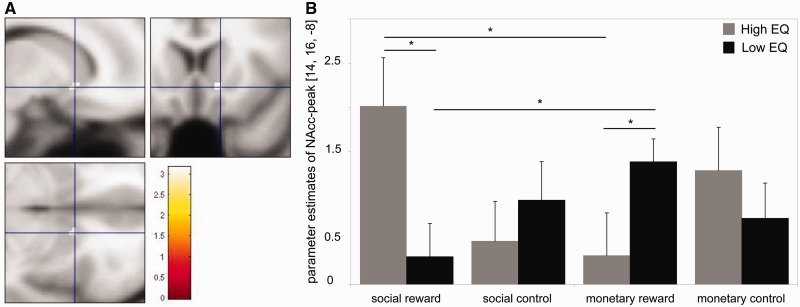
Interaction of condition × cue type × group in the NAcc
At the whole-brain level, no three-way interaction survived our correction threshold. However, in line with our hypothesis, testing for an interaction effect of ‘group × condition × cue type’ in the a priori defined ROI of the bilateral NAcc revealed significant activation in the right NAcc [peak activation: x = 14, y = 16, z = −8, P (FWE) < 0.05; see Table 3 and Figure 4a]. Follow-up-analyses on parameter estimates extracted at peak activation (Figure 4b) revealed that activation in response to cues of social reward was significantly higher in the high-EQ than the low-EQ group (P = 0.011). In contrast, NAcc activation to cues of monetary reward was stronger in the low-EQ than the high-EQ group (P < 0.014). No group differences were observed for control cues (P > 0.05). The direct comparison of NAcc activation during anticipation of social vs monetary reward within each group showed that in the low-EQ group, cues of social reward yielded significantly less activation than cues of monetary reward (P < 0.019), whereas in the high-EQ group, the opposite pattern was observed: cues of social reward elicited a greater response than cues of monetary reward (P < 0.024).
Fig. 4.
Interaction of ‘group × condition × cue type’ shows right NAcc reactivity for anticipated social reward vs. anticipated monetary reward between groups. (A) NAcc activation in ‘high-EQ vs. low-EQ, (social reward – social control) vs. (monetary reward – monetary control)’ axial, coronal and sagittal views [ROI-corrected, P (FWE) < 0.05]. (B) Parameter estimates derived from NAcc peak activation show significant differences between anticipation of social and monetary reward within and between groups (*P < 0.05).
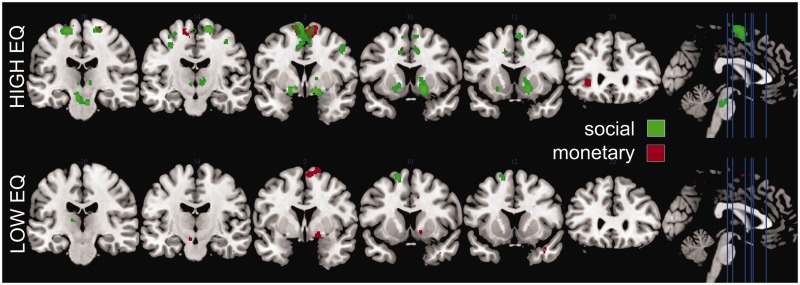
For the interested reader, we included statistical whole-brain maps of the condition-specific reward contrasts for the high-EQ and the low-EQ group, at an uncorrected level of P < 0.001 (Figure 5 and Supplementary Material Supplement 3). Please note that these contrasts are only meant to illustrate differential patterns of activation between conditions within each group but do not necessarily reflect quantifiable differences between conditions or groups.
Fig. 5.
Contrasts of reward anticipation (vs. control) for the high-EQ (top) and the low-EQ (bottom) group on whole-brain level (P < 0.001, uncorrected).
DISCUSSION
The present study provided evidence that individual differences in social proficiency are related to activation differences of reward systems in the brain. In line with our hypothesis, we found a significant group by reward type (by cue type) interaction in the right NAcc, reflecting diminished NAcc activation during processing of cues that were predictive of social reward compared with cues of monetary reward in men with low social proficiency (measured through the EQ), whereas the opposite pattern emerged in the other group: men with a high EQ showed significantly greater NAcc activation during anticipation of social compared to monetary reward.
NAcc activation during reward anticipation has been suggested to reflect positive affect at the prospect of receiving reward (Knutson and Greer, 2008). Further, differences in NAcc activation during anticipation of different types of reward have been interpreted as reflecting varying degrees of incentive salience associated with these rewards (Kirsch et al., 2003; Spreckelmeyer et al., 2009). We therefore suggest that the observed group differences in NAcc activation patterns during anticipation of social vs monetary reward in our sample reflect group-specific differences in the perceived salience to social vs non-social rewards. This finding is in line with our expectation that self-reported social proficiency correlates with sensitivity toward the motivational value of social stimuli.
Data from developmental psychology suggest that early differences in social interest may account for differences in social skills and social competence later in life (Vaughan Van Hecke et al., 2007; Parlade et al., 2009), and that a lack of social interest in young infants may be responsible for severe social impairments in developmental disorders such as ASD (Mundy and Neal, 2000; Schultz et al., 2000; Dawson et al., 2005; Schultz, 2005). Specifically, it has been suggested that the heightened attention paid to social stimuli (i.e. social interest) results in the specialized processing of these stimuli (Schultz et al., 2000; Grelotti et al., 2002; Mundy and Jarrold, 2010). According to this account, reduced social interest results in less specialized processing of social cues and hampers social ability (reviewed by Chevalier et al., 2012 and Dawson et al., 2012). This account predicts that even in adulthood, low social interest is associated with low social proficiency. We provide support for this assumption by showing that reward-related NAcc activation during anticipation of social reward is blunted in individuals with self-reported low social proficiency relative to participants with self-reported high social proficiency.
However, it must be kept in mind that the present findings are correlational. Hence, it cannot be ruled out that the causality is inversed, i.e. that participants with low social proficiency are less motivated to approach social stimuli because of a negative learning trajectory whereby previous social encounters were unrewarding for them (ostensibly as a consequence of their social deficits). However, such an interpretation would predict greater rejection sensitivity in the low-EQ group than the high-EQ group, which was not found in our sample (as measured by the rejection sensitivity questionnaire). Also noteworthy, both groups rated the social stimuli as equally rewarding and showed no difference in behavioral measures of motivation; it is therefore unlikely that participants in the low-EQ group were trying to avoid social stimuli. The fact that groups did not differ in their rating of the outcome stimuli is in discordance with the effects on the neural level. However, ratings of stimuli are more likely to be subject to social desirability effects than neural responses to reward predictive cues. Moreover, preference ratings are believed to capture the ‘liking’ component of face reward processing more than the ‘wanting’ component. Hence, it is possible that individuals with low EQ feel the same pleasure when seeing a smiling face as individuals with high EQ but do not have the same urge to orient to social stimuli.
Whereas NAcc activation to social cues was more pronounced in the high-EQ group, men in the low-EQ group showed greater NAcc activation than men in the high-EQ group during anticipation of monetary incentives. This finding speaks against a general deficit in incentive processing in the low-EQ group. Instead, this interaction indicates that the incentive salience of social stimuli in particular was attenuated in men with low-EQ scores. It has been suggested that insensitivity to the rewarding component of social stimuli may arise from deficits already at the level of face perception (Grelotti et al., 2002). For example, ASD patients show behavioral deficits in face recognition and identification (Klin et al., 1999; Joseph and Tanaka, 2003; Wolf et al., 2008). Although participants in our low-EQ group scored significantly higher than the high-EQ group on the alexithymia scale, indicating difficulties in recognizing their own emotions, they performed equally well in the (emotional) facial expression recognition test. Hence, it seems unlikely that reduced reactivity to cues of social reward followed from difficulties in recognizing the facial expressions of the social stimuli.
The present study has some limitations that should be addressed in future research. One limitation is that we only collected data from individuals scoring at the extreme ends of the EQ dimensions. Future studies need to cover the entire spectrum of social proficiency to corroborate the assumption that social proficiency and social interest are linearly related. Another issue concerns the fact that in our ROI analysis of the NAcc, anticipation of monetary reward did not yield significantly greater activation than anticipation of the monetary control stimuli across groups. This finding is in contrast to other studies on monetary reward anticipation using similar designs (Thut et al., 1997; Knutson et al., 2001b; Spreckelmeyer et al., 2009). One explanation for this result is that in our study, task performance did not affect the amount of money participants could earn. Hence, the motivational value of the monetary stimuli was reduced relative to other studies. Also, and perhaps more importantly, we used dynamic stimuli to represent the outcome for both hit and control outcomes, whereas previous studies typically used static stimuli. It is possible that our dynamic control stimulus was more salient than static baseline stimuli in other studies and therefore less discriminative. Although this finding casts some doubt on the suitability of dynamic stimuli studying monetary reward anticipation, it does not diminish the relevance of our finding that men with a low EQ showed significantly lower NAcc activation during anticipation of social reward than monetary reward, whereas high-EQ men showed the opposite pattern. Another potential limitation of our design was an imbalance in the number of different stimuli presented in the social or monetary condition. Whereas 20 different reward videos were potentially available in the social condition, reward presentation in the monetary condition consisted of only one video. This imbalance in stimulus repetition might have caused different levels of habituation between the two conditions, potentially confounding the results. However, performing an analysis on reaction time changes over time (i.e. mean reaction time of four isochronous intervals throughout the experiment) did not reveal any significant changes between conditions, making differences in habituation unlikely. Finally, our finding of an association between social proficiency and sensitivity to social reward is correlational in nature. Hence, interpretations with regard to the causality of this association have to be treated with caution. Unfortunately, studies that are better equipped to establish causality (i.e. experimentally providing or withdrawing rewarding social stimuli) typically suffer from methodological and ethical limitations. More promising might be the use of computational learning models (e.g. Triesch et al., 2006). Another interesting approach for future studies might be to test if behavioral training of social proficiency alters neural incentive processing of social cues.
Together, our results demonstrate for the first time that individual differences in social proficiency can be linked to differences in neural sensitivity toward the motivational salience of social incentives in typically developing men. Although preliminary, this finding supports the theory that social interest is an important prerequisite for social proficiency (Dawson et al., 2005; Schultz, 2005). The results underscore the importance of identifying deficient social interest in early infancy as potential markers for social communication disorders and/or ASD (Pierce et al., 2011; Elsabbagh et al., 2012).
SUPPLEMENTARY DATA
Supplementary data are available at SCAN Online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This study was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, IRTG 1328), RWTH Aachen medical faculty funding program START (K.N.S., L.W., S.G.), the National Institutes of Health (P30HD026979) (R.T.S., G.K.) and the Robert Wood Johnson Foundation #66727 (R.T.S.).
REFERENCES
- Aharon I, Etcoff N, Ariely D, Chabris CF, O'Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32(3):537–51. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia Scale–I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research. 1994;38(1):23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Banissy MJ, Garrido L, Kusnir F, Duchaine B, Walsh V, Ward J. Superior facial expression, but not identity recognition, in mirror-touch synesthesia. Journal of Neuroscience. 2011;31(5):1820–4. doi: 10.1523/JNEUROSCI.5759-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. Journal of Autism and Developmental Disorders. 2004;34(2):163–75. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Barton JJS, Hefter RL, Cherkasova MV, Manoach DS. Investigations of face expertise in the social developmental disorders. Neurology. 2007;69(9):860–70. doi: 10.1212/01.wnl.0000267842.85646.f2. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Wessa M, Kedia G, Wicker B, Grezes J. Cross-cultural validation of the empathy quotient in a French-speaking sample. Canadian Journal of Psychiatry. 2008;53(7):469–77. doi: 10.1177/070674370805300712. [DOI] [PubMed] [Google Scholar]
- Chakrabarti B, Bullmore E, Baron-Cohen S. Empathizing with basic emotions: Common and discrete neural substrates. Social Neuroscience. 2006;1(3-4):364–84. doi: 10.1080/17470910601041317. [DOI] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Sciences. 2012;16(4):231–9. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero JA, Reeck C, Carter RM, Smith DV, Huettel SA. Nucleus accumbens mediates relative motivation for rewards in the absence of choice. Frontiers Human Neuroscience. 2011;5:87. doi: 10.3389/fnhum.2011.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD. The Temperament and Character Inventory: A Guide to its Development and Use. St. Louis, MO: Center for Psychobiology of Personality, Washington University; 1994. [Google Scholar]
- Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1983;44(1):113–26. [Google Scholar]
- Davis MH. Empathy: A Social Psychological Approach. Boulder, CO: Westview Press; 1994. [Google Scholar]
- Dawson G, Bernier R, Ring RH. Social attention: a possible early indicator of efficacy in autism clinical trials. Journal of Neurodevelopmental Disorders. 2012;4(1):11. doi: 10.1186/1866-1955-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb S, Wijsman E, et al. Neurocognitive and electrophysiological evidence of altered face processing in parents of children with autism: implications for a model of abnormal development of social brain circuitry in autism. Development and Psychopathology. 2005;17(3):679–97. doi: 10.1017/S0954579405050327. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behavioral and Cognitive Neuroscience Reviews. 2004;3(2):71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Deutsch F, Madle RA. Empathy: historic and current conceptualizations, measurement, and a cognitive theoretical perspective. Human Development. 1975;18(4):267–87. doi: 10.1159/000271488. [DOI] [PubMed] [Google Scholar]
- Dichter G, Richey J, Rittenberg A, Sabatino A, Bodfish J. Reward circuitry function in autism during face anticipation and outcomes. Journal of Autism and Developmental Disorders. 2011;42(2):147–60. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW. Reward circuitry function in autism spectrum disorders. Social Cognitive and Affective Neuroscience. 2012;7(2):160–72. doi: 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey G, Feldman SI. Implications of rejection sensitivity for intimate relationships. Journal of Personality and Social Psychology. 1996;70(6):1327–43. doi: 10.1037//0022-3514.70.6.1327. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Mercure E, Hudry K, et al. Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Current Biology. 2012;22(4):338–42. doi: 10.1016/j.cub.2011.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Grelotti DJ, Gauthier I, Schultz RT. Social interest and the development of cortical face specialization: what autism teaches us about face processing. Developmental Psychobiology. 2002;40(3):213–25. doi: 10.1002/dev.10028. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Tanaka J. Holistic and part-based face recognition in children with autism. Journal of Child Psychology and Psychiatry. 2003;44(4):529–42. doi: 10.1111/1469-7610.00142. [DOI] [PubMed] [Google Scholar]
- Josephs O, Turner R, Friston KJ. Event-related fMRI. Human Brain Mapping. 1997;5:243–8. doi: 10.1002/(SICI)1097-0193(1997)5:4<243::AID-HBM7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kim H, Adolphs R, O'Doherty JP, Shimojo S. Temporal isolation of neural processes underlying face preference decisions. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(46):18253–8. doi: 10.1073/pnas.0703101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee SJ. Reliability and validity of the Korean version of the empathy quotient scale. Psychiatry Investigation Psychiatry Investigation. 2010;7(1):24–30. doi: 10.4306/pi.2010.7.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Schienle A, Stark R, et al. Anticipation of reward in a nonaversive differential conditioning paradigm and the brain reward system: an event-related fMRI study. Neuroimage. 2003;20(2):1086–95. doi: 10.1016/S1053-8119(03)00381-1. [DOI] [PubMed] [Google Scholar]
- Klin A, Sparrow SS, de Bildt A, Cicchetti DV, Cohen DJ, Volkmar FR. A normed study of face recognition in autism and related disorders. The Journal of Autism and Developmental Disorders. 1999;29(6):499–508. doi: 10.1023/a:1022299920240. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001a;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001b;12(17):3683–7. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18(2):263–72. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363(1511):3771–86. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Peltzer J, Herpertz-Dahlmann B, Konrad K. Differential effects of social and non-social reward on response inhibition in children and adolescents. Developmental Science. 2009;12(4):614–25. doi: 10.1111/j.1467-7687.2009.00816.x. [DOI] [PubMed] [Google Scholar]
- Kohls G, Peltzer J, Schulte-Rüther M, et al. Atypical brain responses to reward cues in autism as revealed by event-related potentials. Journal of Autism and Developmental Disorders. 2011;41(11):1523–33. doi: 10.1007/s10803-011-1177-1. [DOI] [PubMed] [Google Scholar]
- Kohls G, Schulte-Rüther M, Nehrkorn B, et al. Reward system dysfunction in autism spectrum disorders. Social Cognitive and Affective Neuroscience. 2013;8(5):565–72. doi: 10.1093/scan/nss033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping. 2000;10(3):120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence EJ, Shaw P, Baker D, Baron-Cohen S, David AS. Measuring empathy: reliability and validity of the Empathy Quotient. Psychological Medicine. 2004;34(5):911–9. doi: 10.1017/s0033291703001624. [DOI] [PubMed] [Google Scholar]
- Lin A, Adolphs R, Rangel A. Impaired learning of social compared to monetary rewards in autism. Frontiers in Neuroscience. 2012;6:143. doi: 10.3389/fnins.2012.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubben J, Gironda M. Measuring social networks and assessing their benefits. In: Phillipson C, Allan G, Morgan D, editors. Social Networks and Social Exclusion: Sociological and Policy Perspectives. Hampshire, United Kingdom: Ashgate; 2004. pp. 20–35. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mundy P, Jarrold W. Infant joint attention, neural networks and social cognition. Neural Network. 2010;23(8-9):985–97. doi: 10.1016/j.neunet.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Neal RA. Neural plasticity, joint attention, and a transactional social-orienting model of autism. In: Glidden LM, editor. International Review of Research in Mental Retardation. 2000. Autism Vol. 23, pp. 139–68., San Diego, Academic Press. [Google Scholar]
- O'Doherty JP, Buchanan TW, Seymour B, Dolan RJ. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006;49(1):157–66. doi: 10.1016/j.neuron.2005.11.014. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33(5):815–26. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Parlade MV, Messinger DS, Delgado CE, Kaiser MY, Van Hecke AV, Mundy PC. Anticipatory smiling: linking early affective communication and social outcome. Infant Behavior and Development. 2009;32(1):33–43. doi: 10.1016/j.infbeh.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Conant D, Hazin R, Stoner R, Desmond J. Preference for geometric patterns early in life as a risk factor for autism. Archives of General Psychiatry. 2011;68(1):101–9. doi: 10.1001/archgenpsychiatry.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Gründer G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage. 2010;49(4):3276–85. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Schneider D, Regenbogen C, Kellermann T, et al. Empathic behavioral and physiological responses to dynamic stimuli in depression. Psychiatry Research. 2012;200(2-3):294–305. doi: 10.1016/j.psychres.2012.03.054. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience. 2005;23(2-3):125–41. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57(4):331–40. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Research. 2010;3(2):53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims TB, Van Reekum CM, Johnstone T, Chakrabarti B. How reward modulates mimicry: EMG evidence of greater facial mimicry of more rewarding happy faces. Psychophysiology. 2012;49(7):998–1004. doi: 10.1111/j.1469-8986.2012.01377.x. [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer K, Krach S, Kohls G, et al. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience. 2009;4(2):158–65. doi: 10.1093/scan/nsn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreckelmeyer K, Rademacher L, Paulus F, Gründer G. Neural activation during anticipation of opposite-sex and same-sex faces in heterosexual men and women. Neuroimage. 2012;66C:223–31. doi: 10.1016/j.neuroimage.2012.10.068. [DOI] [PubMed] [Google Scholar]
- Staebler K, Helbing E, Rosenbach C, Renneberg B. Rejection sensitivity and borderline personality disorder. Clinical Psychology and Psychotherapy. 2010;18(4):275–83. doi: 10.1002/cpp.705. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34(13):2655–66. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka JW, Wolf JM, Klaiman C, et al. The perception and identification of facial emotions in individuals with autism spectrum disorders using the Let's Face It! Emotion Skills Battery. Journal of Child Psychology and Psychiatry. 2012;53(12):1259–67. doi: 10.1111/j.1469-7610.2012.02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Schultz W, Roelcke U, et al. Activation of the human brain by monetary reward. Neuroreport. 1997;8(5):1225–8. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- Triesch J, Teuscher C, Deák GO, Carlson E. Gaze following: why (not) learn it? Developmental Science. 2006;9(2):125–47. doi: 10.1111/j.1467-7687.2006.00470.x. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others' actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48(3):564–84. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Vaughan Van Hecke A, Mundy PC, Acra CF, et al. Infant joint attention, temperament, and social competence in preschool children. Child development. 2007;78(1):53–69. doi: 10.1111/j.1467-8624.2007.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Horn A, Backman L, Davidsson T, Hansen S. Empathizing, systemizing and finger length ratio in a Swedish sample. Scandinavian Journal of Psychology. 2010;51(1):31–7. doi: 10.1111/j.1467-9450.2009.00725.x. [DOI] [PubMed] [Google Scholar]
- Wakabayashi A, Baron-Cohen S, Uchiyama T, Yoshida Y, Kuroda M, Wheelwright S. Empathizing and systemizing in adults with and without autism spectrum conditions: cross-cultural stability. Journal of Autism and Developmental Disorders. 2007;37(10):1823–32. doi: 10.1007/s10803-006-0316-6. [DOI] [PubMed] [Google Scholar]
- Weigelt S, Koldewyn K, Kanwisher N. Face identity recognition in autism spectrum disorders: a review of behavioral studies. Neuroscience and Biobehavioral Reviews. 2012;36(3):1060–84. doi: 10.1016/j.neubiorev.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Wiseman T. A concept analysis of empathy. Journal of Advanced Nursing. 1996;23(6):1162–7. doi: 10.1046/j.1365-2648.1996.12213.x. [DOI] [PubMed] [Google Scholar]
- Wolf JM, Tanaka JW, Klaiman C, et al. Specific impairment of face-processing abilities in children with autism spectrum disorder using the Let's Face It! skills battery. Autism Research. 2008;1(6):329–40. doi: 10.1002/aur.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.