Abstract
Leukocyte adhesion deficiency type II is a hereditary disorder of neutrophil migration caused by mutations in the guanosine diphosphate-fucose transporter gene (SLC35C1). In these patients, inability to generate key fucosylated molecules including sialyl Lewis X leads to leukocytosis and recurrent infections, in addition to short stature and developmental delay. We report two brothers with short stature and developmental delay who are compound heterozygotes for novel mutations in SLC35C1 resulting in partial in vivo defects in fucosylation. Specifically, plasma glycoproteins including immunoglobulin G demonstrated marked changes in glycoform distribution. While neutrophil rolling on endothelial selectins was partially impeded, residual adhesion proved sufficient to avoid leukocytosis or recurrent infection. These findings demonstrate a surprising degree of immune redundancy in the face of substantial alterations in adhesion molecule expression, and show that short stature and developmental delay may be the sole presenting signs in this disorder.
INTRODUCTION
Innate immunity critically depends on the capacity of circulating granulocytes to migrate efficiently to sites of infection. Recruitment begins with tethering and rolling interactions between granulocytes and target tissue endothelium. These shear–resistant interactions are principally mediated by the selectins, a family of calcium-dependent lectins. The prototypical binding determinant for selectins is the fucosylated glycan known as sialyl-Lewis X (sLex/CD15s).
A hereditary deficiency of fucosylation has been described in humans. In this condition, known as leukocyte adhesion deficiency type II (LADII) (MIM 266265), affected individuals bear mutations in the Golgi transporter that shuttles the nucleotide sugar precursor, guanosine diphosphate (GDP) fucose, from the cytosol where it is made, to the Golgi where it is used to create fucosylated glycoconjugates (1–6). Accordingly, LADII patients are unable to generate fucosylated glycans including CD15s. This results in the inability of granulocytes to bind selectins which manifests with marked leukocytosis, an inability to generate pus, and recurrent bacterial infections. These clinical manifestations of impaired immunity are the cardinal signs of LADII. Besides deficiencies in host defense, LADII patients lack the fucosylated Lewis blood group and ABO antigens resulting in the rare Bombay blood type (7). Importantly, though deficits in host defense prompted the initial discovery of this condition, patients with LADII also suffer from growth retardation, developmental delay and dysmorphic features (8), but it is not clear how lack of fucosylation yields these phenotypes. Due to these diverse manifestations, this disorder has also been termed congenital disorder of glycosylation type 2c (CDG2c). From prior clinical observations, it is believed that all physical manifestations of LADII require a severe defect of glyconjugate fucosylation. We report here on brothers who have developmental delay and significant growth retardation and on the genetic, structural and functional studies undertaken to elucidate the biochemical basis of their condition. The results of our studies of these patients, at the nexus of genomics and glycomics, provide new perspectives on the molecular basis of defects of leukocyte migration and host defense, and force reconsideration of the assessment of children who present with growth and developmental compromise.
Case presentations
Our patients are two brothers, now 21 (Proband 1) and 19 (Proband 2) years of age, born to non-consanguineous parents of British heritage with unremarkable family history. The pregnancies and births were uncomplicated. Proband 1 had a birth weight of 2.5 kg (<2nd %) and length of 49.5 cm (60th %), and Proband 2 had a birth weight of 3.0 kg (25th %) and length of 51 cm (70th %). Their 13-year-old sister has had normal growth and development.
The brothers have global developmental delay; however, expressive language and cognition are the more significant challenges. Proband 1 currently has an ∼150 word vocabulary, speaking in three word phrases with echolalia; he has anxiety and is on the autism/pervasive developmental disorder spectrum. Proband 2 has less global delays, milder autistic features and obsessive–compulsive disorder.
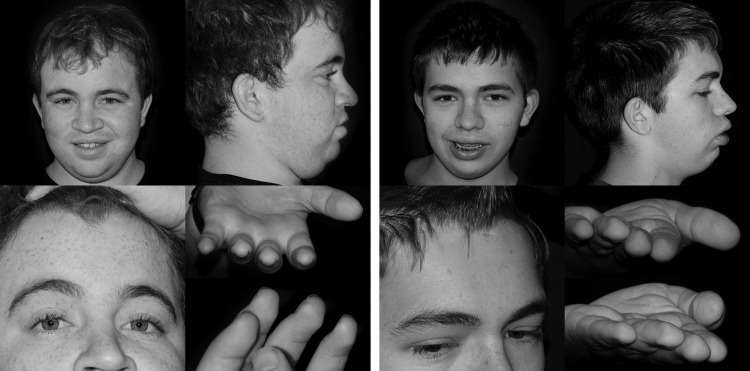
Their past medical history is significant for multiple episodes of otitis media as infants which responded to oral antimicrobial therapy. Neither had placement of tympanostomy drainage tubes, but Proband 2 had an adenoidectomy secondary to episodic sinus infections. Proband 1 had a hospitalization at age 12 weeks for bronchiolitis and at age 10 months for a febrile seizure, but otherwise neither brother had any serious infections. Comprehensive endocrine and metabolic evaluation revealed normal serum IGF-I and IGFBP-3 levels, plasma amino acids, urine amino acids and organic acids, urine sialic acid, mucopolysaccharides and urine oligosaccharides. Chromosomal microarrays showed no copy number variants or areas with loss of heterozygosity. Notably, white blood cell counts and absolute neutrophil counts were within the normal range on two occasions (Supplementary Material, Table S1) and both brothers had the O+ blood type. Phenotypically, both brothers have short stature with heights of −2.8 and −3.3 standard deviations, coarse facial features with a bulbous nose, widow's peak, small hands and feet with brachydactyly and persistent fetal finger pads (Fig. 1).
Figure 1.
Photos of Proband 1 (left) and 2 (right).
RESULTS
Targeted sequencing of the exons of 1077 candidate genes was performed on Proband 1 as part of a short stature research study (9). Proband 1 was found to carry two novel non-synonymous variants in SLC35C1, the gene which encodes the Golgi GDP-fucose transporter 1 (UniProt Q96A29). Sanger sequencing was performed in both subjects, their parents and unaffected sister. The subjects' mother carries the p.Glu31* (c.91G>T) nonsense variant while the father carries the p.Phe168del (c.501_503delCTT) variant. Both subjects were confirmed to be compound heterozygotes for the two variants and the unaffected sister does not carry either variant. The nonsense variant abrogates essentially the entire protein including all the transmembrane domains while the Phe168del variant leads to loss of a single phenylalanine which is the second amino acid in the 5th transmembrane domain (10).
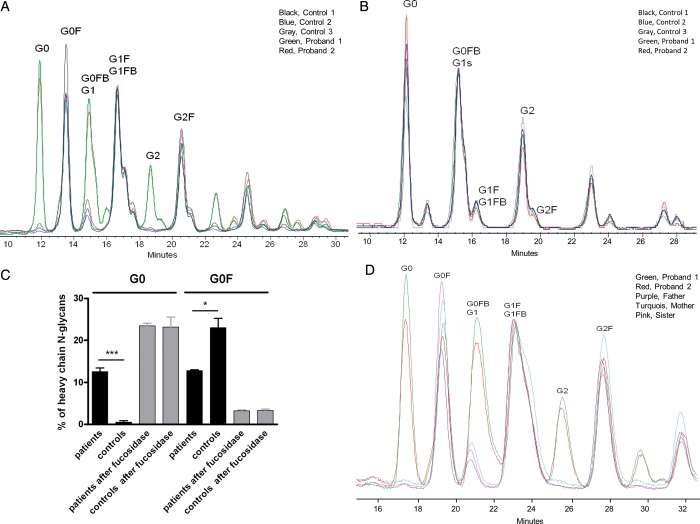
As the subjects were missing some of the key features of LADII, namely leukocytosis, recurrent infections and the Bombay blood type, we sought to ascertain whether their variants in SLC35C1 were associated with abnormalities in glycosylation. To this end, we evaluated an abundant circulating glycoprotein, immunoglobulin G (IgG). Approximately 95% of the glycans on IgG feature a fucose attached to the first N-acetylglucosamine linked to asparagine in N-glycans (11,12). We hypothesized that defective GDP-fucose transportation from the cytosol into the Golgi would alter this pattern. While glycans from control subjects showed the expected marked predominance of fucosylated forms, both probands exhibited approximately equal proportions of fucosylated and non-fucosylated IgG heavy chain glycans, consistent with a marked but partial fucosylation defect (Fig. 2). To assess whether this abnormality reflected defective fucosylation alone and not a broader abnormality in IgG glycosylation, we treated patient and control glycans with α-l-fucosidase. After enzymatic defucosylation, patient and control glycans were indistinguishable, confirming that the defect was selective to fucosylation (Fig. 2; Supplementary Material, Fig. S1). To assess whether this fucosylation defect was selective to the affected family members, we tested IgG heavy chain glycans from the unaffected father, mother and sister. These resembled controls and were markedly distinct from the affected cases, showing that presence of both mutant alleles was required to elicit the phenotype (Fig. 2).
Figure 2.
Characterization of N-glycans from purified IgG heavy chains by normal-phase HPLC. (A) N-Glycans were extracted from purified heavy chains of Probands 1 and 2 (green and red) and from male age matched pediatric controls undergoing evaluation for short stature (black, blue and gray). HPLC tracings normalized to the G1F/G1FB peak reveal that patients 1 and 2 show a striking abundance of afucosylated species (G0, G1, G2), whereas these species represent only a minor fraction of IgG glycans among controls. The letters reflect the specific glycan species shown, including the number of galactoses (G, 0–2), core-fucosylation if present (F) and the presence of a bisecting N-acetylglucosamine if present (B). For example, G1FB defines a monogalactosylated structure with both a core fucose and a bisecting N-acetylglucosamine. (B) HPLC tracings from the same patients and controls following defucosylation, demonstrating normalization of differences; see Figure S1 for before and after tracings from representative patients. (C) Quantitation of non-fucosylated and fucosylated species among agalactosyl (G0 + G0F) IgG heavy chain glycans. *P < 0.05, ***P < 0.001 by Student's t-test. (D) Glycan profiles from patients compared with unaffected family members (mother M, turquoise; father F, purple; sister S, pink). A TSKgel amide-80 column (4.6 mm ID × 15 cm, 3 µm) was used for the separation in (A)–(C), while a TSKgel amide-80 column (4.6 mm ID × 25 cm, 5 µm) was used for separation in (D), accounting for differences in trace profiles.
We then proceeded to perform a global plasma N-glycan analysis using mass spectrometry (Supplementary Material, Fig. S2). This confirmed that, in contrast to their unaffected family members, the two subjects had global increases in non-fucosylated glycans, but each was able to make fucosylated glycans. Peaks consistent with the H2 antigen and sialyl-Lewis X were present (Supplementary Material, Fig. S2), explaining the absence of the Bombay blood type and leukocytosis.
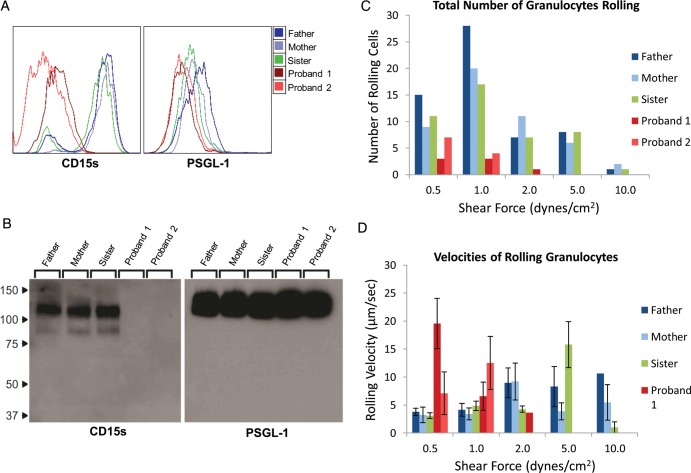
To further explore the hematological phenotype, we performed flow cytometry and western blot analysis, as well as parallel plate flow chamber assays of peripheral blood granulocytes obtained from affected and unaffected family members. Granulocytes were analyzed for expression of CD15s, and were also analyzed for expression of P-selectin glycoprotein ligand-1 (PSGL-1), the principal CD15s-bearing glycoprotein on the granulocyte surface. Whereas >95% of granulocytes from unaffected family members expressed CD15s, 88 and 50% of granulocytes from the two probands displayed CD15s with a mean expression level per cell profoundly lower (>20-fold) than in unaffected family members. However, there was no appreciable difference in expression of PSGL-1 among family members. Western blot confirmed the relative absence of CD15s display on PSGL-1 (Fig. 3) on granulocytes of probands.
Figure 3.
Biochemical and functional analysis of granulocytes. (A) Live peripheral blood granulocytes from family members were stained for surface expression of CD15s and PSGL-1. Granulocytes from the probands had profoundly less CD15s expression than the unaffected family members. Conversely, no appreciable difference was observed in expression of PSGL-1 among the family members. (B) Lysates of equivalent granulocyte numbers from all family members were analyzed by western blot, staining for both CD15s and PSGL-1. Granulocytes from the unaffected family members express a 120 kDa CD15s band that corresponds to a 120 kDa PSGL-1 band. Conversely, granulocytes from the affected subjects, while maintaining the PSGL-1 band, are notably lacking CD15s expression. (C) Live peripheral blood granulocytes were perfused over activated HUVEC monolayers under increasing shear stress. Rolling events were totaled over four individual FOV for each shear stress. (D) The velocities of each observed rolling event were also evaluated. At a low shear stress (0.5 dynes/cm2), the total number of rolling granulocytes from the affected individuals were not different from the unaffected family members. However, the rolling velocities of the probands' granulocytes at this shear stress level are significantly faster than the velocities of the unaffected family members.
Granulocytes from all family members were perfused over TNFα-stimulated human umbilical vein endothelial cell (HUVEC) monolayers to assess E-selectin-mediated tethering and rolling under increasing shear stress. In each case, specificity for binding to E-selectin was assessed by use of anti-E-selectin blocking antibody. While granulocytes of probands were capable of tethering and rolling adhesive interactions, the total numbers of granulocytes from unaffected family members which tethered and rolled at all shear stresses (0.5–10 dyne/cm2) were considerably higher. Notably, at shear stress of ≥2 dyne/cm2, granulocytes from unaffected family members maintained rolling interactions with the HUVEC monolayers while those from affected subjects did not. Furthermore, analysis of rolling velocities revealed that granulocytes from the probands rolled significantly faster, indicating weaker attachment to E-selectin (Fig. 3; Supplementary Material, Movie).
DISCUSSION
In 1992, Etzioni et al. first reported two cases of LADII (2,8). These two patients presented with recurrent severe bacterial infections in infancy. In total, there have been seven reported cases of LADII (2,7,8,10,13,14), and while this disorder is quite rare, it has provided fundamental insights into the glycobiology of leukocyte migration and fucose metabolism. To date, all subjects with LADII share the key clinical characteristics of significant neutrophilia, the Bombay blood type, as well as short stature and marked mental retardation (15). In each case, the presenting clinical symptom prompting evaluation was recurrent bacterial infection, thus drawing attention to deficits in innate immunity. Here we report two brothers in whom the presenting signs prompting evaluation were short stature and developmental delay. Though each manifested episodes of otitis media, neither the frequency nor intensity of bacterial infections raised a suspicion of neutrophil dysfunction. Genomic studies revealed that they are compound heterozygotes for two non-synonymous variants in SLC35C1, the locus encoding the GDP-fucose transporter. Consistent with classic LADII, they have short stature and mental retardation but they lack the distinctive Bombay blood type and neutrophilia. N-Glycan analysis demonstrates a global decrease but not absolute lack of fucosylation with the presence of the H (Bombay) antigen and CD15s. Likewise, granulocyte flow cytometry studies show a marked decrease in surface CD15s, resulting in diminished but not absent granulocyte rolling on vascular E-selectin. Notably, the canonical leukocyte P- and E-selectin ligand, PSGL-1, showed normal expression by western blot but no expression of the CD15s determinant.
The Bombay blood type, one of the key diagnostic features for LADII, is due to the absence of the fucosylated H antigen. All patients reported to date with classic LADII presented with the Bombay blood type. We hypothesize that our patients' lack of the Bombay blood type is due to a less severe partial defect in fucose transport. This presumably allows them to produce some amount of the H antigen, as seen in the global N-glycan analysis, which leads to lack of anti-ABO blood group antibodies which characterize the Bombay blood type.
It is striking that despite low CD15s expression and clear defects in granulocyte rolling, the subjects did not present with clinical features of severe recurrent infections or neutrophilia. There are two possible explanations for this finding. The simplest explanation is that the minimal expression of CD15s present in the brothers allows for sufficient leukocyte rolling to achieve immune surveillance. This possibility is most likely, as the results of parallel plate flow assays show that proband granulocytes are capable of tethering and rolling interactions at the lower end of physiologic shear stress (1–4 dynes/cm2) found within post-capillary venules, the vascular portal of extravasation (16). The other possibility is that adaptive immunity was sufficient to protect these patients from infections and plays a more prominent role in preventing infections as these patients age. Notably, patients with classic LADII do seem to outgrow their recurrent infections presumably due to compensatory mechanisms, although the granulocytosis never completely resolves (15). Nonetheless, our data suggest that considerable immune competence can be achieved with significantly depressed levels of E-selectin ligand activity challenging existing paradigms in leukocyte trafficking.
The data presented here draw attention to the spectrum of biologic changes engendered by fucosylation defects. In contrast to canonical LADII, the probands lacked the hematological phenotype, but did present with growth and mental retardation. The mechanism of short stature and development delay is not well understood in LADII. Fucosylated glycans are known to play a role in brain development and presumably disruption of these glycans could lead to developmental delay (17,18). Notch signaling is critical for brain development and N-fucosylation has been shown to be severely disrupted in fibroblasts from a patient with LADII (19). Additionally, both the insulin-like growth factor 1 receptor (20,21) and the epidermal growth factor receptor (22) are known to be fucosylated. Defects of fucosylation in both of these pathways which are related to growth have been demonstrated to lead to post-receptor signaling defects in mouse models (22,23).
Previously, identification of immune deficits was key for consideration of the diagnosis of LADII. Our studies unveil a previously unappreciated spectrum of presentation of fucosylation defects, indicating that the prior emphasis on immune deficits may underestimate the prevalence of this condition within the general population. Short stature and developmental delay may be the sole presenting signs of this disorder. The identification of fucosylation defects in our patients has critical ramifications in so far as two patients with classic LADII responded to fucose supplementation (7,14) including one young child with improvement in cognitive development (7). Had this diagnosis been made in infancy, it is possible that our subjects' growth and mental retardation could have been improved with fucose supplementation. Quantitation of granulocyte CD15s expression via flow cytometry is a readily translatable low-cost diagnostic approach for this disorder. While likely to be a rare cause of short stature, our case demonstrates that children who present with growth and developmental compromise should raise consideration of screening for fucosylation defects even in the absence of signs and symptoms of classic LADII.
MATERIALS AND METHODS
This study was approved by the institutional review board at Boston Children's Hospital. Written informed consent was obtained from subjects or their legal guardians.
Sequencing methods
DNA samples from multiple subjects were pooled for DNA sequencing using previously described methods available at the Broad Institute (24). The targeted exons of the 1077 candidate genes were enriched using a custom Agilent SureSelect hybrid selection system. The candidate genes selected comprise genes from identified genome-wide association loci associated with height as well as genes known to cause skeletal dysplasias or syndromic conditions which include short stature as previously described (9). After hybrid selection, barcoded libraries were prepared for each pool. Sequencing was performed on the Illumina HiSeq platform. Syzygy software (24) was used to call variants. Variants were annotated for functional effect using SnpEff 2.0.5 (http://snpeff.sourceforge.net/). Variant allele frequency data were obtained from two publicly available data sets: (i) integrated variant call set of 1000 Genomes Phase 1 samples (25) (February 2012 release) and (ii) National Heart Lung and Blood Institute Exome Variant Server (26). Novel variants were not observed in these two datasets or in dbSNP. SLC35C1 variants were annotated using reference sequences NM_018389.4 and NP_060859.4. Primers for Sanger sequencing are available on request.
Characterization of IgG heavy chain N-glycans using normal-phase HPLC separation
N-Glycan analysis was performed according to the procedure detailed by Royle et al., with slight modifications (27,28). IgG heavy chains were isolated from the plasma of patients and relevant controls. IgG was purified using a protein G column and then reduced, denatured and separated on a 12% discontinuous SDS–PAGE gel. Heavy bands were excised and N-glycans released by overnight incubation in PNGase F at 37°C. The released N-glycans were collected and labeled with 2-aminobenzylamine (2AB). Excess label was removed by absorbing the mixture of free and glycan-conjugated 2AB on 3MM Whatman chromatography paper. After washing away the unreacted 2AB with acetonitrile, labeled glycans were released with water. Dried and resuspended samples were separated on a Waters 1525 binary HPLC pump equipped with a Waters 2475 fluorescence detector with inline TSKgel amide-80 column (4.6 mm ID × 15 cm, 3 µm beads or 4.6 mm ID × 25 cm, 5 µm beads) with a linear gradient of 35–47% 50 mm formic acid (pH 4.4) while decreasing acetonitrile after 48 min.
Fucosidase treatment
The 2AB-labled glycans in water were supplemented with phosphate buffer (pH 5.5) to a final concentration of 50 mm. The mixture (20 µl) was treated with α-l-fucosidase from bovine kidney (specific activity, ≥2 units per mg protein, Sigma-Aldrich) overnight at 37°C. Defucosylated glycans were characterized as described above.
Plasma global N-glycan analysis using mass spectrometry
For plasma analysis, 200 µl was added to a glass test tube. Glycosphingolipids were extracted with 2:1 chloroform/methanol. The protein pellet was digested with trypsin/chymotrypsin as described (29). N-Linked glycans were released using N-glycanase (Prozyme) and separated via C18 and porous graphitized carbon solid phase extraction (30). The purified N-glycans were reduced and permethylated as described (31). Mass spectrometry analysis was performed on an LTQ (ThermoFisher, San Jose, CA, USA) equipped with a Nanomate nanoelectrospray source (Advion, Ithaca, NY, USA). Samples were dissolved in 1:1 methanol/water. All ions are sodium adducts. Peaks were selected manually for MSn analysis.
Peripheral blood granulocyte isolation
Whole peripheral blood from all the family members was obtained. The components of the peripheral blood were separated using gradient centrifugation over Histopaque-1077 (Sigma). After centrifugation at 400 g for 30 min, the granulocytes were collected from the buffy coat layer above the red blood cell pellet. The granulocytes were treated briefly (<30 s) with Red Blood Cell Lysing Buffer (Sigma) with further washes in PBS w/o Ca2+/Mg2+.
Western blot
Granulocytes were lysed with 2% NP-40 in 150 mm NaCl, 50 mm Tris–HCI, pH 7.4, 20 μg/ml PMSF, 0.02% sodium azide and protease inhibitor cocktail tablet (Roche Molecular Biochemicals). The cell lysates were normalized based on cell numbers and were diluted with Laemmli reducing sample buffer and resolved using 4–20% SDS–PAGE gels (Bio-Rad). Membrane proteins were then transferred to Sequi-blot polyvinylidene difluoride membrane (Bio-Rad), blocked and stained with either HECA452 (Biolegend) or KPL-1 (BD Biosciences), then washed with Tris-buffered saline/0.1% Tween 20. Blots were then incubated with appropriate horse radish peroxidase-conjugated secondary antibodies (anti-RatIgM for HECA452 or anti-MsIgG for KPL-1), washed and visualized with chemiluminescence using Lumi-Light Western Blotting Substrate (Roche Diagnostics).
Flow cytometry
Aliquots of cells were washed with PBS/2% FBS and incubated with primary mAbs or with isotype control mAbs. The cells were washed in PBS/2% FBS and, for indirect immunofluorescence, incubated with appropriate secondary fluorochrome-conjugated antibody. After washing the cells, fluorescence intensity was determined using a Cytomics FC 500 MPL flow cytometer (Beckman Coulter).
Parallel plate flow chamber assay
A parallel plate flow chamber (GlycoTech) was mounted on top of a confluent monolayer of TNF-α-activated HUVEC (obtained from the Vascular Biology Core, Brigham & Women's Hospital). Purified granulocytes, resuspended in binding buffer (HBSS, 10 mm HEPES, 2 mm CaCl2) at a concentration of 1 × 106cells/ml, were then perfused over the HUVEC monolayer under defined shear stress conditions by a syringe pump (Harvard Apparatus). After introduction of the granulocytes into the chamber at 1 ml/min, the flow was momentarily suspended, allowing for cells to contact the HUVEC monolayer. Next, the flow rate was then increased incrementally to exert shear stress at levels from 0.25 to 10 dynes/cm2. Cell–cell interactions were monitored and recorded in real time with an inverted phase contrast microscope, connected to a video camera, an SMPTE time code generator, a monitor and a DVD recorder. Recorded videos were analyzed offline. In each case, specificity for binding to E-selectin was assessed by use of anti-E-selectin blocking monoclonal antibody. The number of leukocytes rolling were quantified under ×100 magnification at shear stresses of 0.5, 1, 2, 5 and 10 dynes/cm2 with four-independent fields of view (FOV) of 15 s intervals for each shear stress. The rolling velocities were determined by measuring the rolling distance and time traveled by a granulocyte.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Institutes of Health Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic healthcare centers). Sequencing experiments were performed by the Sequencing Core Facility of the Molecular Genetics Core Facility at Boston Children's Hospital supported by National Institutes of Health (grant number P30-HD18655). This work was also supported by the NIH at the National Institutes of Health (1K23HD073351 to A.D.), the Pediatric Endocrine Society Clinical Scholar Award to AD, support from the Translational Research Program at Boston Children's Hospital to J.N.H., March of Dimes (6-FY09-507 to J.N.H.), National Institutes of Health (1R21AI099435 to P.A.N., 2K12HD051959 to A.E. and P01 HL107146 NIH Program of Excellence in Glycosciences to R.S.), the Cogan Family Fund to P.A.N. and the Arthritis National Research Foundation to A.E. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, the NIH or the National Institutes of Health.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the subjects and their family members for their dedicated participation in this work. The authors thank Jennifer Moon for technical assistance.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Etzioni A., Tonetti M. Leukocyte adhesion deficiency II-from A to almost Z. Immunol. Rev. 2000;178:138–147. doi: 10.1034/j.1600-065x.2000.17805.x. [DOI] [PubMed] [Google Scholar]
- 2.Etzioni A., Frydman M., Pollack S., Avidor I., Phillips M.L., Paulson J.C., Gershoni-Baruch R. Brief report: recurrent severe infections caused by a novel leukocyte adhesion deficiency. N. Engl. J. Med. 1992;327:1789–1792. doi: 10.1056/NEJM199212173272505. [DOI] [PubMed] [Google Scholar]
- 3.Lubke T., Marquardt T., Etzioni A., Hartmann E., von Figura K., Korner C. Complementation cloning identifies CDG-IIc, a new type of congenital disorders of glycosylation, as a GDP-fucose transporter deficiency. Nat. Genet. 2001;28:73–76. doi: 10.1038/ng0501-73. [DOI] [PubMed] [Google Scholar]
- 4.Luhn K., Wild M.K., Eckhardt M., Gerardy-Schahn R., Vestweber D. The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat. Genet. 2001;28:69–72. doi: 10.1038/ng0501-69. [DOI] [PubMed] [Google Scholar]
- 5.Lubke T., Marquardt T., von Figura K., Korner C. A new type of carbohydrate-deficient glycoprotein syndrome due to a decreased import of GDP-fucose into the Golgi. J. Biol. Chem. 1999;274:25986–25989. doi: 10.1074/jbc.274.37.25986. [DOI] [PubMed] [Google Scholar]
- 6.Liu L., Hirschberg C.B. Developmental diseases caused by impaired nucleotide sugar transporters. Glycoconj. J. 2013;30:5–10. doi: 10.1007/s10719-012-9375-4. [DOI] [PubMed] [Google Scholar]
- 7.Marquardt T., Brune T., Luhn K., Zimmer K.P., Korner C., Fabritz L., van der Werft N., Vormoor J., Freeze H.H., Louwen F., et al. Leukocyte adhesion deficiency II syndrome, a generalized defect in fucose metabolism. J. Pediatr. 1999;134:681–688. doi: 10.1016/S0022-3476(99)70281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frydman M., Etzioni A., Eidlitz-Markus T., Avidor I., Varsano I., Shechter Y., Orlin J.B., Gershoni-Baruch R. Rambam-Hasharon syndrome of psychomotor retardation, short stature, defective neutrophil motility, and Bombay phenotype. Am. J. Med. Genet. 1992;44:297–302. doi: 10.1002/ajmg.1320440307. [DOI] [PubMed] [Google Scholar]
- 9.Wang S.R., Carmichael H., Andrew S.F., Miller T.C., Moon J.E., Derr M.A., Hwa V., Hirschhorn J.N., Dauber A. Large scale pooled next-generation sequencing of 1077 genes to identify genetic causes of short stature. J. Clin. Endocrinol. Metab. 2013;98:E1428–E1437. doi: 10.1210/jc.2013-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helmus Y., Denecke J., Yakubenia S., Robinson P., Luhn K., Watson D.L., McGrogan P.J., Vestweber D., Marquardt T., Wild M.K. Leukocyte adhesion deficiency II patients with a dual defect of the GDP-fucose transporter. Blood. 2006;107:3959–3966. doi: 10.1182/blood-2005-08-3334. [DOI] [PubMed] [Google Scholar]
- 11.Raju T.S. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr. Opin. Immunol. 2008;20:471–478. doi: 10.1016/j.coi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Pucic M., Knezevic A., Vidic J., Adamczyk B., Novokmet M., Polasek O., Gornik O., Supraha-Goreta S., Wormald M.R., Redzic I., et al. High throughput isolation and glycosylation analysis of IgG – variability and heritability of the IgG glycome in three isolated human populations. Mol. Cell. Proteomics. 2011;10:1–15. doi: 10.1074/mcp.M111.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etzioni A., Tonetti M. Fucose supplementation in leukocyte adhesion deficiency type II. Blood. 2000;95:3641–3643. [PubMed] [Google Scholar]
- 14.Hidalgo A., Ma S., Peired A.J., Weiss L.A., Cunningham-Rundles C., Frenette P.S. Insights into leukocyte adhesion deficiency type 2 from a novel mutation in the GDP-fucose transporter gene. Blood. 2003;101:1705–1712. doi: 10.1182/blood-2002-09-2840. [DOI] [PubMed] [Google Scholar]
- 15.Gazit Y., Mory A., Etzioni A., Frydman M., Scheuerman O., Gershoni-Baruch R., Garty B.Z. Leukocyte adhesion deficiency type II: long-term follow-up and review of the literature. J. Clin. Immunol. 2010;30:308–313. doi: 10.1007/s10875-009-9354-0. [DOI] [PubMed] [Google Scholar]
- 16.Sackstein R. The lymphocyte homing receptors: gatekeepers of the multistep paradigm. Curr. Opin. Hematol. 2005;12:444–450. doi: 10.1097/01.moh.0000177827.78280.79. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda T., Hashimoto H., Okayasu N., Kameyama A., Onogi H., Nakagawasai O., Nakazawa T., Kurosawa T., Hao Y., Isaji T., et al. Alpha1,6-fucosyltransferase-deficient mice exhibit multiple behavioral abnormalities associated with a schizophrenia-like phenotype: importance of the balance between the dopamine and serotonin systems. J. Biol. Chem. 2011;286:18434–18443. doi: 10.1074/jbc.M110.172536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murrey H.E., Gama C.I., Kalovidouris S.A., Luo W.I., Driggers E.M., Porton B., Hsieh-Wilson L.C. Protein fucosylation regulates synapsin Ia/Ib expression and neuronal morphology in primary hippocampal neurons. Proc. Natl. Acad. Sci. USA. 2006;103:21–26. doi: 10.1073/pnas.0503381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sturla L., Rampal R., Haltiwanger R.S., Fruscione F., Etzioni A., Tonetti M. Differential terminal fucosylation of N-linked glycans versus protein O-fucosylation in leukocyte adhesion deficiency type II (CDG IIc) J. Biol. Chem. 2003;278:26727–26733. doi: 10.1074/jbc.M304068200. [DOI] [PubMed] [Google Scholar]
- 20.Carlberg M., Dricu A., Blegen H., Wang M., Hjertman M., Zickert P., Hoog A., Larsson O. Mevalonic acid is limiting for N-linked glycosylation and translocation of the insulin-like growth factor-1 receptor to the cell surface. Evidence for a new link between 3-hydroxy-3-methylglutaryl-coenzyme a reductase and cell growth. J. Biol. Chem. 1996;271:17453–17462. doi: 10.1074/jbc.271.29.17453. [DOI] [PubMed] [Google Scholar]
- 21.Masnikosa R., Baricevic I., Jones D.R., Nedic O. Characterisation of insulin-like growth factor receptors and insulin receptors in the human placenta using lectin affinity methods. Growth Horm. IGF Res. 2006;16:174–184. doi: 10.1016/j.ghir.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Wang X., Gu J., Ihara H., Miyoshi E., Honke K., Taniguchi N. Core fucosylation regulates epidermal growth factor receptor-mediated intracellular signaling. J. Biol. Chem. 2006;281:2572–2577. doi: 10.1074/jbc.M510893200. [DOI] [PubMed] [Google Scholar]
- 23.Vanhooren V., Dewaele S., Kuro O.M., Taniguchi N., Dolle L., van Grunsven L.A., Makrantonaki E., Zouboulis C.C., Chen C.C., Libert C. Alteration in N-glycomics during mouse aging: a role for FUT8. Aging Cell. 2011;10:1056–1066. doi: 10.1111/j.1474-9726.2011.00749.x. [DOI] [PubMed] [Google Scholar]
- 24.Rivas M.A., Beaudoin M., Gardet A., Stevens C., Sharma Y., Zhang C.K., Boucher G., Ripke S., Ellinghaus D., Burtt N., et al. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat. Genet. 2011;43:1066–1073. doi: 10.1038/ng.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Exome Variant Server. Seattle: National Heart , Lung, and Blood Institute Exome Sequencing Project http://eversusgs.washington.edu/EVS , accessed March 22, 2012.
- 27.Royle L., Campbell M.P., Radcliffe C.M., White D.M., Harvey D.J., Abrahams J.L., Kim Y.G., Henry G.W., Shadick N.A., Weinblatt M.E., et al. HPLC-based analysis of serum N-glycans on a 96-well plate platform with dedicated database software. Anal. Biochem. 2008;376:1–12. doi: 10.1016/j.ab.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Ercan A., Barnes M.G., Hazen M., Tory H., Henderson L., Dedeoglu F., Fuhlbrigge R.C., Grom A., Holm I.A., Kellogg M., et al. Multiple juvenile idiopathic arthritis subtypes demonstrate pro-inflammatory IgG glycosylation. Arthritis Rheum. 2012;64:3025–3033. doi: 10.1002/art.34507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aoki K., Perlman M., Lim J.M., Cantu R., Wells L., Tiemeyer M. Dynamic developmental elaboration of N-linked glycan complexity in the Drosophila melanogaster embryo. J. Biol. Chem. 2007;282:9127–9142. doi: 10.1074/jbc.M606711200. [DOI] [PubMed] [Google Scholar]
- 30.Ashline D.J., Lapadula A.J., Liu Y.H., Lin M., Grace M., Pramanik B., Reinhold V.N. Carbohydrate structural isomers analyzed by sequential mass spectrometry. Anal. Chem. 2007;79:3830–3842. doi: 10.1021/ac062383a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y., Mechref Y. Comparing MALDI-MS, RP-LC-MALDI-MS and RP-LC-ESI-MS glycomic profiles of permethylated N-glycans derived from model glycoproteins and human blood serum. Electrophoresis. 2012;33:1768–1777. doi: 10.1002/elps.201100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.