Abstract
This study investigates the spatiotemporal brain dynamics of emotional information processing during reading using a combination of surface and intracranial electroencephalography (EEG). Two different theoretical views were opposed. According to the standard psycholinguistic perspective, emotional responses to words are generated within the reading network itself subsequent to semantic activation. According to the neural re-use perspective, brain regions that are involved in processing emotional information contained in other stimuli (faces, pictures, smells) might be in charge of the processing of emotional information in words as well. We focused on a specific emotion—disgust—which has a clear locus in the brain, the anterior insula. Surface EEG showed differences between disgust and neutral words as early as 200 ms. Source localization suggested a cortical generator of the emotion effect in the left anterior insula. These findings were corroborated through the intracranial recordings of two epileptic patients with depth electrodes in insular and orbitofrontal areas. Both electrodes showed effects of disgust in reading as early as 200 ms. The early emotion effect in a brain region (insula) that responds to specific emotions in a variety of situations and stimuli clearly challenges classic sequential theories of reading in favor of the neural re-use perspective.
Keywords: reading, emotion, disgust, ERP, word recognition
INTRODUCTION
Words are more than abstract linguistic symbols; they evoke thoughts, feelings and actions (Niedenthal, 2007). Although much is known about the basic processes in reading and their neural substrates (Perry et al., 2010; Jobard et al., 2003; Price, 2012), the question of how emotions are processed during reading has received much less attention (for review, see Citron, 2012).
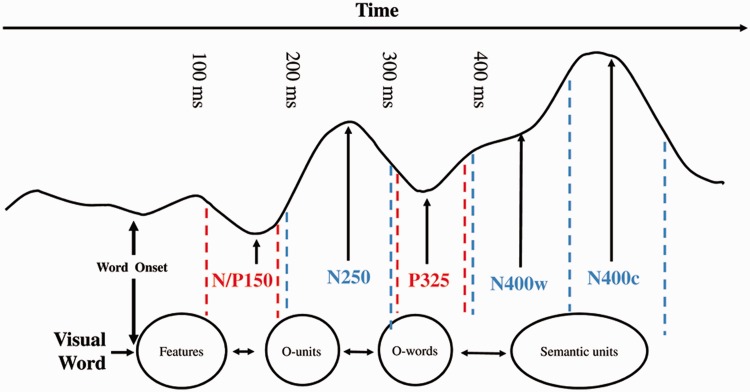
Two different theoretical views can be opposed. According to the standard psycholinguistic perspective, emotional responses to words are generated within the reading network itself, which is hosted mostly in the left hemisphere and includes the occipitotemporal visual word-form area for perceptual analysis of letter strings (Dehaene et al., 2005), the inferior parietal and superior temporal cortices for print-to-sound translation (van Atteveldt et al., 2007) and lateral temporal cortices and inferior frontal gyrus for processing meaning (Devlin et al., 2004). According to this view, the retrieval of emotional content follows abstract semantic activation. Grainger and Holcomb (2009) proposed a time-course model of visual word recognition based on over 10 years of research using event-related brain potentials (ERPs). As can be seen in Figure 1, visual word recognition proceeds sequentially, and meaning is activated no earlier than 400 ms (but see Hauk et al., 2006).
Fig. 1.
Time-course model of visual word recognition proposed by Grainger and Holcomb (2009, Figure 12).
Alternatively, according to the neural re-use perspective (Anderson, 2010), brain regions that are involved in processing emotional information in a variety of stimuli (faces, pictures, odors) might be in charge of the processing of emotional information in words as well. The neural re-use perspective assumes that newly acquired cognitive functions (e.g. language) are not the product of entirely new networks, but result from novel combinations of ‘ancient’ components that are already involved in other cognitive functions. According to this perspective, emotion words would activate those brain areas outside the classic reading network (e.g. amygdala, insula, orbitofrontal cortex) that process emotions in domains other than reading. The goal of this study was to contrast these theoretical views by investigating the spatiotemporal brain dynamics of emotion processing during reading using a combination of surface and intracranial electroencephalography [stereoelectroencephalography (sEEG)]. This combination offers a unique way to associate high temporal resolution with accurate spatial localization. We focused on a specific emotion—disgust—which has a clear locus in the brain, the anterior insula (see below).
Disgust is believed to have evolved to protect against noxious substances that may endanger health (Darwin, 1872; Rozin and Fallon, 1987). The experience of disgust specifically signals the threat of contamination, thereby encouraging withdrawal (Rozin and Fallon, 1987; Olatunji et al., 2005; Rozin, et al., 2009). Along evolution, feeling disgust may have assisted humans in the identification of rotten smells signaling toxins, preventing infection and disease or even in the reproduction of the species, by preventing incestuous relationships (Lieberman et al., 2007). The results of brain imaging (Phillips et al., 1997; Wicker et al., 2003; Wright et al., 2004), lesion studies (Calder et al., 2000; Borg et al., 2013) and intracranial recordings in humans (Krolak-Salmon et al., 2003) all point to the anterior insula as the likely neural locus for processing disgust information across a variety of modalities although it is fair to say that this complex multimodal structure is involved in a number of other cognitive functions (e.g. Cauda et al., 2012).
If we were to take the anterior insula as a likely locus for the processing of disgust in the brain, the neural re-use theory would predict a rapid activation of this structure for words with disgusting content. The standard psycholinguistic view would not necessarily predict the activation of the anterior insula but rather that of the classic reading network. If anything at all, the anterior insula would become activated only after abstract semantic processing, which tends to occur rather late, at least according to the time-course model displayed in Figure 1 (Grainger and Holcomb, 2009).
Previous ERP studies have provided some insights about the time course of the activation of emotional information during reading (for review, see Citron, 2012). Both early and late emotion-related effects on word processing have been reported (Kanske and Kotz, 2007; Kissler et al., 2007, 2009; Herbert et al., 2008; Bayer et al., 2010, 2012). Although emotional effects on early time windows such as the P1-N1 and P2-N2 have been somewhat inconsistent (Bernat et al., 2001; Kissler, et al., 2006; Hofmann et al., 2009; Scott et al., 2009), a rather robust effect has been found to peak around 200–300 ms, mainly at left occipitotemporal sites, also referred to as the early posterior negativity (EPN, Kissler et al., 2007, 2009; Herbert et al., 2008; Schacht and Sommer, 2009a, 2009b). It has been suggested that the EPN reflects an initial allocation of visual attention toward emotional words (Herbert et al., 2008) and/or some kind of tagging mechanism that gives priority to emotional stimuli during early perceptual processing (Schupp et al., 2004a). In addition to these early effects, emotional information has been shown to affect the N400 (Herbert et al., 2008; Kanske and Kotz, 2007) and, more frequently, the late positive complex (LPC; Fischler and Bradley, 2006; Herbert et al., 2006; Kanske and Kotz, 2007; Schacht and Sommer, 2009a, 2009b; Bayer et al., 2010). These late effects are thought to reflect deeper emotional analysis and semantic integration.
In this study, ERPs were recorded while participants performed a Go-NoGo semantic categorization task (e.g. Pattamadilok et al., 2009), which allows emotional information to be manipulated on the NoGo trials and therefore be processed at an implicit level (i.e. no decision is required on the NoGo trials). The stimuli included disgust and neutral words, matched for emotional arousal, as well as other linguistic properties known to affect ERPs in visual word processing, such as imageability, length and frequency (for details see Supplementary Material).
On the basis of the literature reviewed above, we expected to find emotion effects on both early and late ERP components (EPN, N400, LPC). If the processing of disgust words were to make demands on brain areas involved in processing disgust in other domains, we would expect to see the involvement of the insular cortex in the source localization of cortical responses. This hypothesis was further tested through the intracranial recordings from two pharmacoresistant epileptic patients who had a deep electrode in the insula and the orbitofrontal cortex (OFC). The OFC is yet another brain region outside the classic reading network known to be involved in the processing of emotional valence and arousal (Schafer et al., 2009). Therefore, intracranial recordings provided a strong test for the hypothesis that regions outside the classic reading network, such as the insula or the OFC, should become quickly activated during the processing of written words, well before the typical effects of semantic access.
METHODS
Electrophysiological study in healthy participants
Participants
Twenty-one healthy individuals (10 women), aged 18–33 years (M = 22.6; s.d. = 4.42), participated in this study. They were all native French speakers, right handed (Annett, 1970), with normal or corrected-to-normal vision and without any psychiatric or neuropsychological disorder, as provided by self-report. The study was approved by the local ethics committee. All participants gave informed written consent and received 15€ for their participation. Four participants were excluded from further analysis, one because of excessively high error rates (>87%) and the others because of noisy electroencephalography (EEG).
Stimulus material
A first set of 213 French words was selected from the online database LEXIQUE3.55 (New et al., 2001). This set included 93 neutral (e.g. motivation, giant, statue) and 120 disgust-related words (e.g. infection, vomit, vermin). Eighteen participants who did not participate in the present experiment rated all stimuli for emotional valence (from −3, very negative, to +3, very positive), arousal (from 1, not stimulating, to 5, very stimulating) and imageability (from 1, very imageable, to 7, not imageable at all). From this list of 213 rated words, we then selected 35 neutral and 35 disgust-related words that were matched for arousal and standard word recognition variables (Table 1). All items along with their translations and item characteristics can be found in the supplementary material. The two groups only differed on valence because disgust-related words are typically of negative valence (t(68) = 7.704, P < 0.0001). For the Go trials, 24 words describing means of transportation were used (e.g. car, bike, taxi).
Table 1.
Characteristics of the word stimuli
| Variable | Word category |
||||
|---|---|---|---|---|---|
| Neutral |
Disgust related |
||||
| M | s.d. | M | s.d. | P | |
| Valence | −0.1 | 0.80 | −1.3 | 0.53 | <0.0001 |
| Arousal | 1.2 | 0.53 | 1.2 | 0.40 | >0.90 |
| Imageability | 5.0 | 1.40 | 5.0 | 1.24 | >0.90 |
| Word length (letters) | 6.7 | 1.68 | 6.3 | 1.54 | >0.30 |
| Word length (syllables) | 2.1 | 0.68 | 1.9 | 0.59 | >0.50 |
| Frequency of use | 17.9 | 30.74 | 16.1 | 16.32 | >0.80 |
Procedure
Participants were instructed to silently read the presented words and decide as quickly and accurately as possible whether a given word referred to a ‘means of transportation’. If so, they were asked to press a response button with the index finger of their right hand (Go trials). If not, they were asked to withhold from responding (NoGo trials). All words were pseudorandomized across different presentation lists. Each subject was given a total of 94 experimental trials corresponding to the presentation of 35 neutral words, 35 disgust-related words and 24 transportation words. Stimuli were presented in white lowercase (Arial font, size 18) on a black background, during 1000 ms in the center of the screen. Each word was preceded by a fixation cross presented for 500 ms and followed by a blank screen for 500 ms. To allow for eye blinks during the experiment, a blinking signal [(- -)] was presented for 2500 ms, right after the blank screen. Before doing the task, participants completed 20 training trials. E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA) was used for stimulus presentation.
EEG acquisition
Electrical activity was continuously recorded with a sampling rate of 256 Hz using the Biosemi ActiveTwo system (BioSemi, Amsterdam, Netherlands). Horizontal and vertical electrooculograms were recorded using two facial electrodes placed, respectively, above the left eye and to the outer canthi of the right eye. Scalp EEG was recorded from 64 electrodes attached to an electrode cap and positioned according to the extended 10–20 EEG system. All sites were re-referenced offline to the algebraic average of the left and right mastoids. The data were filtered using a high pass of 0.1 Hz and a low pass of 30 Hz. Each trial lasted for 1250 ms including a 250 ms prestimulus time period. Data were baseline corrected using the first 250 ms of the epoch, which corresponded to the presentation of the fixation cross. Once artifacts and eye blinks were removed, epochs were averaged separately for each of the 64 electrodes, 2 conditions (disgust and neutral) and 17 participants. Grand-average ERPs were calculated by computing the mean ERPs across participants in the two conditions.
Measures and statistical analysis
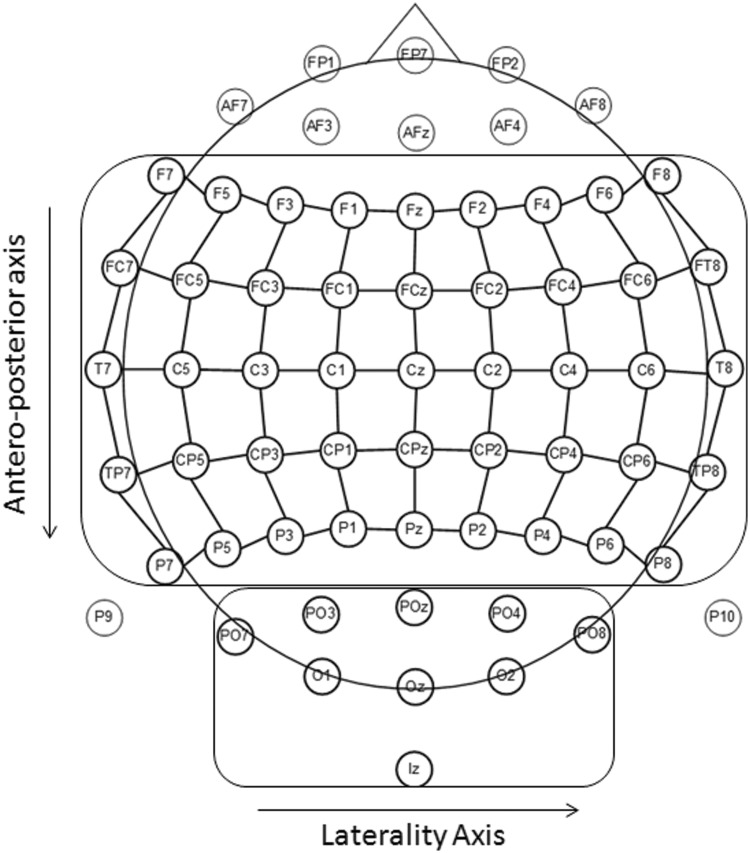
ERP data were analyzed by calculating the mean amplitudes in several time windows. After visual inspection of the signal at posterior sites, we chose three time windows corresponding to three early components, N100 (63–86 ms), P100 (113–152 ms) and EPN (207–266 ms). For these early components, we selected nine posterior electrodes that exhibited particularly clear-cut components in the time windows of interest. As shown in Figure 2, these nine electrodes covered the occipital region and were organized in three areas (left lateral, midline and right lateral) of three electrodes each (PO7-PO3-O1, POz-Oz-Iz and PO4-O2-PO8, respectively). Thus, for the early components, data were analyzed in a three-factorial analysis of variance (ANOVA) with emotion, area and electrode as factors.
Fig. 2.
Schematic view of the scalp electrode montage used for the analysis.
For the later and more anterior components, three time windows were chosen to capture the P200 (184–277 ms), N400 (344–465 ms) and LPC (492–656 ms) components. These time segments were chosen because their shape, polarity and timing corresponded to the classical components described in related studies (e.g. Kissler et al., 2006). To cover a large part of the scalp in the analyses, we defined a grid that was composed of nine columns going from left lateral to right lateral and five rows going from frontal to parietal (Figure 2). Thus, using this 9 × 5 grid, the data were analyzed in three-way ANOVAs with emotion (disgust vs neutral), laterality (nine columns) and anterior–posterior extent (five rows) as within-subject factors.
Source localization
Source analyses were performed using low-resolution brain electromagnetic tomography (sLORETA, version 3, Pascual-Marqui et al., 1994), which allowed us to estimate the position of the neural sources underlying the electrical field configurations for a given component. sLORETA is based on an inverse solution technique that estimates the distribution of coherent co-activation of electrical neuronal activity of neighboring cortical areas and, accordingly, computes the “smoothest” of all possible activity distributions. Importantly, sLORETA does not make a priory assumption on the number and locations of the sources, and the estimation is based on information acquired from the electrodes positioned over the entire scalp. sLORETA solutions are computed within a three-shell spherical head model co-registered to the MNI152 template. Therefore, the source locations were given as (x, y, z) coordinates (x from left to right; y from posterior to anterior; z from inferior to superior). sLORETA estimates the three-dimensional intracerebral current density distribution in 6239 voxels (5-mm resolution). For all participants, sLORETA images corresponding to EPN, N400 and LPC components were defined as the mean current density values for previously described time windows intervals. Statistical significance of differences for those images elicited by the disgust and neutral stimuli was assessed with statistical nonparametric mapping tests for paired samples with correction for multiple comparisons, as implemented in sLORETA. The level of significance was set to P = 0.05, corrected for multiple comparisons. The coordinates of the foci of significant differences between conditions were transformed from the MNI to the Talaraich coordinates. The Talaraich daemon was then used to identify the brain structures involved.
Electrophysiological study in epileptic patients
Intracranial recording procedure
Electrophysiological responses were recorded in two epileptic patients both suffering from left frontotemporal epilepsy. These patients were implanted for presurgical investigation with depth cortical electrodes. sEEG recording was performed to define the epileptogenic zone (for a description of the method, see McGonigal et al., 2007). The choice of electrode location was based on pre-sEEG clinical and video-EEG recordings and made independently of this study. This study did not add any invasive procedure to depth EEG recordings. Analysis of sEEG concluded that the epileptogenic zone was located outside the regions analyzed here. In addition, no subject had seizures in the 12 h before ERP recordings. Both patients provided informed consent to the protocol (agreement IRB0000388 and FWA00005831).
Patient 1 was 23 years old, right handed, female and her native language was French. Her IQ was slightly below average as assessed by the Wechsler Adult Intelligence Scale, third edition (WAIS-III), with no visuoverbal dissociation (total IQ: 82, verbal IQ: 81 and performance IQ: 87). Spoken language tests showed a relatively weak level of semantic knowledge. Word production (object naming) was normal.
Patient 2 was 33 years old, left handed, male and his native language was French. His IQ (WAIS-III) was in the normal range with no visuoverbal dissociation (total IQ: 104, verbal IQ: 99 and performance IQ: 109). Verbal efficiency, working memory and spontaneous language were also preserved. He showed normal results in the oral object naming and verbal fluency tests.
In both patients, each electrode contained 4–10 contacts 2–3 mm long, 10 mm apart, mounted on a 1-mm-wide flexible plastic probe. The cerebral structures explored by intracerebral electrodes were defined according to clinical exploration priorities. For patient 1, the contacts located in the insula showed normal activity, suggesting that the insula did not belong to the epileptogenic zone. Similarly, for patient 2, the contacts situated in the left OFC did not show any epileptic activity.
sEEG signals were acquired using Brain Amps amplifiers (Brain Products GmbH, Munich, Germany), with the sampling rate of 1000 Hz (bandpass 0.15–200 Hz). The reference was a surface electrode located at position Fz in the 10–20 international EEG system. sEEG evoked potentials were obtained by averaging the electrical signal time locked to the stimulus onset for each word category separately. Single-trial EEG epochs were computed offline using BrainVision Analyzer 2 from 0 to 1500 ms after stimulus with a prestimulus baseline of 500 ms.
Individual trials were inspected visually to reject trials with slow waves or epileptic spikes. Time windows, which showed a significant effect of emotion, were determined by comparing the amplitudes of disgust words with those of neutral words for each time point using a two-tailed t-test. Performing tests on multiple time points increases the probability of a false positive. Therefore, we considered only significant differences that were present for 20 consecutive time points, because the likelihood of getting 20 false-positives in a row is considerably low (Molholm et al., 2006).
RESULTS
Global task performance
Analysis of the Go trials showed that participants had a high level of accuracy (M = 95.1% correct; s.d. = 5.15) with an average response times of 735.7 ms (s.d. = 110.15).
Electrophysiological results
For 17 participants, the mean number of averaged trials, after removal of artifacts and eye blinks, was 33.6 ± 1.53 and 34.11 ± 1.49 for disgust and neutrals words, respectively. The Go trials were not further analyzed due to the small number of trials (<24). ERP components were identified based on their distinctive polarities, latencies and topographic properties.
Early event-related components
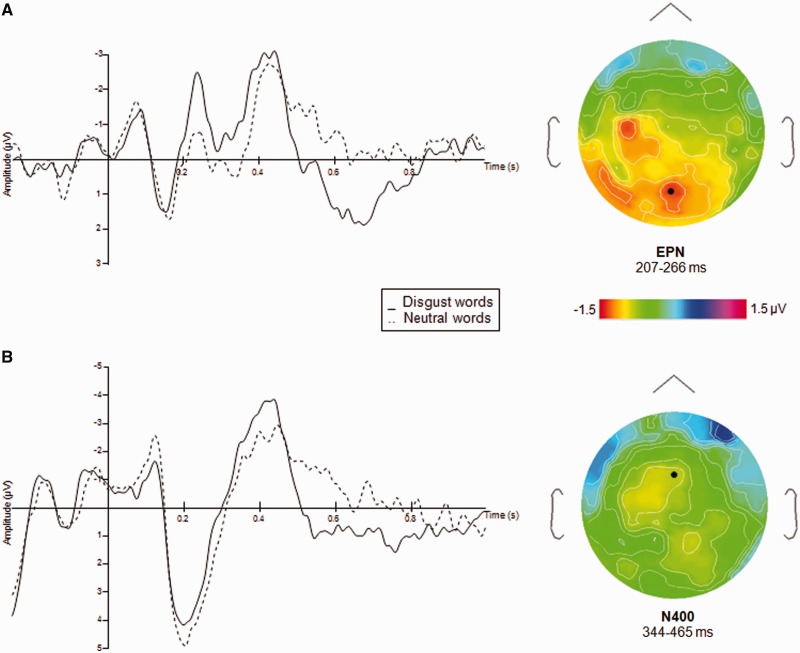
Statistical analyses were performed on the mean amplitudes comprised in the three early time windows (see Methods). No significant effect of emotion was found on the N100 and the P100 (F(1, 16) = 0.57, P = 0.45 and F(1, 16) = 0.24, P = 0.62, respectively). A significant main effect of emotion was found for the EPN (F(1, 16) = 6.10, P = 0.02), which consisted of a larger bilateral posterior negativity for the disgust relative to the neutral words. The EPN occurred around 250 ms poststimulus (Figure 3). None of the interactions was significant.
Fig. 3.
(A) ERPs (mean amplitudes in µV) to the NoGo trials for disgust and neutral word categories at POz. Right, topography of ERPs at the averaged time window of the EPN latency, (B) ERPs (mean amplitudes in µV) to the NoGo trials for the disgust and neutral word categories at FCz. Right, topography of ERPs at the averaged time window of the N400 latency.
Late event-related components
Visual examination of grand average ERP data revealed three major components over frontoparietal regions, bilaterally. For each of the three components (P200, N400 and LPC), statistical analyses were performed on the mean amplitudes using the 9 × 5 grid described above (laterality vs anterior–posterior extent).
P200
No significant effect of emotion was found in the P200 time window (F(1, 16) = 2.75, P = 0.11). None of the interactions reached significance.
N400
The main effect of emotion failed to reach significance in the N400 time window (F(1, 16) = 2.72, P = 0.11). However, the emotion × column interaction was highly significant (F(8, 128) = 4.40, P = 0.00009). Pairwise comparisons performed for each column separately showed that the emotion effect was restricted to a column in the left hemisphere (left 4, F(1, 16) = 5.13, P = 0.03), which showed greater negativity for disgust than for neutral words. Main effects of columns (F(8, 128) = 3.41, P = 0.001) and anterior–posterior axis (F(4, 64) = 4.87, P = 0.001) were significant, as well as the interaction between the two variables (F(32, 512) = 1.67, P = 0.001), implying a specific scalp distribution of the component. The signal amplitude tended to be overall more negative over left centroparietal sites.
LPC
Analysis in the LPC time window (490–654 ms) revealed a main effect of emotion (F(1, 15) = 6.64, P = 0.02). Disgust words generated greater positive amplitudes than neutral words. Emotion × column and emotion × anterior–posterior axis were both significant (F(8, 120) = 3.48, P = 0.001 and F(4, 60) = 6.34, P = 0.0002, respectively), implying that the emotion effect was stronger over centroparietal sites.
Source localization
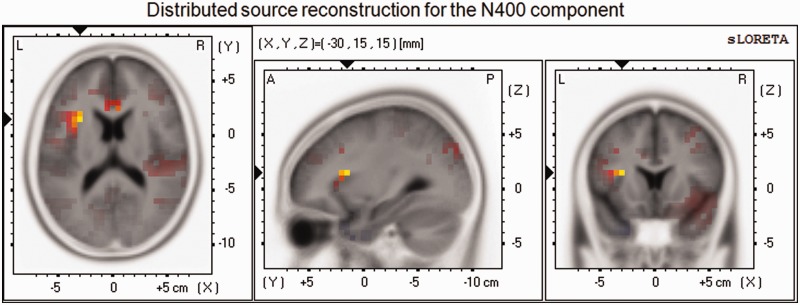
sLORETA was used to perform source localization of the main surface components as previously identified in the ERPs. The disgust-minus-neutral-word contrast was analyzed in the selected time windows described above. Source localizations in the EPN and LPC time windows failed to converge on a stable result. However, as shown in Figure 4, current density for the N400 component was higher for disgust than neutral words in left insula (BA13, t(16) = 4.40, P = 0.05), the right middle temporal gyrus (BA21, t(17) = 4.31, P = 0.05) and the right superior temporal gyrus (BA21, t(17) = 4.30, P = 0.05).
Fig. 4.
sLORETA maps for the N400 component for the contrast between Disgust and Neutral (NoGo) words, centered on the maximum peak activity, in the left insula.
sEEG results
Patient 1
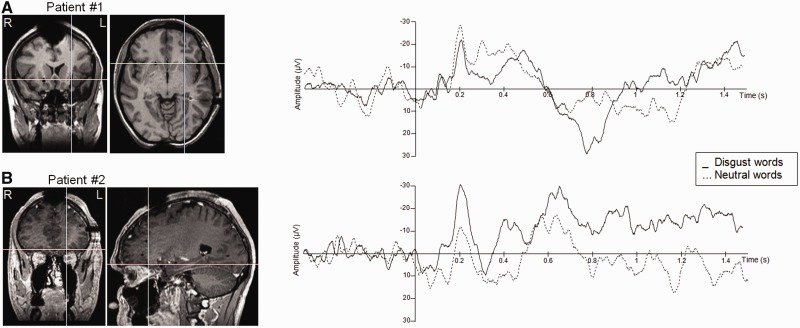
The patient had an electrode implanted in the left anterior insula (Figure 5). Due to a technical failure, accuracy data in the Go-trials were not recorded. Statistical analyses showed that one contact showed a selective response to a word’s emotional category. It should be noted that the other contacts on the same electrode showed similar differences, but these differences failed to reach significance. Three time windows exhibited significant differences between neutral and disgust words, namely an early time window between 186 and 225 ms (t(44) = 3.77, P = 0.001), a N400 time window between 437 and 500 ms (t(44) = 2.46, P = 0.005) and a late time window between 727 and 865 ms (t(44) = 4.26, P = 0.001).
Fig. 5.
Intracranial recordings. Left panel: Anatomical MRIs showing the recording site. R: right hemisphere; L: left hemisphere. On the right panel: Time course of the intracranial potentials in response to disgust (plain line) and neutral (dotted line) words.
Patient 2
The patient had an electrode implanted in the left OFC (Figure 5). Accuracy for the Go trials was 100%. Reaction times were comparable with those of the participants in the ERP experiment (M = 742.96 ms; SD = 81.96 ms). Statistical analyses of the sEEG signal performed on the difference between disgust and neutral words showed significant effects for one particular contact in three time windows. A first effect was found between 180 and 248 ms (t(46) = 5.52, P = 0.001), with a greater negativity for disgust compared with neutral words. A second effect was found around 400 ms with greater negativity for disgust than neutral words (t(46) = 2.33, P = 0.05). Finally, disgust words produced more positive-going waves than neutral words around 565 ms, an effect that lasted for almost 200 ms (t(46) = 3.55, P = 0.001).
To summarize, the sEEG recordings from the two patients showed early emotion effects in insular and orbitofrontal electrodes. The almost perfect overlap of the surface ERP at POz (Figure 3) and the orbitofrontal sEEG of patient 2 (Figure 5) is rather striking. To the extent that the EPN has been localized in extrastriate cortex, including the temporooccipital visual association area (Franken et al., 2009; Fruhholz et al., 2011; Kissler et al., 2007; Schupp et al., 2007), the present data suggest that early emotion effects in reading emerge within a distributed network, which includes both occipital and frontal regions.
DISCUSSION
The present results are straightforward and can be summarized as follows. In terms of the temporal dynamics of emotion effects in reading, disgust words elicited cortical responses as soon as 200 ms after stimulus onset in more posterior sites, as reflected by a large bilateral EPN. This finding replicates previous studies showing early modulations of the EPN by emotional stimuli (Herbert et al., 2008; Kissler et al., 2009). No emotion effect was found on the P2 in more central and anterior locations. As noted by Kanske and Kotz (2007), the absence of a P2 emotion effect might have been due to the task because they found a P2 in a lexical decision but not a Go–NoGo task that was similar to ours. Importantly, the early emotion effects were replicated in the intracranial recordings of two patients in more anterior regions (insula and OFC), which both showed differences between disgust and neutral words starting around 200 ms. These findings stand in contrast with a previous sEEG study showing amygdala activation to threatening words only around 600 ms for consciously perceived words and even later for subliminally presented words (Naccache et al., 2005).
An additional disgust effect was revealed on a later ERP component, the N400. Disgust words elicited larger N400 amplitudes than neutral words (see below for Discussion). This effect started around 350 ms and was nicely replicated in the intracranial recordings of the two patients. Note that the sEEG results are not discussed in terms of the polarity of the differences between disgust and neutral words (i.e. greater negativity/greater positivity), because the polarity depends on the location of the electrode with respect to the underlying generator. Interestingly, the N400 provides a reference point in time, suggesting that the emotion effects obtained before the N400 are likely to reflect a rapid emotional analysis that occurs before a deeper semantic analysis.
Following the N400, we found robust emotion effects on the LPC, starting around 500 ms. As for the N400, the sensitivity of this component to emotional valence was found in both surface and intracranial potentials. It has been suggested that although cortical activity measured at the N400 might represent initial stages of a semantic memory–based analysis, the centroposteriorly distributed LPC component might reflect the engagement of additional cognitive processes aiming at further semantic refinement and contextual integration (Fischler and Bradley, 2006) and sustained attention to emotional stimuli (Schupp et al., 2004b). The LPC was found in literally all studies that manipulated the emotional valence of words or faces (Fischler and Bradley, 2006; Herbert et al., 2006; Kissler et al., 2006), including earlier studies using intracranial recordings (Naccache et al., 2005).
In terms of the spatial distribution of the disgust effect, we had a strong a priori hypothesis about the implication of the anterior insula in reading disgust words (e.g. Wicker et al., 2003; Wright et al., 2004). Indeed, we found an activation of the left insula both in sLORETA performed on the N400 component of the surface EEG and in sEEG recordings of an epileptic patient with an electrode located in the insula. The latter effect occurred as early as 200 ms following the onset of the word.
The theoretical implications of the present findings are straightforward. As discussed in the Introduction, two perspectives on emotional information processing on reading can be identified. According to the first perspective, the psycholinguistic perspective, the activation of emotional content in reading occurs within the reading network itself and happens rather late in time because it requires a minimal level of semantic analysis. Alternatively, according to the second perspective, the neural re-use perspective (Anderson, 2010), brain regions that are involved in processing emotional information in faces or odors might be in charge of processing emotions in words as well. Thus, according to this perspective, emotion words would rapidly activate brain areas outside the classic reading network that process emotional information in other modalities. The present data clearly support the second prediction because the insula is one of these structures that has been found to process emotions in other modalities (e.g. Wicker et al., 2003; Wright et al., 2004). Moreover, the insula is activated early enough (∼200 ms) to claim that such an activation must occur before a deeper semantic analysis, which is typically indexed by the N400 (Kutas and Federmeier, 2011).
The present results have implications for both models of reading and the larger debate on grounded cognition, that is, the idea that semantic content is grounded in perceptual-motor and emotional processing (Niedenthal, 2007; Barsalou, 2008). As concerns models of visual word recognition, our results challenge the commonly held view according to which word recognition proceeds in a neatly sequential fashion going from low-level orthographic processing that takes place in occipitotemporal areas of the brain to phonological and semantic processing in more frontal areas (Grainger and Holcomb, 2009; Jobard et al., 2003). The fact that the frontal cortex and the anterior insula play a role in the emotional analysis of written words as soon as 200 ms after the presentation of a written word is hard to reconcile with such a sequential view.
This interpretation is further supported by the results of a series of studies conducted by Pulvermüller and colleagues who showed that semantic activation can occur earlier than 400 ms and is not restricted to the left hemispheric network but spreads to non-linguistic brain regions (for review, see Pulvermüller, 2005). For instance, hearing or reading action verbs, such as lick, pick and kick, activates not only the classic language areas but also the motor cortex, a region that is not dedicated to language: ‘lick’ activates the tongue area, ‘pick’ the hand area and ‘kick’ the foot area of the motor cortex.
In defense of the classic psycholinguistic perspective, it has been suggested that the activation of motor or emotion circuits in language processing could be the result of postsemantic spreading activation and not indicative of responsibility for semantic processing itself (Mahon and Caramazza, 2008). The present time-course data speak against this interpretation because emotional information located in frontal areas of the brain seems to be activated very early (around 200 ms). Thus, as far as the relative timing of events is concerned, the present effects are hard to explain by postsemantic spreading activation.
One issue that needs to be discussed is the fact that we found larger N400 amplitudes to emotion over neutral words, whereas previous studies showed the opposite pattern, that is, reduced N400 amplitudes to emotion words (Herbert et al., 2008; Kanske and Kotz, 2007). We believe that task differences can explain this seemingly conflicting pattern. In our Go–NoGo task, participants were asked to detect words that refer to means of transportation and they had to ignore all other types of words. We suggest that because of their high emotional valence, disgust words were harder to ignore than neutral words. Thus, in these cases, the N400 may index semantic inhibition rather than semantic integration (see Debruille et al., 2008). This interpretation is supported by the findings of Trauer et al. (2012) who manipulated emotional valence in an implicit word recognition task. As in our study, negative emotion words produced larger N400 amplitudes than neutral words, suggesting that emotion words were harder to ignore than neutral words. This situation is different from other tasks, in which the reduced N400 amplitudes to emotion words might reflect easier semantic access and integration. For example, if participants were to make lexical decisions, emotional content might facilitate lexical access (but see Briesemeister et al., 2011; Silva et al., 2012), which should then result in reduced N400 amplitudes (e.g. Kanske and Kotz, 2007).
In summary, contrary to previous reports (Naccache, et al., 2005), the brain seems to rapidly distinguish disgusting from non-disgusting orthographic stimuli, which is consistent with the view that potential threats must be recognized quickly (Ohman et al., 2001; Ohman and Mineka, 2001). Although this is obviously important when dealing with phylogenetically meaningful stimuli, such as faces or smells (Ohman and Mineka, 2001; Pourtois et al., 2004; Schupp et al., 2004b), it is striking that similar mechanisms seem to be at play when processing linguistic stimuli, that are ontogenetically learned and culturally mediated.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The authors thank Amel Mosbah for assistance in data collection and analysis with patients and Romain Duprat for assistance in scalp EEG data acquisition. This research was supported by a French-German ANR/DFG grant [ANR-10-FRAL-005-01 awarded to J.C.Z. and A.M.J.]; and CLC was supported by a CPER Telius Inserm-Inria grant.
References
- Anderson ML. Neural reuse: a fundamental organizational principle of the brain. Behavioral Brain Sciences. 2010;33:245–66; discussion 266–313. doi: 10.1017/S0140525X10000853. [DOI] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association analysis. British Journal of Psychology. 1970;61:303–21. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Grounded Cognition Annual Review of Psychology. Vol. 59. Palo Alto: Annual Reviews; 2008. pp. 617–45. [DOI] [PubMed] [Google Scholar]
- Bayer M, Sommer W, Schacht A. Reading emotional words within sentences: the impact of arousal and valence on event-related potentials. International Journal of Psychophysiology. 2010;78:299–307. doi: 10.1016/j.ijpsycho.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Bayer M, Sommer W, Schacht A. P1 and beyond: functional separation of multiple emotion effects in word recognition. Psychophysiology. 2012;49:959–69. doi: 10.1111/j.1469-8986.2012.01381.x. [DOI] [PubMed] [Google Scholar]
- Bernat E, Bunce S, Shevrin H. Event-related brain potentials differentiate positive and negative mood adjectives during both supraliminal and subliminal visual processing. International Journal of Psychophysiology. 2001;42:11–34. doi: 10.1016/s0167-8760(01)00133-7. [DOI] [PubMed] [Google Scholar]
- Borg C, Bedoin N, Peyron R, et al. Impaired emotional processing in a patient with a left posterior insula-SII lesion. Neurocase. 2013;19(6):592–603. doi: 10.1080/13554794.2012.713491. [DOI] [PubMed] [Google Scholar]
- Briesemeister BB, Kuchinke L, Jacobs AM. Discrete emotion effects on lexical decision response times. PLoS One. 2011;6:e23743. doi: 10.1371/journal.pone.0023743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manes F, Antoun N, Young AW. Impaired recognition and experience of disgust following brain injury. Nature Neuroscience. 2000;3:1077–8. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- Cauda F, Costa T, Torta DM, Sacco K, D'Agata F, Duca S. Meta-analytic clustering of the insular cortex: characterizing the meta-analytic connectivity of the insula when involved in active tasks. Neuroimage. 2012;62:343–55. doi: 10.1016/j.neuroimage.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron FM. Neural correlates of written emotion word processing: a review of recent electrophysiological and hemodynamic neuroimaging studies. Brain & Language. 2012;122:211–26. doi: 10.1016/j.bandl.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Darwin C. The Expression of the Emotions in Man and Animals. 3rd edn. New York: Oxford University Press; 1872/1998. [Google Scholar]
- Debruille JB, Ramirez D, Wolf Y, et al. Knowledge inhibition and N400: a within- and a between-subjects study with distractor words. Brain Research. 2008;1187:167–83. doi: 10.1016/j.brainres.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: a proposal. Trends Cognitive Sciences. 2005;9:335–41. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Jamison HL, Matthews PM, Gonnerman LM. Morphology and the internal structure of words. Proceedings of the National Academy of Sciences USA. 2004;101:14984–8. doi: 10.1073/pnas.0403766101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischler I, Bradley M. Event-related potential studies of language and emotion: words, phrases, and task effects. Progress in Brain Research. 2006;156:185–203. doi: 10.1016/S0079-6123(06)56009-1. [DOI] [PubMed] [Google Scholar]
- Franken IH, Gootjes L, van Strien JW. Automatic processing of emotional words during an emotional Stroop task. Neuroreport. 2009;20:776–81. doi: 10.1097/WNR.0b013e32832b02fe. [DOI] [PubMed] [Google Scholar]
- Fruhholz S, Jellinghaus A, Herrmann M. Time course of implicit processing and explicit processing of emotional faces and emotional words. Biological Psychology. 2011;87:265–74. doi: 10.1016/j.biopsycho.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Grainger J, Holcomb PJ. Watching the word go by: on the time-course of component processes in visual word recognition. Language and Linguistics Compass. 2009;3:128–56. doi: 10.1111/j.1749-818X.2008.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauk O, Davis MH, Ford M, Pulvermüller F, Marslen-Wilson WD. The time course of visual word recognition as revealed by linear regression analysis of ERP data. Neuroimage. 2006;30:1383–400. doi: 10.1016/j.neuroimage.2005.11.048. [DOI] [PubMed] [Google Scholar]
- Herbert C, Junghofer M, Kissler J. Event related potentials to emotional adjectives during reading. Psychophysiology. 2008;45:487–98. doi: 10.1111/j.1469-8986.2007.00638.x. [DOI] [PubMed] [Google Scholar]
- Herbert C, Kissler J, Junghofer M, Peyk P, Rockstroh B. Processing of emotional adjectives: evidence from startle EMG and ERPs. Psychophysiology. 2006;43:197–206. doi: 10.1111/j.1469-8986.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- Hofmann MJ, Kuchinke L, Tamm S, Vo ML, Jacobs AM. Affective processing within 1/10th of a second: high arousal is necessary for early facilitative processing of negative but not positive words. Cognitive Affective Behavioral Neuroscience. 2009;9:389–97. doi: 10.3758/9.4.389. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Kanske P, Kotz SA. Concreteness in emotional words: ERP evidence from a hemifield study. Brain Research. 2007;1148:138–48. doi: 10.1016/j.brainres.2007.02.044. [DOI] [PubMed] [Google Scholar]
- Kissler J, Assadollahi R, Herbert C. Emotional and semantic networks in visual word processing: insights from ERP studies. Progress in Brain Research. 2006;156:147–83. doi: 10.1016/S0079-6123(06)56008-X. [DOI] [PubMed] [Google Scholar]
- Kissler J, Herbert C, Peyk P, Junghofer M. Buzzwords: early cortical responses to emotional words during reading. Psychological Science. 2007;18:475–80. doi: 10.1111/j.1467-9280.2007.01924.x. [DOI] [PubMed] [Google Scholar]
- Kissler J, Herbert C, Winkler I, Junghofer M. Emotion and attention in visual word processing: an ERP study. Biological Psychology. 2009;80:75–83. doi: 10.1016/j.biopsycho.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Krolak-Salmon P, Henaff MA, Isnard J, et al. An attention modulated response to disgust in human ventral anterior insula. Annals of Neurology. 2003;53:446–53. doi: 10.1002/ana.10502. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP) Annual Review of Psychology. 2011;62:621–47. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman D, Tooby J, Cosmides L. The architecture of human kin detection. Nature. 2007;445:727–31. doi: 10.1038/nature05510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. Journal of Physiology Paris. 2008;102:59–70. doi: 10.1016/j.jphysparis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- McGonigal A, Bartolomei F, Regis J, et al. Stereoelectroencephalography in presurgical assessment of MRI-negative epilepsy. Brain. 2007;130:3169–83. doi: 10.1093/brain/awm218. [DOI] [PubMed] [Google Scholar]
- Molholm S, Sehatpour P, Mehta AD, et al. Audio-visual multisensory integration in superior parietal lobule revealed by human intracranial recordings. Journal of Neurophysiology. 2006;96:721–9. doi: 10.1152/jn.00285.2006. [DOI] [PubMed] [Google Scholar]
- Naccache L, Gaillard R, Adam C, et al. A direct intracranial record of emotions evoked by subliminal words. Proceedings of the National Academy of Sciences USA. 2005;102:7713–7. doi: 10.1073/pnas.0500542102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New B, Pallier C, Ferrand L, Matos R. Une base de données lexicales du français contemporain sur internet: LEXIQUE. L'Année Psychologique. 2001;101:447–62. [Google Scholar]
- Niedenthal PM. Embodying emotion. Science. 2007;316:1002–5. doi: 10.1126/science.1136930. [DOI] [PubMed] [Google Scholar]
- Ohman A, Lundqvist D, Esteves F. The face in the crowd revisited: a threat advantage with schematic stimuli. Journal of Personality and Social Psychology. 2001;80:381–96. doi: 10.1037/0022-3514.80.3.381. [DOI] [PubMed] [Google Scholar]
- Ohman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Williams NL, Lohr JM, Sawchuk CN. The structure of disgust: domain specificity in relation to contamination ideation and excessive washing. Behavioral Research Therapy. 2005;43:1069–86. doi: 10.1016/j.brat.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. International Journal of Psychophysiology. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Pattamadilok C, Perre L, Dufau S, Ziegler JC. On-line orthographic influences on spoken language in a semantic task. Journal of Cognitive Neuroscience. 2009;21:169–79. doi: 10.1162/jocn.2009.21014. [DOI] [PubMed] [Google Scholar]
- Perry C, Ziegler JC, Zorzi M. Beyond single syllables: large-scale modeling of reading aloud with the Connectionist Dual Process (CDP++) model. Cognitive Psychology. 2010;61:106–51. doi: 10.1016/j.cogpsych.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–8. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Grandjean D, Sander D, Vuilleumier P. Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cerebral Cortex. 2004;14:619–33. doi: 10.1093/cercor/bhh023. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F. Brain mechanisms linking language and action. Nature Review Neuroscience. 2005;6:576–82. doi: 10.1038/nrn1706. [DOI] [PubMed] [Google Scholar]
- Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62:816–47. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozin P, Fallon AE. A perspective on disgust. Psychological Review. 1987;94:23–41. [PubMed] [Google Scholar]
- Rozin P, Haidt J, Fincher K. Psychology. From oral to moral. Science. 2009;323:1179–80. doi: 10.1126/science.1170492. [DOI] [PubMed] [Google Scholar]
- Schacht A, Sommer W. Emotions in word and face processing: early and late cortical responses. Brain & Cognition. 2009a;69:538–50. doi: 10.1016/j.bandc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Schacht A, Sommer W. Time course and task dependence of emotion effects in word processing. Cognitive Affective Behavioral Neuroscience. 2009b;9:28–43. doi: 10.3758/CABN.9.1.28. [DOI] [PubMed] [Google Scholar]
- Schafer A, Leutgeb V, Reishofer G, Ebner F, Schienle A. Propensity and sensitivity measures of fear and disgust are differentially related to emotion-specific brain activation. Neuroscience Letters. 2009;465:262–266. doi: 10.1016/j.neulet.2009.09.030. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghofer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: an ERP analysis. Psychophysiology. 2004a;41:441–9. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Ohman A, Junghofer M, Weike AI, Stockburger J, Hamm AO. The facilitated processing of threatening faces: an ERP analysis. Emotion. 2004b;4:189–200. doi: 10.1037/1528-3542.4.2.189. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Stockburger J, Codispoti M, Junghofer M, Weike AI, Hamm AO. Selective visual attention to emotion. Journal of Neuroscience. 2007;27:1082–9. doi: 10.1523/JNEUROSCI.3223-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GG, O'Donnell PJ, Leuthold H, Sereno SC. Early emotion word processing: evidence from event-related potentials. Biological Psychology. 2009;80:95–104. doi: 10.1016/j.biopsycho.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Silva C, Montant M, Ponz A, Ziegler JC. Emotions in reading: disgust, empathy and the contextual learning hypothesis. Cognition. 2012;125:333–8. doi: 10.1016/j.cognition.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Trauer SM, Andersen SK, Kotz SA, Muller MM. Capture of lexical but not visual resources by task-irrelevant emotional words: a combined ERP and steady-state visual evoked potential study. Neuroimage. 2012;60:130–8. doi: 10.1016/j.neuroimage.2011.12.016. [DOI] [PubMed] [Google Scholar]
- van Atteveldt NM, Formisano E, Goebel R, Blomert L. Top-down task effects overrule automatic multisensory responses to letter-sound pairs in auditory association cortex. Neuroimage. 2007;36:1345–60. doi: 10.1016/j.neuroimage.2007.03.065. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–64. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Wright P, He G, Shapira NA, Goodman WK, Liu Y. Disgust and the insula: fMRI responses to pictures of mutilation and contamination. Neuroreport. 2004;15:2347–51. doi: 10.1097/00001756-200410250-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.