Abstract
Social rejection often increases aggression, but the neural mechanisms underlying this effect remain unclear. This experiment tested whether neural activity in the dorsal anterior cingulate cortex (dACC) and anterior insula in response to social rejection predicted greater subsequent aggression. Additionally, it tested whether executive functioning moderated this relationship. Participants completed a behavioral measure of executive functioning, experienced social rejection while undergoing functional magnetic resonance imaging and then completed a task in which they could aggress against a person who rejected them using noise blasts . We found that dACC activation and executive functioning interacted to predict aggression. Specifically, participants with low executive functioning showed a positive association between dACC activation and aggression, whereas individuals with high executive functioning showed a negative association. Similar results were found for the left anterior insula. These findings suggest that social pain can increase or decrease aggression, depending on an individual’s regulatory capability.
Keywords: social pain, social rejection, aggression, executive functioning, fMRI, dACC
INTRODUCTION
Why people behave aggressively continues to be an important question for scientists and laypersons alike. The world is less violent now than ever before (Pinker, 2011). But people continue to scuffle, fistfight and brawl. One potent cause of aggression is social rejection (e.g. Twenge et al., 2001; Leary et al., 2006; Gaertner et al., 2008). Given the basic need for social acceptance (Baumeister and Leary, 1995), one might expect that people would respond to social rejection by trying very hard to gain re-inclusion from those who rejected them. However, people often do the opposite—they respond to social rejection with high levels of aggression. The present experiment focused on why this might occur.
Despite some early successes, researchers have struggled to identify the psychological and neural processes through which social rejection increases aggression, and who is most likely to respond to social rejection with aggression. To fill this gap, the present experiment integrated behavioral and functional neuroimaging methods to test the hypothesis that social rejection increases aggression through greater activation in two brain regions associated with the pain of rejection, namely the dorsal anterior cingulate cortex (dACC) and anterior insula (Eisenberger et al., 2003). Crucially, we predicted that the relationship between activation in these regions and aggression would depend on individual differences in executive functioning.
Consistent with the appraisal and decision-making component of the General Aggression Model (GAM; Anderson and Bushman, 2002; DeWall et al., 2011), we hypothesized that greater dACC and anterior insula activation would predict greater aggression among participants relatively low in executive functioning because they have a propensity toward impulsive actions. Conversely, we hypothesized that greater dACC and anterior insula activation would relate to lesser aggression among participants relatively high in executive functioning because of their propensity toward thoughtful actions.
THE GAM: AFFECT AND APPRAISAL
The GAM (Anderson and Bushman, 2002) posits that provocative social encounters, such as rejection, can increase aggression through emotions such as anger. However, the GAM also states that provocation and its associated emotions do not inevitably lead to aggression. In the GAM, cognitive appraisals and decision-making strategies influence whether emotions produce impulsive, aggressive actions or thoughtful, non-aggressive ones. Such ‘thoughtful’ responses to provocation require the ability of individuals to control any prepotent responses to respond aggressively and, simultaneously, to consciously and effortfully reappraise the situation (Anderson and Bushman, 2002). Therefore, aggression may occur as a result of the interaction between people’s affective reactions and their ability to control and reappraise them.
SOCIAL PAIN AND AGGRESSION
Social pain—the aversive emotional response that accompanies social injuries such as rejection, ostracism and exclusion (MacDonald and Leary, 2005)—is a likely candidate for the affective construct that promotes rejection-related aggression. Underscoring its importance for human survival, social pain mirrors physical pain in several ways. Social rejection is associated with activation of neural regions involved in the affective component of physical pain (Eisenberger, 2012; Eisenberger et al., 2003). Specifically, rejection is associated with activation of the dACC and the anterior insula. But why would social pain promote aggression?
Physical pain reliably increases aggression as a defensive reaction to bodily harm (Berkowitz, 1983, 1993). This ability of physical pain to promote aggression is relevant to the rejection–aggression link because social and physical pain share neural substrates: the dACC and anterior insula (Eisenberger et al., 2003). But social pain does not imply the subjective co-occurrence of physical pain. Given this overlap between physical and social pain, we predicted that activation in the dACC and anterior insula in response to a socially painful experience, social rejection, would directly relate to increased aggression.
THE ROLE OF EXECUTIVE FUNCTIONING
As predicted by the GAM, the effect of social pain on aggression should be modulated by ‘top-down’ or ‘cold’ inhibitory and reappraisal processes (Anderson and Bushman, 2002). Pain is a ‘hot’ affect-laden psychological process (e.g. Anderson et al., 1998). The GAM posits that such affective processes promote aggression, but inhibitory and reappraisal processes can deem the expression of such affect as not useful (e.g. lashing out at your boss after he or she berates you) and limit their expression. Such aggression-related inhibitory and reappraisal processes, and their ability to regulate aggression, rely on executive functioning (Giancola, 2000). Executive functioning is a cognitive ability that regulates goal-oriented behaviors (Milner, 1995).
The relationship between social pain and aggression may depend on the amount of executive functioning individuals bring with them to aggressive situations. Indeed, individuals who perform poorly on behavioral measures of executive functioning are more aggressive than their high executive-functioning counterparts (e.g. Hoaken et al., 1998). Individuals who are low in executive functioning are often unable to inhibit the ‘hot’ affective processes that promote aggression (Hoaken et al., 2003). As the pain of rejection increases, the affect-driven impulse to aggress increases (Anderson et al., 1998). As such, we predicted that for individuals low in executive functioning, greater dACC and anterior insula activation in response to social rejection would relate to more post-rejection aggression because they favor impulsive over thoughtful actions. More specifically, individuals low in executive functioning are unable to engage in the inhibitory and reappraisal processes that underpin thoughtful, non-aggressive action.
In contrast, individuals high in executive functioning are readily capable of engaging in the deliberative appraisal processes that inhibit ‘hot’ affective processes and subsequently aggression (Giancola, 2000). Regulatory functions associated with executive functioning are recruited in an increasing manner as pain and its associated affect increase (Eccleston and Crombez, 1999). As such, we predicted that greater dACC and anterior insula activation in response to social rejection would relate to less post-rejection aggression among individuals high in executive functioning because they favor thoughtful over impulsive actions. This is because individuals high in executive functioning are readily able to engage in the inhibitory and reappraisal processes that underpin thoughtful, non-aggressive action.
These predictions were only expected to hold under conditions in which aggression would not be a useful strategy for the aggressor. Specifically, when aggression is a useful option (e.g. fending off an attacker), individuals across the executive functioning spectrum are likely to aggress. As the GAM states, aggression is sometimes an adaptive response that is thoughtfully chosen (Anderson and Bushman, 2002). In the context of purely emotional and reactive aggression, however, we predicted that individual differences in executive functioning would manifest as shifts in rejection-associated aggression.
METHODS
Participants
Participants were 40 healthy, right-handed undergraduate students who received course credit and $65. For safety reasons, participants were excluded from our study if they reported any history of claustrophobia, seizures, head trauma or an injury involving a metallic object. Additionally, participants were excluded if they reported a body mass index >30 lb/in2 (as individuals exceeding this cutoff might be uncomfortable in the confined MRI scanner), pregnancy or suspected pregnancy, color blindness, psychoactive medication use or psychological/neurological pathology. One participant was excluded from analyses because of behavioral issues in the MRI environment that resulted in distorted fMRI data. Four participants did not complete the aggression measure because the MRI scan exceeded the allotted time. Analyses were therefore performed on the 35 remaining participants (17 females; meanAge = 19.03, s.d. = 1.36).
Procedure
Pre-scan measure of executive functioning
To measure executive functioning, participants completed a computerized version of the Stroop (1935) color-naming task. The Stroop color-naming task is a widely used, reliable and valid measure of executive functioning because performance on the task requires attention maintenance, working memory and the rapid and accurate inhibition of prepotent responses (see Engle, 2002). Participants were instructed to identify the font color of words as fast as possible, while ignoring the word’s meaning. There were 40 congruent trials in which the word’s meaning matched the font color (e.g. the word RED in red font) and 40 incongruent trials in which the word’s meaning did not match the font-color (e.g. the word RED in green font), ordered randomly. This 80-trial design has been effectively used in previous research to measure individual differences in executive functioning (e.g. Dinn and Harris, 2000; Kiefer et al., 2005). This task was performed several days before the MRI portion of the experiment, as the depletion of executive functioning from this task may have impacted subsequent neural activation and aggressive behavior (DeWall et al., 2007).
Scanner task
Participants were informed that they would play three rounds of a computerized ball-tossing game (Cyberball) in an MRI scanner with two same-sex partners located in nearby scanners (Williams et al., 2000). In reality, participants played with a preset computer program that was designed to produce a within-participants experience of both social acceptance and rejection. Cyberball was implemented in the MRI scanner as a block design with three rounds (60 s each). Before each round, participants were presented with instructions to rest for 10 s. This was followed by a screen instructing them to ‘get ready’ for the upcoming round (2 s). In rounds 1 and 2, participants were accepted for the entire duration of the task, receiving one-third of all ball tosses. In round 3, participants received the ball three times, after which their partners only threw the ball to each other.
Acceptance was operationalized as occurring throughout rounds 1 and 2, as well as throughout the first part of round 3, in which participants received the ball three times. Rejection was operationalized as occurring during the second part of round 3, after participants had received the ball three times and then witnessed three more ball tosses without receiving a toss themselves (30 s duration). After playing Cyberball and a series of anatomical MRI scans, participants were removed from the MRI scanner in order to complete the Need Threat Scale (van Beest and Williams, 2006), a 20-item questionnaire intended to assess current social distress due to social rejection.
Post-scan aggression measure
Participants then completed a behavioral measure of aggression. Participants were told they would play a computerized game against one of their partners from Cyberball. This game took the form of a competitive reaction-time task in which the winner could deliver aversive and prolonged noise to the loser through headphones. The aggression task consisted of nine trials. Prior to each trial, participants set the volume of the noise blast their partner would receive if the participant won the round, ranging from Level 1 (60 dB) to Level 10 (105 dB) in 5 dB intervals. A non-aggression option, Level 0, was also provided. Participants also controlled how long their opponent suffered by setting the duration of the noise blast, which could range from 0 to 5 s in half-second intervals. After each trial, participants saw whether they won or lost, as well as the volume and intensity settings their partners had ostensibly set for them. Participants won five trials and lost four trials (determined randomly, despite being told that their performance was what determined the outcome of each trial). Basically, within the ethical limits of the laboratory, participants controlled a weapon that could be used to blast their opponent with unpleasant noise. The construct validity of this task is well established (Bernstein et al.,1987; Giancola and Zeichner, 1995; Anderson and Bushman, 1997). It has been used for decades as a reliable and valid measure of laboratory aggression (Taylor, 1967).
fMRI data acquisition
All images were collected on a 3T Siemens Magnetom Trio scanner. Functional images were acquired with a T2-weighted gradient echo sequence with the following parameters: 2.5 s repetition time, 28 ms echo time, 64 × 64 matrix, 224 × 224 mm field of view, 40 3.5 mm axial slices acquired in interleaved order. A 3D shim was applied before functional data acquisition. These parameters allowed for whole-brain coverage with 3.5 mm cubic voxels. A high-resolution, T1-weighted image was also acquired from each participant so that functional data could be registered to native anatomical space and then normalized to the Montreal Neurological Institute (MNI) atlas space.
fMRI preprocessing
All preprocessing and statistical analyses were conducted using FSL [Oxford Center for Functional Magnetic Resonance Imaging (FMRIB); Smith et al., 2004; Woolrich et al., 2009]. Functional volumes were reconstructed from k-space using a linear time interpolation algorithm to double the effective sampling rate, the first of which was removed to allow for signal equilibration. Remaining functional volumes were corrected for head movement to the median volume using MCFLIRT (Jenkinson et al., 2002), corrected for slice-timing skew using temporal sinc interpolation, pre-whitened using FILM and smoothed with a 5 mm FWHM Gaussian kernel. To remove drifts within sessions, a high-pass filter with a cutoff period of 120 s was applied. Non-brain structures were stripped from functional and anatomical volumes using FSL’s Brain Extraction Tool (Smith, 2002).
fMRI data analysis
FMRI analysis was performed using FSL’s FMRI Expert Analysis Tool (FEAT version 5.98). A fixed-effects analysis modeled event-related responses for each run of each participant. Acceptance and Rejection blocks were modeled as events using a canonical double-gamma hemodynamic response function with a temporal derivative. Pre-block instructions were modeled as a nuisance regressor while rest blocks were left un-modeled. The contrast of interest was rejection > acceptance. Functional volumes and first-level contrast images from this analysis were first registered to corresponding structural volumes using 7 degrees of freedom, and then spatially normalized to an MNI stereotaxic space template image using 12 degrees of freedom with FMRIB’s Linear Image Registration Tool (Jenkinson and Smith, 2001; Jenkinson et al., 2002). FMRIB’s Local Analysis of Mixed Effects module (Beckmann et al., 2003; Woolrich, Behrens, Beckmann, Jenkinson, & Smith, 2004) was used to perform top-level, mixed-effects analysis, which created group average maps for contrasts of interest. Z (Gaussianized T/F) statistic images were thresholded using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of P < 0.005 in an a priori regions of interest (ROI; Worsley, 2001; Heller et al., 2006). Functional data from the activated main effect cluster from our contrast and ROI were converted to units of percent signal change, averaged across each participant and extracted (as outlined by J.A. Mumford, http://mumford.bol.ucla.edu/perchange_guide.pdf).
Construction of ROI
ROIs of the dACC and anterior insula were created by Way et al. (2009) from the automated anatomical atlas using MNI coordinates (Tzourio-Mazoyer et al., 2002). The dACC ROI used a rostral boundary of y = 33 based on criteria established by Vogt et al. (2003) and a caudal boundary of y = 0. The anterior insula ROIs used a caudal boundary of y = 8 to correspond to the agranular insula.
Behavioral data analysis
Response times from correct trials on the Stroop task were averaged for each participant, separately for congruent and incongruent trials. Response times on congruent trials were subtracted from those on incongruent trials to create an index of how much participants were able to utilize executive functioning. Higher values indicated greater interference from incongruent trials. To enhance interpretation, these values were then reverse-scored so that higher values corresponded to higher executive functioning.
In the aggression task, noise intensity and volume levels had high internal reliabilities (Cronbach α = 0.91 and 0.88, respectively) and were significantly correlated, r(34) = 0.79, P < 0.001. Thus, we standardized and summed intensity and duration levels across the nine trials to create a more reliable measure of aggression.
RESULTS
Stroop task results
Stroop task responses had an accuracy rate of 96.7%, across all participants. Stroop task response times were characterized by a main effect of congruency, t(34) = −3.71, P = 0.001, d = 0.33. Replicating the classic Stroop effect, participants were faster at correctly identifying congruent word-color pairings (mean = 466.90 ms, s.d. = 107.95 ms) than incongruent pairings (mean = 521.99 ms, s.d. = 149.27 ms).
Imaging results
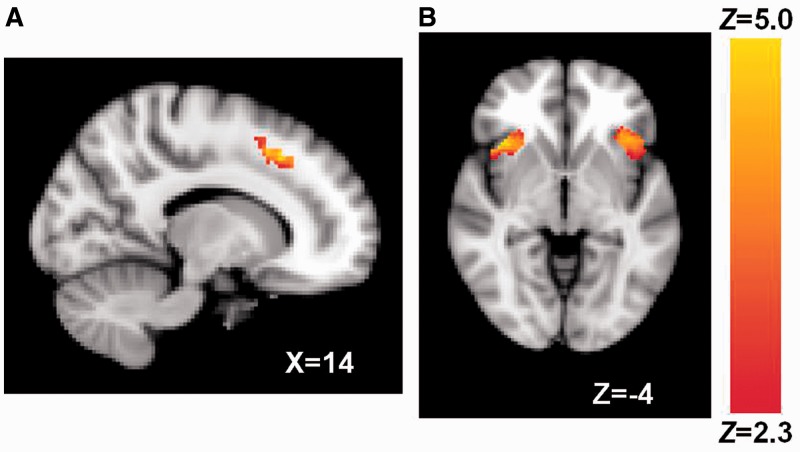
Social rejection, compared with social acceptance, led to increased activity in the dACC and bilateral anterior insula (Figure 1 and Table 1; rejection > acceptance contrast). This finding replicates prior social rejection research on the dACC and anterior insula as indicators of social pain (Eisenberger et al., 2003; DeWall et al., 2010; Kross et al., 2011).
Fig. 1.
(A) dACC and (B) bilateral anterior insula activation associated with rejection > acceptance. Coordinates are in MNI space.
Table 1.
ROIs associated with rejection > acceptance
| ROI | Contiguous voxels | Peak Z | Peak MNI coordinates (x, y, z) |
|---|---|---|---|
| dACC | 470 | 4.96 | 14, 22, 44 |
| Anterior insula | 477 | 5.27 | 40, 22, 0 |
| 542 | 4.75 | −42, 22, −6 |
Post-rejection social distress, as measured by mean scores from the Need Threat Scale (Cronbach α = 0.90) taken 60 min after the social rejection task, was uncorrelated or marginally correlated with percent signal change units from activated clusters (obtained from rejection > acceptance contrast) in the dACC [r(34) = 0.14, P = 0.40], left insula [r(34) = 0.28, P = 0.09] and right insula [r(34) = 0.27, P = 0.10]. These null effects are similar to those obtained in previous studies that delayed giving the Need Threat Scale to participants (Zadro et al., 2006).
Participants high and low in executive functioning did not differ in activation of the social pain network in response to social rejection, as correlations between executive functioning and each of the three neural regions of the social pain network were non-significant: dACC activation, r(34) = 0.08, P = 0.65; left anterior insula activation, r(34) = −0.03, P = 0.87; right anterior insula activation, r(34) = 0.09, P = 0.60.
Moderation analysis
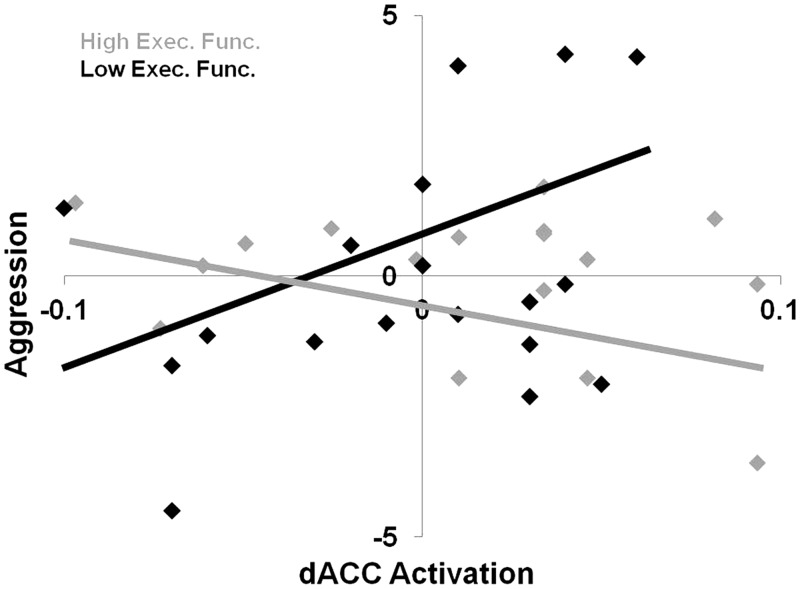
As predicted, dACC activation interacted with executive functioning to predict aggression, β = 0.63, t(30) = 3.82, P = 0.001 (Figure 2). At low levels (−1 s.d.) of executive functioning, dACC activation was positively associated with aggression, β = 0.55, t(34) = 2.89, P < 0.01. In contrast, at high levels (+1 s.d.) of executive functioning, dACC activation was negatively associated with retaliatory aggression, β = −0.47, t(34) = −2.26, P < 0.05.
Fig. 2.
Interactive effect of dACC activation and executive functioning on aggression. Aggression units are in Z-scores with higher values representing greater aggression on the Taylor aggression paradigm. dACC activation is in units of percent signal change from the rejection > acceptance contrast.
Subsequent analyses examined the effect of executive functioning on aggression at low and high levels of dACC activation. At low levels (−1 s.d.) of dACC activation, executive functioning did not correspond with aggression, β = 0.31, t(34) = 1.68, P = 0.11. But at high levels (+1 s.d.) of dACC activation, lower levels of executive functioning corresponded to greater aggression, β = −0.71, t(34) = −3.31, P < 0.005.
As with the dACC, anterior insula activation interacted with executive functioning to predict aggression, though only in the left hemisphere; left insula: β = 0.39, t(30) = 2.12, P < 0.05; right insula: β = 0.32, t(30) = 1.68, P = 0.10. At low levels (−1 s.d.) of executive functioning, left insula activation was marginally, positively associated with aggression, β = 0.40, t(34) = 2.00, P = 0.055, whereas at high levels (+1 s.d.) of executive functioning, left insula activation was unassociated with retaliatory aggression, β = −0.41, t(34) = −1.31, P > 0.2. Subsequent analyses examined the effect of executive functioning on aggression at low and high levels of left anterior insula activation. At low levels (−1 s.d.) of left anterior insula activation, executive functioning did not correspond with aggression, β = 0.32, t(34) = 1.21, P = 0.23. But at high levels (+1 s.d.) of left anterior insula activation, lower levels of executive functioning marginally corresponded to greater aggression, β = −0.49, t(34) = −2.03, P = 0.051.
DISCUSSION
People often react aggressively when they are socially rejected. This experiment sought to understand why this effect occurs, and who is most likely to respond to social rejection with aggression. Utilizing fMRI, we replicated the finding that social rejection elicits activation of the dACC and anterior insula. We also offered a novel extension by showing that dACC and left anterior insula activation significantly interacted with executive functioning to predict aggression. Among individuals low in executive functioning, greater dACC and left anterior insula activation were associated with more aggression. In contrast, individuals high in executive functioning showed a negative association between dACC activation and aggression.
These findings are the first examination of the neural substrates of the rejection–aggression link. They support the GAM’s predictions regarding affective processes underlying the relationship between rejection and aggression, along with how individual differences in the tendency toward impulsive and thoughtful actions influence aggression. Our results strongly suggest that social pain is a means through which rejection causes reactive aggression. Although there are other rejection-related mechanisms that facilitate the rejection–aggression link, such as hostile cognitions (DeWall et al., 2009), we have implicated pain-related neural activity as a potential contributing factor as well. This similarity provides further evidence for Pain Overlap Theory (Eisenberger and Lieberman, 2005). Building this diverse model of the neural and psychological underpinnings of the rejection–aggression link is a necessary step in understanding how to reduce aggression.
Despite the consistency of our results, the current experiment had limitations that may inform future research. First, the dACC was not significantly associated with social distress and the anterior insula was only marginally significantly associated with social distress. This was likely due to the fact that ∼1 h elapsed between our rejection manipulation and the post-scan assessment of social distress (due to various anatomical MRI scans and safe removal of the participant from the scanner), and self-reports of post-rejection social distress diminish after ∼45 min (Zadro et al., 2006). Because of this, we cannot be certain that neural activity in these regions was indicative of underlying social pain. However, given prior research showing that these regions respond to social exclusion and tend to correlate with self-reported social distress in response to this particular task (Eisenberger, 2012), we think that the lack of a significant relationship between self-reported social distress and neural activity is more likely to be due to the delay in the self-report assessment rather than due to a completely different psychological process taking place during the fMRI scan. Future work will be needed, however, to more carefully probe this issue. In addition, this lack of a correlation between self-reported social pain and dACC and anterior insula activation does not mean that participants were not still being affected by the rejection episode, as rejected individuals still show evidence of social pain on implicit behavioral measures well after an hour (Zadro et al., 2006).
A second limitation is that our aggression measure was direct. It is an open question as to whether our findings apply to displaced aggression, in which individuals harm innocent bystanders. Third, we relied on individual differences in executive functioning instead of experimentally manipulating them. Hence, we cannot make causal claims regarding the interactive effect of executive functioning and dACC and anterior insula activation in predicting aggression. Future research should assess whether depleting and strengthening participants’ levels of executive functioning would increase and decrease the relationship between dACC and anterior insula activation and aggression, respectively.
Fourth, although dACC activity was observed in response to social rejection, activation of the dACC has also been associated with expectancy violations (e.g. Botvinick et al., 2004). Because our rejection manipulation inherently involved such a violation of participants’ expectations (i.e. not receiving any ball tosses in an ostensibly equitable ball-toss game), the dACC activation we observed during rejection may merely be due to this phenomenon and not representative of the distress associated with social rejection. A recent study made such a possibility unlikely, as it showed that comparing the rejection condition in Cyberball to an ‘over-inclusion’ condition (i.e. an expectancy violation) still yields activation of the dACC (Kawamoto et al., 2012).
Fifth, participants’ aggression toward their rejecter may have been affected by the lack of an expectation of future interaction with that individual. Had participants expected to interact with their partner again, individuals high in executive functioning may have aggressed in a similar fashion to their low executive functioning counterparts. Translating their social pain into aggression, individuals both high and low in executive functioning could stand to gain from retaliating against their provocateur under such conditions. Sixth, because rejection always occurred later in time than acceptance, our fMRI contrast between acceptance and rejection conditions was confounded with the inevitable changes in the MRI signal that occur over the length of a scan. To reduce the impact of this confound, we utilized several widely accepted preprocessing strategies. Specifically, our data were highpass filtered to remove low frequency shifts in the data over time, prewhitened to remove temporal autocorrelation, and a temporal derivative was included in the statistical model to account for time-based shifts in the hemodynamic response function (see Poldrack et al., 2011; Woolrich, Ripley, Brady, & Smith, 2001). Finally, because we only measured a single outcome variable, aggression, we cannot be sure that our interactive effect of social pain and executive functioning is specific to aggression or extends to other variables.
Notwithstanding these limitations, our findings have substantial implications for theory and practice. To our knowledge, this experiment is the first to demonstrate the neural underpinnings of the rejection–aggression link. Furthermore, our findings are the first to show that social pain-related neural activity is associated with increased aggression under certain conditions. If high levels of executive functioning can reverse the rejection–aggression link, researchers may design interventions aimed at bolstering executive functioning among socially rejected people. Although global violence appears to be on the decline, such interventions may help to reduce it further.
Acknowledgments
This experiment was funded by a grant from the University of Kentucky’s Center for Drug Abuse Research Translation (Sponsor: National Institute on Drug Abuse, Grant number: DA005312) to the last author and a grant from the National Science Foundation (Grant number: BCS1104118) to the fifth and last authors.
REFERENCES
- Anderson CA, Bushman BJ. External validity of “trivial” experiments: the case of laboratory aggression. Review of General Psychology. 1997;1:19–41. [Google Scholar]
- Anderson CA, Bushman BJ. Human aggression. Annual Review of Psychology. 2002;53(1):27–51. doi: 10.1146/annurev.psych.53.100901.135231. [DOI] [PubMed] [Google Scholar]
- Anderson KB, Anderson CA, Dill KE, Deuser WE. The interactive relations between trait hostility, pain, and aggressive thoughts. Aggressive Behavior. 1998;24(3):161–71. [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117(3):497–529. [PubMed] [Google Scholar]
- Beckmann C, Jenkinson M, Smith SM. General multi-level linear modeling for group analysis in fMRI. NeuroImage. 2003;20(2):1052–63. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Berkowitz L. Aversively stimulated aggression: some parallels and differences in research with animals and humans. American Psychologist. 1983;38:1135–44. doi: 10.1037//0003-066x.38.11.1135. [DOI] [PubMed] [Google Scholar]
- Berkowitz L. Pain and aggression: some findings and implications. Motivation and Emotion. 1993;17(3):277–93. [Google Scholar]
- Bernstein S, Richardson D, Hammock G. Convergent and discriminant validity of the Taylor and Buss measures of physical aggression. Aggressive Behavior. 1987;13(1):15–24. [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8(12):539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- DeWall CN, Anderson CA, Bushman BJ. The general aggression model: theoretical extensions to violence. Psychology of Violence. 2011;1(3):245–58. [Google Scholar]
- DeWall CN, Baumeister RF, Stillman TF, Gailliot MT. Violence restrained: effects of self-regulation and its depletion on aggression. Journal of Experimental Social Psychology. 2007;43:62–76. [Google Scholar]
- DeWall CN, Twenge JM, Gitter SA, Baumeister RF. It’s the thought that counts: the role of hostile cognition in shaping aggressive responses to social exclusion. Journal of Personality and Social Psychology. 2009;96(1):45–59. doi: 10.1037/a0013196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWall CN, MacDonald G, Webster GD, et al. Acetaminophen reduces social pain behavioral and neural evidence. Psychological Science. 2010;21(7):931–37. doi: 10.1177/0956797610374741. [DOI] [PubMed] [Google Scholar]
- Dinn WM, Harris CL. Neurocognitive function in antisocial personality disorder. Psychiatry Research. 2000;97(2–3):173–90. doi: 10.1016/s0165-1781(00)00224-9. [DOI] [PubMed] [Google Scholar]
- Eccleston C, Crombez G. Pain demands attention: a cognitive–affective model of the interruptive function of pain. Psychological Bulletin. 1999;125(3):356–66. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nature Reviews Neuroscience. 2012;13:421–34. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why it hurts to be left out: the neurocognitive overlap between physical and social pain. In: Williams KD, Forgas JP, von Hippel W, editors. The Social Outcast: Ostracism, Social Exclusion, Rejection, and Bullying. New York: Psychology Press; 2005. pp. 109–27. [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302(5643):290–92. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Engle RW. Working memory capacity as executive attention. Current Directions in Psychological Science. 2002;11(1):19–23. [Google Scholar]
- Gaertner L, Iuzzini J, O’Mara EM. When rejection by one fosters aggression against many: multiple-victim aggression as a consequence of social rejection and perceived groupness. Journal of Experimental Social Psychology. 2008;44(4):958–70. doi: 10.1016/j.jesp.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR. Executive functioning: a conceptual framework for alcohol-related aggression. Experimental and Clinical Psychopharmacology. 2000;8(4):576–97. doi: 10.1037//1064-1297.8.4.576. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Zeichner A. Construct validity of a competitive reaction-time aggression paradigm. Aggressive Behavior. 1995;21:199–204. [Google Scholar]
- Heller R, Stanley D, Yekutieli D, Rubin N, Benjamini Y. Cluster-based analysis of fMRI data. NeuroImage. 2006;33(2):599–608. doi: 10.1016/j.neuroimage.2006.04.233. [DOI] [PubMed] [Google Scholar]
- Hoaken PNS, Giancola PR, Pihl RO. Executive cognitive functions as mediators of alcohol-related aggression. Alcohol and Alcoholism. 1998;33(1):47–54. doi: 10.1093/oxfordjournals.alcalc.a008347. [DOI] [PubMed] [Google Scholar]
- Hoaken PNS, Shaughnessy VK, Pihl RO. Executive cognitive functioning and aggression: is it an issue of impulsivity? Aggressive Behavior. 2003;29(1):15–30. [Google Scholar]
- Jenkinson M, Smith SM. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Onoda K, Nakashima K, Nittono H, Yamaguchi S, Ura M. Is dorsal anterior cingulate cortex activation in response to social exclusion due to expectancy violation? An fMRI study. Frontiers in Evolutionary Neuroscience. 2012;4:11. doi: 10.3389/fnevo.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M, Ahlegian M, Spitzer M. Working memory capacity, indirect semantic priming, and stroop interference: pattern of interindividual prefrontal performance differences in healthy volunteers. Neuropsychology. 2005;19(3):332–44. doi: 10.1037/0894-4105.19.3.332. [DOI] [PubMed] [Google Scholar]
- Kross E, Berman MG, Mischel W, Smith EE, Wager TD. Social rejection shares somatosensory representations with physical pain. Proceedings of the National Academy of Sciences. 2011;108(15):6270–75. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary MR, Twenge JM, Quinlivan E. Interpersonal rejection as a determinant of anger and aggression. Personality and Social Psychology Review. 2006;10(2):111–32. doi: 10.1207/s15327957pspr1002_2. [DOI] [PubMed] [Google Scholar]
- MacDonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychological Bulletin. 2005;131(2):202–23. doi: 10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- Milner B. Aspects of human frontal lobe function. In: Jasper H, Riggio S, Goldman-Rakic P, editors. Epilepsy and the Functional Anatomy of the Frontal Lobe. New York: Raven Press; 1995. pp. 67–84. [Google Scholar]
- Pinker S. The Better Angels of Our Nature: Why Violence Has Declined. New York: Penguin; 2011. [Google Scholar]
- Poldrack RA, Mumford JA, Nichols TE. Handbook of Functional MRI Data Analysis. New York: Cambridge University Press; 2011. [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology: General. 1935;18(1):643–62. [Google Scholar]
- Taylor SP. Aggressive behavior and physiological arousal as a function of provocation and the tendency to inhibit aggression. Journal of Personality. 1967;35(2):297–310. doi: 10.1111/j.1467-6494.1967.tb01430.x. [DOI] [PubMed] [Google Scholar]
- Twenge JM, Baumeister RF, Tice DM, Stucke TS. If you can’t join them, beat them: effects of social exclusion on aggressive behavior. Journal of Personality and Social Psychology. 2001;81(6):1058–69. doi: 10.1037//0022-3514.81.6.1058. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Beest I, Williams KD. When inclusion costs and ostracism pays, ostracism still hurts. Journal of Personality and Social Psychology. 2006;91(5):918–28. doi: 10.1037/0022-3514.91.5.918. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SWG. Structural and functional dichotomy of human midcingulate cortex. European Journal of Neuroscience. 2003;18(11):3134–44. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BM, Taylor SE, Eisenberger NI. Variation in the μ-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proceedings of the National Academy of Sciences. 2009;106(35):15079–84. doi: 10.1073/pnas.0812612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KD, Cheung CKT, Choi W. Cyberostracism: effects of being ignored over the internet. Journal of Personality and Social Psychology. 2000;79(5):748–62. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004;21(4):1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady JM, Smith SM. Temporal autocorrelation in univariate linear modelling of fMRI data. NeuroImage. 2001;14(6):1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, et al. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45(1):S173–86. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Testing for signals with unknown location and scale in a χ2 random field, with an application to fMRI. Advances in Applied Probability. 2001;33(4):773–93. [Google Scholar]
- Zadro L, Boland C, Richardson R. How long does it last? The persistence of the effects of ostracism in the socially anxious. Journal of Experimental Social Psychology. 2006;42(5):692–97. [Google Scholar]