Abstract
In everyday life, people adaptively prepare for the future by simulating dynamic events about impending interactions with people, objects and locations. Previous research has consistently demonstrated that a distributed network of frontal–parietal–temporal brain regions supports this ubiquitous mental activity. Nonetheless, little is known about the manner in which specific regions of this network contribute to component features of future simulation. In two experiments, we used a functional magnetic resonance (fMR)-repetition suppression paradigm to demonstrate that distinct frontal–parietal–temporal regions are sensitive to processing the scenarios or what participants imagined was happening in an event (e.g. medial prefrontal, posterior cingulate, temporal–parietal and middle temporal cortices are sensitive to the scenarios associated with future social events), people (medial prefrontal cortex), objects (inferior frontal and premotor cortices) and locations (posterior cingulate/retrosplenial, parahippocampal and posterior parietal cortices) that typically constitute simulations of personal future events. This pattern of results demonstrates that the neural substrates of these component features of event simulations can be reliably identified in the context of a task that requires participants to simulate complex, everyday future experiences.
Keywords: future event simulation, fMRI, repetition suppression, default network
INTRODUCTION
A growing number of neuroimaging studies have delineated neural correlates of the capacity to imagine or simulate future events. These studies of future event simulation have revealed that a distributed network of frontal–parietal–temporal brain regions underlies the flexible capacity to simulate hypothetical events that may one day come to pass in the personal future (Schacter et al., 2008; Szpunar, 2010). In a recent review of the literature, Schacter et al. (2012) noted that an important limitation of existing studies has been the use of relatively unconstrained task designs that do not readily allow the identification of component processes of future event simulation. For instance, simulated future events typically consist of scenarios that involve interactions with familiar people, objects and locations (D’Argembeau and Van der Linden, 2012), yet there has been little progress in identifying and distinguishing among these key components of future event simulations. A more complete and detailed understanding of future event simulation will require the development of paradigms that can systematically isolate the contributions of specific brain regions to specific features of future simulation (for initial attempts, see Hassabis et al., 2007; Szpunar et al., 2009; Andrews-Hanna et al., 2010a).
Here, we present a paradigm in which the content and frequency of simulated future events were systematically varied in order to evoke content-specific repetition-related reductions in neural activity. Functional magnetic resonance (fMR)-repetition suppression is a technique that evokes repetition-related reductions in neural activity to demonstrate that specific regions of the brain are sensitive to processing specific classes of stimuli (Grill-Spector et al., 2006; Schacter et al., 2007b). For instance, fMR-repetition suppression has been used to demonstrate that distinct regions of the medial temporal lobe are sensitive to the initial, relative to repeated, processing of objects and scenes (Litman et al., 2009), or items and their context (Diana et al., 2012). Although much of this research has been conducted within the domain of perceptual processing, the technique has been extended to identify processes involved in making self-other judgments (Jenkins et al., 2008), and more recently to distinguish between novel and repeated future event simulations (V. van Mulukom et al., submitted for publication). We propose that manipulating the content of future event simulation (e.g. whether a future event involves interacting with another person or object) and the frequency with which specific features of that content are simulated (e.g. people, objects and locations) can elucidate which brain regions support which aspects of future event simulation in the context of a complex simulation task.
Across two experiments, participants simulated future social (Exp. 1) or non-social (Exp. 2) events, and we manipulated the frequency with which familiar people (Exp. 1), objects, (Exp. 2) and locations (Exps 1 and 2) were included in those simulations. An advantage of directly manipulating the presentation of people, objects and locations in the context of future simulation is that the extensive research on these component features in non-simulation contexts allowed us to make informed predictions about how various frontal–parietal–temporal brain regions would contribute to the construction of complex event simulations. In particular, we made three predictions. First, simulated social (Exp. 1), but not non-social (Exp. 2), scenarios (i.e. what the participant imagines happening in an event) should preferentially engage a distributed set of medial prefrontal, parietal and lateral temporal regions commonly activated during tasks that focus attention on socially relevant interactions (Hari and Kujala, 2009; Van Overwalle, 2009). Second, simulations involving people (Exp. 1) and objects (Exp. 2) should preferentially engage regions commonly activated by tasks that focus attention on conceptual features of people and objects (such as middle and inferior frontal gyrus, respectively; Wig et al., 2009; Raposo et al., 2011). Third, simulated locations (Exps 1 and 2) should preferentially engage regions commonly activated by tasks that focus attention on scenes (i.e. parahippocampal and retrosplenial cortices; Epstein, 2008). However, activity in parahippocampal cortex should be more pronounced during non-social events as simulations of object use (Exp. 2), more so than interpersonal interactions (Exp. 1), direct attention to one’s immediate surroundings (i.e. objects typically require the individual to be aware of their immediate surroundings; e.g. when bouncing a basketball or writing with a pen; Mullally and Maguire, 2011).
To date, most studies of future simulation map specific frontal–parietal–temporal regions to specific processes on the basis of existing research concerning mental imagery of individual components of simulation (e.g. people or locations). To our knowledge, no paradigm has yet demonstrated the capacity to extract the neural signature of these various components of future thinking in the context of a simulation task involving multiple event components. However, the ability to distinguish the contributions of these regions in the context of a complex simulation task represents an important step toward the ability to conduct direct hypothesis-driven studies of future event simulation.
METHODS
Participants
Participants were 60 right-handed adults with normal or corrected-to-normal vision and with no prior history of neurological or psychiatric impairment. Fourteen participants were excluded from data analysis (six due to scanner error, six due to insufficient responding and two due to excessive movement). The remaining 46 participants [23 in Exp. 1 (14 female; mean age, 21.1 years); and 23 in Exp. 2 (13 female; mean age, 20.1 years)] were included in all subsequent statistical analyses. All participants provided informed written consent in accordance with the guidelines set by the Harvard University Institutional Review Board.
Experiment 1
Stimulus collection and preparation
One week before the fMRI session, participants visited the laboratory and generated a list of 72 familiar people (using Facebook friends lists) and 72 familiar locations that they were most likely to visit in the near future. The following restrictions directed the manner in which participants described their locations (maximum of five words per location): (i) for buildings with more than one room (e.g. My Apartment, William James Hall), participants were instructed to provide the name of a specific room within those buildings (e.g. My Apartment Kitchen; William James Lobby); (ii) participants were permitted to provide the name of only one room per building; (iii) for large areas of open space (e.g. JFK Park; Shaw’s Grocery Store), participants were instructed to name a particular subsection within those areas (e.g. Gates of JFK Park; Shaw’s Express Checkout); and (iv) participants were permitted to name only one subsection per large area of open space. The resulting lists of locations compiled by participants were diverse and tended to include: rooms in friends’ homes, locations on school campus, familiar meeting places and familiar stores. Prior to returning for the fMRI portion of the experiment, a randomized set of location–person pairs were created for each participant (i.e. names of locations were randomly paired with names of people; assignment of location–person pairs to various conditions of the experiment was also completely randomized in order to further offset any uncontrolled effects of stimulus familiarity).
Procedure
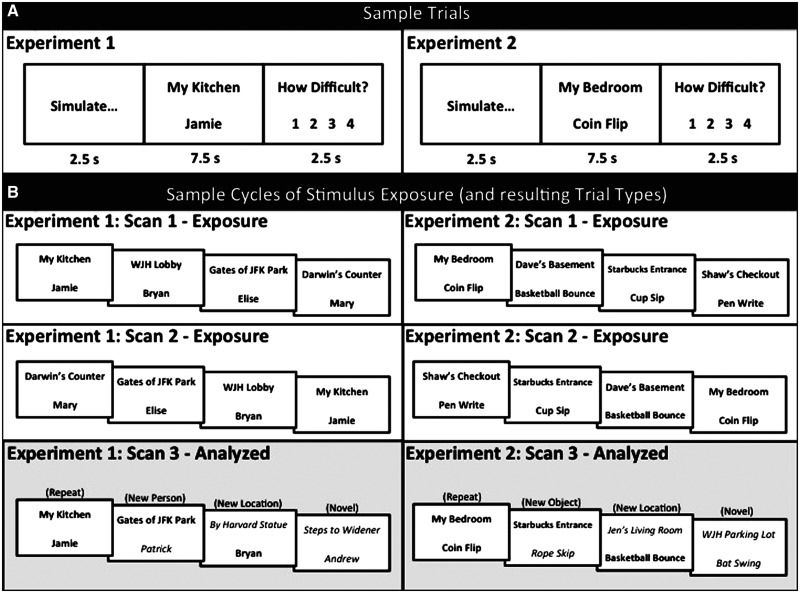
Immediately prior to scanning, participants were given practice with sample experimental trials, and the experimenter made sure that all instructions were fully understood. In the scanner, participants were presented with experimental trials in an event-related manner across nine separate scans. Each trial lasted 12.5 s and consisted of the following sequence (left panel of Figure 1A): (i) the word ‘simulate’ presented in the center of the computer screen for 2.5 s, alerting participants that they were about to simulate a future event; (ii) a location–person pair presented for 7.5 s (location above person), indicating the specific future event participants were to simulate (i.e. use the full trial to imagine a future event in the given location interacting with the given person); and (iii) the question ‘how difficult?’ presented above a four-point rating scale for 2.5 s, alerting participants to rate, with a button press, how difficult it was for them to simulate the future event; 1 = easy, 4 = difficult. The presentation of each trial was randomly interleaved with 2.5, 5, 7.5 or 10 s of fixation, so as to introduce temporal jitter into the experimental design and thereby allow for event-related analyses.
Fig. 1.
Sample trials and cycles of stimulus exposure for Experiments 1 and 2. (A) Left panel: Sample trial for Experiment 1. For each trial, participants first saw the word ‘simulate’ for 2.5 s (alerting participants to simulate a future event), a location–person pair (location above person) for 7.5 s (indicating the event participants were to simulate; i.e. imagine being in the given location interacting with the given person), and a question for 2.5 s (during which participants rated how difficult it was for them to bring the event to mind). Right panel: Trials were identical for participants in Experiment 2, except that people were replaced with objects (i.e. object–action pairs; participants simulated events in which they were in the given location using the given object). (B) Left panels: Sample cycle of stimulus exposure for Experiment 1. Specific events were simulated once in each of the first two ‘exposure’ scans. During a third scan, some of the events were simulated for a third time (repeat trials), some of the events involved a location that was being simulated for a third time, but paired with a new person (new person trials), some of the events involved a person that was being simulated for a third time, but paired with a new location (new location trials), some events involved a new location and a new person (novel trials) and some events involved a location and a person that had been simulated as part of separate pairs, but that were now paired together (repair trials; not pictured). Statistical analyses were based on the five trial types presented during these third (or ‘analysed’) scans. Right panels: Sample cycles of stimulus exposure were identical for Experiment 2, except that people were replaced with objects (i.e. object–action pairs).
The nine scans involving experimental trials were divided into exposure scans and analysed scans (left panels of Figure 1B). During exposure scans (1, 2, 4, 5, 7 and 8) participants simulated future events based on a series of location–person pairs that were systematically related to location–person pairs that would later be presented during analysed scans (3, 6 and 9). For instance, during the first (exposure) scan participants were presented with and simulated a future event in response to 16 location–person pairs. During the second (exposure) scan, participants were presented with and simulated a future event in response to the same 16 location–person pairs from the first scan (presented in a new random order; informal interviews following the experiment indicated that the relatively small number of events that were repeated across scans enabled participants to comfortably simulate the same events). During the third (analysed) scan, participants were presented with and simulated a future event in response to 20 location–person pairs. Four of the analysed scan pairs had been presented in the first and second exposure scans (repeat trials). Four of the analysed scan pairs involved a location that had been presented in the first and second exposure scans, but that was now paired with a new person that had not previously been encountered during the course of a cycle of stimulus exposure (i.e. during the previous two exposure scans; new person trials). Four of the analysed scan pairs involved a person that had been presented in the first and second exposure scans, but that was now paired with a new location that had not previously been encountered in the course of a cycle of stimulus exposure (new location trials). Four of the analysed scan pairs involved both a location and person that had not been previously encountered during the course of a cycle of stimulus exposure (novel trials). Finally, four of the analysed scan pairs involved a location and a person that had been presented in the first and second exposure scans, but as part of separate pairs and were now paired together (repair trials). The 20 analysed scan pairs were presented in random order.
This procedure was repeated during scans 4–6 and scans 7–9 using locations and people that had not previously been encountered during the course of the scanning session. Exposure scans lasted 5 m and 40 s. Analysed scans lasted 6 m and 55 s. All subsequent data analyses are based on data from analysed scans. That is, the effects of repeated simulation on various component features of events were assessed between trials that occurred within the same scan (e.g. the comparison of novel trials against repeat trials was expected to reveal the frontal–parietal–temporal network of brain regions commonly associated with simulating future events). This method of analysis helped to avoid potential problems associated with timing of trial presentations and fatigue that might have arisen if estimates of repetition suppression had been assessed across scans (e.g. examining the neural response associated with repeated presentations of repeat trials).1
Data acquisition and analysis
fMRI acquisition
Imaging was conducted on a 3T Siemens Magnetom TimTrio Scanner, equipped with a 12-channel head coil. A laptop computer running E-Prime software controlled stimulus display via an LCD projector, which projected onto a screen placed at the head of the bore. Participants viewed the screen through a mirror fastened to the head coil. Cushions were used to minimize head movement and earplugs dampened scanner noise. Participants made responses using a button box placed in their right hand.
Anatomical images were acquired using a high-resolution three-dimensional magnetization-prepared rapid gradient echo sequence (MPRAGE; 176 sagittal slices, echo time [TE] = 1.64 ms, repetition time [TR] = 2530 ms, flip angle = 7°, voxel size = 1 mm × 1 mm × 1 mm). Functional images (136 for exposure scans, 166 for analysed scans) were collected using a T2* gradient echo, echo-planar imaging (EPI) sequence sensitive to blood oxygen level-dependent (BOLD) contrast (TR = 2500 ms, TE = 30 ms, flip angle = 90°, 3 mm × 3 mm in-plane resolution). Whole-brain coverage was obtained with 39 contiguous slices, acquired parallel to the anterior–posterior commissure plane (3 mm slice thickness, 0.5 mm skip between slices).
Imaging analyses
Imaging data acquired during analysed scans were preprocessed and statistically evaluated using SPM8 (Wellcome Department of Imaging Neuroscience, London, UK). First, these data were preprocessed to remove sources of noise and artifact. The first four volumes (10 s) of each scan were excluded from analyses to account for T1 saturation effects. Preprocessing included slice-time correction to correct for differences in acquisition time between slices for each whole-brain volume; realignment within and across runs to correct for head movement; spatial normalization to the Montreal Neurological Institute (MNI) template (resampled at 2 mm × 2 mm × 2 mm voxels); and spatial smoothing [8 mm full-width at half maximum (FWHM)] using a Gaussian kernel.
Preprocessed data were analysed using the general linear model. For each participant (i.e. fixed effects models), the BOLD response to each trial type (i.e. repeat trials, novel trials, new person trials, new location trials and repair trials) was modeled using SPM8’s canonical hemodynamic response function over a 12.5 s time window (i.e. an epoch) that immediately followed trial onset. Because the only difference across trials was the type of event that was being simulated (e.g. novel trials vs repeat trials), it was assumed that statistical differences emerging as a result of contrasts between trial types could be safely attributed to differences in the makeup of events rather than to the processing of the initial simulate cue or the subsequent difficulty rating (no reaction time differences across conditions; see Results).
The results of the fixed effects analyses were moved forward to a group-level (i.e. random effects) analysis. These analyses involved planned contrasts across the five trial types (within the context of a One-Way Analysis of Variance) that were meant to identify regions involved in constructing simulated representations of future social events, the scenarios that make up future social events (i.e. what the participant imagines happening in an event), the people involved in future social events, and the locations where future social events take place. Additionally, our experimental design allowed us to isolate neural correlates associated with event novelty and repetition. The underlying logic of each of the planned contrasts were as follows: (i) simulated representations of future social events [novel trials (i.e. events simulated for the first time) > repeat trials (i.e. events simulated a third time)]; (ii) simulated representations of the scenarios that make up future social events (i.e. what the participant imagines happening in an event) [(new person trials) + (new location trials) > repeat trials, simulating a new person or a new location necessarily required participants to generate new scenarios, whereas repeat trials involved re-simulating a scenario for a third time (this analysis was further supplemented by comparing repair trials against repeat trials; repair trials involved locations and people that had separately been encountered over the course of a cycle of stimulus exposure, but that were now paired together, and hence also required participants to generate a new scenario)]; (iii) the people involved in future social events [(new person trials > repeat trials, holding constant the number of times a location had been simulated, new person trials required participants to simulate a person for the first time, whereas repeat trials involved re-simulating a person for a third time) exclusively masked with (new location trials > repeat trials, to ensure that activations were unique to people)]; (iv) the locations where future social events take place [(new location trials > repeat trials, holding constant the number of times a person had been simulated, new location trials required participants to simulate a location for the first time, whereas repeat trials involved re-simulating a location for a third time) exclusively masked with (new person trials > repeat trials, in order to assure that activations were unique to locations)]; (v) novelty [(novel trials > repeat trials) (i.e. trials involving both a new person and a new location) exclusively masked with (new location + new person trials > repeat trials) (i.e. trials involving only a new person or only a new location]; and (vi) repetition [repeat trials (i.e. events simulated for a third time) > novel trials (i.e. events simulated for the first time)].
Each contrast (i.e. direct contrasts, conjunctions and masked contrasts) was whole-brain corrected (FWE) at P < 0.05. An extent threshold of five contiguously activated voxels (2 mm × 2 mm × 2 mm) was applied. Finally, the peak MNI coordinates of active regions were converted to Talairach space, and regions of activations were localized in reference to a standard stereotaxic atlas.
Experiment 2
Stimulus collection and preparation
One week before the fMRI session, participants visited the laboratory and generated a list of 72 familiar locations according to the parameters outlined in Experiment 1. Additionally, a list of 72 object–action pairs (e.g. pen–write, basketball–bounce) that were most familiar to the sample of participants from whom we were drawing (i.e. Boston University undergraduates) was compiled in a pilot experiment (N = 20) and subsequently used for each participant in the fMRI study. Prior to returning for the fMRI portion of the experiment, a randomized set of location–object–action pairs were created for each participant. Although participants in Experiment 2 did not generate the list of object–action pairs, post-scan interviews determined that participants had imagined using personal objects from their own lives (e.g. when prompted by the cue pen–write, participants imagined using a pen of their own).
Procedure, data acquisition and analysis
All aspects of Experiment 2 were identical to that of Experiment 1 except that names of familiar people were replaced by names of familiar objects (i.e. object–action pairs; see right panels of Figure 1A and B). As a result the contrasts of interest were designed to isolate: non-social future events, non-social scenarios, objects, locations, novelty and repetition.
RESULTS
Experiments 1 and 2
Behavioral results
Analysis of participants’ ratings of difficulty demonstrated a behavioral priming effect in Experiment 1 [χ2 (4, N = 23) = 18.25, P < 0.001] and Experiment 2 [χ2 (4, N = 23) = 14.23, P = 0.006], such that repeat trials (Mdn = 1) came to mind more easily than all other trial types (Medians = 2), lowest z = 2.16, P = 0.031; no other differences emerged between trial types. Importantly, reaction times for difficulty ratings did not differ as a function of trial type in Experiment 1 (Grand M = 1058 ms; F < 1) or Experiment 2 [Grand M = 1009 ms; F (4, 88) = 1.23, P = 0.304], and ratings of difficulty (all zs < 1) and reaction times (F < 1) did not differ across the two experiments.
Simulating future social and non-social events
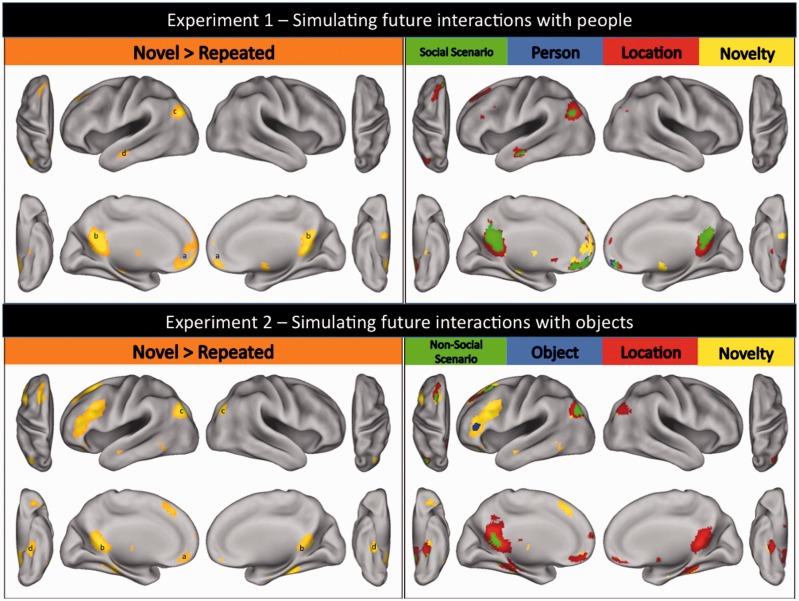
Initial, relative to repeated, simulations (i.e. novel trails > repeat trials) of future social (Exp. 1; see top left panel of Figure 2; Table 1) and non-social (Exp. 2; see bottom left panel of Figure 2; Table 2) events recruited distributed sets of frontal–parietal–temporal brain regions commonly associated with future event simulation (Schacter et al., 2007b, 2008; Szpunar, 2010). Specifically, simulations of future social events were associated with peaks of activity in (a) medial prefrontal; (b) posterior cingulate; (c) left angular; and (d) left middle temporal (extending to superior temporal sulcus) cortices, whereas simulations of future non-social events were associated with peaks of activity in (a) medial prefrontal; (b) retrosplenial; (c) bilateral angular (extending to occipital); and (d) bilateral parahippocampal cortices. While informative, these general contrasts do not reveal which regions are involved in constructing scenarios, people, objects or locations that constitute simulations of future social and non-social events. To elucidate which regions underlie these processes, we further compared and contrasted new person (Exp. 1), new object (Exp. 2), new location (Exps 1 and 2), and repair (Exps 1 and 2) trials against repeat trials (as outlined in the Methods).
Fig. 2.
Results for Experiments 1 and 2. Top left panel: Regions of the brain demonstrating repetition-related reductions in neural activity for simulated future social events in Experiment 1 [i.e. greater activity when participants simulated future social events for the first time (i.e. novel trials) relative to a third time (i.e. repeat trials); whole-brain corrected (FWE) at P < 0.05; prominent activations include (a) medial prefrontal; (b) posterior cingulate; (c) left angular; and (d) left middle temporal cortices]. Top right panel: Regions involved in constructing simulated representations of future social events that are preferentially associated with processing details about: (1) social scenarios (prominent activations include medial prefrontal, posterior cingulate, left temporal–parietal and left middle temporal cortices); (2) people (prominent activations include medial prefrontal cortex); (3) locations (prominent activations include posterior cingulate cortex and left angular gyrus); and (4) novelty (prominent activations include anterior cingulate cortex and left hippocampus) [all activations whole-brain corrected (FWE) at P < 0.05]. Bottom left panel: Regions of the brain demonstrating repetition-related reductions in neural activity for simulated future non-social events in Experiment 2 [i.e. greater activity when participants simulated future non-social interactions for the first time (i.e. novel trials) relative to a third time (i.e. repeat trials); whole-brain corrected (FWE) at P < 0.05; prominent activations include (a) medial prefrontal; (b) retrosplenial; (c) bilateral angular; and (d) bilateral parahippocampal cortices]. Bottom right panel: Regions involved in constructing simulated representations of future non-social events that are preferentially associated with processing details about: (1) non-social scenarios [prominent activations include retrosplenial cortex and left angular gyrus (extending to occipital cortex)]; (2) objects (prominent activations include left inferior prefrontal and left premotor cortices); (3) locations (prominent activations include retrosplenial, bilateral parahippocampal and right angular cortices); and (4) novelty (prominent activations include left lateral prefrontal cortex) [all activations whole-brain corrected (FWE) at P < 0.05].
Table 1.
Simulating future interactions with people
| Novel > Repeated | Social scenario | Person | Location | Novelty | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | BA | x | y | z | t | Voxels | Region | BA | x | y | z | t | Voxels | Region | BA | x | y | z | t | Voxels | Region | BA | x | y | z | t | Voxels | Region | BA | x | y | z | t | Voxels |
| FRONTAL | FRONTAL | FRONTAL | FRONTAL | FRONTAL | ||||||||||||||||||||||||||||||
| mPFC | 10 | −6 | 55 | 19 | 7.04 | 1580 | a/v mPFC | 10 | −4 | 44 | −9 | 6.88 | 434 | a/v mPFC | 10 | 1 | 50 | −9 | 5.86 | 28 | L mid. FG | 8 | −21 | 33 | 42 | 6.45 | 745 | ACC | 32 | −10 | 40 | 9 | 5.92 | 451 |
| dmPFC | 8 | −12 | 44 | 41 | 5.36 | 58 | −8 | 46 | −3 | 5.52 | 22 | ACC | 32 | −4 | 42 | 10 | 5.62 | 161 | 32 | −10 | 28 | −4 | 5.69 | 54 | ||||||||||
| −4 | 56 | 15 | 4.96 | 21 | a/v mPFC | 11 | 0 | 38 | −14 | 5.53 | 145 | dmPFC | 8 | −15 | 38 | 50 | 5.48 | 6 | ||||||||||||||||
| 10 | −2 | 52 | 0 | 5.28 | 161 | amPFC | 10 | 9 | 44 | −9 | 5.07 | 10 | ||||||||||||||||||||||
| L dlPFC | 9 | −37 | 8 | 30 | 5.04 | 6 | ||||||||||||||||||||||||||||
| 46 | −42 | 21 | 22 | 5.01 | 12 | |||||||||||||||||||||||||||||
| PARIETAL | PARIETAL | PARIETAL | PARIETAL | PARIETAL | ||||||||||||||||||||||||||||||
| PCC | 31 | −6 | −58 | 22 | 8.35 | 1476 | PCC | 31 | −5 | −54 | 24 | 8.09 | 944 | L AG | 39 | −40 | −71 | 28 | 8.29 | 815 | Rsp | 29 | −1 | −47 | 7 | 5.94 | 54 | |||||||
| L AG | 39 | −40 | −76 | 30 | 7.56 | 430 | L TPJ | 39 | −42 | −66 | 26 | 5.73 | 55 | L PCC | 30 | −13 | −54 | 17 | 7.51 | 1094 | ||||||||||||||
| R preC | 19 | 39 | −74 | 37 | 5.86 | 116 | ||||||||||||||||||||||||||||
| TEMPORAL | TEMPORAL | TEMPORAL | TEMPORAL | TEMPORAL | ||||||||||||||||||||||||||||||
| L mid. TG | 21 | −58 | −14 | −10 | 6.65 | 183 | L mid. TG | 21 | −58 | −14 | −10 | 5.82 | 110 | L mid. TG | 21 | −62 | −18 | −8 | 6.34 | 193 | L HF | −25 | −27 | −3 | 6.19 | 131 | ||||||||
| L HF | −25 | −27 | −3 | 6.19 | 152 | L HF | −28 | −35 | −6 | 5.74 | 42 | L PHG | 28 | −17 | −14 | −10 | 5.37 | 35 | ||||||||||||||||
| L PHG | 28 | −17 | −14 | −10 | 5.37 | 35 | R PHG | 36 | 25 | −31 | −11 | 5.37 | 35 | |||||||||||||||||||||
| SUB-GYRAL | SUB-GYRAL | SUB-GYRAL | SUB-GYRAL | SUB-GYRAL | ||||||||||||||||||||||||||||||
| Midbrain | −1 | −21 | −8 | 5.79 | 115 | Midbrain | 0 | −31 | −16 | 5.1 | 7 | Midbrain | 0 | −31 | −14 | 5.21 | 7 | Midbrain | −3 | −23 | −8 | 5.44 | 58 | Midbrain | 15 | −10 | −1 | 6.04 | 85 | |||||
| 15 | −10 | −1 | 5.6 | 85 | L Caudate | −6 | 9 | 0 | 5.22 | 17 | 1 | −19 | −6 | 5.67 | 32 | |||||||||||||||||||
| Thalamus | −3 | −9 | 8 | 5.36 | 47 | L Putamen | −16 | 7 | 1 | 4.93 | 7 | Thalamus | −3 | −9 | 8 | 5.36 | 47 | |||||||||||||||||
| L Caudate | −11 | 8 | 18 | 5.01 | 7 | Midbrain | −1 | −29 | −16 | 5.32 | 17 | |||||||||||||||||||||||
| L GP | −18 | 1 | 0 | 4.93 | 9 | L Caudate | −11 | 8 | 18 | 5.01 | 7 | |||||||||||||||||||||||
| L GP | −18 | 1 | 0 | 4.93 | 6 | |||||||||||||||||||||||||||||
| CEREBELLUM | CEREBELLUM | CEREBELLUM | CEREBELLUM | CEREBELLUM | ||||||||||||||||||||||||||||||
| R | 4 | −53 | −32 | 7.97 | 745 | R | 6 | −56 | −36 | 6.21 | 93 | R | 4 | −51 | −27 | 6.47 | 323 | R | 6 | −57 | −27 | 6.4 | 347 | |||||||||||
| 17 | −71 | −19 | 5.66 | 244 | 17 | −73 | −21 | 5.64 | 191 | 9 | −81 | −24 | 5.29 | 64 | ||||||||||||||||||||
| 43 | −70 | −27 | 5.36 | 24 | 43 | −69 | −25 | 5.02 | 7 | 39 | −71 | −27 | 5.17 | 18 |
BA, Brodmann's area; L, left; R, right; a, anterior; d, dorsal; l, lateral; m, medial; mid, middle; v, ventral; ACC, anterior cingulate cortex; AG, angular gyrus; FG, frontal gyrus; GP, globus pallidus; HF, Hippocampal Formation; PCC, posterior cingulate cortex; PFC; prefrontal cortex; PHG, parahippocampal gyrus; preC, precuneus; Rsp, retrosplenial cortex; TG, temporal gyrus; TPJ, temporal–parietal junction
Table 2.
Simulating future interactions with objects
| Novel > Repeated | Non-social scenario | Object | Location | Novelty | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | BA | x | y | z | t | Voxels | Region | BA | x | y | z | t | Voxels | Region | BA | x | y | z | t | Voxels | Region | BA | x | y | z | t | Voxels | Region | BA | x | y | z | t | Voxels | |||
| FRONTAL | FRONTAL | FRONTAL | FRONTAL | FRONTAL | |||||||||||||||||||||||||||||||||
| L sFG | 8 | −17 | 20 | 46 | 7.39 | 1295 | L sFG | 8 | −19 | 20 | 44 | 5.48 | 99 | L iFG | 46 | −43 | 28 | 13 | 5.46 | 61 | a/v mPFC | 10 | −10 | 41 | −10 | 6.7 | 491 | L sFG | 8 | −15 | 18 | 46 | 6.8 | 765 | |||
| L iFG | 47 | −27 | 22 | −4 | 6.18 | 273 | L pMC | 6 | −24 | 12 | 46 | 5.02 | 14 | −8 | 54 | 15 | 5.48 | 70 | L iFG | 47 | −27 | 22 | −4 | 6.18 | 273 | ||||||||||||
| L mPFC | 10 | −12 | 39 | −8 | 6.03 | 292 | L sFG | 8 | −20 | 38 | 38 | 6.1 | 499 | L ACC | 32 | −12 | 37 | −3 | 5.54 | 22 | |||||||||||||||||
| 9 | −6 | 54 | 15 | 4.95 | 8 | R | 24 | 27 | 36 | 5.4 | 32 | 0 | 35 | −8 | 4.97 | 10 | |||||||||||||||||||||
| R mid. FG | 8 | 24 | 27 | 34 | 5.1 | 15 | R dlPFC | 9 | 25 | 27 | 32 | 4.99 | 5 | ||||||||||||||||||||||||
| PARIETAL | PARIETAL | PARIETAL | PARIETAL | PARIETAL | |||||||||||||||||||||||||||||||||
| L AG | 39 | −40 | −76 | 31 | 8.14 | 701 | L AG | 19 | −42 | −77 | 30 | 6.08 | 204 | Rsp | 29 | −3 | −47 | 9 | 8.29 | 2090 | L preC | 19 | −28 | −79 | 37 | 5.78 | 117 | ||||||||||
| R | 41 | −74 | 31 | 6.42 | 312 | L Rsp | 29 | −5 | −51 | 11 | 5.94 | 152 | L preC | 39 | −33 | −75 | 33 | 8.13 | 449 | R iPL | 40 | 35 | −69 | 31 | 5.13 | 9 | |||||||||||
| L Rsp | 29 | −3 | −51 | 11 | 7.5 | 904 | R PCC | 30 | 16 | −47 | 16 | 5.22 | 34 | R AG | 39 | 41 | −74 | 31 | 6.7 | 386 | |||||||||||||||||
| TEMPORAL | TEMPORAL | TEMPORAL | TEMPORAL | TEMPORAL | |||||||||||||||||||||||||||||||||
| L PHG | 36 | −21 | −39 | −8 | 7.37 | 403 | L FusG | 37 | −28 | −48 | −2 | 5.18 | 21 | L PHG | 36 | −19 | −39 | −8 | 8.13 | 578 | L mid. TG | 37 | −47 | −52 | −1 | 6.24 | 171 | ||||||||||
| 35 | −19 | −19 | −10 | 4.94 | 7 | R | 25 | −35 | −11 | 6.98 | 348 | L PHG | 36 | −26 | −33 | −13 | 5.9 | 86 | |||||||||||||||||||
| R | 36 | 25 | −25 | −11 | 7.21 | 280 | R | 28 | 19 | −16 | −16 | 4.97 | 6 | ||||||||||||||||||||||||
| 28 | 19 | −16 | −16 | 4.97 | 6 | R FusG | 37 | 27 | −39 | −13 | 5.52 | 29 | |||||||||||||||||||||||||
| L mid. TG | 37 | −47 | −52 | −1 | 6.24 | 171 | L sTG | 22 | −51 | −11 | −10 | 5.1 | 12 | ||||||||||||||||||||||||
| L sTG | 22 | −51 | −11 | −10 | 5.1 | 12 | |||||||||||||||||||||||||||||||
| OCCIPITAL | OCCIPITAL | OCCIPITAL | OCCIPITAL | OCCIPITAL | |||||||||||||||||||||||||||||||||
| R sOG | 19 | 41 | −80 | 28 | 5.33 | 24 | |||||||||||||||||||||||||||||||
| R mid. OG | 39 | 53 | −66 | 25 | 5.03 | 10 | |||||||||||||||||||||||||||||||
| SUB-GYRAL | SUB-GYRAL | SUB-GYRAL | SUB-GYRAL | SUB-GYRAL | |||||||||||||||||||||||||||||||||
| L Insula | 13 | −38 | 18 | 18 | 7.34 | 1608 | L Insula | 13 | −38 | 20 | 16 | 4.85 | 7 | L Insula | 13 | −37 | 18 | 18 | 4.97 | 6 | Thalamus | 21 | −26 | 15 | 5.4 | 31 | Insula | 13 | −37 | 18 | 20 | 715 | 1538 | ||||
| L Caudate | −21 | 16 | 13 | 5.65 | 60 | −2 | −7 | 8 | 5.13 | 9 | L Caudate | −21 | 16 | 13 | 5.65 | 60 | |||||||||||||||||||||
| Thalamus | 13 | −13 | 10 | 4.9 | 12 | −9 | −26 | 15 | 5.12 | 13 | Thalamus | 13 | −13 | 10 | 4.9 | 12 | |||||||||||||||||||||
| −5 | −17 | 10 | 4.87 | 6 | R GP | 9 | −4 | 0 | 4.99 | 7 | −5 | −17 | 10 | 4.87 | 6 | ||||||||||||||||||||||
| CEREBELLUM | CEREBELLUM | CEREBELLUM | CEREBELLUM | CEREBELLUM | |||||||||||||||||||||||||||||||||
| R | 12 | −70 | −21 | 6.73 | 750 | R | 10 | −48 | −37 | 6.97 | 288 | R | 12 | −70 | −21 | 6.73 | 666 | ||||||||||||||||||||
| 17 | −38 | −32 | 5.73 | 125 | 13 | −85 | −23 | 5.63 | 99 | 10 | −53 | −27 | 5.28 | 13 | |||||||||||||||||||||||
| 33 | −68 | −34 | 5.01 | 8 | 37 | −70 | −34 | 5.5 | 127 | ||||||||||||||||||||||||||||
| 17 | −71 | −17 | 5.1 | 12 | |||||||||||||||||||||||||||||||||
| L | −15 | −44 | −34 | 5.33 | 27 | ||||||||||||||||||||||||||||||||
BA, Brodmann's area; L, left; R, right; a, anterior; d, dorsal; i, inferior; l, lateral; m, medial; mid, middle; s, superior; v, ventral; ACC, anterior cingulate cortex; AG, angular gyrus; FG, frontal gyrus; FusG, Fusiform gyrus; GP, globus pallidus; OG, occipital gyrus; PL; parietal lobule; PCC, posterior cingulate cortex; PFC; prefrontal cortex; pMC, premotor cortex; PHG, parahippocampal gyrus; preC, precuneus; Rsp, retrosplenial cortex; TG, temporal gyrus
Simulating scenarios in social and non-social events
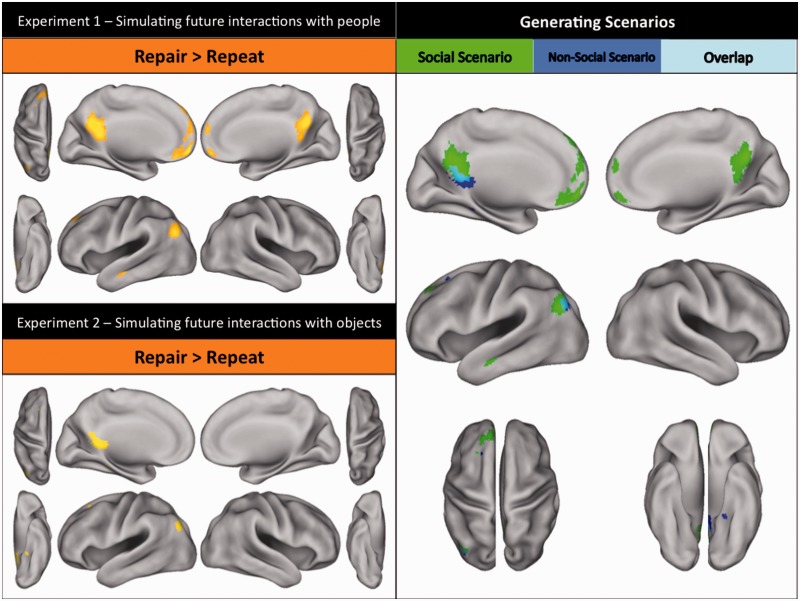
As predicted on the basis of functional brain imaging studies of social interaction (e.g. Hari and Kujala, 2009; Van Overwalle, 2009), initial, relative to repeated, simulations of social scenarios (i.e. what the event is about; Exp. 1; see top right panel of Figure 2; Table 1) engaged dorsal/anterior/ventral medial prefrontal, posterior cingulate, left temporal–parietal and left middle temporal (extending to superior temporal sulcus) cortices. Conversely, initial, relative to repeated, simulations of non-social scenarios (Exp. 2; bottom right panel of Figure 2; Table 2) most prominently engaged retrosplenial cortex and left angular gyrus (extending to occipital cortex). Importantly, regions associated with simulated representations of social (Exp. 1) and non-social (Exp. 2) scenarios demonstrated little overlap, suggesting that simulations of social and non-social scenarios are largely subserved by distinct neural substrates (Figure 3).
Fig. 3.
Regions involved in constructing simulated representations of scenarios in Experiments 1 and 2 as identified by the contrast of (new person trials + new location trials) > repeat trials, i.e. trials requiring the generation of a new scenario against those that involved re-simulating a previous scenario. Notably, an identical pattern of results arose when repair trials (also requiring the generation of a new scenario) were contrasted against repeat trials. Top left panel: Regions involved in constructing simulated representations of social scenarios in Experiment 1 [activations include bilateral medial prefrontal, bilateral posterior cingulate, left temporal–parietal, and left middle temporal cortices; whole-brain corrected (FWE) at P < 0.05]. Bottom left panel: Regions involved in constructing simulated representations of non-social scenarios in Experiment 2 [activations include left frontal, left retrosplenial, left fusiform and left posterior parietal cortices; whole-brain corrected (FWE) at P < 0.05]. Right panel: A descriptive visualization of the anatomical overlap between regions involved in constructing social and non-social scenarios.
It is possible, however, that new person, new location and repair trials (Exp. 1) did not evoke entirely new scenarios; new person trials might have simply involved imagining the same scenario in a particular location, but with a new person. If so, then processes related to elaboration of prior scenarios could explain this particular aspect of the results. This possibility also applies to new object, new location and repair trials in Experiment 2. Hence, future work may need to more clearly discriminate between the generation of novel scenarios and elaboration of prior scenarios.
Simulating people in social events and objects in non-social events
As predicted on the basis of functional brain imaging studies of person and object representation, initial, relative to repeated, simulations of people (Exp. 1; see top right panel of Figure 2; Table 1) engaged a number of anterior/ventral medial prefrontal regions (e.g. Raposo et al., 2011), whereas initial, relative to repeated, simulations of objects (Exp. 2; see bottom right panel of Figure 2; Table 2) engaged regions of left inferior prefrontal and left premotor cortices (e.g. Wig et al., 2009).
Simulating locations in social and non-social events
As predicted on the basis of functional brain imaging studies of location representation (e.g. Epstein, 2008), initial, relative to repeated, simulations of locations in the context of social events (Exp. 1; see top right panel of Figure 2; Table 1) engaged a distributed set of medial parietal and temporal brain regions, including most prominently left posterior cingulate (extending to right) and left angular gyrus, and some activity in the medial temporal lobe (left hippocampus and right parahippocampal gyrus). Similarly, initial simulations of locations in the context of non-social events (bottom right panel of Figure 2; Table 2) also engaged a distributed set of medial parietal and temporal brain regions, including most prominently retrosplenial, bilateral parahippocampal and right angular cortices. Importantly, medial temporal lobe activity (particularly in bilateral parahippocampal gyri) was considerably more extensive when simulated future events revolved around interactions with objects (Exp. 2) than with people (Exp. 1) (Figure 2), suggesting that the manner in which features of the simulated environment are processed differs across the two contexts.
Novelty and repetition
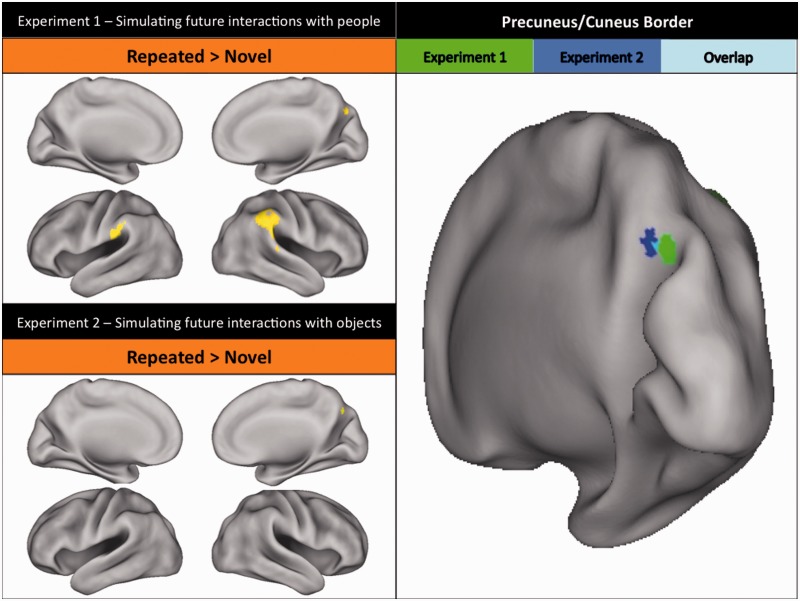
Initial simulations of completely novel social events (top right panel of Figure 2; Table 1) were associated with activity in a distributed set of brain regions including anterior cingulate cortex, left hippocampus and right cerebellum. On the other hand, initial simulations of completely novel non-social events (bottom right panel of Figure 2; Table 2) most prominently activated left lateral prefrontal cortex, left insula and right cerebellum. Repeated simulations of future social and non-social events were both associated with activity in right precuneus/cuneus border (Figure 4; BA 7, Exp. 1, xyz = 14, −68, 32, Exp. 2, xyz = 10, −70, 34). Moreover, repeated future social events were associated with activity in bilateral posterior parietal lobule (Figure 4; BA 40; xyz = −64, −32, 31 and 61, −31, 40). We elaborate further on the potential significance of our findings in relation to event novelty and repetition in the Discussion section.
Fig. 4.
Regions demonstrating greater activity for repeated events than for novel events (i.e. repetition enhancement). Top left panel: Regions demonstrating repetition enhancement for events that involved interactions with people in Experiment 1 [i.e. repeat trials > novel trials; whole-brain corrected (FWE) at P < 0.05]. Significant activations include bilateral inferior parietal lobule and right precuneus/cuneus border. Bottom left panel: Regions demonstrating repetition enhancement for events that involved interactions with objects in Experiment 2 [i.e. repeat trials > novel trials; whole-brain corrected (FWE) at P < 0.05]. Significant activations include right precuneus/cuneus border. Right panel: A descriptive visualization of the anatomical overlap in right precuneus/cuneus border across Experiments 1 and 2.
DISCUSSION
A rapidly growing line of research has demonstrated that a distributed set of frontal–parietal–temporal brain regions support the capacity to simulate personal future events (Schacter et al., 2007a; Buckner et al., 2008; Schacter et al., 2008, 2012; Spreng et al., 2009; Szpunar, 2010). The present results show that repetition-related reductions in neural activity can be used to identify the contributions of specific regions to specific features of future event simulation in the context of a task that requires simulation of complex, everyday future experiences. By manipulating the content of future event simulation and the frequency with which that content was simulated, we showed that distinct regions support the simulated social scenarios (medial prefrontal, posterior cingulate, temporal–parietal and middle temporal cortices), people (medial prefrontal cortex), objects (inferior frontal and premotor cortices) and locations (posterior cingulate/retrosplenial, parahippocampal and lateral parietal cortices) that typically constitute simulations of the personal future.
This pattern of results provides a powerful demonstration that functional brain imaging techniques can be used to reliably dissociate component features of multi-faceted mental simulations in the context of an experimental task that invokes complex imagined scenarios that are similar to those that characterize future thinking in everyday settings. It is important to note, however, that the features of future event simulation identified here (i.e. scenarios, people, objects and locations) also represent multidimensional constructs, and future research will need to further elucidate the contributions of specific regions. For instance, what unique or overlapping processes do medial prefrontal, posterior cingulate, temporal–parietal and middle temporal cortices contribute to the generation of simulated social scenarios? The present results suggest that repetition suppression techniques represent a fruitful avenue for answering such questions.
Our results also elucidate the neural correlates of integrated future events, as opposed to individual features of events, in terms of both novelty and repetition. Although various regions were found to be sensitive to specific features of future event simulation, other regions were only sensitive to events that were novel in all respects (i.e. events that involved a novel scenario, location and person/object). Notably, the identity of regions sensitive to event novelty differed depending on whether participants had simulated future interactions with people (most prominent activations in anterior cingulate cortex and left hippocampus; V. van Mulukom et al., submitted for publication) or objects (most prominent activations in left lateral prefrontal cortex and left insula). This pattern suggests that the neural signature of event novelty may be systematically related to the content of simulation, as was the case with specific features of simulation. Although the present experiments were not designed to isolate processes that have previously been associated with hippocampus (for review and discussion, see Addis and Schacter, 2012), it is noteworthy that this region was responsive to event novelty (V. van Mulukom et al., submitted for publication).
With regard to event repetition, precuneus/cuneus border was found to track the frequency with which future events had been simulated (right panel of Figure 4). Indeed, previous studies have shown that a region similar to precuneus/cuneus border observed in the current study was preferentially engaged by repeated stimuli in a variety of contexts (e.g. identifying environmental sounds, Bergerfest et al., 2004; repeated recall of word pairs, Hashimoto et al., 2011; sentence judgments, Hasson et al., 2006; facial identification, Ida Gobbini and Haxby, 2006; and continuous recognition of colored photos, Suzuki et al., 2011). One possibility is that precuneus/cuneus border processes information that correlates with repetition (Szpunar and Schacter, 2013), such as subjective detail (Fletcher et al., 1995; Cavanna and Trimble, 2006) and/or plausibility (Weiler et al., 2010). This suggestion, however, raises an interesting puzzle. Given that activity in precuneus was dissociated from activity in regions sensitive to specific features of future event simulations (see also Buckner et al., 2008; Margulies et al., 2009; Andrews-Hanna et al., 2010b), how is information related to frequency (or perhaps detail or plausibility) of simulation transferred to precuneus? The development of a more complete understanding of the relations between frontal–parietal–temporal regions involved in representing various features of future event simulation and precuneus represents an exciting avenue for future research.
Notably, the frontal–parietal–temporal regions associated with future event simulation in this and other experiments (Addis et al., 2007; Szpunar et al., 2007) closely resemble the default network that has become synonymous in the literature with cognitive tasks that require attention to be directed inwards, and away from the external environment (Shulman et al., 1997; Raichle et al., 2001; Buckner et al., 2008; Andrews-Hanna et al., 2010b; Andrews-Hanna, 2012; Spreng, 2012). Nonetheless, a recent meta-analysis demonstrated that although default regions are similar to those involved in future event simulation, they do not overlap completely (Spreng et al., 2009). Moreover, recent studies have shown that regions not typically associated with the default network are commonly engaged by tasks such as autobiographical memory retrieval and future event simulation (Spreng et al., 2010; St. Jacques et al., 2011). Accordingly, although it will be important for future research to examine the extent to which activity associated with integrated brain networks, such as the default network, can be deconstructed using techniques such as the one presented here, we stress that complex cognitions such as future event simulation draw on a number of brain regions that are likely distributed across various brain networks. Moving forward, it will be important not only to understand the manner in which such networks interact with one another, but also to avoid limiting our understanding of complex cognitive functions such as future event simulation by attempting to isolate them to the domain of singular networks. As an example, Margulies et al. (2009) demonstrated that ventral precuneus possesses transitory connectivity with posterior cingulate/retrosplenial cortex, a hub of the default network; an observation that potentially helps to elucidate how precuneus interacts with regions of the brain responsible for representing various aspects of simulated events.
CONCLUSION
In summary, future event simulation represents a dynamic cognitive activity that typically revolves around a number of closely interrelated features, including simulated scenarios, people, objects and locations. Such simulations constitute an adaptive feature of cognition that serve important functions in everyday life (Ingvar, 1979, 1985; Suddendorf and Corballis, 1997, 2007; Schacter et al., 2008; Szpunar, 2010; Schacter, 2012; Hassabis et al., in press). Understanding the neural basis of this adaptive process requires an ability to distinguish among the brain regions that support component features of mental simulations. In two experiments, we have demonstrated that manipulations of the content and frequency of future event simulation can reliably bring about content-specific repetition-related reductions in neural activity that help to identify the contributions of specific regions to complex simulations of the future. Functional brain imaging techniques that can be used to identify the components of complex future event simulations, as shown here, should not only broaden and deepen our understanding of future thinking in healthy individuals, but could also provide novel insights into the breakdown of simulation-related processes in various disorders of future thinking, including such conditions as amnesia, depression and anxiety (for review, see Addis and Schacter, 2012; Schacter et al., 2008, 2012; Szpunar, 2010). We are thus optimistic that the approach described here constitutes only a first step toward a broad understanding of the neural basis of mental simulation and future thinking.
Acknowledgments
This research was supported by National Institute of Mental Health Grant 5R01MH60941-13, awarded to Daniel L. Schacter.
Footnotes
1 We note further that repetition was manipulated across scans in order to minimize cognitive demand (i.e. number of simulated events) within individual scans. Nonetheless, studies of future simulation have also utilized repetitions of events within scans and obtained converging results (V. van Mulukom et al., submitted for publication).
REFERENCES
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–77. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Schacter DL. The hippocampus and imagining the future: where do we stand? Frontiers in Human Neuroscience. 2012;5:173. doi: 10.3389/fnhum.2011.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The adaptive role of the default network in internal mentation. Neuroscientist. 2012;18:251–70. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010a;65:550–62. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network’s role in spontaneous cognition. Journal of Neurophysiology. 2010b;104:322–35. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergerfest D, Ghahremani DG, Gabrieli JD. Neural correlates of auditory repetition priming: reduced fMRI activation in the auditory cortex. Journal of Cognitive Neuroscience. 2004;16:966–77. doi: 10.1162/0898929041502760. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Van der Linden M. Predicting the phenomenology of episodic future thoughts. Consciousness and Cognition. 2012;21:1198–206. doi: 10.1016/j.concog.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Adaptation to cognitive context and item information in the medial temporal lobes. Neuropsychologia. 2012;50:3062–9. doi: 10.1016/j.neuropsychologia.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends in Cognitive Sciences. 2008;12:388–96. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ. The mind’s eye—precuneus activation in memory-related imagery. NeuroImage. 1995;2:195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends in Cognitive Sciences. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Hari R, Kujala MV. Brain basis of human social interaction: from concepts to brain imaging. Physiological Reviews. 2009;89:829–58. doi: 10.1152/physrev.00041.2007. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Usui N, Taira M, Kojima S. Neural enhancement and attenuation induced by repetitive recall. Neurobiology of Learning and Memory. 2011;96:143–9. doi: 10.1016/j.nlm.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. Journal of Neuroscience. 2007;27:14365–74. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Spreng RN, Rusu AA, Robbins CA, Mar RA, Schacter DL. Imagine all the people: How the brain creates and uses personality models to predict behavior. Cerebral Cortex. in press doi: 10.1093/cercor/bht042. doi: 10.1093/cercor/bht042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Nusbaum HC, Small SL. Repetition suppression for spoken sentences and the effect of task demands. Journal of Cognitive Neuroscience. 2006;18:2013–29. doi: 10.1162/jocn.2006.18.12.2013. [DOI] [PubMed] [Google Scholar]
- Ida Gobbini M, Haxby JV. Neural response to the visual familiarity of faces. Brain Research Bulletin. 2006;71:76–82. doi: 10.1016/j.brainresbull.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Ingvar DH. “Hyperfrontal” distribution of the cerebral grey matter flow in resting wakefulness: on the functional anatomy of the conscious state. Acta Neurologica Scandinavica. 1979;60:12–25. doi: 10.1111/j.1600-0404.1979.tb02947.x. [DOI] [PubMed] [Google Scholar]
- Ingvar DH. “Memory of the future”: an essay on the temporal organization of conscious awareness. Human Neurobiology. 1985;4:127–36. [PubMed] [Google Scholar]
- Jenkins AC, Macrae CN, Mitchell JP. Repetition suppression of ventromedial prefrontal activity during judgments of self and others. Proceedings of the National Academy of Sciences USA. 2008;105:4507–12. doi: 10.1073/pnas.0708785105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman L, Awipi T, Davachi L. Category-specificity in the human medial temporal lobe cortex. Hippocampus. 2009;19:308–19. doi: 10.1002/hipo.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proceedings of the National Academy of Sciences USA. 2009;106:20069–74. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally SL, Maguire EA. A new role for the parahippocampal cortex in representing space. Journal of Neuroscience. 2011;31:7441–9. doi: 10.1523/JNEUROSCI.0267-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences USA. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo A, Vicens L, Clithero JA, Dobbins IG, Huettel SA. Contributions of frontopolar cortex to judgments about self, others and relations. Social Cognitive and Affective Neuroscience. 2011;6:260–269. doi: 10.1093/scan/nsq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL. Adaptive constructive processes and the future of memory. American Psychologist. 2012;67:603–13. doi: 10.1037/a0029869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nature Reviews Neuroscience. 2007a;8:657–61. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Current Opinions in Neurobiology. 2007b;17:171–6. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: concepts, data, and applications. Annals of the New York Academy of Sciences. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Schacter DK, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. The future of memory: remembering, imagining, and the brain. Neuron. 2012;76:677–94. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–63. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Spreng RN. The fallacy of a “task-negative” network. Frontiers in Psychology. 2012;3:145. doi: 10.3389/fpsyg.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53:303–17. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques PL, Kragel PA, Rubin DC. Dynamic neural networks supporting memory retrieval. NeuroImage. 2011;57:608–16. doi: 10.1016/j.neuroimage.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. Mental time travel and the evolution of the human mind. Genetic, Social, and General Psychology Monographs. 1997;123:133–67. [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. The evolution of foresight: What is mental time travel, and is it unique to humans? Behavioral Brain Sciences. 2007;30:299–313. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Johnson JD, Rugg MD. Decrements in hippocampal activity with item repetition during continuous recognition: an fMRI study. Journal of Cognitive Neuroscience. 2011;23:1522–32. doi: 10.1162/jocn.2010.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK. Episodic future thought: an emerging concept. Perspectives on Psychological Science. 2010;5:142–62. doi: 10.1177/1745691610362350. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Chan JCK, McDermott KB. Contextual processing in episodic future thought. Cerebral Cortex. 2009;19:1539–48. doi: 10.1093/cercor/bhn191. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Schacter DL. Get real: effects of repeated simulation and emotion on the perceived plausibility of future experiences. Journal of Experimental Psychology: General. 2013;142(2):323–7. doi: 10.1037/a0028877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proceedings of the National Academy of Sciences USA. 2007;104:642–7. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Human Brain Mapping. 2009;30:829–58. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler JA, Suchan B, Daum I. Foreseeing the future: occurrence probability of imagined future events modulates hippocampal activation. Hippocampus. 2010;20:685–690. doi: 10.1002/hipo.20695. [DOI] [PubMed] [Google Scholar]
- Wig GS, Buckner RL, Schacter DL. Repetition priming influences distinct brain systems: evidence from task-evoked data and resting-state correlations. Journal of Neurophysiology. 2009;101:2632–48. doi: 10.1152/jn.91213.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]