Abstract
Users of the illicit drug, 3,4-methylenedioxymethamphetamine (MDMA), show signs of neurotoxicity. However, the precise mechanism of neurotoxicity caused by use of MDMA has not yet been elucidated. Synthetic glutathione (GSH) conjugates of MDMA are transported into the brain by the GSH transporter and subsequently produce neurotoxicity. The objective of this research is to show direct evidence of the formation of GSH adducts of MDMA in human hepatocytes. High-performance liquid chromatography coupled with tandem mass spectrometry was utilized to examine in vitro incubations of MDMA with cryopreserved human hepatocytes. The use of hydrophilic liquid chromatography in combination with linear ion trap mass spectrometry permitted the identification of two possible GSH metabolites. Enhanced product ion scans of m/z = 499 and 487 amu of extracts from hepatocytes treated with 1.0 mM MDMA show a distinct fragmentation pattern (m/z 194.2, 163, 135, 105), suggesting the formation of MDMA–GSH conjugate, MDMA-SG and 3,4-dihydroxymethamphetamine-SG. The formation of an MDMA–GSH conjugate was further supported by the apparent lack of the same fragmentation pattern from hepatocyte samples without MDMA treatment. The results generated from this study yield valuable qualitative and quantitative information about the neurotoxic thioether metabolites formed from MDMA in humans.
Introduction
3,4-Methylenedioxymethamphetamine (MDMA), a relatively simple chemical belonging to the amphetamine family of compounds, has properties of both stimulants and hallucinogens (1). This substance, also known on the streets as “Ecstasy”, is identified chemically as N-methyl-3,4-methylenedioxyamphetamine. Chronic use of MDMA has been shown to cause brain damage in humans (2, 3). Administration of MDMA in primates and rats produces toxic effects on serotonergic neurons of the central nervous system (4, 5). Positron emission topography studies have also demonstrated that human MDMA use results in brain serotonin 5-hydroxytryptamine (5-HT) neurotoxicity (6). In animals, it appears that peripheral administration of MDMA is necessary to generate neurotoxic effects because direct injection of MDMA into the brain does not result in neurotoxicity (7, 8). Thus, metabolism of the drug is a prerequisite for the development of neurotoxicity. The exact identity of the metabolite or metabolites responsible for neurotoxicity has yet to be determined.
The metabolism of MDMA follows two major pathways (see Supplementary data, Figures S1 and S2). In vitro human studies indicate that it is demethylenated to N-methyl-α-methyldopamine (a) primarily by the CYP2D6 enzyme (9, 10). This major oxidative pathway has been studied in animals and leads to reactive catechol metabolites that are further converted by methylation, hydroxylation, glucuronidation, sulfation or glutathione (GSH) conjugation (11–13). A second major metabolic pathway is initiated by N-demethylation to 3,4-methylenedioxyamphetamine (MDA) (b), which can be subsequently demethylenated to α-methyldopamine (c), then followed by conjugation reactions (14–16).
McCann et al. (17, 18) studied the effects of two metabolites of MDMA, α-methyldopamine (c) and 4-hydroxy-3-methoxyamphetamine (d) on brain serotonin neurons. When administered to rats intracerebroventricularly, into brain parenchyma, or systemically, these compounds did not produce long-term depletion of brain serotonin, suggesting that these metabolites do not mediate toxic effects on 5-HT neurons. When monitoring the brain concentrations of N-methyl-α-methyldopamine (a), MDA (b) and another potential metabolite, 6-hydroxy-3,4-methylenedioxymethamphetamine (e) after administration of MDMA in rats, Chu et al. (19) found that there was no correlation between the brain concentrations of these compounds and serotonin depletion. While intracerebroventricular and intrastriatal administration in rats of another putative metabolite of MDMA, 2-(methylamino)-1-(2,4,5-trihydroxyphenyl)propane (f) did deplete serotonin concentrations, it also targeted the dopaminergic system, thus did not demonstrate the serotonergic selectivity characteristic of MDMA (20). These studies all suggest that the neurotoxic effects of MDMA may not be caused by Phase I metabolites.
If metabolic activation of MDMA is indeed a prerequisite for the occurrence of neurotoxicity, it is possible that Phase II metabolites are responsible for axonal damage at the serotonergic nerve terminal. Hiramatsu et al. (12) postulated that the catechol metabolite, N-methyl-α-methyldopamine (a), is readily oxidized to the corresponding ortho-quinone (h) which is capable of forming an adduct with GSH (see Supplementary data, Figure S2). Quinones are electrophiles and can react readily with the nucleophilic sulfhydryl group in GSH (21). The potential for conversion of the catecholamine (a) to a GSH adduct, N-methyl-5-(glutathion-S-yl)-α-methyldopamine (i), was demonstrated in vitro by Hiramatsu et al. (12) using rat liver microsomes. MDMA and GSH were incubated with a microsomal suspension of rat liver. The formation of (i) was confirmed with mass spectrometry by spectral comparison with a synthesized standard of N-methyl-5-(glutathion-S-yl)-α-methyldopamine. The catecholamine (a) was found to be unstable and was rapidly converted to the GSH adduct and other thiol compounds, such as cysteine and or N-acetylcysteine. The oxidation to the o-quinone did not appear to be cytochrome P-450 dependent but required NADPH and microsomal protein.
Bai et al. (22) showed that the thioether conjugates of α-methyldopamine are selective serontonergic neurotoxins. Intracerebroventricular injection of 5-(glutathion-S-yl)-α-methyldopamine (n) and 2,5-bis(glutathion-S-yl)-α-methyldopamine (o) causes serotonergic toxicity similar to that seen following systemic MDMA administration. The recent work shows that these GSH adducts are transported into the brain by the GSH transporter and produce neurotoxicity. The thioether MDMA metabolites are strong neurotoxins; in fact, they are significantly more neurotoxic than their corresponding parent catechols (23).
Direct evidence of formation of GSH adducts in animals and humans after MDMA administration has been clearly demonstrated recently. Administration of MDMA (20 mg/kg SC) at 12 h intervals for a total of four injections led to a significant accumulation of the N-Me-α-MeDA thioether metabolites in rat brain (5). The formation of 5-(N-acetylcystein-S-yl)-3,4-dihydroxymethamphetamine and 5-(N-acetylcystein-S-yl)-3,4-dihydroxyamphetamine in human urine was also reported (24). This paper reports the formation and identification of new thioether conjugates of MDMA in humans, using in vitro testing with human liver hepatocytes.
Materials
HPLC-grade solvents (isopropanol, methanol, water and acetonitrile; CHROMASOLV plus for HPLC, ≥99.9%) were supplied by Sigma–Aldrich (St. Louis, MO, USA). Glacial acetic acid was obtained from Fisher Scientific (Fair Lawn, NJ, USA). Ammonium acetate (Reagent Plus™, 99.99%) and other chemicals were provided by Aldrich Chemical (Milwaukee, WI, USA). All chemicals were analytical grade. (±)-3,4-Methylenedioxymethamphetamine hydrochloride solution (M-5029) or (±)-3,4-methylenedioxymethamphetamine hydrochloride powder from Sigma-Aldrich was used to prepare MDMA standards.
Human liver hepatocytes (female in vitro hepatocytes, lot CHD), hepatocyte incubation medium (InVitroGro HI medium) and hepatocyte thawing medium (InVitroGro HT medium) were obtained from In Vitro Technologies (Baltimore, MD, USA).
Methods
Preparation of standards
HPLC-grade methanol and water were used to prepare MDMA for analysis. In preparing the sample of standard MDMA (10 μg/mL), an aliquot of 10 μL of MDMA standard solution (1.0 mg/mL MDMA in methanol) was added to 990 μL of HPLC-grade methanol (MeOH).
Preparation of MDMA solutions for incubation with hepatocytes
MDMA stock solution (10 mM) was prepared in a sterile 5 mL plastic disposable test tube by combining 11.5 mg of MDMA with 5 mL of hepatocyte incubation medium prewarmed to 37°C in a water bath. The mixture was vortexed (under a sterile hood).
A 2.5 mM MDMA solution was prepared by combining a 1.25 mL aliquot of the 10 mM MDMA stock solution with 3.75 mL of hepatocyte incubation medium in a 5-mL disposable test tube using 2 and 5 mL non-pyrogenic serological pipettes, respectively. A 0.625 mM MDMA solution was prepared by combining 1.25 mL of the 2.5 mM MDMA solution with 3.75 mL of hepatocyte incubation medium in a 5-mL disposable test tube using serological pipettes. The 2.5 and 0.625 mM solutions were then vortexed at 1,000 rpm for 2 min.
Preparation of human hepatocytes for incubation
A vial of human hepatocytes (in vitro, female human hepatocytes, lot CHD) was removed from storage under liquid nitrogen. The cap of the vial was opened to release any liquid nitrogen inside the vial, and then was immediately closed. The cell vial was held in a 37°C water bath while rolling the vial between the hands to thaw the cells. Once the cells had thawed, the exterior of the vial was sprayed with ethanol and placed under a sterile hood.
The hepatocytes were drawn up from the vial using a disposable sterile 1 mL pipette. The hepatocytes were carefully placed into a 50-mL centrifuge tube containing 48 mL of hepatocyte thawing medium. The cells were then transferred drop by drop to avoid damage to the fragile cells. The hepatocyte vial was washed with a small amount (∼1 mL) of hepatocyte thawing medium, and the remaining cells were transferred gently (drop by drop) to the centrifuge tube. The cells were centrifuged for 2 min at 2,000 rpm at 20°C using a Model 5810 Eppendorf® Centrifuge equipped with an A-4-62 swing-bucket rotor.
Under a hood, a Pasteur pipette connected to an aspirator and vacuum pump was used to aspirate the supernatant at the top of the centrifuge tube containing the hepatocytes. The tube was carefully tilted to remove the supernatant. The liquid was removed as carefully as possible without harming the cells. Finally, an aliquot of 7.5 mL of prewarmed incubation medium was gently added to the cells remaining in the centrifuge tube using a 10-mL serological pipette. The medium and cells were slowly drawn in and out of the pipette to break up the cell pellet until it was completely dispersed. The tube was gently shaken in an attempt to stir the dispersed cells.
A 5 µL aliquot of cell suspension was placed in a 2-mL Eppendorf centrifuge tube, using a 10-μL pipette. A 45 µL aliquot of hepatocyte incubation medium and a 5 µL aliquot of trypan blue were added using a 50-μL pipette and a 10-µL pipette, respectively. The contents of the tube were mixed by suctioning the liquid gently in and out of the pipette. The tube was incubated for 1–2 min. A 10 μL sample of these dyed cells was placed under a slide cover; the total cell count and number of viable cells were determined using the trypan blue exclusion method.
Preparation of 48-well plate for incubation
Human liver hepatocytes were incubated for 4 h with MDMA at concentrations of 0, 0.0625, 0.25 and 1 mM MDMA. An aliquot of 500 μL of cell suspension was added to each well (16 wells). A solution of 50 μL of MDMA (0, 0.625, 2.5 and 10 mM) was added using a 50-μL automatic pipette. A clean sterile pipette was used for each MDMA solution. The plate was placed in an incubator at 37°C for 4 h. After 4 h, the 48-well plate was removed from the incubator. The plate was gently shaken to loosen up the cells. The cells were removed from the wells using a 1,000-μL pipette and transferred into prelabeled 2 mL eppendorf centrifuge tubes (16 tubes per treatment group). The cells were drawn in and out of the pipette to dislodge them from the bottom of the wells. A new pipette tip was used for each treatment group. The tubes were centrifuged using a Model 5417R Eppendorf Centrifuge equipped with an FA 45-30-11 rotor for 5 min at 500 rpm at 4°C, followed by centrifugation at 14,000 rpm for10 min. The liquid was drawn up from each centrifuge tube using a 1,000-μL pipette. A new pipette tip was used for each sample. The supernatant from each sample was placed into separate prelabeled centrifuge tubes. All sample tubes were stored at −80°C prior to analysis.
Extraction of metabolites from hepatocyte incubations
After the supernatant was carefully removed, an aliquot of 200 μL of solvent B (95% ACN, 5% H2O, 10 mM ammonium acetate, 10 mM acetic acid) was added to the hepatocyte pellets. The mixtures containing the hepatocytes and solvent B were then sonicated for 10 min to ensure the rupture of cell walls using a Model 500 Fisher Scientific Sonic Dismembrator. The hepatocyte and solvent mixture was centrifuged again at 14,000 rpm for 15 min using a Model 5417R Eppendorf Centrifuge equipped with an FA 45-30-11 rotor (to remove hepatocyte debris). The supernatant was carefully removed and analyzed by HPLC and MS for the identification of potential MDMA metabolites.
Extract delivery—chromatography
All samples were run through a Shimadzu HPLC system (Shimadzu Scientific Instruments, Columbia, MD, USA), consisting of an SCL-10AVP controller, SIL-10AD VP Autoinjector, SPD-10A VP UV-VIS Detector and LC-10AT VP Pump or an UltiMate™ HPLC System (LC Packings, Dionex Corporation, Sunnyvale, CA, USA) with the SWITCHOS mode by passed and no flow sensor.
An Atlantis hydrophilic interaction liquid chromatography (HILIC) column (silica, 3 μM particle size, C18, 50 × 2.0 mm, 25°C) was used. The mobile phase consisted of solvent A (95% isopropyl alcohol, 5% H2O, 10 mM acetic acid, 10 mM ammonium acetate) and solvent B (95% acetonitrile, 5% H2O, 10 mM acetic acid, 10 mM ammonium acetate). A gradient elution program was used consisting of 70–50% B from 0 to 14 min, a 6-min hold at 50% B, followed by a return to 70% B for a 5-min equilibration to recondition the column before the next injection. The column temperature and the flow rate were 25°C and 300 μL/min, respectively. The injection volume was set to 5 μL.
Extract detection-mass spectrometry
A hybrid quadrupole/linear ion trap 4000 QTrap® MS/MS system (AB Sciex, Framingham, MA, USA) equipped with an electrospray ionization source was used to qualitatively and quantitatively analyze metabolites. Effluent from the HPLC was split to a UV detector and the mass spectrometer. MS/MS experiments were performed using both triple quadrupole and linear ion trap scan functions with Analyst® Software 1.4.2. (AB Sciex, Framingham, MA, USA). A 5 µL sample of the each extract from in vitro incubation of MDMA with human hepatocytes was separated by liquid chromatography before introduction into the mass spectrometer. The full spectra of unknown metabolites were compared with the full spectra of standards to determine the structure of the unknown metabolites.
Results and Discussion
HPLC separation of MDMA metabolites
The MDMA metabolites were separated by the HILIC HPLC system. The compounds eluted in order of increasing hydrophobicity. The HPLC was run with mobile phase A: 95% isopropyl alcohol, 5% H2O (10 mM HAc, 10 mM NH4Ac) and mobile phase B: 95% ACN, 5% H2O (10 mM HAc, 10 mM NH4Ac) at a gradient of 75–50% B in 30 min. Each conjugate had a different chemical/structural make-up so the mass spectrometer was able to separate the metabolites.
Metabolic product from MDMA hepatocyte cell incubation
Figure 1 shows the HILIC–HPLC chromatograph of the hepatocyte extract and mass spectrum of the enhanced product ion scan of the parent ion of MDMA at m/z 194. The hepatocyte extract came from the cell incubated with 1.0 mM of MDMA. The eluate with a retention time of 4.16 min shows the presence of MDMA, with distinct product ion peaks at m/z 163, 135, 105 and 77.
Figure 1.
Enhanced product ion scan at 194 amu of HILIC–HPLC eluate of human hepatocyte extract.
Enhanced product ion scans of m/z = 499 amu of extracts from hepatocytes treated with 1.0 mM MDMA (Figure 2A) show a distinct fragmentation pattern (m/z 194.2, 163, 135, 105), suggesting the formation of an MDMA–GSH conjugate and MDMA-SG. The formation of MDMA-SG was further supported by the apparent lack of the same fragmentation pattern from hepatocyte samples without MDMA treatment (Figure 2B).
Figure 2.
(A) Enhanced product ion scan at 499 amu of HILIC–HPLC eluate from extract of hepatocyte incubation with MDMA. (B) Enhanced product ion scan at 499 amu of HILIC–HPLC eluate from extract of hepatocyte incubation without MDMA (control). (C) The proposed pathway of the formation of MDMA-SG.
The metabolic pathway for the formation of a direct GSH conjugate of MDMA is proposed (Figure 2C). The formation of a direct MDMA–GSH conjugate, MDMA-SG, has not been reported before. The studies from other research groups suggest that MDMA will be demethylenated to N-methyl-α-methyldopamine or N-demethylated to MDA first, which can be subsequently demethylenated to α-methyldopamine, followed by GSH conjugation reactions. The preliminary finding of the formation of a direct GSH conjugate of MDMA provides an alternative mechanism for the formation of an MDMA–GSH conjugate.
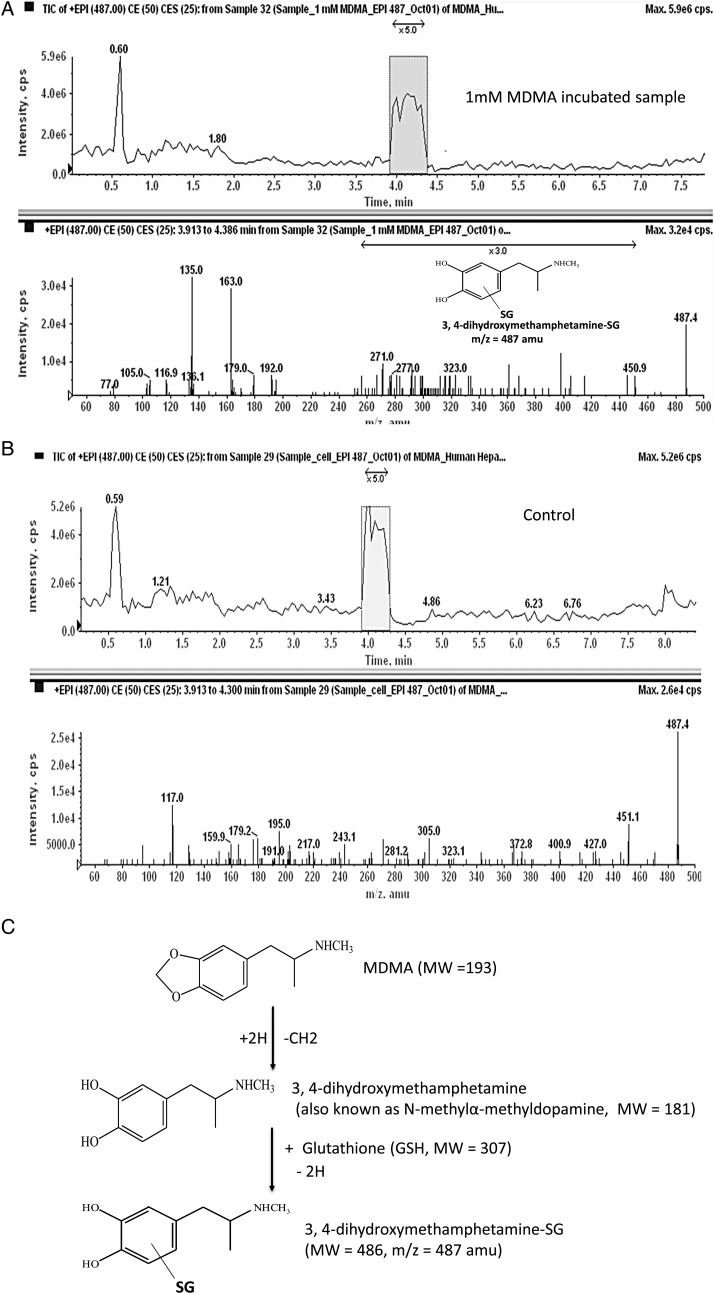
Enhanced product ion scans of m/z = 487 a.m.u. of extracts from hepatocytes treated with 1 mM MDMA also show a distinct fragmentation pattern (m/z 192, 163, 135) that may come from conjugation of an MDMA oxidative metabolite with GSH (Figure 3A). The formation of 3,4-dihydroxymethamphetamine-SG from 3,4-dihydroxymethamphetamine (also known as N-methyl-α-methyldopamine, MW = 181) is supported by the apparent lack of the same fragmentation pattern from hepatocytes without MDMA treatment (Figure 3B). The proposed pathway for the formation of 3,4-dihydroxymethamphetamine-SG is shown in Figure 3C.
Figure 3.
(A) Enhanced product ion scan at 487 amu of HILIC–HPLC eluate from extract of hepatocyte incubation with MDMA. (B) Enhanced product ion scan at 487 amu of HILIC–HPLC eluate from extract of hepatocyte incubation without MDMA (control). (C) The proposed pathway for the formation of 3,4-dihydroxymethamphetamine SG.
Conclusion
Direct injection of MDMA into the brain does not result in neurotoxic effects observed after peripheral administration, suggesting that systemic metabolism of MDMA plays an important role in the development of neurotoxicity. Catecholamine metabolites of MDMA (3,4-dihydroxymethamphetamine; 3,4-dihydroxyamphetamine; 4-hydroxy-3-methoxymethamphetamine and 4-hydroxy-3-methoxyamphetamine), the major metabolites formed from the P450 enzyme system, fail to produce serotonergic neurotoxicity. Catecholamine metabolites of MDMA are believed to be further oxidized to ortho-quinones. The ortho-quinones then react with GSH to form Phase II metabolites. Intracerebroventricular injection of thioether conjugates 2,5-bis(glutathion-S-yl)-α-methyldopamine and 5-(glutathion-S-yl)-α-methyldopamine) causes serotonergic toxicity similar to that seen following systemic MDMA administration. The recent evidence shows that these GSH adducts are transported into the brain by the GSH transporter and produce neurotoxicity. In addition, the metabolites of endogenous dopamine, specifically catechol thioethers, have been shown to induce dopaminergic neurodegeneration in Parkinson's disease.
Direct evidence of formation of GSH adducts in animals and humans after MDMA administration has not been clearly demonstrated previously. Neither the mechanism nor the enzymes involved in the formation of these neurotoxic thioether metabolites have been elucidated. This paper reports direct evidence of the formation of GSH adducts during in vitro incubation of fresh human hepatocytes with MDMA. The analysis of several batch incubations of MDMA with cryopreserved hepatocytes using LC–MS-MS has identified two possible GSH conjugate metabolites. The results generated from this study should yield valuable qualitative and quantitative information about the neurotoxic thioether metabolites formed from MDMA in humans.
Supplementary Data
Supplementary data are available at Journal of Analytical Toxicology online.
Funding
This work was supported by a grant 1R24DA012546-1 from the National Institutes of Health to C.D.
Supplementary Material
References
- 1.Capela J.P., Carmo H., Remiao F., Bastos M.L., Meisel A., Carvalho F. Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: an overview. Molecular Neurobiology. 2009;39:210–271. doi: 10.1007/s12035-009-8064-1. [DOI] [PubMed] [Google Scholar]
- 2.Bolla K.I., McCann U.D., Ricaurte G.A. Memory impairment in abstinent MDMA (“Ecstasy”) users. Neurology. 1998;51:1532–1537. doi: 10.1212/wnl.51.6.1532. [DOI] [PubMed] [Google Scholar]
- 3.McCann U.D., Mertl M., Eligulashvili V., Ricaurte G.A. Cognitive performance in (+/−) 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) users: a controlled study. Psychopharmacology (Berl) 1999;143:417–425. doi: 10.1007/s002130050967. [DOI] [PubMed] [Google Scholar]
- 4.Jones D.C., Duvauchelle C., Ikegami A., Olsen C.M., Lau S.S., de la Torre R., et al. Serotonergic neurotoxic metabolites of ecstasy identified in rat brain. The Journal of Pharmacology and Experimental Therapeutics. 2005;313:422–431. doi: 10.1124/jpet.104.077628. [DOI] [PubMed] [Google Scholar]
- 5.Erives G.V., Lau S.S., Monks T.J. Accumulation of neurotoxic thioether metabolites of 3,4-(+/−)-methylenedioxymethamphetamine in rat brain. The Journal of Pharmacology and Experimental Therapeutics. 2008;324:284–291. doi: 10.1124/jpet.107.128785. [DOI] [PubMed] [Google Scholar]
- 6.Ricaurte G.A., McCann U.D., Szabo Z., Scheffel U. Toxicodynamics and long-term toxicity of the recreational drug, 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) Toxicology Letters. 2000;112–113:143–146. doi: 10.1016/s0378-4274(99)00216-7. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt C.J., Taylor V.L. Direct central effects of acute methylenedioxymethamphetamine on serotonergic neurons. European Journal of Pharmacology. 1988;156:121–131. doi: 10.1016/0014-2999(88)90154-9. [DOI] [PubMed] [Google Scholar]
- 8.Paris J.M., Cunningham K.A. Lack of serotonin neurotoxicity after intraraphe microinjection of (+)-3,4-methylenedioxymethamphetamine (MDMA) Brain Research Bulletin. 1992;28:115–119. doi: 10.1016/0361-9230(92)90237-r. [DOI] [PubMed] [Google Scholar]
- 9.Kreth K., Kovar K., Schwab M., Zanger U.M. Identification of the human cytochromes P450 involved in the oxidative metabolism of “Ecstasy”-related designer drugs. Biochemical Pharmacology. 2000;59:1563–1571. doi: 10.1016/s0006-2952(00)00284-7. [DOI] [PubMed] [Google Scholar]
- 10.Delaforge M., Jaouen M., Bouille G. Inhibitory metabolite complex formation of methylenedioxymethamphetamine with rat and human cytochrome P450. Particular involvement of CYP 2D. Environmental Toxicology and Pharmacology. 1999;7:153–158. doi: 10.1016/s1382-6689(99)00007-1. [DOI] [PubMed] [Google Scholar]
- 11.Lim H.K., Foltz R.L. Ion trap tandem mass spectrometric evidence for the metabolism of 3,4-(methylenedioxy)methamphetamine to the potent neurotoxins 2,4,5-trihydroxymethamphetamine and 2,4,5-trihydroxyamphetamine. Chemical Research in Toxicology. 1991;4:626–632. doi: 10.1021/tx00024a004. [DOI] [PubMed] [Google Scholar]
- 12.Hiramatsu M., Kumagai Y., Unger S.E., Cho A.K. Metabolism of methylenedioxymethamphetamine: formation of dihydroxymethamphetamine and a quinone identified as its glutathione adduct. The Journal of Pharmacology and Experimental Therapeutics. 1990;254:521–527. [PubMed] [Google Scholar]
- 13.Lim H.K., Zeng S., Chei D.M., Foltz R.L. Comparative investigation of disposition of 3,4-(methylenedioxy)methamphetamine (MDMA) in the rat and the mouse by a capillary gas chromatography-mass spectrometry assay based on perfluorotributylamine-enhanced ammonia positive ion chemical ionization. Journal of Pharmaceutical and Biomedical Analysis. 1992;10:657–665. doi: 10.1016/0731-7085(92)80094-4. [DOI] [PubMed] [Google Scholar]
- 14.Maurer H.H., Bickeboeller-Friedrich J., Kraemer T., Peters F.T. Toxicokinetics and analytical toxicology of amphetamine-derived designer drugs (“Ecstasy”) Toxicology Letters. 2000;112–113:133–142. doi: 10.1016/s0378-4274(99)00207-6. [DOI] [PubMed] [Google Scholar]
- 15.Cho A.K., Hiramatsu M., Distefano E.W., Chang A.S., Jenden D.J. Stereochemical differences in the metabolism of 3,4-methylenedioxymethamphetamine in vivo and in vitro: a pharmacokinetic analysis. Drug Metabolism and Disposition: the Biological Fate of Chemicals. 1990;18:686–691. [PubMed] [Google Scholar]
- 16.Fallon J.K., Kicman A.T., Henry J.A., Milligan P.J., Cowan D.A., Hutt A.J. Stereospecific analysis and enantiomeric disposition of 3,4-methylenedioxymethamphetamine (Ecstasy) in humans. Clinical Chemistry. 1999;45:1058–1069. [PubMed] [Google Scholar]
- 17.McCann U.D., Ricaurte G.A. Major metabolites of (+/−) 3,4-methylenedioxyamphetamine (MDA) do not mediate its toxic effects on brain serotonin neurons. Brain Research. 1991;545:279–282. doi: 10.1016/0006-8993(91)91297-e. [DOI] [PubMed] [Google Scholar]
- 18.McCann U.D., Ridenour A., Shaham Y., Ricaurte G.A. Serotonin neurotoxicity after (+/−)3,4-methylenedioxymethamphetamine (MDMA; “Ecstasy”): a controlled study in humans. Neuropsychopharmacology. 1994;10:129–138. doi: 10.1038/npp.1994.15. [DOI] [PubMed] [Google Scholar]
- 19.Chu T., Kumagai Y., DiStefano E.W., Cho A.K. Disposition of methylenedioxymethamphetamine and three metabolites in the brains of different rat strains and their possible roles in acute serotonin depletion. Biochemical Pharmacology. 1996;51:789–796. doi: 10.1016/0006-2952(95)02397-6. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Z.Y., Castagnoli N., Jr, Ricaurte G.A., Steele T., Martello M. Synthesis and neurotoxicological evaluation of putative metabolites of the serotonergic neurotoxin 2-(methylamino)-1-[3,4-(methylenedioxy)phenyl] propane [(methylenedioxy)methamphetamine] Chemical Research in Toxicology. 1992;5:89–94. doi: 10.1021/tx00025a015. [DOI] [PubMed] [Google Scholar]
- 21.Bolton J.L., Trush M.A., Penning T.M., Dryhurst G., Monks T.J. Role of quinones in toxicology. Chemical Research in Toxicology. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- 22.Bai F., Lau S.S., Monks T.J. Glutathione and N-acetylcysteine conjugates of alpha-methyldopamine produce serotonergic neurotoxicity: possible role in methylenedioxyamphetamine-mediated neurotoxicity. Chemical Research in Toxicology. 1999;12:1150–1157. doi: 10.1021/tx990084t. [DOI] [PubMed] [Google Scholar]
- 23.Capela J.P., Macedo C., Branco P.S., Ferreira L.M., Lobo A.M., Fernandes E., et al. Neurotoxicity mechanisms of thioether ecstasy metabolites. Neuroscience. 2007;146:1743–1757. doi: 10.1016/j.neuroscience.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 24.Perfetti X., O'Mathuna B., Pizarro N., Cuyas E., Khymenets O., Almeida B., et al. Neurotoxic thioether adducts of 3,4-methylenedioxymethamphetamine identified in human urine after ecstasy ingestion. Drug Metabolism and Disposition: the Biological Fate of Chemicals. 2009;37:1448–1455. doi: 10.1124/dmd.108.026393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.