Abstract
The purpose of this study was to report the outcomes of high-dose-rate (HDR) brachytherapy and hypofractionated external beam radiotherapy (EBRT) combined with long-term androgen deprivation therapy (ADT) for National Comprehensive Cancer Network (NCCN) criteria-defined high-risk (HR) and very high-risk (VHR) prostate cancer. Data from 178 HR (n = 96, 54%) and VHR (n = 82, 46%) prostate cancer patients who underwent 192Ir-HDR brachytherapy and hypofractionated EBRT with long-term ADT between 2003 and 2008 were retrospectively analyzed. The mean dose to 90% of the planning target volume was 6.3 Gy/fraction of HDR brachytherapy. After five fractions of HDR treatment, EBRT with 10 fractions of 3 Gy was administered. All patients initially underwent ≥6 months of neoadjuvant ADT, and adjuvant ADT was continued for 36 months after EBRT. The median follow-up was 61 months (range, 25–94 months) from the start of radiotherapy. The 5-year biochemical non-evidence of disease, freedom from clinical failure and overall survival rates were 90.6% (HR, 97.8%; VHR, 81.9%), 95.2% (HR, 97.7%; VHR, 92.1%), and 96.9% (HR, 100%; VHR, 93.3%), respectively. The highest Radiation Therapy Oncology Group-defined late genitourinary toxicities were Grade 2 in 7.3% of patients and Grade 3 in 9.6%. The highest late gastrointestinal toxicities were Grade 2 in 2.8% of patients and Grade 3 in 0%. Although the 5-year outcome of this tri-modality approach seems favorable, further follow-up is necessary to validate clinical and survival advantages of this intensive approach compared with the standard EBRT approach.
Keywords: high-dose-rate brachytherapy, prostate cancer, androgen deprivation therapy, high-risk, very high-risk
INTRODUCTION
In the field of radiation oncology, the current strategy for treating high-risk prostate cancer is to combine external beam radiation therapy (EBRT) with long-term androgen deprivation therapy (ADT). Randomized trials have shown not only an improved biochemical control rate, but also an improved overall survival rate (OS) with this combination compared with radiation alone [1, 2] or hormonal therapy alone [3]. Radiation dose escalation without ADT is also well established using 3D conformal beam radiotherapy, intensity-modulated radiotherapy, low- or high-dose-rate (HDR) brachytherapy, and particle beams. Although the combination of dose escalation and ADT has been suggested to offer improved treatment results, little information has been accumulated regarding this approach. Few reports appear to have described the combination of HDR brachytherapy and long-term ADT for high-risk prostate cancer patients.
Among modern radiotherapeutic techniques, brachytherapy is expected to provide an effective approach for delivering radiation doses more safely and precisely compared with 3D conformal radiotherapy or intensity-modulated radiotherapy [4]. In addition, hypofractionated radiotherapy may prove advantageous for treating prostate cancer when compared with other types of cancer, because of the low α:β ratio [5]. We have been treating prostate cancer patients with HDR brachytherapy combined with hypofractionated EBRT using a fractional dose of 3 Gy administered five times per week [6].
The purpose of this study was to report the long-term outcomes of HDR brachytherapy combined with hypofractionated EBRT with long-term ADT for localized prostate cancer.
MATERIALS AND METHODS
Patients
The institutional review board approved this retrospective study. A total of 200 consecutive patients with National Comprehensive Cancer Network (NCCN) criteria-defined high-risk (HR) and very high-risk (VHR) prostate cancer were treated using HDR brachytherapy between December 2003 and January 2008. Clinical Stage T3a, a Gleason score of 8–10, and a prostate-specific antigen (PSA) level >20 ng/ml were defined as HR factors. Clinical stage T3b–T4 was defined as the VHR factor. Patients with a single HR factor were classified as HR, and patients with at least the single VHR factor or two HR factors were classified as VHR. Pretreatment evaluation included clinical history, physical examination, blood laboratory findings, pelvic computed tomography (CT), and a bone scan. Magnetic resonance imaging (MRI) was recommended on request. No lymph node dissection was performed. Patients with positive lymph nodes or distant metastasis were excluded. International Prostate Symptom Score (IPSS), previous transurethral resection, and prostate volume were not considered in the selection criteria.
Of the 200 patients, 6 failed to complete the scheduled protocol, and another 16 were lost to follow-up. Analyses were thus performed for 178 of the 200 patients. Characteristics of these 178 patients are shown in Table 1.
Table 1.
Patients' characteristics
| High–risk | Very–high–risk | |
|---|---|---|
| n | 96 | 82 |
| Age | 72 (57–87) | 72 (54–87) |
| Clinical T stage | ||
| T1c | 38 | 4 |
| T2a | 3 | 1 |
| T2b | 26 | 3 |
| T2c | 8 | 1 |
| T3a | 20 | 43 |
| T3b | 1 | 28 |
| T4 | 0 | 2 |
| Gleason score | ||
| 10 | 2 | 1 |
| 9 | 8 | 21 |
| 8 | 21 | 18 |
| 4 + 3 | 20 | 23 |
| 3 + 4 | 26 | 15 |
| 6 | 14 | 4 |
| 5 | 5 | 0 |
| Initial PSA (ng/ml) | 19.9 (3.3–159) | 40.5 (2.7–337.6) |
| Prostate volume (ml) | 20.7 (9.5–63.5) | 18.8 (6.9–60.8) |
| Neoadjuvant ADT (months) | 12 (8–28) | 13 (7–74) |
Values are number or median (range)
PSA, prostate specific antigen; LNI, lymph-node involvement
ADT; androgen deprivation therapy
Radiotherapy and hormonal therapy
All patients initially underwent ≥6 months (mean, 14 months; median, 12 months; range, 7–74 months) of neoadjuvant ADT, and adjuvant ADT was continued for 36 months after completion of radiotherapy. Neoadjuvant ADT comprised combined androgen blockade with monthly gonadotropin-releasing hormone agonist (GnRHa) injections and 125 mg of flutamide twice daily. Adjuvant ADT consisted of monthly injections of GnRHa. Briefly, patients in the operating room were placed in a lithotomy position under epidural anesthesia. Treatment was initiated using placement of a closed transperineal hollow needle under transrectal ultrasound guidance. Multiple 20- to 25-cm-long, closed-end, 15-G plastic hollow needles were inserted transperineally using a Syed-Neblett plastic template (Alpha-Omega Services, Bellflower, CA). Routinely, 18 needles were implanted. Twelve needles were inserted in the peripheral portion and six needles were inserted in the central portion of the prostate. Flexible cystoscopy was conducted to check that the urethra had not been penetrated by the implanted tubes. The needle tips were left within the urinary bladder, 1.5 cm above the sonographically or cystoscopically defined base of the prostate. Metallic marker seeds were placed transperineally into the base and apex.
After all of these procedures had been completed, the patient underwent CT to obtain scans at 5-mm intervals for CT-based planning. Contours of the planning target volume (PTV), urethra and rectum were outlined according to transverse CT images. The PTV was defined as the prostate gland with or without proximal seminal vesicles, with an additional 3- to 5-mm margin all around. Reference points for the urethra were set on the center of the urethral catheter, and those for the rectal wall were set 5 mm behind the edge of the anterior rectal wall on transverse CT images with 10-mm intervals. Reference points for the PTV were automatically distributed on the surface of the PTV. Dose limitation was set as 10 Gy/fraction for urethral reference points and 4 Gy/fraction for rectal reference points, and we attempted to prescribe 7.5 Gy/fraction to reference points of the PTV (unless the dose limitation was violated) using inverse planning and geometric optimization. Because of urethral and rectal dose limitations, covering the periphery of the PTV with the prescribed dose was difficult in some patients. The mean dose to 90% of the PTV (D90), the prostate volume receiving at least 6 Gy (V6), and the prostate volume receiving at least 9 Gy (V9) were 6.3 ± 0.6 Gy, 91 ± 5%, and 53 ± 9% per fraction, respectively. Five fractions of HDR treatment were administered. After CT-based planning using a Nucletron planning system (Veenendaal, the Netherlands), the first treatment session of HDR brachytherapy was conducted using the Nucletron microSelectron HDR 192Ir remote afterloading system. Dwell positions were activated at 5-mm intervals along each catheter. The deepest dwell position could be set at 6.5 mm from the catheter tip if needed. Catheter positions were checked by fluoroscopy before every treatment session and corrected if interfraction needle movements >5 mm were noted. The first treatment session was conducted on the day of implantation, with the subsequent four treatment sessions administered twice daily with an interval of at least 6 h between fractions. Treatment duration was thus 3 days. At 6 days after completion of HDR brachytherapy, patients received EBRT using a dynamic-arc conformal technique, administered with high-energy photons comprising 10-MV X-rays to a total dose of 30 Gy. Total dose was administered in five weekly fraction doses of 3 Gy. The radiation field was limited to the prostate gland with or without proximal seminal vesicles with a 7-mm leaf margin using multileaf collimators.
Follow-up
Duration of follow-up was calculated from the start of HDR brachytherapy. Toxicities were evaluated using the Radiation Therapy Oncology Group scale [7] at every visit, and all patients were followed up at 3-month intervals during the first year and at 3- to 6-month intervals thereafter. Acute toxicity was defined as toxicity occurring ≤3 months after implantation and late toxicity as that occurring after >3 months. Median follow-up for all patients was 61 months (range, 25–94 months). Biochemical failure was defined according to the Phoenix definition [8]. Biochemical non-evidence of disease rate (bNED) was calculated for all living patients and reflected biochemical failures. Freedom from clinical failure rate (FFcF) was calculated for all living patients and reflected all clinical events (local, regional or distant failure) and salvage ADT. OS reflected all deaths, cancer-related or otherwise.
Statistical analysis
Univariate analysis (log-rank) was used to examine the predictive value of patient-related factors (clinical T stage (≤T2c vs ≥T3a), Gleason score (≤7 vs ≥8), initial PSA (≤20 ng/ml vs >20 ng/ml), NCCN criteria (HR vs VHR), prostate volume (≤20 ml vs >20 ml), age (≤70 years vs >70 years)) and treatment-related factors (D90 (≤6.3 Gy/fraction vs >6.3 Gy/fraction) and duration of neoadjuvant ADT (<12 months vs ≥12 months)). To evaluate interactions and independent influences on factors, multivariate analysis was performed using Cox regression analysis. Differences were regarded as statistically significant at the P < 0.05 level.
RESULTS
Efficacy
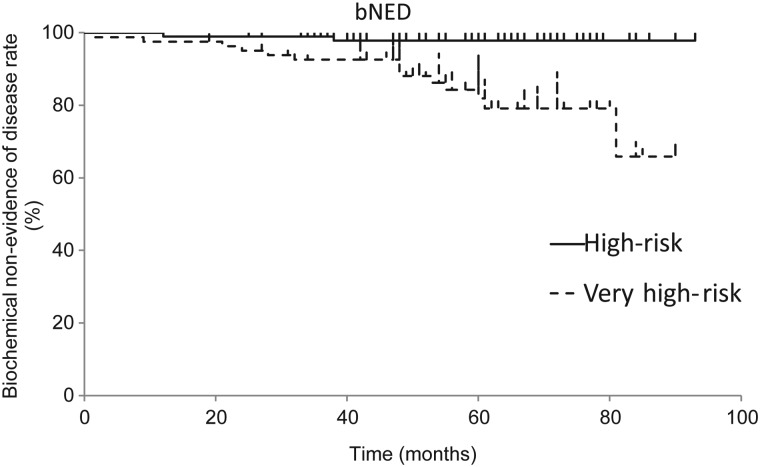
The 3- and 5-year bNED were 96.0% (HR, 98.9%; VHR, 92.6%) and 90.6% (HR, 97.8%; VHR, 81.9%), respectively (Fig. 1). The corresponding values for FFcF were 97.4% (HR, 99.0%; VHR, 96.3%) and 95.2% (HR, 97.7%; VHR, 92.1%), respectively. The 3- and 5-year OS rates were 97.7% (HR, 100%; VHR, 95.1%) and 96.9% (HR, 100%; VHR, 93.3%), respectively. Nine patients experienced clinical progression, including 4 patients with bone metastasis, 1 patient with lung metastasis, 1 patient with distant lymph-node metastasis, 1 patient with regional lymph-node metastasis, 1 patient with positive biopsy, and 1 patient who underwent salvage ADT. Five patients died during follow-up including 1 patient who died of interstitial pneumonitis, 1 patient who died of bladder cancer, 2 patients who died of prostate cancer, and 1 patient who died due to an accident.
Fig. 1.
Biochemical non-evidence of disease rates (bNED) for high-risk and very high-risk patients.
Toxicity
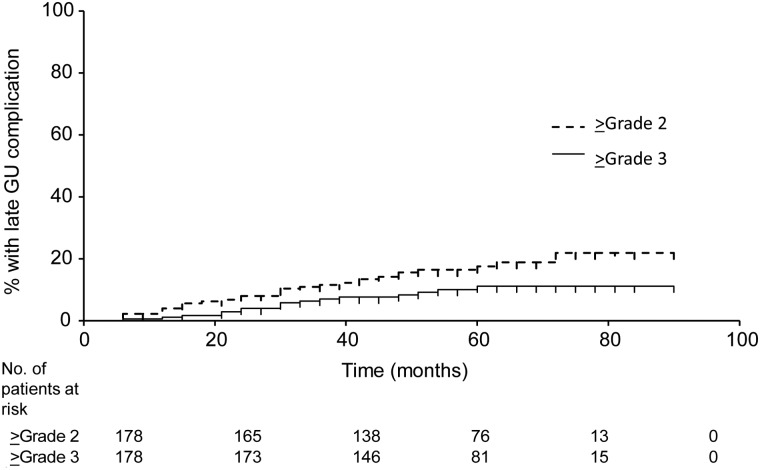
The highest Radiation Therapy Oncology Group (RTOG)-defined acute genitourinary (GU) toxicities were Grade 2 in 19 patients (10.7%) and Grade 3 in 10 patients (5.6%). No patients experienced ≥Grade 2 acute gastrointestinal (GI) toxicities. The highest RTOG-defined late GU toxicities were Grade 2 in 13 patients (7.3%) and Grade 3 in 17 patients (9.6%). Most Grade 3 GU toxicities were urinary retention or urethral stricture, which were managed successfully by temporary catheterization or internal urethrotomy. The highest late GI toxicities were Grade 2 in 5 patients (2.8%). No patients showed Grade 3 toxicities. No patients experienced acute Grade 4 or 5 toxicity. Actuarial rates of late GU toxicity are shown in Fig. 2.
Fig. 2.
Actuarial incidence of late genitourinary (GU) complications.
Predictive factors for bNED and FFcF
Table 2 shows the results of uni- and multivariate analyses for factors predicting bNED and FFcF. On univariate analysis, clinical T stage, Gleason score and NCCN risk-group were detected as predictive factors for bNED. Clinical T stage, NCCN risk-group and prostate volume were significant predictors for FFcF. On multivariate analysis, however, no risk factor reached the level of statistical significance.
Table 2.
Univariate and multivariate analysis of biochemical and clinical disease control rates
| Variable | bNED |
FFcF |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
||||||
| p | HR | p | HR | p | HR | p | HR | ||
| Stage | ≤T2c ≥T3a |
0.014* | 0.234 | 0.316 | 2.165 | 0.020* | 0.127 | 0.187 | 5.242 |
| Gleason score | ≤7 ≥8 |
0.047* | 0.374 | 0.215 | 2.003 | 0.283 | 0.494 | ||
| PSA level (ng/ml) | ≤20 >20 |
0.309 | 0.562 | − | 0.688 | 1.308 | |||
| NCCN criteria | High–risk Very–high–risk | <0.001* | 0.109 | 0.083 | 4.842 | 0.044* | 0.229 | 0.687 | 1.465 |
| Age (y.o) | ≤70 >70 |
0.043* | 2.813 | 0.058 | 0.355 | 0.922 | 1.068 | ||
| Prostate volume (ml) | ≤20 >20 |
0.218 | 1.937 | − | 0.021* | 7.759 | 0.094 | 0.168 | |
| D90 (Gy per fraction) | ≤6.3 >6.3 |
0.909 | 0.945 | − | 0.631 | 0.726 | |||
| NeoADT duration (months) | ≤12 >12 |
0.403 | 0.632 | − | 0.129 | 0.304 | |||
* statistical significance
bNED, biochemical non–evidence of disease rate; FFcF, freedom from clinical failure rate; HR, hazard ratio;
PSA, prostate specific antigen; ADT, androgen deprivation therapy; NCCN, national comprehansive cancer network
DISCUSSION
With more than 5 years of follow-up, HDR and hypofractionated EBRT combined with long-term ADT produced encouraging results. Even with VHR patients, a 5-year bNED of 81.9% could be achieved with this combination. Although continued failures will likely occur with additional follow-up, our result was encouraging compared with the reported bNED of HDR brachytherapy for HR prostate cancer (Table 3), which varies within the range of 60–80% at a 5-year median follow-up with or without hormonal therapy[9–25].
Table 3.
Selected reports of HDR for high–risk localized prostate cancer (n ≥ 100, median follow-up ≥5 years)
| Auther | Year | n | HDR | EBRT | Follow-up (median) | OS | bDFS or bNED | Definition | Hx | H × length |
|---|---|---|---|---|---|---|---|---|---|---|
| Yoshioka | 2011 | 112 (high, 68) | 54Gy in 9fx | none | 5.4 years | 5-year 97% | 5-year bNED Low, 85% Int, 93% High, 79% |
Phoenix | 84% | Median 36 months (2–131 months) |
| Agoston | 2011 | 100 (high, 61) | 10Gy in 1fx | 60Gy | 61.5 months | 5-year 93.3% | 7-year bNED Int, 84.2% High, 81.6% |
Phoenix | 30% | Mean 17.7 months (4–60 months) |
| Phan | 2007 | 309 (high, 133) | 15–26Gy in 3–4fx | 36–50.4Gy | 59 months | 5-year 91% | 5-year bNED Low, 98% Int, 90% High, 78% |
ASTRO | 36% | NA |
| Hoskin | 2012 | 197 (high, 86) | 34–36Gy in 4fx 31.5Gy in 3fx 26Gy in 2fx |
none | 4.5-5-years | NA | 4-year bNED Int, 95% High, 87% |
Phoenix | 80% | Median 6.3 months (1–40 months) |
| Hoskin | 2012 | 110 (high, 54) | 2 × 8.5Gy | 35.75Gy in 13fx | 85 months | 5-year 88% | bDFS 5-year, 75% 7-year, 66% 10-year, 46% |
Phoenix | 77% | Low and int, 6 months High, up to 3 years |
| Martinez | 2011 | 472 (high, NA) | 5.5Gy–11.5Gy × 2–3fx | 46Gy | 8.2 years | NA | 10-year bNED 70.6% |
Phoenix | 51% | <6 months |
| Khor | 2013 | 344 (high, 141) | 19.5Gy in 3 fractions | 46Gy | 60.5 months | NA | 5-year bNED 79.8% |
Phoenix | 59% | NA |
| Kaprealian | 2012 | 165 (high, 156) | 18Gy in 3fx 19Gy in 2fx |
45Gy | 105 months 45 months |
5-year 92% 97% |
5-year bNED 93.5% 87.3% |
Phoenix | 76% 92% |
Mean 9.5 months Mean 19.2 months |
| Prada | 2012 | 313 (high, 238) | 23Gy in 2fx | 46Gy | 68 months | 5-year 92% |
10-year bNED Low, 100% Int, 88% High, 91% Very high, 79% |
Phoenix | 70% | 12 months |
| Prada | 2012 | 252 (high, 252) | 23Gy in 2fx | 46Gy | 74 months | 5-year 88% | bNED 5-year, 84% 10-year, 78% |
Phoenix | 69% | 12 months |
| Kotecha | 2012 | 229 | 5.5–7.5Gy × 3 | 45–50.4Gy | 61 months | NA | 7-year bDFS Low, 95% Int, 90% High, 57% |
Phoenix | 42% | 9 months |
| Pellizzon | 2008 | 209 (high, 67) | Median 20Gy (16–24Gy) in 4fx |
Median 45Gy (36–54Gy) |
5.3 years | 5-year 95.7% |
3.3-year bNED Low, 91.5% Int, 90.2% High, 88.5% |
Phoenix | 48% | 3–6 months |
| Galalae | 2004 | 611 (high, 359) | 3–4Gy × 4fx 8–9Gy × 2fx 5.5–11.5Gy × 2–3fx |
45.6–50Gy | mean 5 years | 5-year 85% |
5-year bNED Low, 96% Int, 88% High, 69% |
ASTRO | 29% | Median 4 months |
| Galalae | 2006 | 324 (high, 80) | 5.5–6.5Gy × 3fx 8.25–15Gy × 2fx |
45.6–50Gy | 5.3 years | 5-year 90% |
5-year bNED Low, 85% Int, 81% High, 69% |
ASTRO | 0% | NA |
| Deger | 2005 | 411 (high, 295) | 9–10Gy × 2fx | 40–50.4Gy | 5 years | 5-year 87% |
5-year bDFS Low, 81% Int, 65% High, 59% |
ASTRO | NA | NA |
| Kalkner | 2007 | 154 (high, 66) | 20Gy in 2fx | 50Gy | median 6 years | NA | 5-year bNED or bDFS Low, 97% Int, 83% High, 83% Very high, 51% |
Phoenix | 100% | 6–9 months |
| Demanes | 2005 | 209 (high, 47) | 22–24Gy in 4fx | 36Gy in 20fx | 7.25 years | Crude 79% |
5-year bDFS Low, 93% Int, 93% High, 83% |
Phoenix | 0% | NA |
| Present study | 178 (high, 178) | 31.5Gy in 5fx | 30Gy in 10fx | 61 months | 5-year 96.6% |
5-year bNED 90.6% |
Phoenix | 100% | ≥42 months |
EBRT, external radation therapy; bDFS, biochemical dsease free survival rate; bNED, biochmical non–evidence of disease rate; Hx, hormonal therapy; ASTRO, american society of radation oncology OS, overall survival rate
On the other hand, Japanese-specific high-sensitivity to hormonal therapy may have some impacts on our outcomes [26]. Table 4 shows selected Japanese series of high-risk prostate cancer patients treated with conventional EBRT and hormonal therapy [27–31]. These favorable outcomes seem to be equal to our HDR approach. From the data of the 5-year follow up, it seems that our tri-modality therapy did not show any advantages over conventional EBRT with hormonal therapy.
Table 4.
Selected reports of ERRT for high-risk localized Japanese prostate cancer patients
| Auther | Year | n | ERRT | Follow-up (median) | OS | bDFS | Definition | Hx | Hx length |
|---|---|---|---|---|---|---|---|---|---|
| Takaha | 2011 | 75 (High, 100%) |
70Gy | 59 months | 5-year High, 98.3% | 5-year High, 87.4% | bDFS Phoenix |
100% | median 27months (8–63 months) |
| Sakamoto | 2010 | 70 (High, 100%) |
median 70Gy (60–70Gy) |
64.9 months | 5-year High, 90.3% |
5-year High, 60.5% |
bDFS Phoenix |
100% | median 4 months (3–16 months) |
| Nakamura | 2008 | 679 (High, 66.3%) |
>60Gy | 46 months | 5-year 93.0% | 5-year Low, 90.8% Int, 75.7%High, 67.6% |
bDFS ASTRO |
82.90% | Neoadjuvant, median 6months (1–68 months) Adjuvant, median 38 months (1–109 months) |
| Mitsumori | 2006 | 27 (High, 100%) |
70Gy | 63 months | 5-year High, 83.0% |
5-year High, 43.0% |
bDFS Phoenix |
100% | 3 months |
| Saito | 2006 | 78 (High, 100%) |
median 70Gy (60–70Gy) |
55 months | 5-year High, 94.9% |
NA | NA | 100% | 1–36 months or longer |
ERRT, external radiation therapy; bDFS, biochemical disease free survival rate; Hx, hormonal therapy; ASTRO, american society of radiation oncology OS, overall survival rate
Regarding late toxicities, 9.6% of our patients suffered from Grade 3 urethral toxicities after treatment. Meanwhile, the incidence of Grade 3 toxicity after EBRT was very rare [28]. HDR brachytherapy is associated with relatively severe GU toxicities, such as urethral stricture [11, 23, 24, 32]. Although the severity of GU toxicities in our study population was relatively high, all cases were manageable by medical or surgical intervention. Compared with patients (n = 298) treated before June 2008 when more strict urethral dose limitation was applied, the patients treated after that month (n = 279) show a significantly lower rate of Grade 3 late GU toxicity (11% vs 1%), although follow-up duration for the latter group was immature (54 months vs 30 months). Detailed data about updated DVH analysis is planned for publication in another study.
The biological equivalent dose (BED) for our protocol was a total of 205.7 Gy2 (130.7 Gy2 for D90 of HDR; 75 Gy2 for the isocenter of EBRT) based on α/β = 2. Compared with other institutions [9–15], our total dose was not excessive.
Although the data for ADT toxicities were not available in our study, ADT may lead to numerous toxicities, such as osteoporosis, obesity, sarcopenia, lipid alterations, insulin resistance, and increased risk for diabetes and cardiovascular morbidity [33]. These potential side-effects may reduce quality of life and overall survival. Combining ADT with EBRT is warranted because it will improve overall survival in high-risk patients [1]. However, there has been no randomized trial of the combination of ADT and HDR brachytherapy. Further trial is needed for exploring whether ADT really improves overall survival when it is combined with HDR brachytherapy.
Without whole pelvic irradiation, only 1 patient in our study population showed regional lymph node recurrence. The probability of lymph node involvement based on the Roach equation [34] was >30% for half of our patients. It is generally considered that whole pelvic irradiation may have potential benefit for these HR patients [35]. In our population, however, potential lymph-node metastasis was controlled by ADT alone, without irradiation. Unknown mechanisms and/or tumor-specific immune responses such as cytotoxic T lymphocyte (CTL) activity might be evoked through our protocol, although this remains to be established [36, 37].
Several limitations must be considered when interpreting the results of this study. First, our protocol did not apply a uniform duration of neoadjuvant ADT. Many of our patients had been on waiting lists for >6 months because of our limited capacity; they were treated with ADT while on the waiting list, and half of our patients received neoadjuvant ADT for ≥12 months. Second, the lack of testosterone data may confound the interpretation of the bNED results. After long-term ADT, delays in PSA recovery may lead to delays in biochemical failure. Third, our median follow-up of 61 months may have been insufficient, considering the provision of long-term adjuvant ADT for 36 months. Fourth, the excludion of 22 patients from the analysis (due o failure to complete the scheduled protocol or loss to follow-up) might have led to some degree of selection bias in the present study.
CONCLUSION
Although the 5-year outcome of this tri-modality approach seems favorable, further follow-up is necessary to validate clinical and survival advantages of this intensive approach compared with the standard EBRT approach.
FUNDING
This work was supported by JSPS KAKENHI Grant Number 24791334.
REFERENCES
- 1.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–6. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 2.Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 3.Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373:301–8. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 4.Challapalli A, Jones E, Harvey C, et al. High dose rate prostate brachytherapy: an overview of the rationale, experience and emerging applications in the treatment of prostate cancer. Br J Radiol. 2012;85 Spec. No. 1 doi: 10.1259/bjr/15403217. S18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner DJ. The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction. Semin Radiat Oncol. 2008;18:234–9. doi: 10.1016/j.semradonc.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishiyama H, Kitano M, Satoh T, et al. Genitourinary toxicity after high-dose-rate (HDR) brachytherapy combined with Hypofractionated External beam radiotherapy for localized prostate cancer: an analysis to determine the correlation between dose-volume histogram parameters in HDR brachytherapy and severity of toxicity. Int J Radiat Oncol Biol Phys. 2009;75:23–8. doi: 10.1016/j.ijrobp.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–6. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 8.Roach M, III, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–74. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 9.Yoshioka Y, Konishi K, Sumida I, et al. Monotherapeutic high-dose-rate brachytherapy for prostate cancer: five-year results of an extreme hypofractionation regimen with 54 Gy in nine fractions. Int J Radiat Oncol Biol Phys. 2011;80:469–75. doi: 10.1016/j.ijrobp.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Agoston P, Major T, Frohlich G, et al. Moderate dose escalation with single-fraction high-dose-rate brachytherapy boost for clinically localized intermediate- and high-risk prostate cancer: 5-year outcome of the first 100 consecutively treated patients. Brachytherapy. 2011;10:376–84. doi: 10.1016/j.brachy.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Phan TP, Syed AM, Puthawala A, et al. High dose rate brachytherapy as a boost for the treatment of localized prostate cancer. J Urol. 2007;177(123–7; discussion 127) doi: 10.1016/j.juro.2006.08.109. [DOI] [PubMed] [Google Scholar]
- 12.Hoskin PJ, Rojas AM, Bownes PJ, et al. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol. 2012;103:217–22. doi: 10.1016/j.radonc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Hoskin P, Rojas A, Lowe G, et al. High-dose-rate brachytherapy alone for localized prostate cancer in patients at moderate or high risk of biochemical recurrence. Int J Radiat Oncol Biol Phys. 2012;82:1376–84. doi: 10.1016/j.ijrobp.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 14.Martinez AA, Gonzalez J, Ye H, et al. Dose escalation improves cancer-related events at 10 years for intermediate- and high-risk prostate cancer patients treated with hypofractionated high-dose-rate boost and external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:363–70. doi: 10.1016/j.ijrobp.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 15.Khor R, Duchesne G, Tai KH, et al. Direct 2-arm comparison shows benefit of high-dose-rate brachytherapy boost vs external beam radiation therapy alone for prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85:679–85. doi: 10.1016/j.ijrobp.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Kaprealian T, Weinberg V, Speight JL, et al. High-dose-rate brachytherapy boost for prostate cancer: comparison of two different fractionation schemes. Int J Radiat Oncol Biol Phys. 2012;82:222–7. doi: 10.1016/j.ijrobp.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Prada PJ, Gonzalez H, Fernandez J, et al. Biochemical outcome after high-dose-rate intensity modulated brachytherapy with external beam radiotherapy: 12 years of experience. BJU Int. 2012;109:1787–93. doi: 10.1111/j.1464-410X.2011.10632.x. [DOI] [PubMed] [Google Scholar]
- 18.Prada PJ, Mendez L, Fernandez J, et al. Long-term biochemical results after high-dose-rate intensity modulated brachytherapy with external beam radiotherapy for high risk prostate cancer. Radiat Oncol. 2012;7:31. doi: 10.1186/1748-717X-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotecha R, Yamada Y, Pei X, et al. Clinical outcomes of high-dose-rate brachytherapy and external beam radiotherapy in the management of clinically localized prostate cancer. Brachytherapy. 2013;12:44–9. doi: 10.1016/j.brachy.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Pellizzon AC, Salvajoli J, Novaes P, et al. Updated results of high-dose rate brachytherapy and external beam radiotherapy for locally and locally advanced prostate cancer using the RTOG-ASTRO Phoenix definition. Int Braz J Urol. 2008;34:293–301. doi: 10.1590/s1677-55382008000300006. [DOI] [PubMed] [Google Scholar]
- 21.Galalae RM, Martinez A, Mate T, et al. Long-term outcome by risk factors using conformal high-dose-rate brachytherapy (HDR-BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:1048–55. doi: 10.1016/j.ijrobp.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Galalae RM, Martinez A, Nuernberg N, et al. Hypofractionated conformal HDR brachytherapy in hormone naive men with localized prostate cancer. Is escalation to very high biologically equivalent dose beneficial in all prognostic risk groups? Strahlenther Onkol. 2006;182:135–41. doi: 10.1007/s00066-006-1448-5. [DOI] [PubMed] [Google Scholar]
- 23.Deger S, Boehmer D, Roigas J, et al. High dose rate (HDR) brachytherapy with conformal radiation therapy for localized prostate cancer. Eur Urol. 2005;47:441–8. doi: 10.1016/j.eururo.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Kalkner KM, Wahlgren T, Ryberg M, et al. Clinical outcome in patients with prostate cancer treated with external beam radiotherapy and high dose-rate iridium 192 brachytherapy boost: a 6-year follow-up. Acta Oncol. 2007;46:909–17. doi: 10.1080/02841860601156140. [DOI] [PubMed] [Google Scholar]
- 25.Demanes DJ, Rodriguez RR, Schour L, et al. High-dose-rate intensity-modulated brachytherapy with external beam radiotherapy for prostate cancer: California endocurietherapy's 10-year results. Int J Radiat Oncol Biol Phys. 2005;61:1306–16. doi: 10.1016/j.ijrobp.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Fukagai T, Namiki TS, Carlile RG, et al. Comparison of the clinical outcome after hormonal therapy for prostate cancer between Japanese and Caucasian men. BJU Int. 2006;97:1190–3. doi: 10.1111/j.1464-410X.2006.06201.x. [DOI] [PubMed] [Google Scholar]
- 27.Sakamoto M, Mizowaki T, Mitsumori M, et al. Long-term outcomes of three-dimensional conformal radiation therapy combined with neoadjuvant hormonal therapy in Japanese patients with locally advanced prostate cancer. Int J Clin Oncol. 2010;15:571–7. doi: 10.1007/s10147-010-0109-y. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura K, Mizowaki T, Imada H, et al. External-beam radiotherapy for localized or locally advanced prostate cancer in Japan: a multi-institutional outcome analysis. Jpn J Clin Oncol. 2008;38:200–4. doi: 10.1093/jjco/hyn008. [DOI] [PubMed] [Google Scholar]
- 29.Takaha N, Okihara K, Kamoi K, et al. Optimal duration of androgen deprivation in combination with radiation therapy for Japanese men with high-risk prostate cancer. Urol Int. 2011;87:28–34. doi: 10.1159/000324478. [DOI] [PubMed] [Google Scholar]
- 30.Mitsumori M, Sasaki Y, Mizowaki T, et al. Results of radiation therapy combined with neoadjuvant hormonal therapy for stage III prostate cancer: comparison of two different definitions of PSA failure. Int J Clin Oncol. 2006;11:396–402. doi: 10.1007/s10147-006-0600-7. [DOI] [PubMed] [Google Scholar]
- 31.Saito T, Kitamura Y, Komatsubara S, et al. Outcomes of locally advanced prostate cancer: a single institution study of 209 patients in Japan. Asian J Androl. 2006;8:555–61. doi: 10.1111/j.1745-7262.2006.00175.x. [DOI] [PubMed] [Google Scholar]
- 32.Mate TP, Gottesman JE, Hatton J, et al. High dose-rate afterloading 192Iridium prostate brachytherapy: feasibility report. Int J Radiat Oncol Biol Phys. 1998;41:525–33. doi: 10.1016/s0360-3016(98)00097-2. [DOI] [PubMed] [Google Scholar]
- 33.Isbarn H, Boccon-Gibod L, Carroll PR, et al. Androgen deprivation therapy for the treatment of prostate cancer: consider both benefits and risks. Eur Urol. 2009;55:62–75. doi: 10.1016/j.eururo.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roach M, III, Marquez C, Yuo HS, et al. Predicting the risk of lymph node involvement using the pre-treatment prostate specific antigen and Gleason score in men with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 1994;28:33–7. doi: 10.1016/0360-3016(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 35.Morikawa LK, Roach M., III Pelvic nodal radiotherapy in patients with unfavorable intermediate and high-risk prostate cancer: evidence, rationale, and future directions. Int J Radiat Oncol Biol Phys. 2011;80:6–16. doi: 10.1016/j.ijrobp.2010.11.074. [DOI] [PubMed] [Google Scholar]
- 36.Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–47. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 37.Ciernik IF, Romero P, Berzofsky JA, et al. Ionizing radiation enhances immunogenicity of cells expressing a tumor-specific T-cell epitope. Int J Radiat Oncol Biol Phys. 1999;45:735–41. doi: 10.1016/s0360-3016(99)00226-6. [DOI] [PubMed] [Google Scholar]