Abstract
The purpose of this study was to investigate the efficacy of metformin as a radiosensitizer for use in combination therapy for human hepatocellular carcinoma (HCC). Three human HCC cell lines (Huh7, HepG2, Hep3B) and a normal human hepatocyte cell line were treated with metformin alone or with radiation followed by metformin. In vitro tests were evaluated by clonogenic survival assay, FACS analysis, western blotting, immunofluorescence and comet assay. Metformin significantly enhanced radiation efficacy under high and low Linear Energy Transfer (LET) radiation conditions in vitro. In combination with radiation, metformin abrogated G2/M arrest and increased the cell population in the sub-G1 phase and the ROS level, ultimately increasing HCC cellular apoptosis. Metformin inhibits the repair of DNA damage caused by radiation. The radiosensitizing effects of metformin are much higher in neutron (high LET)-irradiated cell lines than in γ (low LET)-irradiated cell lines. Metformin only had a moderate effect in normal hepatocytes. Metformin enhances the radiosensitivity of HCC, suggesting it may have clinical utility in combination cancer treatment with high-LET radiation.
Keywords: Metformin, high-LET radiation, radiosensitivity, hepatocarcinoma cells, DNA damage
INTRODUCTION
The standard treatment for hepatocellular carcinoma (HCC) is surgery, with 5-year survival rates of 30–70% [1]. However, surgery is suitable for fewer than 20% of patients with HCC, and conventional cytotoxic chemotherapy does not provide significant clinical benefits to patients with advanced HCC [1]. Among the possible treatments, radiotherapy can produce sustained local control in select cases [1], and the use of radiosensitizing agents may improve the radiotherapeutic efficacy.
Metformin (1,1-dimethylbiguanide hydrochloride), the most widely used treatment for type 2 diabetes, provides a good tolerability profile at low cost and has recently sparked keen interest as a potential anticancer agent [2–6]. Recent evidence has suggested metformin provides a synergistic benefit with chemotherapy or radiotherapy against certain cancers in several clinical cohort studies [7]. Treatment response rates are higher in patients treated with metformin in cohort studies of breast cancer treated with neoadjuvant chemotherapy [8], in head and neck cancer treated with radiation [9], and in esophageal cancer treated with chemoradiotherapy [10].
As the sensitizing effect of metformin in HCC has been characterized in vitro and in vivo [11], we investigated the radiosensitizing effect of metformin in HCC cells in combination with γ-ray (low-LET) and neutron (high-LET) radiation.
MATERIALS AND METHODS
Antibodies and chemicals
Anti-cyclin A, anti-cyclin B, and β-actin were purchased from Santa Cruz Biotechnology. Anti-ATM and anti-cleaved PARP1 antibodies were purchased from Cell Signaling Technology (Danver, MA, USA). γH2AX (Millipore) and anti-p ATM (S1981) (Upstate Company) were also used. Metformin (marketed as Nexavar by Bayer) was purchased from Sigma, dissolved in DMSO to make a 10 mmol/ l stock solution and stored at 4°C.
Cell culture
Human hepatocarcinoma Hep3B cancer cells were cultured in Dulbecco's minimal essential medium (DMEM; GIBCO, Gaithersburg, MD, USA) supplemented with heat-inactivated 10% fetal bovine serum (FBS; GIBCO), 0.1 mM non-essential amino acids, glutamine, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and antibiotics at 37°C in a 5% CO2-humidified incubator. Human hepatocarcinoma Huh7 and human hepatocarcinoma HepG2 cells were grown in RPMI 1640 medium supplemented with 10% FBS, glutamine, HEPES and antibiotics at 37°C in a 5% CO2 humidified incubator. Human normal hepatocytes were cultured in Hepatocyte Medium supplemented with 0.5% FBS (Science Cell), 0.1% hepatocyte growth supplement, and 0.1% penicillin/streptomycin solution (P/S; Science Cell).
Irradiation
Cells were plated in 60-mm dishes and incubated at 37°C under humidified conditions and 5% CO2 to 70–80% confluence. Cells were irradiated with a 137Cs γ-ray source (Atomic Energy of Canada, Ltd, Ontario, Canada) at a dose rate of 3.81 Gy/min. Fast neutrons (9.8 MeV, 30–40 keV/µm) were produced by the bombardment of beryllium by proton 9Be(p,n)10B, as a nuclear reaction in the cyclotron (MC-50; Scanditronix, Uppsala, Sweden). For measuring the absorbed dose and dose distribution of fast neutron beams or γ-rays, we used the dosimetry method of paired ionization chambers [12]. Dosimetry data was measured before in vitro study and the neutron dose was calculated using RBE, 2.2, which has been used for neutron therapy in our institute and represents an equivalent cell-killing efficacy to γ-rays, as determined by clonogenic assay [13].
Colony-forming assay
Cells (500–1000) were seeded into 6-well plates in triplicate and maintained at the indicated doses of metformin before IR. After 14–20 days, colonies were stained with 0.4% crystal violet (Sigma, St Louis, MO, USA). The plating efficiency (PE) represents the percentage of seeded cells that grew into colonies under the specific culture conditions of a given cell line. The survival fraction, expressed as a function of irradiation, was calculated as follows:survival fraction = colonies counted/(cells seeded × PE/100).
The plating efficiency of Huh7, Hep3B and HepG2 were 0.48 ± 0.18, 0.42 ± 0.15 and 0.45 ± 0.15, respectively. To evaluate the radiosensitizing effects of metformin, the ratio for radiation alone and radiation plus metformin was calculated as the dose (Gy) for radiation alone divided by the dose for radiation plus metformin at a surviving fraction of 10%.
Detection of apoptotic cells by annexin vs staining
After metformin exposure, the cells were irradiated and incubated for 48 h. Cells were washed with ice-cold PBS, trypsinized, and resuspended in × 1 binding buffer [10 mM HEPES/NaOH (pH 7.4), 140 mM NaCl, and 2.5 mM CaCl2] at 1 × 106 cells/ml. Aliquots (100 μl) of cell solution were mixed with 5 μl annexin V FITC (PharMingen) and 10 µl propidium iodide stock solution (50 µg/ml in PBS) by gentle vortexing, followed by 15 min incubation at room temperature in the dark. Buffer (400 μl × 1) was added to each sample and analyzed on a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). A minimum of 10 000 cells was counted for each sample, and data analysis was performed in CellQuest software (BD Biosciences).
Caspase activity
Caspase-3, caspase-8 and caspase-9 activities were determined using detection kits (R&D Systems) as described previously [14]. The assay is based on spectrophotometric detection of the chromophore p-nitroanilide (pNA) after cleavage from the labeled substrates of DEVD-pNA (for caspase-3), IETD-pNA (for caspase-8), and LEHD-pNA (for caspase-9). The pNA light emission can be quantified using a spectrophotometer or microtiter plate reader at 405 nm. Comparison of the pNA absorbance of apoptotic and control samples allows determination of the fold increase in caspase activity.
Fluorescence measurement of intracellular ROS
The fluorescent probe 2′, 7′-dichlorofluorescin diacetate (DCFH-DA) was used to assess intracellular ROS. For fluorocytometry analysis, cells were plated in 60-mm dishes (1 × 105 cells/dish) and loaded for 30 min at room temperature with 10 µM DCFH-DA in 5 ml PBS. Unincorporated DCFH-DA was removed by two washes in PBS. DCFH-DA-loaded cells were stimulated with metformin, radiation or both. Fluorescence was measured using a flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
Immunohistochemistry
Immunohistochemistry was performed to determine the nuclear distribution of γ-H2AX and p-ATM in individual cells. Cells were grown on chambered slides 1 d prior to irradiation or metformin treatments. After metformin exposure, cells were irradiated and incubated for 6 and 24 h. All treatments were performed while cells remained attached to the slides, followed by fixation with 4% paraformaldehyde and permeabilization with 0.2% solution Triton X-100 in PBS. Detection was performed after blocking the slides in 10% FBS/1% bovine serum albumin (BSA) for 1 h with a 1:1000 dilution of fluorescein isothiocyanate (FITC)-labeled mouse monoclonal antibody against γ-H2AX (Millipore, Billerica, MA, USA) in background-reducing antibody diluent (DAKO plus S3022; Millipore).
Western blotting
After metformin exposure, HCC and normal hepatocytes were irradiated and incubated for 6 and 24 h. The cells were lysed with RIPA buffer; proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes. The membranes were blocked with 1% (v/v) nonfat dry milk in Tris-buffered saline with 0.05% Tween20 and incubated with the indicated antibodies. Blots were reacted with primary antibody (1:1000) and secondary antibody (1:5000) dilutions. Immunoreactive protein bands were visualized by Enhanced Chemi-luminescence (Amersham Biosciences) and scanned.
Neutral comet assay
To detect double-strand breaks (DSBs), a neutral comet (single cell gel electrophoresis assay) was performed according to the manufacturer's instructions (Trevigen, Gaithersburg, MD). Cells were plated in 100-mm tissue culture dishes at 1 × 106/dish and incubated overnight. After metformin exposure, cells were irradiated and incubated for 6 and 24 h. The cells were immediately lysed at 4°C for 1 h in lysis buffer (2.5 M NaCl, 100 mM ethylenediamine tetraacetic acid, 10 mM Tris-HCl, 1% N-lauroylsarcosine, 1% Triton X-100, 10% DMSO, pH 10.0) and subjected to neutral electrophoresis buffer at 4°C. To detect DNA, the slides were stained with ethidium bromide and examined for fluorescence emission using an excitation filter of 515–560 nm and a barrier filter of 590 nm. DNA damage was quantified by computer-assisted image analysis (Komet analysis software, ver. 3.1; Kinetic Imaging, Liverpool, UK) to integrate fluorescence intensity.
Statistical analysis
All data were plotted as the mean ± standard error of the mean (SEM). Results of colony forming assays were analyzed by paired t-test with SPSS 18.0 software (SPSS, Chicago, IL, USA). All other data were analyzed by parametric repeated measure one-way ANOVA followed by Tukey's-HSD test (SPSS 18.0). Statistical significance was set at P < 0.05.
RESULTS
Metformin sensitizes HCC cells to radiation
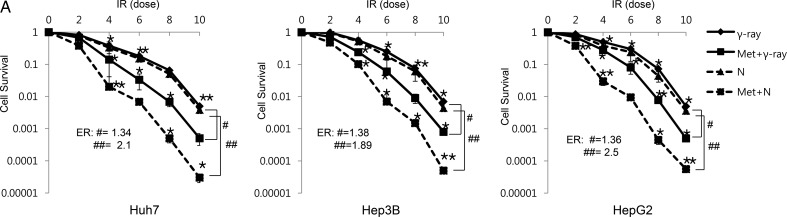
To determine the effects of metformin on radiation-induced cytotoxicity, clonogenic survival was assessed in Huh7, Hep3B and HepG2. In this study, we used the MTT assay to decide the optimal metformin concentration (data not shown). Among variable doses, 10 mM showed the most effective cytotoxicity, corresponding to an inhibitory concentration of 25% at 48 h exposure. This concentration was used by another research investigation in HCC cell lines, and a similar level of sensitization was observed [11]. Figure 1A shows the dose–response curves of three HCC cell lines irradiated with γ-ray and neutron beams in the presence of metformin. Survival decreased in metformin-treated cells after γ-ray and neutron irradiation in comparison with cells irradiated without metformin. Equivalent doses of γ-ray and neutron radiation yielded similar cytotoxic effects. In the presence of metformin, the neutron beam was more effective than γ-ray irradiation (Fig. 1A, Table 1). The metformin effect expressed as the enhancement ratio by radiation was calculated at D10 (Fig. 1A).
Fig. 1.
The radiosensitizing effects of metformin on HCC cells. (A) Radiosensitivity of Huh7, HepG2 and Hep3B cell lines with and without metformin (10 mM) after various doses of γ-ray and neutron radiation was measured by colony-forming assay. Asterisks indicate values that are statistically significant in comparison with radiation-treated cells. Values represent means of three experiments ± SE; *P < 0.05, **P < 0.001. ER(#) = enhancement ratio.
Table 1.
Detection of apoptotic cells by annexin V staining of three HCC cell-lines
| Treatments | % apoptotic cells |
||
|---|---|---|---|
| Huh7 | Hep3B | HepG2 | |
| Control | 0.10 | 0.12 | 0.13 |
| Met | 0.90 | 1.00 | 1.18 |
| γ-ray | 2.30 | 1.38 | 1.20 |
| N | 2.70 | 1.80 | 1.30 |
| Met + γ-ray | 5.00 | 4.18 | 4.00 |
| Met + N | 9.50 | 6.80 | 6.02 |
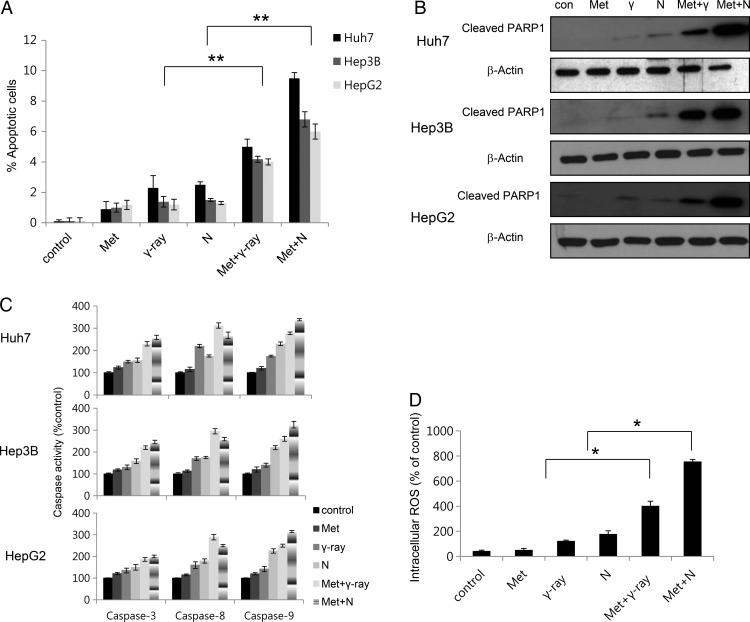
Metformin enhances radiation-induced apoptosis
To investigate whether metformin and radiation initiate cellular apoptosis, we assessed early apoptosis by annexin V and PI staining. In HCC cell lines, 48 h metformin and radiation exposure significantly increased the percentage of early apoptotic cells (Fig. 2A, Table 2). Flow cytometry and colony-forming assays yielded similar results, showing a more sensitive effect of metformin and neutron irradiation in comparison with metformin and γ-ray irradiation (Fig. 2A, Table 2). Metformin and neutron irradiation yielded the most apoptotic cells. We next investigated whether metformin-enhanced neutron cytotoxicity resulted from further activation of caspase, resulting in apoptotic cell death. Caspase activation is a unique characteristic of apoptotic cell death [14]. We measured metformin and radiation-induced activation of caspase-3, caspase-8 and caspase-9 protease activities. As shown in Fig. 2c, radiation produced a metformin-dependent increase (% control) in caspase-3, caspase-8 and caspase-9 activities in HCC cells. Neutron radiation enhances metformin-induced cytotoxicity more effectively than γ-ray radiation, and neutron radiation combined with metformin significantly enhanced caspase-9 activity more than caspase-8 activity. Caspase-3 activation leads to cleavage of PARP-1, which can be detected in western blots by the appearance of a distinct band at 89 kDa, indicating cleaved PARP-1 [15]. The intensity of the cleaved PARP-1 band increased for up to 48 h after exposure to metformin and neutron radiation (Fig. 2B). Annexin V staining and caspase assays indicated that neutron enhancement of metformin-induced cell death is dependent on apoptosis induction. To investigate the relationship between ROS production and enhancement of radiation-induced apoptosis by metformin, the ROS-sensitive dye DCFH-DA was used for flow cytometry to detect ROS generation. ROS production was induced more by the combination of metformin and neutron radiation than metformin and γ-ray treatment. These results indicate that ROS generated by combined treatment regulates the apoptotic process, including intracellular caspase signaling.
Fig. 2.
Effects of metformin and radiation on apoptosis in HCC cells. (A) Huh7, HepG2 and Hep3B cells were exposed to metformin (10 mM) and/or 5 Gy γ-ray or neutron radiation for 48 h for Annexin V staining. Values represent means of three experiments ± SE; *P < 0.05, **P < 0.001. (B) Cell lysates (30 µg) were immunoblotted (IB) with indicated antibodies with cleaved PARP1 and β-actin (C) Caspase activities in HCC were determined (See Materials and Methods). Data are expressed as percentage of control and are means ± SE for three or four experiments. (D) Huh7, HepG2 and Hep3B cells were treated with metformin, radiation or both, and ROS levels were determined by DCF-DA flow cytometry. Values represent means of three experiments ± SE; *P < 0.05.
Table 2.
Detection of apoptotic cells by annexin V staining of normal hepatocytes
| Treatments | % apoptotic cells |
|---|---|
| normal hepatocytes | |
| Control | 0.10 |
| Met | 0.50 |
| γ-ray | 0.53 |
| N | 0.60 |
| Met + γ-ray | 0.98 |
| Met + N | 1.00 |
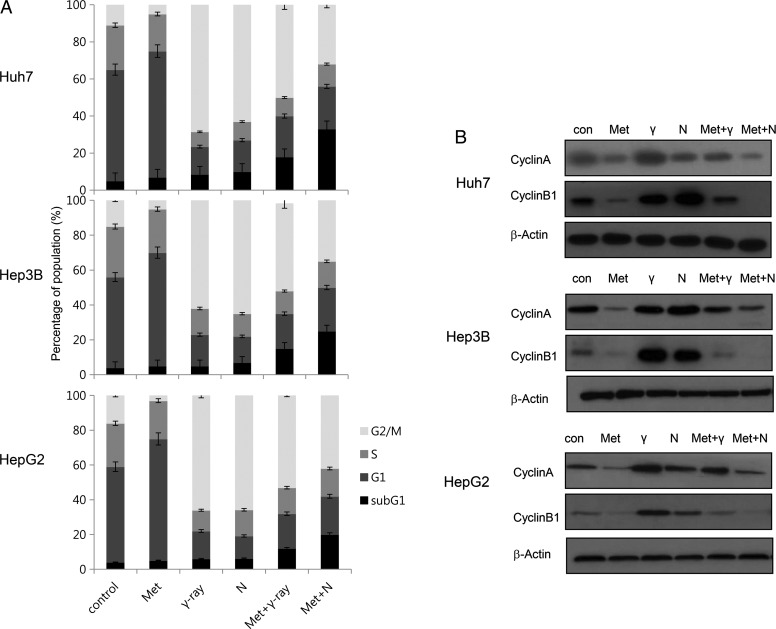
Effects of metformin and radiation on cell cycle phase distribution
We next investigated whether differences between γ-ray- and neutron-induced cytotoxicity in combination with metformin resulted from differences in cell cycle regulation. As shown in Fig. 3, metformin alone increased the number of cells in G1 phase, while decreasing the number of cells in G2/M phase. Treatment with γ-rays or neutron irradiation alone markedly increased the number of cells in G2/M phase (Fig. 3A) and reduced the number of cells in G1 phase. When metformin was added, the radiation-induced changes predominated. Combined metformin and radiation (γ-ray or neutron) treatment reduced G2-M and increased subG1 distribution in comparison with radiation alone. The effect was greater with neutron than with γ-ray irradiation. We also examined the expression of cell cycle regulators following combined treatment with metformin and radiation. Western blotting showed that radiation (γ-ray or neutron) alone yielded a significant accumulation of cyclin A and cyclin B, key cell cycle regulators involved in the G2/M transition, whereas metformin alone reduced expression of these regulators (Fig. 3B). Combined metformin and γ-ray or neutron treatment reduced radiation-induced accumulation of cyclin A and cyclin B.
Fig. 3.
Combined treatment with metformin and radiation modulated cell-cycle progression and expression of cell cycle regulators. (A) Huh7, HepG2 and Hep3B cells were treated with 10 mM metformin and/or 5 Gy γ-ray and neutron radiation for 24 h. The cell-cycle distribution was analyzed quantitatively. *P < 0.05 vs radiation-treated cells. (B) Cyclin expression was analyzed by western blotting. Huh7, HepG2 and Hep3B cells were treated with metformin before radiation and incubated for 24 h. Equal amounts of cell lysates (30 µg) were separated by electrophoresis and analyzed by western blotting for cyclin A and cyclin B1.
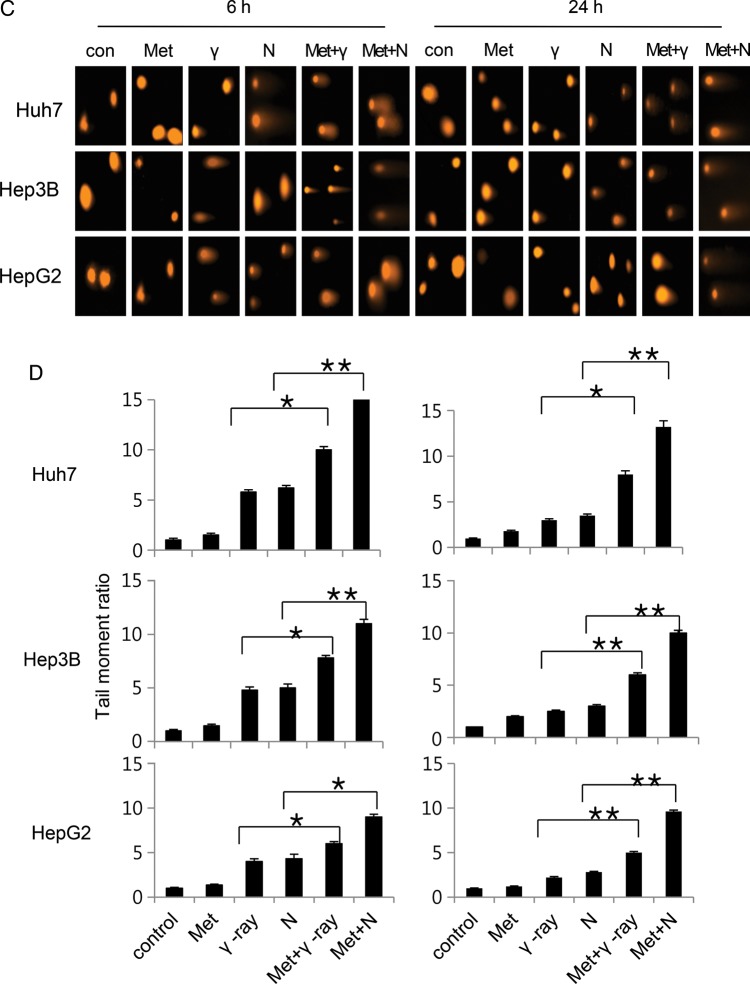
Influence of metformin on radiation-induced DNA damage
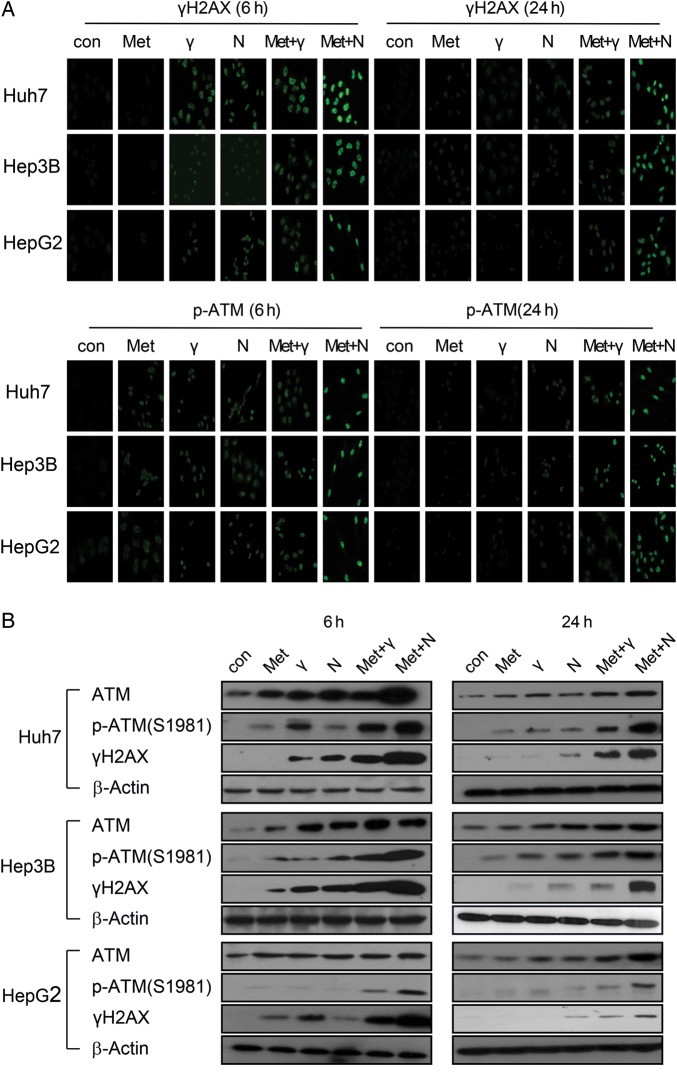
To analyze the effect of metformin on DSB processing, the level of phosphorylated H2AX (γ H2AX), a marker for DSB, was examined by immunofluorescence and western blotting at 0, 6 and 24 h after treatment. Three HCC cells treated with metformin/radiation exhibited damaged DNA foci, which appeared 6 h after treatment exposure, and these were retained even 24 h after exposure; the effect was greater with neutron than γ-ray irradiation (Fig. 4A). Thus, combined treatment delayed the disappearance of γ H2AX, suggesting that metformin maintains DNA damage and increases radiosensitivity. In contrast, metformin alone did not yield foci, even 24 h after exposure (Fig. 4A). Cell lysates were immunoblotted with γ H2AX antibody (Fig. 4B). With metformin, no substantial difference in γ H2AX protein was observed in comparison with the control group at 24 h; this result was consistent with that of the immunofluorescence assay. Ionizing radiation activates the ATM (Ataxia telangiectasia mutated) kinase throughout the cell cycle [16]. Metformin mediates an ATM-mediated DDR (DNA damage response)-like response [17]. To determine whether the ATM pathway mediates the effect of combined metformin and radiation, ATM activity was measured using immunofluorescence and western blotting (Fig. 4A and B). Metformin and radiation induced ATM phosphorylation and activity (detected as γ H2AX) [18] and the effect was greater with neutron than with γ-ray irradiation. DNA damage increased in HCC cells exposed to metformin and radiation, yielding long ‘comet tails’ in comparison with cells treated with metformin alone (Fig. 4C and D). Thus, combined metformin/γ-ray or neutron treatment, but not metformin alone, caused greater DNA damage in HCC cells than γ-ray or neutron beam radiation alone.
Fig. 4.
(Continued)
Fig. 4.
The effect of metformin and radiation on the DNA damage response. (A) Immunocytochemistry staining for H2AX phosphorylation (Ser 139, green) and p-ATM in Huh7, HepG2 and Hep3B cells treated with γ-ray/neutron irradiation or metformin after 6 h and 24 h. (B) Cell lysates of metformin, γ-ray/neutron irradiation, and metformin + γ-ray/neutron irradiation experiments were immunoblotted with the indicated antibodies. (C) Neutral comet assay results in Huh7, HepG2 and Hep3B cell lines treated with γ-ray/neutron irradiation or metformin compared with untreated cells after 6 h and 24 h. (D) Tail was measured after 6 h and 24 h treatment of Huh7, Hep3B and HepG2 cells with metformin alone, γ-ray/neutron irradiation alone, or combination treatment. *P < 0.05, **P < 0.001.
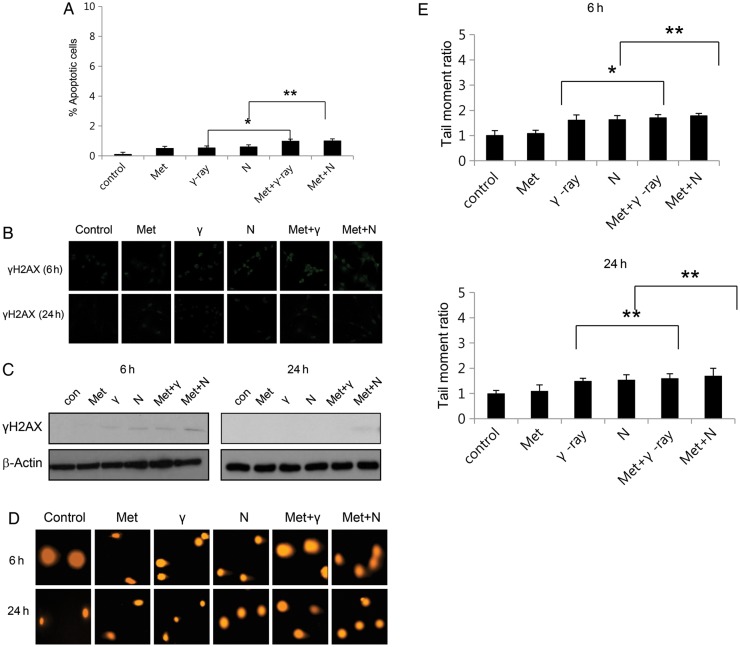
The effects of metformin on normal hepatocyte radiosensitivity
To evaluate the effects of metformin on the in vitro radiosensitivity of normal human cells, normal hepatocytes were exposed to metformin before irradiation, according to the same treatment protocol applied to the tumor cell lines (Fig. 5A, Table 2). In contrast to the effect on tumor cell sensitivity, metformin had only a moderate effect in normal hepatocytes. DDRs (γ H2AX foci assay and neutral comet assay) were minimal in normal hepatocytes after 24 h exposure to metformin and radiation (Fig. 5B, C, D and E).
Fig. 5.
The effect of metformin on the radiosensitivity of normal hepatocytes. (A) Cell death of normal hepatocytes after exposure to metformin, γ-ray/neutron irradiation, and metformin + γ-ray/neutron irradiation for 48 h was measured using Annexin V staining. Values represent means of three experiments ± SE; *P < 0.05, **P < 0.001. (B) Immunocytochemistry staining for H2AX phosphorylation (Ser 139, green) in normal hepatocytes treated with γ-ray/neutron irradiation or metformin after 6 h and 24 h. (C) Cell lysates after treatment with metformin, γ-ray/neutron irradiation, and metformin + γ-ray/neutron irradiation were immunoblotted with the indicated antibodies. (D) Neutral comet assay results in normal hepatocytes treated with γ-ray/neutron irradiation or metformin compared with untreated cells after 6 h and 24 h. (E) Tail moment was measured after 6 h and 24 h treatment of normal hepatocytes with metformin alone, γ-ray/neutron irradiation alone, or combination treatment. *P < 0.05, **P < 0.001.
DISCUSSION
We investigated the radiosensitizing effects of metformin in three HCC and a normal hepatocyte cell line using two different types of radiation. Metformin significantly enhanced high- and low-LET radiation efficacy in HCC cells in vitro. The radiosensitizing effect of metformin was much higher in neutron-irradiated than in γ-irradiated cell lines. Recent studies have revealed that metformin enhances the cell-killing effect of ionizing radiation (X-ray or γ-ray) in several cancer cell lines and provides an anticancer effect. Song et al. demonstrated that metformin radiosensitized breast cancer cells and fibrosarcoma cells, preferentially cytotoxic to cancer stem cells, and effectively eradicated radioresistant tumors [19]. Skinner et al. showed that metformin reduced the surviving fraction of head and neck cancer cells [9]. Sanli et al. also suggested metformin is effective against lung cancer cells in combination with radiation treatment [20]. Colquhoun et al. suggested that metformin is an effective radiosensitizing agent in the treatment of prostate cancer [21]. Storozhuk et al. revealed that clinically achievable low doses of metformin provided a radiosensitizing effect in lung cancer cells [22]. This radiosensitization occurs through several mechanisms: induction of apoptosis, modulation of DDR, regulation of expression and activity of the ATM-AMPK/Akt-mTOR pathways, and modulation of tumor vasculature [22]. Liu et al. found that the enhanced cytotoxic effect on hepatoma cells of low doses of metformin combined with ionizing radiation occurred via ATP deprivation and inhibition of DNA repair [23]. And Zhou et al. [24] suggested that metformin activates AMPK in hepatocytes; AMPK is a key sensor of radiation signals [20], and AMPK subunit expression is tightly regulated by radiation. The absence of AMPK establishes the Akt-mTOR and DDR pathways, inhibiting radiation responsiveness [25]. In our study, the radiation-sensitizing effect of metformin occurred through enhanced apoptosis, cell cycle arrest, and increased DNA damage, consistent with previous reports. And our data showed that apoptosis induced by combination treatment in HCC cells was associated with enhanced ROS generation and caspase activation.
Though the radiosensitizing effect of metformin has been investigated in several tumor cells including HCC, the studies have used low-, not high-LET radiation. Our results showed metformin radiosensitization is more effective with high-LET (neutron) than with low-LET (γ-ray) irradiation. This effect was consistent in enhancing apoptosis and cell cycle arrest, and in increasing DNA damage, which we (and others) have observed with low-LET radiation. Neutron, high-LET radiation, results in stable free radicals and causes more double-strand DNA breaks than γ-rays do [26, 27], while Moritake showed that a carbon-ion beam produced smaller hydroxyl radicals (*OH) than low-LET radiation in solution [28]. These unique findings enable further understanding of the indirect effect of high-LET radiation. Therefore, high-LET radiation, including neutron and carbon ion, must produce different, more complicated cellular effects; indeed, the molecular biological mechanism of the effect of high-LET radiation remains a topic of investigation.
In Japan, where increasingly popular radiation therapy using carbon ion high-LET radiation has been used to obtain dramatic local control in clinical settings, several researchers have revealed that molecular mechanisms differ between carbon ion and low-LET irradiation. They have suggested that low-LET and high-LET radiation trigger different apoptotic pathways. While low-LET radiation mediated p53-induced caspase-8-dependent apoptosis, damage from high-LET radiation could induce mitochondria-dependent and p53-independent apoptosis through caspase-9 [29]. This result correlates with our data showing higher caspase-9 than caspase-8 activity in cells treated with combined metformin and neutron radiation. The fact that metformin is an inhibitor of the mitochondrial complex I [4] and selectively inhibits growth or induces radiosensitization in cancer cells lacking functional p53 [9] would be one clue to explain why the metformin effect on apoptosis is more synergistic with high-LET than low-LET irradiation.
Sanli et al. [20] revealed that ionizing radiation activates AMPK and transduces signals through a DDR-mediated ATM-AMPK-p53-p21cip1 axis, mediating cell cycle arrest and radiosensitization. Because there is no evidence that metformin directly induces DSBs, it seems to produce DNA damage mediated through ATM activity [17]. The sustained increase in γ H2AX and the longer comet tail after combined metformin with γ-ray or neutron radiation were likely the result of enhanced ATM activity related to potential replication stress. This may explain why metformin yielded a more enhanced effect on DNA damage with neutron than γ-ray irradiation. High-LET radiation induces more clustered DNA damage, the complexity of which makes DNA repair more difficult [26]. ATM mediates the non-homologous end-joining repair pathway and therefore plays a more important role in high-LET radiation damage [30]. Our data showed that ATM phosphorylation and activity (detected as γ H2AX) [18] is increased further in high-LET radiation combined treatment, likely due to enhanced ATM activation by metformin and high-LET radiation to mediate damage repair. Future studies should investigate the mechanism of ATM regulation by metformin.
Fortunately, metformin had little effect on normal tissues. Our studies revealed no interaction between metformin and radiation in normal hepatocytes. A similar phenomenon was observed in normal prostate cells, astrocytes, and non-transformed mammary epithelial cells [6, 31, 32]. These results show the potential utility of metformin as a radiosensitizer to high-LET radiation therapy (such as carbon beam treatment).
CONCLUSION
In conclusion, metformin provided a stronger radiosensitizing effect with high-LET neutron than low-LET γ-ray radiation by increasing apoptosis, cell cycle arrest and sustained DNA damage in HCC cells. This phenomenon was not observed in normal hepatocytes. This work provides a preclinical basis to begin investigation of metformin in combination with high-LET radiation.
FUNDING
This work was supported by a grant of Korea Heavy Ion Medical Accelerator Project (code 2013K000083) from the Ministry of Science, ICT and Future Planning, Republic of Korea.
REFERENCES
- 1.Lau WY, Lai EC, Lau SH. The current role of neoadjuvant/adjuvant/chemoprevention therapy in partial hepatectomy for hepatocellular carcinoma: a systematic review. Hepatobiliary Pancreat Dis Int. 2009;8:124–33. [PubMed] [Google Scholar]
- 2.Bodmer M, Meier C, Krähenbuhl S, et al. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care. 2010;33:1304–8. doi: 10.2337/dc09-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53–deficient tumor cell growth. Cancer Res. 2007;67:6745–52. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 4.El-Mir MY, Nogueira V, Fontaine E, et al. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–8. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 5.Evans JM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isakovic A, Harhaji L, Stevanovic D, et al. Dual antiglioma action of metformin: cell cycle arrest and mitochondria-dependent apoptosis. Cell Mol Life Sci. 2007;64:1290–302. doi: 10.1007/s00018-007-7080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby G, Donnelly LA, Donnan PT, et al. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–5. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skinner HD, Sandulache VC, Ow TJ, et al. TP53 disruptive mutations lead to head and neck cancer treatment failure through inhibition of radiation-induced senescence. Clin Cancer Res. 2012;18:290–300. doi: 10.1158/1078-0432.CCR-11-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi M, Kato K, Iwama H, et al. Antitumor effect of metformin in esophageal cancer: in vitro study. Int J Oncol. 2013;42:517–24. doi: 10.3892/ijo.2012.1722. [DOI] [PubMed] [Google Scholar]
- 11.Qu Z, Zhang Y, Liao M, et al. In vitro and in vivo antitumoral action of metformin on hepatocellular carcinoma. Hepatol Res. 2012;42:922–33. doi: 10.1111/j.1872-034X.2012.01007.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee DH, Seo SH, Ji YH, et al. Characteristics of radiation generated by BNCT irradiator of Hanaro nuclear reactor. J Korean Soc Ther Radiol Oncol. 2006;24:s158. [Google Scholar]
- 13.Keun-Yong Eom, Hong-Gyun Wu, Hye Jin Park, et al. Evaluation of biological characteristics of neutron beam generated from MC50 cyclotron. J Korean Soc Ther Radiol Oncol. 2006;24:280–4. [Google Scholar]
- 14.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Med. 2013;3 doi: 10.1101/cshperspect.a008656. a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Los M, Mozoluk M, Ferrari D, et al. Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol Biol Cell. 2002;13:978–88. doi: 10.1091/mbc.01-05-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandita TK, Lieberman HB, Lim DS, et al. Ionizing radiation activates the ATM kinase throughout the cell cycle. Oncogene. 2000;19:1386–91. doi: 10.1038/sj.onc.1203444. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez-Martin A, Oliveras-Ferraros C, Cufi S, et al. Metformin activates an ataxia telangiectasia mutated (ATM)/Chk2-regulated DNA damage-like response. Cell Cycle. 2011;10:1499–501. doi: 10.4161/cc.10.9.15423. [DOI] [PubMed] [Google Scholar]
- 18.Menendez JA, Cufi S, Oliveras-Ferraros C, et al. Metformin and the ATM DNA damage response (DDR): accelerating the onset of stress-induced senescence to boost protection against cancer. Aging. 2011;3:1063–77. doi: 10.18632/aging.100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song CW, Lee H, Dings RP, et al. Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Sci Rep. 2012;2:1–9. doi: 10.1038/srep00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanli T, Rashid A, Liu C, et al. Ionizing radiation activates AMP-activated kinase (AMPK): a target for radiosensitization of human cancer cells. Int J Radiat Oncol Biol Phys. 2010;78:221–9. doi: 10.1016/j.ijrobp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Colquhoun A, Venier N, Vandersluis A, et al. Utilizing metformin as a radiosensitizing agent in the treatment of prostate cancer. J Urol. 2011;185 e296. [Google Scholar]
- 22.Storozhuk Y, Hopmans SN, Sanli T, et al. Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J Cancer. 2013;108:2021–32. doi: 10.1038/bjc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Hou M, Yuan T, et al. Enhanced cytotoxic effect of low doses of metformin combined with ionizing radiation on hepatoma cells via ATP deprivation and inhibition of DNA repair. Oncol Rep. 2012;28:1406–12. doi: 10.3892/or.2012.1932. [DOI] [PubMed] [Google Scholar]
- 24.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanli T, Storozhuk Y, Linher-Melville K, et al. Ionizing radiation regulates the expression of AMP-activated protein kinase (AMPK) in epithelial cancer cells: modulation of cellular signals regulating cell cycle and survival. Radiother Oncol. 2012;102:459–65. doi: 10.1016/j.radonc.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka K, Gajendiran N, Endo S, et al. Neutron energy-dependent initial DNA damage and chromosomal exchange. J Radiat Res. 1999;40:36–44. doi: 10.1269/jrr.40.s36. [DOI] [PubMed] [Google Scholar]
- 27.Simmons JA, Bewley DK. The relative effectiveness of fast neutrons in creating stable free radicals. Radiat Res. 1976;65:197–201. [PubMed] [Google Scholar]
- 28.Moritake T, Tsuboi K, Anzai K, et al. ESR spin trapping of hydroxyl radicals in aqueous solution irradiated with high-LET carbon-ion beams. Radiat Res. 2003;159:670–5. doi: 10.1667/0033-7587(2003)159[0670:estohr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Mori E, Takahashi A, Yamakawa N, et al. High LET heavy ion radiation induces p53–independent apoptosis. J Radiat Res. 2009;50:37–42. doi: 10.1269/jrr.08075. [DOI] [PubMed] [Google Scholar]
- 30.Xue L, Yu D, Furusawa Y, et al. Regulation of ATM in DNA double strand break repair accounts for the radiosensitivity in human cells exposed to high linear energy transfer ionizing radiation. Mutat Res. 2009;670:15–23. doi: 10.1016/j.mrfmmm.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Ben Sahra I, Laurent K, Loubat A, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–86. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang Y, Miskimins WK. Metformin induces both caspase-dependent and poly(ADP-ribose) polymerase-dependent cell death in breast cancer cells. Mol Cancer Res. 2011;9:603–15. doi: 10.1158/1541-7786.MCR-10-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]