Abstract
A growing body of evidence shows that long non-coding RNAs (lncRNAs) are involved in multiple human diseases than previously realized. However, no information is available now about lncRNAs in cardiac fibroblasts. The expression profile of lncRNAs was analyzed in Ang II-treated cardiac fibroblasts using lncRNAs arrays. The analysis showed that 282 of 4376 detected lncRNAs demonstrated >2-fold differential expression in response to the treatment with Ang II (100 nm) for 24 h. Among of them, 22 lncRNAs showed a greater than 4-fold changes. Meanwhile, Ang II also induced a widely expression changes in protein-coding genes in cardiac fibroblasts. Quantitative real time PCR confirmed the changes of six lncRNAs (AF159100, BC086588, MRNR026574, MRAK134679, NR024118, AX765700) and mRNAs (IL6, RGS2, PRG4, TIMP1, Cdkn1c, TIMP3, Col I, Col III and Fibronectin) in cardiac fibroblasts. Bioinformatic analysis indicated the process of cell proliferation. Further studies revealed that the down-regulating of Ang II on the expression of lncRNA-NR024118 was time-dependent, that the level of NR024118 was lowest at 24 h and back at 48 h. Ang II also dynamically down regulated the expression of Cdkn1c in cardiac fibroblasts. Ang II at a range from 10-9 M to 10-6 M induced a decrease of NR024118 and Cdkn1c in cardiac fibroblasts. In conclusion, the expression profile of lncRNAs was significantly altered in the Ang II-treated cardiac fibroblasts and Ang II dynamically regulated the expression of lncRNA-NR024118 and Cdkn1c in cardiac fibroblasts, indicating the potential role of NR024118 in cardiac fibroblasts.
Keywords: Angiotensin Π, cardiac fibroblasts, long non-coding RNA
Introduction
Cardiac fibrosis is the excess accumulation of extracellular matrix in the heart, which is closely associated with numerous cardiovascular diseases [1,2]. Cardiac fibroblasts play a pivotal role in the development of cardiac fibrosis through the synthesis of extracellular matrix (ECM) proteins, the degradation of ECM by producing matrix metalloproteinases (MMPs) and their endogenous inhibitors (TIMPs), and the secretion of cytokines including interleukin (IL)-6 [3,4]. Angiotensin II (Ang II) is considered to be a major factor in the pathogenesis of cardiac remodeling [5,6]. Ang II has been shown to induce cardiac fibrosis by stimulation of cell proliferation, ECM synthesis and cytokines secretion in cardiac fibroblasts [7,8]. At the present time, the molecular mechanisms underlying the effects of Ang II on cardiac fibroblasts are still not completely understood.
Currently, it is known that only ~2% of the mammalian genome encodes proteins, and that the over-whelming majority of the remaining genome is transcribed into noncoding RNAs (ncRNAs). These ncRNAs can be divided into housekeeping RNAs and regulatory ncRNAs, which are further grouped into short and long ncRNAs [9]. MicroRNAs (miRNA), as the short ncRNAs, have been demonstrated to be involved in the development of various diseases [10]. Several miRNAs have been confirmed to be closely associated with cardiac fibrosis [4]. The long ncRNAs (lncRNAs) are defined as a kind of ncRNAs which are longer than ~200 nucleotides and lacking of protein-encoding capacity. The ratio of lncRNAs in total ncRNAs is beyond 80% but is the least well-understood ncRNAs now [10]. Although initially thought to be transcriptional noise, recent evidence suggests that the expression of lncRNAs is cell- and developmental stage-specific and regulated by common transcription factors [11-13].
Although lncRNAs have been studied in different types of human cancer and neural diseases, the research of lncRNAs in cardiovascular disease is clearly in its infancy [14]. Only several lncRNAs were reported in cardiovascular system. LncRNA-MIAT has been identified to confer risk of myocardial infarction and lncRNA-ANRIL was considered to be a risk factor of coronary artery diseases [14,15]. LncRNA-AK143260 (Braveheart) was reported to be necessary for cardiac development [16]. Recently, lncRNAs were reported to be able to be regulated by Ang II in vascular smooth cells [17]. However, no information is available now about lncRNAs in cardiac fibroblasts. In this study, we found that Ang II (100 nm) for 24 h simultaneously induced widely changes of lncRNAs and protein-coding RNAs in adult rat cardiac fibroblasts. Bioinformatic analysis indicated the process of cell proliferation. Further studies revealed that Ang II dynamically downregulated the expression of lncRNA-NR024118, companying the decrease of Cdkn1c in cardiac fibroblasts. Ang II at a range from 10-9 M to 10-6 M induced a decrease of NR024118 and Cdkn1c in cardiac fibroblasts. Our current studies indicated the potential role of NR024118 in cardiac fibroblasts.
Methods
Materials and animals
Collagenase, trypsin and Ang II were obtained from Sigma Chemical (St Louis, MO, USA); Dulbecco’s modified Eagle’s medium (DMEM) and TRIzol were obtained from Life Technologies (Invitrogen, Carlsbad, CA, USA). Rat 4x44K LncRNA expression arrays were purchased from Arraystar (Rockville, USA). Sprague-Dawley (SD) rats were supplied from the Experimental Animal Center of Xian Jiaotong University (Xian, China). The animal experiments were approved by the University Committee of Laboratory Animal Care and Use and followed the guidelines of the National Animal Research Center.
Isolation and culture cardiac fibroblasts
Cardiac ventricular fibroblasts were obtained from hearts of adult male SD rats weighing 250~300 g as described previously [18]. In brief, following rapid excision of the hearts, the fibroblasts were prepared by enzymatic digestion with a collagenase/trypsin solution. After a 2h period of attachment to uncoated culture plates, the cells which were weakly attached or unattached were rinsed free and attached cells (mostly fibroblasts) were washed and grown in DMEM with 10% fetal bovine serum. The cardiac fibroblasts (passages 3~5) were grown to 80-90% confluence and serum starved for 24 h before treatment.
Preparation of RNA
Following 24 h serum starvation, cardiac fibroblasts were treated with Ang II (100 nM) for 24 h. Total RNAs were extracted using the TRIZOL reagent as previously described and RNAs were dissolved in RNase-free water [18]. The RNA quantity was determined spectrophotometrically as A260 and A260/A280 ratio using NanoDrop spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA) and RNA quality were checked by electrophoresis on a 1.2% agarose/formaldehyde gel. Isolated RNAs were stored at -70°C prior to lncRNAs arrays analysis and real time-PCR.
Microarray analysis of long ncRNAs and mRNAs expression
The rat LncRNA 4x44K Arrays from Arraystar (Rockville, USA) were used to analyze the expression profile of long non-coding RNAs and mRNAs in adult rat cardiac fibroblasts. The array contains probes of lncRNAs (~9300) and protein-coding genes (~15,200). The microarray hybridization was performed based on the manufacturer’s standard protocols [19]. Briefly, total RNAs from three pairs of control cardiac fibroblasts and Ang II-treated cardiac fibroblasts were extracted and pooled. Next, 1 μg of total RNAs were amplified and transcribed into fluorescent cRNA using Agilent’s Quick Amp Labeling protocol (version 5.7, Agilent Technologies). The labeled cRNAs were hybridized onto the Rat LncRNA 4x44K Array (Arraystar, Rockville, USA), washed and the microarrays scanned using an Agilent Scanner G2505B. Agilent Feature Extraction software (version 10.7.3.1) were used to analyze acquired array images. Median normalization and subsequent data processing were performed using the GeneSpring GX v11.5.1 software package (Aglent Technologies).
Quantitative real time-PCR
Quantitative real time-PCR (qPCR) was performed to quantify the levels of lncRNAs and mRNAs as previously described [18]. Briefly, total RNAs of cardiac fibroblasts were extracted using TRIzol Reagent. cDNAs were synthesized using the First Strand cDNA Synthesis kit (Fermentas Life Science, Burling, ON, Canada). Reactions were incubated for 60 mins at 42°C, 5 mins at 70°C, and then stored at -20°C. Quantitative PCR was then performed by using SYBR Premix Ex TaqTM II (TaKaRa, Ohtsu, Shiga, Japan) in iQ5 realtime PCR detection system (Bio-Rad, Hercules, CA, USA). PCR reactions were performed at 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and at 60°C for 30 s. The specificity of PCR products was assessed by melting curve analysis. Primers sequences of lncRNAs and mRNAs for qPCR are listed in Table 1. Gene expression in each sample was normalized to GAPDH and actin expression. Relative quantitation of lncRNAs and mRNAs expression was evaluated by the 2(−ΔΔCt) methods.
Table 1.
Primers sequences for long non-coding RNAs and protein-coding RNAs
| Gene name | Primer sequences |
|---|---|
| GAPDH | Forward: 5’GGGAAACTGTGGCGTGAT3’ |
| Reverse: 5’GAGTGGGTGTCGCTGTTGA3’ | |
| AF159100 | Forward: 5’GTTGCTCCTCGCTGGTTTC3’ |
| Reverse: 5’CAGCTGCCTTTATTCAGATGTA3’ | |
| BC086588 | Forward: 5’CCATAGTAGAAACAGGCAGGAC3’ |
| Reverse: 5’CCAGGCAACAATCAAATCAG3’ | |
| MRNR026574 | Forward: 5’GGCCACCTGCCTTACCTAC3’ |
| Reverse: 5’AGCCCACGGGACCACAAC3’ | |
| MRAK134679 | Forward: 5’ACCATGAGGCGGGACTGAC3’ |
| Reverse: 5’TCTGGTTAAACGAAAGGCAAAT3’ | |
| NR024118 | Forward: 5’GCTGCCCACCTCACTCAC3’ |
| Reverse: 5’CTTTATTGCTCCATTTCCCTC3’ | |
| AX765700 | Forward: 5’TTCCGAGCAGCCATTGACA3’ |
| Reverse: 5’CATCATCTAGCTCAGGGTTTCC3’ | |
| IL6 | Forward: 5’GCCTATTGAAAATCTGCTCTGGT3’ |
| Reverse: 5’GTCTTGGTCCTTAGCCACTCCT3’ | |
| RGS2 | Forward: 5’TCTGGTTGGCTTGCGAAGAC3’ |
| Reverse: 5’TCTCTTTGGGAGCTTCCTTC3’ | |
| PRG4 | Forward: 5’CGGGACGTTAGTTGCATTCG-3’, |
| Reverse: 5’TCAGTGATTCTGCGTGGTGGA-3’ | |
| Cdkn1c | Forward: 5’CCCCCACACATTCATCTTCA3’ |
| Reverse: 5’GGGCAGTACAGGAACCATTTT3’ | |
| TIMP3 | Forward: 5’CCTTTGGCACTCTGGTCT3’ |
| Reverse: 5’TCAGCAGGTACTGGTATTTGT3’ | |
| Fibronectin | Forward: 5’GTGAAGAACGAGGAGGATGTG3’ |
| Reverse: 5’GTGATGGCGGATGATGTAGC3’ | |
| TIMP1 | Forward: 5’GTTGCTCCTCGCTGGTTTC3’ |
| Reverse: 5’CAGCTGCCTTTATTCAGATGTA3’ | |
| Collagen I | Forward: 5’GTTGCTCCTCGCTGGTTTC3’ |
| Reverse: 5’CAGCTGCCTTTATTCAGATGTA3’ | |
| β-actin | Forward: 5’CCTGTACGCCAACACAGTGC3’ |
| Reverse: 5’ATACTCCTGCTTGCTGATCC3’ |
Bioinformatic analysis
The Gene Ontology project provides a controlled vocabulary to describe gene and gene product attributes in any organism (http://www.geneontology.org). The ontology covers three domains: Biological Process, Cellular Component and Molecular Function. Fisher’s exact test is used to find if there is more overlap between the DE list and the GO annotation list than would be expected by chance. The p-value denotes the significance of GO terms enrichment in the DE genes. The lower the p-value, the more significant the GO Term (p-value ≤0.05 is recommended). Pathway analysis is a functional analysis that maps genes to KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways (http://www.genome.jp/kegg/). The p-value (Fisher-P value) denotes the significance of the Pathway correlated to the conditions. Lower the p-value, more significant is the Pathway (The recommend p-value cut-off is 0.05).
Statistical analysis
Data were presented as the means ± SEM. The Student’s t-test was used to compare data between the two groups and one-way ANOVA for more than three groups. P<0.05 was considered to indicate a statistically significant difference. *p<0.05, **p<0.01 and ***p<0.001.
Results
Arrays analysis of lncRNAs and mRNAs expression in cardiac fibroblasts
Initial studies determined the overall numbers and quantity of lncRNAs and mRNAs that could be detected using an Arraystar microarray (Rockville, USA). This showed that 4376 (~47%) of the 9300 lncRNAs could be detected in untreated cells which is lower fraction than the protein coding mRNAs, for which 9553 (~63%) of the 15200 could be detected. The average intensity of 4376 lncRNAs was 2841 while the average intensity of 9553 protein-coding genes was 5467. These results are consistent with other studies showing that lncRNAs were generally expressed at lower levels than protein-coding genes (Figure 1) [20].
Figure 1.
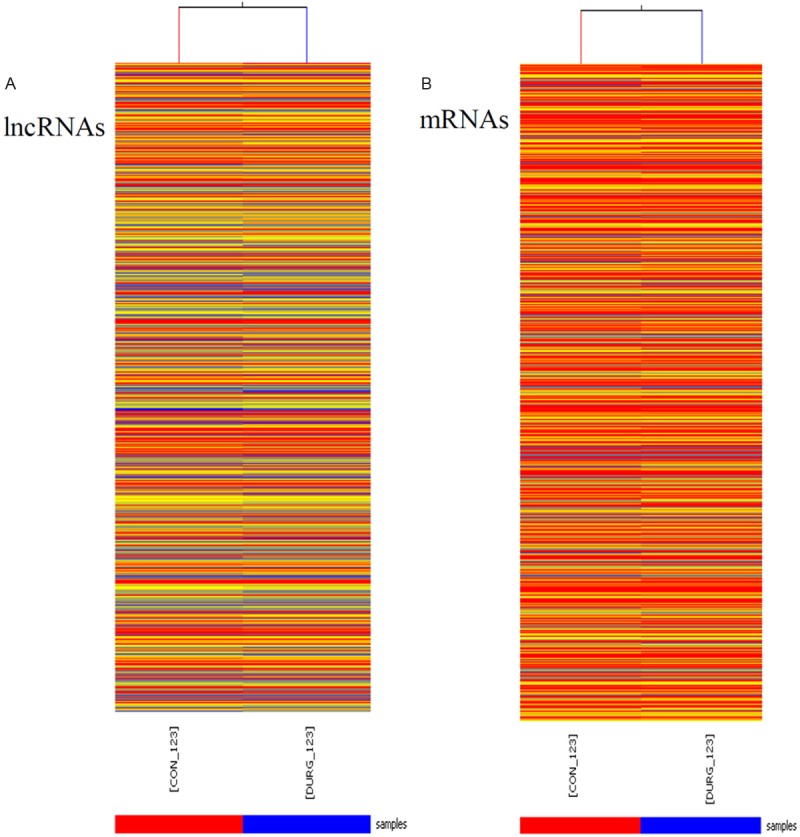
Heat map presentation of the expression profile of lncRNAs and mRNAs in angiotensin II-treatment and control cardiac fibroblasts. “Red” indicates high relative expression, and “blue” indicates low relative expression. CON-123 indicates cardiac fibroblasts. DRUG-123 indicates cardiac fibroblasts treated by angiotensin II (100 nM) for 24 h.
To gain further insights into the putative biological relevance of lncRNAs in cardiac fibroblasts, we compared the levels of lncRNAs and mRNAs in cardiac fibroblasts with/without Ang II-treatment for 24 h. We found that 282 of 4376 detected lncRNAs demonstrated >2-fold differential expression with 178 lncRNAs showing up-regulated and 106 lncRNAs showing down-regulated. When the cut-off was set at 4-fold, 12 lncRNAs were up-regulated while 10 lncRNAs were down-regulated (Table 2). Meanwhile, 882 mRNAs showed beyond a 2-fold differential expression in cardiac fibroblasts when compared to control cells. 521 mRNAs were up-regulated while 361 mRNAs were down-regulated. When the cut-off was set at 10-fold, 27 mRNAs were up-regulated while 9 mRNAs down-regulated (Table 3).
Table 2.
Differentially expressed long non-coding RNAs in cardiac fibroblasts (fold change >4)
| Probe Name | Expression | chromosome | strand | Start | End | Fold change |
|---|---|---|---|---|---|---|
| MRAK034346 | up | chr2 | + | 120115104 | 120115164 | 4.25 |
| AF167308 | up | chr16 | - | 73031874 | 73031931 | 4.26 |
| XR007499 | up | chr13 | - | 39625623 | 39625683 | 4.26 |
| XR006412 | up | chr12 | + | 1021078 | 1021138 | 4.61 |
| U57362 | up | chr8 | - | 84641434 | 84641494 | 4.71 |
| U57361 | up | chr8 | - | 84643465 | 84643525 | 4.74 |
| BC158638 | up | chr3 | - | 113780927 | 113801831 | 5.15 |
| U78517 | up | chr3 | + | 54716706 | 54716766 | 5.40 |
| AF239157 | up | chr10 | - | 46291442 | 46291502 | 5.92 |
| BC086588 | up | chr2 | - | 186049351 | 186049411 | 8.92 |
| MRNR026574 | up | chr1 | + | 159013775 | 159013835 | 12.20 |
| AF159100 | up | chr16 | - | 70640308 | 70640368 | 47.16 |
| MRAK134679 | down | chr3 | - | 62648034 | 62648094 | -8.34 |
| NR_024118 | down | chr20 | - | 4128070 | 4128130 | -7.97 |
| AX765700 | down | chr9 | + | 60980297 | 60980357 | -7.17 |
| MRAK053938 | down | chr15 | + | 109052626 | 109052699 | -6.42 |
| MRAK031289 | down | chr16 | + | 64128370 | 64128430 | -6.26 |
| AY973245 | down | chr16 | + | 64124051 | 64124111 | -5.46 |
| AJ005396 | down | chr2 | + | 210193318 | 210193378 | -5.33 |
| MRAK162711 | down | chr5 | + | 28340911 | 28340971 | -5.15 |
| MRAK132609 | down | chr2 | + | 210192978 | 210193038 | -5.09 |
| NR_027324 | down | chr1 | - | 202822980 | 202823040 | -4.63 |
Table 3.
Differentially expressed protein-coding RNAs in cardiac fibroblasts (fold change >10)
| Probe Name | Gene Symbol | Description | Fold change |
|---|---|---|---|
| CUST3948 | Rgs2 | Rattus norvegicus regulator of G-protein signaling 2 | 10.16 |
| CUST10456 | Nefh | Rattus norvegicus neurofilament, heavy polypeptide | 10.38 |
| CUST8969 | Spp1 | Rattus norvegicus secreted phosphoprotein 1 | 10.48 |
| CUST12405 | Gch1 | Rattus norvegicus GTP cyclohydrolase 1 | 11.92 |
| CUST4425 | Cd55 | Rattus norvegicus decay accelerating factor 1 (Daf1) | 12.69 |
| CUST12794 | Ccdc19 | Rattus norvegicus coiled-coil domain containing 19 | 13.079 |
| CUST14423 | Pde2a | phosphodiesterase 2A isoform 1 | 13.219 |
| CUST15179 | Pde2a | phosphodiesterase 2A, cGMP-stimulated | 13.28 |
| CUST4963 | Gjb2 | gap junction membrane channel protein beta 2 | 13.46 |
| CUST2183 | Elf5 | E74-like factor 5 | 13.51 |
| CUST11179 | Rnase1 | Rattus norvegicus ribonuclease, RNase A family, 1 | 13.73 |
| CUST1306 | Ptprn | Rattus norvegicus protein tyrosine phosphatase, receptor type, N | 14.33 |
| CUST11361 | Hspb7 | cardiovascular heat shock protein | 14.65 |
| CUST1958 | Esm1 | Rattus norvegicus endothelial cell-specific molecule 1 | 15.10 |
| CUST10736 | Cldn3 | Rattus norvegicus claudin 3 | 17.50 |
| CUST11657 | Prg4 | proteoglycan 4 | 18.11 |
| CUST14921 | Lcn2 | Rattus norvegicus lipocalin 2 | 19.78 |
| CUST10982 | Hp | Rattus norvegicus haptoglobin | 20.20 |
| CUST6098 | Ccl11 | Rattus norvegicus chemokine (C-C motif) ligand 11 | 21.15 |
| CUST5899 | Gja5 | gap junction membrane channel protein alpha 5 | 22.59 |
| CUST6613 | RGD1562551 | hypothetical protein LOC311760 | 24.421 |
| CUST10878 | Pnoc | Rattus norvegicus prepronociceptin | 25.39 |
| CUST3944 | Cldn11 | Rattus norvegicus claudin 11 | 25.89 |
| CUST11937 | Star | Rattus norvegicus steroidogenic acute regulatory protein | 31.11 |
| CUST5850 | Slco4a1 | Rattus norvegicus solute carrier organic anion transporter family, member 4a1 | 47.80 |
| CUST10972 | Il6 | Rattus norvegicus interleukin 6 | 49.94 |
| CUST13799 | Slc16a3 | Rattus norvegicus solute carrier family 16, member 3 | 55.36 |
| CUST7261 | Cilp | cartilage intermediate layer protein, nucleotide | -30.32 |
| CUST7758 | Ces1d | Rattus norvegicus carboxylesterase 3 | -20.97 |
| CUST4333 | Adh7 | Rattus norvegicus alcohol dehydrogenase 7 (class IV) | -15.41 |
| CUST7819 | Cdkn1c | Rattus norvegicus cyclin-dependent kinase inhibitor 1C | -13.86 |
| CUST8965 | Timp3 | Rattus norvegicus tissue inhibitor of metalloproteinase 3 | -13.85 |
| CUST8211 | Arhgap20 | Rattus norvegicus Rho GTPase activating protein 20 | -13.49 |
| CUST10750 | Olfml2a | olfactomedin-like 2A | -10.41 |
| CUST9855 | Flrt3 | fibronectin leucine rich transmembrane protein | -10.29 |
| CUST4116 | Mtss1 | metastasis suppressor 1 | -10.15 |
Quantitative real time-PCR analysis of lncRNAs and mRNAs expression
Quantitative real time PCR was used to re-measure the abundance of six lncRNAs (AF159100, BC086588, MRNR026574, MRAK134679, NR024118 and AX765700) and 9 mRNAs associated with fibrosis (IL6, RGS2, PRG4, TIMP1, Cdkn1c, TIMP3, Col I, Col III and Fibronectin). qPCR analysis revealed that the levels of AF159100, BC086588 and MRNR026574 in Ang II-treated cells were up-regulated to 27.42 fold (p=0.0041), 5.50 fold (p<0.001) and 4.37 fold (p=0.0058) compared to control cells (Figure 2). qPCR showed the levels of MRAK134679, NR024118 and AX765700 were decreased to 7.59 fold (p=0.0057), 8.05 fold (p=0.004) and 6.36-fold (p=0.001) compared to control cells (Figure 2).
Figure 2.
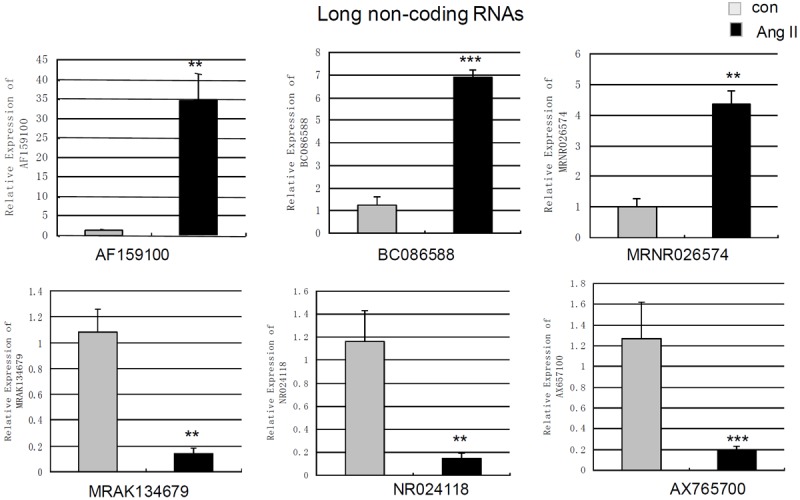
Measurement of changes in long non-coding RNAs using qPCR. The expression levels of AF159100, BC086588, MRNR026574, MRAK134679, NR024118, AX765700 in cardiac fibroblasts treated by angiotensin II-treatment (100 nm 24 h) and control cells were measured by qPCR. Expression of lncRNAs was normalized to GAPDH expression. Data are the mean ± SEM (n=6). P<0.05 was considered to indicate a statistically significant difference compared with control fibroblasts.*p<0.05, **p<0.01 and ***p<0.001.
The levels of 8 mRNAs were also verified by qPCR. It was revealed that the levels of IL6, regulator of G-protein signaling 2 (RGS2) and proteoglycan 4 (PRG4) were increased to 46.93 fold (p=0.0057), 19.28 fold (p=0.001) and 4.69-fold (p=0.0044) in cardiac fibroblasts treated by Ang II (Figure 3). qPCR showed that Ang II decreased the levels of cyclin-dependent kinase inhibitor 1C (Cdkn1c) and TIMP3 to 7.22 fold (p<0.001) and 8.16-fold (p=0.001) in cardiac fibroblasts compared to control (Figure 3). qPCR also revealed that Ang II up-regulated the level of TIMP1 to 2.41 fold (p=0.0048) (Figure 3). However, the changes of collagen I, collagen III and Fibronectin did not show statistical significance using qPCR (Figure 3).
Figure 3.
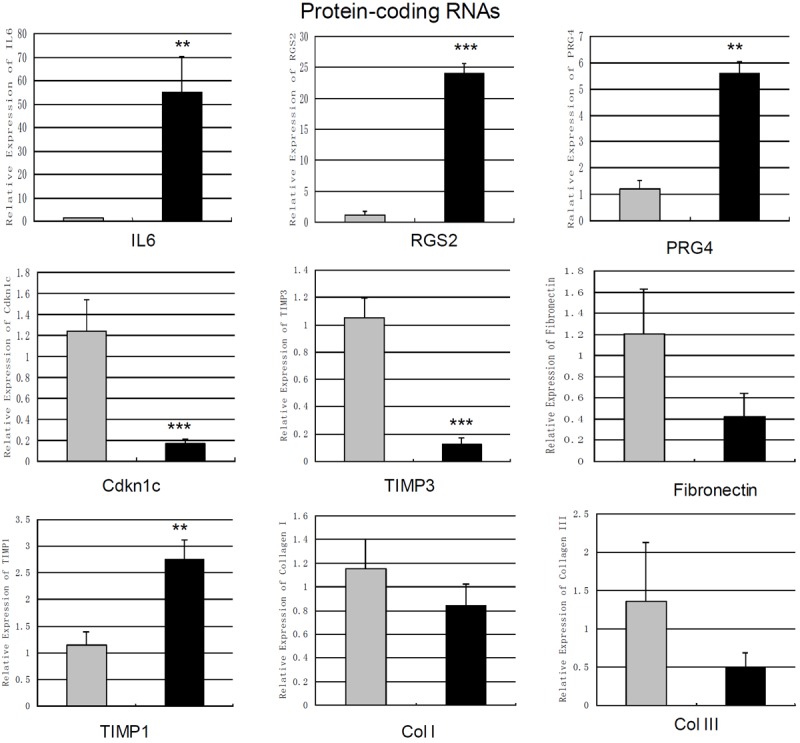
Measurement of changes in protein coding mRNAs using qPCR. The expression levels of IL6, RGS2, PRG4, TIMP1, Cdkn1c, TIMP3, Col I, Col III and Fibronectin in cardiac fibroblasts treated by angiotensin II-treatment (100 nm 24 h) and control cells were measured by qPCR. Expression of mRNAs was normalized to GAPDH expression. Data are the mean ± SEM (n=6). P<0.05 was considered to indicate a statistically significant difference compared with control fibroblasts.*p<0.05, **p<0.01 and ***p<0.001.
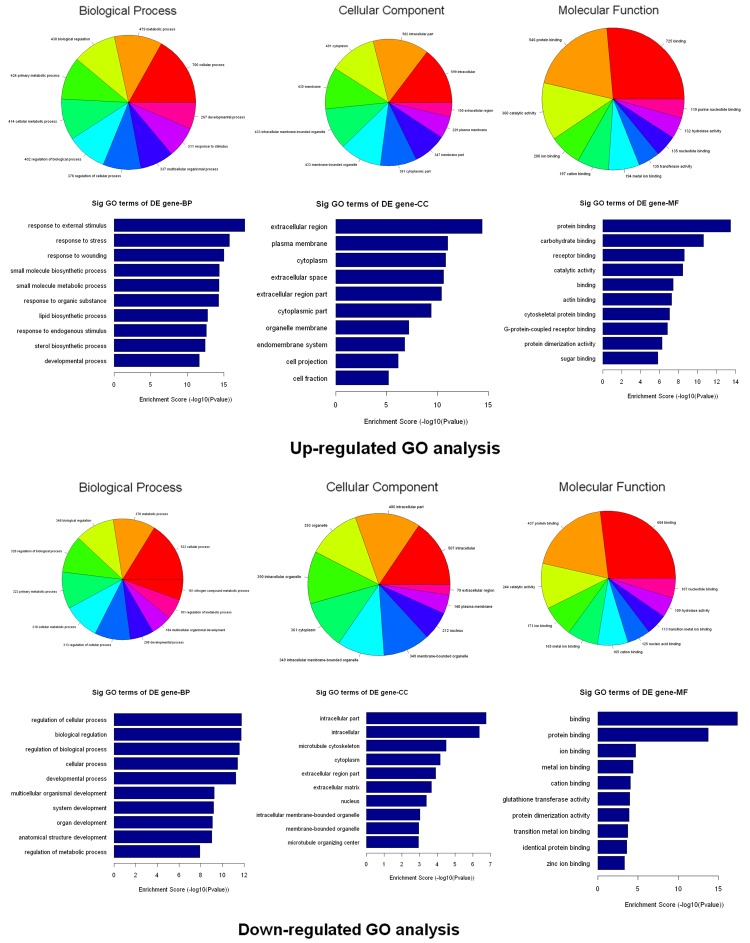
Go analysis and pathway analysis
The number (Top ten) of genes associated with GO term and the significance of GO term (Top ten) were shown (Figure 4). The upregulated genes were involved in 843 biological process, 110 cellular components and 165 molecular functions. In the biological process category, the most significant term was the response to external stimulus (p=1.33509E-18). In the cellular component category, the most represented GO term was the extracellular region (p=4.20796E-15). Within the molecular component category, protein binding (p=3.38671E-14) as the most highly represented term. The downregulated genes were involved in 501 biological process, 40 cellular components and 85 molecular functions. In the biological process category, the regulation of cellular process was enriched most. Within the cellular component category, intracellular part was the most represented GO terms. Among the various molecular functions, binding were most highly represented term.
Figure 4.
Bioinformatic analysis of the differentially expressed genes. The Gene Ontology (GO) analysis provides a controlled vocabulary to describe differentially expressed transcript attributes in all organisms. The ontology covers three domains: Biological Process, Cellular Component and Molecular Function. The p-value denotes the significance of GO terms enrichment in the DE genes. The lower the p-value, the more significant the GO Term (p-value ≤0.05 is recommended).
Following Go analysis, KEGG was used to do a pathway enrichment analysis. The downregulated genes were involved in 18 pathways while upregulated genes were involved in 30 pathways. The top 10 pathways of downregulated and upregulated genes were shown (Figure 5). The pathways of downregulated genes include cell cycle, P53 signaling pathway, MAPK signaling pathway, indicating the activation of cell proliferation. Among the ten pathways, the most significant pathway was the Cell cycle pathway (p=0.000013705).
Figure 5.
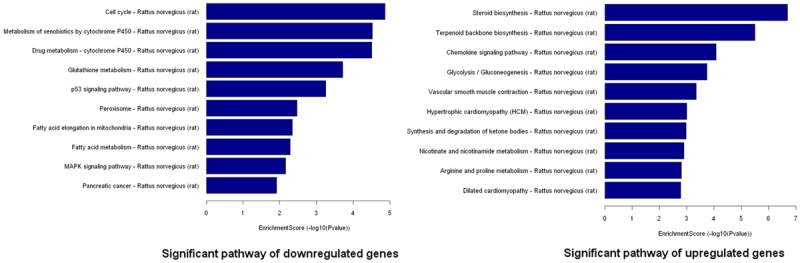
Pathway analysis of the differentially expressed genes. Pathway analysis is a functional analysis that maps genes to KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways (http://www.genome.jp/kegg/). The p-value (Fisher-P value) denotes the significance of the Pathway correlated to the conditions. Lower the p-value, more significant is the Pathway (The recommend p-value cut-off is 0.05).
The regulation of Ang II on the expression of NR024118 and Cdkn1c
In order to investigate how Ang II regulate the expression of lncRNA-NR024118 and Cdkn1c, we determined the levels of NR024118 and Cdkn1c in cardiac fibroblasts when treated by Ang II at different time and different concentration using quantitative real time PCR. We found that the level of NR024118 was gradually decreasing with the exposure time to Ang II in cardiac fibroblasts. The level of NR024118 reached to the lowest at 24 h but back at 48 h in cardiac fibroblasts (Figure 6A). Meanwhile, the level of Cdkn1c in cardiac fibroblasts was gradually decreasing as the extension of time of treatment by Ang II with the lowest level at 48 h (Figure 6B). All of treatment by Ang II at a range from 10-9 M to 10-6 M induced a decrease of both NR024118 and Cdkn1c in cardiac fibroblasts (Figure 6C and 6D).
Figure 6.
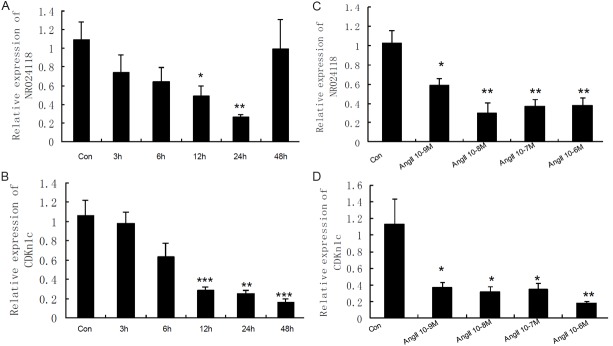
The regulation of angiotensin II on the expression of NR024118 and Cdkn1c in cardiac fibroblasts. The expression of NR024118 and Cdkn1c in cardiac fibroblasts treated by angiotensin II (Ang II 100 nm) at different time and at different concentration for 24 h was measured by qPCR. Expression of transcripts was normalized to actin expression. Data are the mean ± SEM (n=6). P<0.05 was considered to indicate a statistically significant difference compared with control fibroblasts. *p<0.05, **p<0.01 and ***p<0.001.
Discussion
The current literature on lncRNAs mainly focused on cancer and neural diseases [21-25]. Up to now, only a few lncRNAs were reported in cardiovascular system. In our study, the clear changes of lncRNAs (AF159100, BC086588, MRNR026574, MRAK134679, NR024118, AX765700) in adult cardiac fibroblasts in response to Ang II were associated with the changes of several protein-coding RNAs. We suspected that the decrease of Cdkn1c indicated that cell proliferation. The upregulated PRG4 induced by Ang II, an extracellular matrix molecule, indicated the enhancing of the synthesis of extracellular matrix. The changes of TIMP1 and TIMP3 showed that the degradation of ECM might be affected the activity of MMPs. The obvious increase of IL 6 indicated the inflammation mechanism of cytokines in the developing of cardiac fibrosis. It was reported that production of IL6 in cardiac fibroblasts will lead to TGF-β1 production and stimulates cardiac fibrosis that induced by Ang II [26]. RGS2 has been showed to be a regulator of Ang II-effects in cardiac fibroblast that might have a role in Ang II-induced fibrosis [8].
Go analysis indicated cell response to external stimulus and cell division. Pathways analysis showed several significant pathways related to cell proliferation including cell cycle, P53 signaling pathway and MAPK signaling pathway. Further studies revealed that Ang II at a range from 10-9 M to 10-6 M decreased the expression of NR024118 and Cdkn1c in cardiac fibroblasts. Moreover, the regulation of Ang II on the expression of NR024118 and Cdkn1c was in a time-dependent pattern. Moreover, Cdkn1c (p57/KIP2) was common down-regulated in different human cancers, indicating the role of Cdkn1c in cell proliferation [32]. Ang II dynamically down regulated the level of NR024118 and Cdkn1c in cardiac fibroblasts, strongly suggesting the potential role of NR024118 in cardiac fibroblasts.
Bioinformatic analysis reveal that LncRNA-NR024118 (793 bp), located in chromosome 20, is defined as Rattus norvegicus tenascin XA, pseudogene 1 (Tnxa-ps1), non-coding RNA. The protein-coding RNA of tenascin X has been reported to facilitate myocardial fibrosis through transforming growth factor-β1 and peroxisome proliferator-activated receptor γ [27]. Pseudogenes have long been neglected because of being considering nonfunctional. However, recent advances have established that the RNA transcribed from a pseudogene can have diverse functions not only to their parental genes but also to unrelated genes [28]. Pseudogenes are considered not to be pseudo any more now [29]. The pseudogene (PTENP1) of the tumor suppressor PTEN has been reported to regulate the expression of PTEN in mRNA and protein levels [30]. Recently, it was reported that a pseudogene lncRNAs network (PTENpg1 α and β) regulates PTEN transcription and translation in human cells [31]. We anticipate that the next functional studies of Tnxa-ps1 will reveal the roles of Tnxa-ps1 in cardiac fibroblasts.
In conclusion, our current studies showed that the expression profile of lncRNAs was significantly altered in the Ang II-induced cardiac fibroblasts and Ang II dynamically regulated the expression of lncRNA-NR024118 and Cdkn1c in adult cardiac fibroblasts, strongly indicating the potential role of NR024118 in adult cardiac fibroblasts.
Acknowledgements
This study was supported by National Science Foundation of China (31100834) and the International Cooperation Founds of Shaanxi Province (2012KW-32-02).
Disclosure of conflict of interest
None.
References
- 1.Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, Shindo T, Sano M, Otsu K, Snider P, Conway SJ, Nagai R. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 2010;120:254–265. doi: 10.1172/JCI40295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Díez J. Do microRNAs regulate myocardial fibrosis? Nat Clin Pract Cardiovasc Med. 2009;6:88–89. doi: 10.1038/ncpcardio1415. [DOI] [PubMed] [Google Scholar]
- 4.Jiang X, Tsitsiou E, Herrick SE, Lindsay MA. MicroRNAs and the regulation of fibrosis. FEBS J. 2010;277:2015–21. doi: 10.1111/j.1742-4658.2010.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwata M, Cowling RT, Yeo SJ, Greenberg B. Targeting the ACE2-Ang-(1-7) pathway in cardiac fibroblasts to treat cardiac remodeling and heart failure. J Mol Cell Cardiol. 2011;51:542–547. doi: 10.1016/j.yjmcc.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren J, Yang M, Qi G, Zheng J, Jia L, Cheng J, Tian C, Li H, Lin X, Du J. Proinflammatory protein CARD9 is essential for infiltration of monocyticfibroblast precursors and cardiac fibrosis caused by Angiotensin II infusion. Am J Hypertens. 2011;24:701–707. doi: 10.1038/ajh.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lijnen PJ, Van Pelt JF, Fagard RH. Stimulation of Reactive Oxygen Species and Collagen Synthesis by Angiotensin II in Cardiac Fibroblasts. Cardiovasc Ther. 2012;30:e1–e8. doi: 10.1111/j.1755-5922.2010.00205.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang P, Su J, King ME, Maldonado AE, Park C, Mende U. Regulator of G protein signaling 2 is a functionally important negative regulator of angiotensin II-induced cardiac fibroblast responses. Am J Physiol Heart Circ Physiol. 2011;301:H147–H156. doi: 10.1152/ajpheart.00026.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS. Non-coding RNAS: regulator of diseases. J Pathol. 2010;220:126–139. doi: 10.1002/path.2638. [DOI] [PubMed] [Google Scholar]
- 10.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 12.Mercer TR, Dinger ME, Sunkin SM, Mehler MF, Mattick JS. Specific expression of long noncoding RNAs in the mouse brain. Proc Natl Acad Sci U S A. 2008;105:716–721. doi: 10.1073/pnas.0706729105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishii N, Ozaki K, Sato H, Mizuno H, Saito S, Takahashi A, Miyamoto Y, Ikegawa S, Kamatani N, Hori M, Saito S, Nakamura Y, Tanaka T. Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J Hum Genet. 2006;51:1087–1099. doi: 10.1007/s10038-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhuang J, Peng W, Li H, Wang W, Wei Y, Li W, Xu Y. Methylation of p15INK4b and expression of ANRIL on chromosome 9p21 are associated with coronary artery disease. PLoS One. 2012;7:e47193. doi: 10.1371/journal.pone.0047193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, Ding H, Butty VL, Torrey L, Haas S, Abo R, Tabebordbar M, Lee RT, Burge CB, Boyer LA. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152:570–583. doi: 10.1016/j.cell.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung A, Trac C, Jin W, Lanting L, Akbany A, Sætrom P, Schones DE, Natarajan R. Novel Long Noncoding RNAs Are Regulated by Angiotensin II in Vascular Smooth Muscle Cells. Circ Res. 2013;113:266–278. doi: 10.1161/CIRCRESAHA.112.300849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang X, Ning Q, Wang J. Angiotensin II induced differentially expressed microRNAs in adult rat cardiac fibroblasts. J Physiol Sci. 2013;63:31–38. doi: 10.1007/s12576-012-0230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang F, Zhang L, Huo XS, Yuan JH, Xu D, Yuan SX, Zhu N, Zhou WP, Yang GS, Wang YZ, Shang JL, Gao CF, Zhang FR, Wang F, Sun SH. Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology. 2011;54:1679–1689. doi: 10.1002/hep.24563. [DOI] [PubMed] [Google Scholar]
- 20.Guttman M, Garber M, Levin JZ, Donaghey J, Robinson J, Adiconis X, Fan L, Koziol MJ, Gnirke A, Nusbaum C, Rinn JL, Lander ES, Regev A. Ab initio reconstruction of cell type specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat Biotechnol. 2010;28:503–510. doi: 10.1038/nbt.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bian S, Sun T. Functions of noncoding RNAs in neural development and neurological diseases. Mol Neurobiol. 2011;44:359–373. doi: 10.1007/s12035-011-8211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui Z, Ren S, Lu J, Wang F, Xu W, Sun Y, Wei M, Chen J, Gao X, Xu C, Mao JH, Sun Y. The prostate cancer-up-regulated long noncoding RNA PlncRNA-1 modulates apoptosis and proliferation through reciprocal regulation of androgen receptor. Urol Oncol. 2013;31:1117–1123. doi: 10.1016/j.urolonc.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 24.Silva JM, Boczek NJ, Berres MW, Ma X, Smith DI. LSINCT5 is over expressed in breast and ovarian cancer and affects cellular proliferation. RNA Biol. 2011;8:496–505. doi: 10.4161/rna.8.3.14800. [DOI] [PubMed] [Google Scholar]
- 25.Meola N, Pizzo M, Alfano G, Surace EM, Banfi S. The long noncoding RNA Vax2os1 controls the cell cycle progression of photoreceptor progenitors in the mouse retina. RNA. 2012;18:111–123. doi: 10.1261/rna.029454.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma F, Li Y, Jia L, Han Y, Cheng J, Li H, Qi Y, Du J. Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGFβ/Smad activation and cardiac fibrosis induced by angiotensin II. PLoS One. 2012;7:e35144. doi: 10.1371/journal.pone.0035144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jing L, Zhou LJ, Zhang FM, Li WM, Sang Y. Tenascin-x facilitates myocardial fibrosis and cardiac remodeling through transforming growth factor-β1 and peroxisome proliferator-activated receptor γ in alcoholic cardiomyopathy. Chin Med J (Engl) 2011;124:390–395. [PubMed] [Google Scholar]
- 28.Polisenol L. Pseudogenes: newly discoved players in human cancer. Sci Signal. 2012;5:re5. doi: 10.1126/scisignal.2002858. [DOI] [PubMed] [Google Scholar]
- 29.Wen YZ, Zheng LL, Qu LH, Ayala FJ, Lun ZR. Pseudogenes are not pseudo any more. RNA Biol. 2012;9:27–32. doi: 10.4161/rna.9.1.18277. [DOI] [PubMed] [Google Scholar]
- 30.Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnsson P, Ackley A, Vidarsdottir L, Lui WO, Corcoran M, Grandér D, Morris KV. A pseudogene long-noncoding-RNA network regulates PTEN transcription and translation in human cells. Nat Struct Mol Biol. 2013;20:440–446. doi: 10.1038/nsmb.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giovannini C, Gramantieri L, Minguzzi M, Fornari F, Chieco P, Grazi GL, Bolondi L. CDKN1C/P57 is regulated by the Notch target gene Hes1 and induces senescence in human hepatocellular carcinoma. Am J Pathol. 2012;181:413–22. doi: 10.1016/j.ajpath.2012.04.019. [DOI] [PubMed] [Google Scholar]