Abstract
Endometrial carcinoma (EC) is the most common gynecologic cancer worldwide and is one of the leading causes of death in women. Therefore, it is urgent to elucidate the pathological mechanisms of EC. SERPINA3 is a member of the serpin super-family of protease inhibitors. Its aberrant expression has been observed in various tumor cells. However, its clinical significance and biological function in endometrial cancer remains unknown. In the present study, we demonstrated that SERPINA3 expression was significantly up-regulated in EC samples and was closely correlated with lower differentiation, higher stage, positive lymph node or vascular thrombosis and negative estrogen receptor (ER), indicating a poor prognosis. We then demonstrated that SERPINA3 promoted EC cells proliferation by regulating G2/M checkpoint in cell cycle and inhibited cells apoptosis, and we further uncovered that the pro-proliferative effect of SERPINA3 on EC was likely ascribed to the activation of MAPK/ERK1/2 and PI3K/AKT signaling. The results of our study may provide insight into the application of SERPINA3 as a novel predictor of clinical outcomes and a potential therapeutic target of EC.
Keywords: SERPINA3, endometrial cancer, proliferation, G2/M checkpoint, apoptosis
Introduction
Endometrial cancer (EC) is the sixth most common malignancy among females worldwide with an estimated incidence of about 288,000 new cases in the year 2008 [1]. And it is the most common gynecologic malignancy of the female genital tract and the fourth most common neoplasia in women [2]. The most important prognostic factors at diagnosis are: stage, grade, depth of invasive disease, lymphovascular space invasion (LVSI) and histological subtype [1,3]. Traditionally, endometrial cancer has been classified into two types [4]. Type I endometrial carcinoma comprises the endometrioid adenocarcinomas that express the estrogen receptor (ER) and progesterone receptor (PR) and usually low grade and rarely metastasize [5]. The prognosis of type I cancers is favorable if diagnosed at an early stage, with a 5 year survival rate higher than 80% [3,5]. Type II endometrial carcinomas are those of non-endometrioid histology, in particular serous or clear-cell morphology. These tumors are considered to be of high histological grade, arise in the background of atrophic endometrium and do not seem to be related to the ER pathway [6]. Despite the higher prevalence of type I cancers, type II tumors account for a high proportion of endometrial cancer-related deaths [7]. In spite of advances in radiotherapy, surgery and chemotherapeutic strategies, the prognosis of women with recurrent or advanced endometrial cancer is still poor with a median overall survival (OS) of approximately 7-10 months [8-10].
Aggressive phenotypes characterized by lymphovascular invasion, high histological grade, and myometrial invasion, led to the poor prognosis of EC. The mechanisms involved in this aggressive transformation are largely unknown, however, interactions between the primary tumor mass and the surrounding stroma and molecular events likely play a role in this transformation [11,12].
SERPINA3 is a member of the serpin super-family of protease inhibitors also known as α1-Antichymotrypsin (α1-ACT) in human beings and SERPINA3n in mice [13]. SERPINA3 expression has been observed in various tumor cells, such as in breast carcinoma [14], hepatocellular carcinoma [15], prostate carcinoma [16] and adenocarcinoma of the lung [17]. The existence of intracellular SERPINA3 in carcinoma and non-carcinoma cells has been known for a long time [18-20]. However, while the serine protease inhibitors with functions in the extra-cellular space are well studied [21,22], remarkably limited information is available on exact intracellular mechanism of action. Recent reports showed that high SERPINA3 is a marker of poor prognosis in some types of cancer. For example, as one of the inflammatory response pathways protein, SERPINA3 expression is up-regulated in recurrent ovarian cancer, which may play an important role in the progression and chemo-resistance [23]. In human placental diseases SERPINA3 up-regulation related with hypomethylation of promoter region and overexpression of SERPINA3 would protect human choriocarcinoma JEG-3 cells from apoptosis [24].
In this study, we found that SERPINA3 was highly expressed in EC. In an immunohistochemical tissue microarray analysis (TMA), we observed that SERPINA3 protein expression was up-regulated in EC tissues and was closely associated with adverse clinicopathological behaviors. By in vitro cells experiments, we found that silencing of SERPINA3 significantly inhibited EC cells proliferation with cells cycle arrested in G2/M phase and led to apoptosis. Further investigations indicated that the growth-promoting and apoptosis-inhibition effects of SERPINA3 might be ascribed to the activation of MAPK/ERK1/2 and PI3K/AKT signaling pathways.
Materials and methods
Cell culture
Human EC cell lines AN3CA, KLE, HEC-1A ECC-1, and Ishikawa were purchased from Cell Bank of the Chinese Academy of Sciences. AN3CA, KLE, Ishikawa Cells were cultured in Dulbecco’s modified Eagle medium (DMEM)/F12; HEC-1A Cells were cultured in McCoy’5A; ECC-1 Cells were cultured in RPMI-1640; and all of these cells were supplemented with 10% (v/v) fetal bovine serum (FBS), 100 U/ml penicillin and 100 ug/ml streptomycin, and incubated at 37°C in a humidified incubator under 5% CO2 condition.
Clinical tissue samples
We recruited consecutive patients with endometrial carcinomas to a discovery cohort, from October 2004 to May 2013. The fresh endometrial specimens were immediately frozen at -80°C until RNA extraction. 217 human endometrial tissue samples in tissue microarrays as well as the fresh specimens were obtained from Department of Gynecology, Changzhou Maternal and Child Care Hospital and Department of Gynecology and Fengxian Hospital, Southern Medical University. The cases of endometrial carcinomas were selected in this study only if follow up was obtained and clinical data were available. All patients with endometrial carcinomas underwent a modified radical hysterectomy or complete hysterectomy, bilateral salpingo-oophorectomy and pelvic lymphadenectomy with or without para-aortic lymph node sampling. None of them had received radiotherapy, chemotherapy, hormone therapy or other related anti-tumor therapies before surgery. 30 cases of normal proliferative endometria and 30 cases of secretory endometria were selected as the control group. The diagnosis and histologic classification of the endometrial carcinomas was made using the criteria proposed by World Health Organization. The patients’ clinical characteristics are shown in Table 1. All tissue samples were obtained with informed consent and all procedures were performed in accordance with the Human Investigation Ethical Committee of the Fengxian Hospital, Southern Medical University.
Table 1.
Clinical characteristics of patients with endometrial carcinoma
| Variable | Tissue Group | ||
|---|---|---|---|
|
| |||
| Cancer (157) | Normal (60) | ||
| Age | Mean (Std) | 58.98 (10.46) | 42.33 (7.95) |
| Min, Max | 34.00, 88.00 | 20.00, 55.00 | |
| Median | 58.00 | 44.00 | |
| Stage | I | 94 (59.87%) | |
| II | 27 (17.20%) | ||
| III-IV | 36 (22.93%) | ||
| Histology Type* | G1 | 46 (29.49%) | |
| G2 | 62 (39.74%) | ||
| G3+UPSC+CC | 49 (30.77%) | ||
UPSC: Uterine serous papillary carcinoma; CC: Clear cell carcinoma.
Immunohistochemical staining
All tissue samples were fixed in phosphate-buffered neutral formalin and routinely embedded in paraffin, and then cut into 5-μm-thick sections. Tissue sections were incubated with 0.3% hydrogen peroxide/phosphate-buffered saline for 30 minutes and blocked with 10% BSA (Sangon, Shanghai, China), then were detected with primary polyclonal antibody for SERPINA3 (Abcam, Cambridge, UK), Estrogen Receptor α (ERα, Epitomics, Burlingame, US) or Progesterone Receptor (PR, Epitomics, Burlingame, US) overnight at 4°C in a moist chamber. After incubated with the second antibody (Thermo Scientific, US) labeled by HRP (rabbit) for 1 hour at room temperature, the sections were treated with diaminobenzidine and counterstained with hematoxylin. All the sections were observed and photographed with a microscope (Carl Zeiss) and scored was conducted according to the ratio and intensity of positive-staining cells as followed: strongly stained (score 1) designated as high expression and weakly stained (score 0) designated as low expression. All the SERPINA3, ERα or PR expression level was quantified two-blindly by two independent pathologists.
Quantitative real-time PCR
Total RNA was extracted from cells and tissues using Trizol reagent (Takara, Dalian, China) and reverse transcribed by PrimeScript RT reagent kit (Takara, Dalian, China) according to the manufacturer’s instruction. The quantitative real-time polymerase chain reaction (qRT-PCR) was subsequently performed with SYBR Premix Ex Taq (Takara, Dalian, China) using an ABI7300 instrument (Applied Biosystems). And the primers for SERPINA3 were as follows, forward: 5’-TGCCAGCGCACTCTTCATC-3’; reverse: 5’-TGTCGTTCAGGTTATAGTCCCTC-3’. The relative expression of SERPINA3 was analyzed by the comparative cycle threshold method (ΔΔCt method) which was normalized to 18s RNA (forward: 5’-TGCGAGTACTCAACACCAACA-3’, reverse: 5’-GCATATCTTCGGCCCACA-3’).
Western blotting
Whole cell lysates were prepared by lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton-X 100, 1 mM each MgCl2, MnCl2 and CaCl2, 1 mM PMSF and 10 mM sodium fluoride). Proteins were separated by SDS-PAGE and were transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). Then the electroblotted membranes were blocked in phosphate-buffered saline/Tween-20 containing 1% BSA. The primary antibodies for SERPINA3 (Abcam Cambridge, UK), Focal adhesion kinase (FAK, Abcam, Cambridge, UK), p-FAK397 (Abcam, Cambridge, UK), proto-oncogene tyrosine-protein kinase (Src, Cell Signaling Technology, Beverly, MA), p-Src416 (Cell Signaling Technology, Beverly, MA), Extracellular signal-regulated kinase 1/2 (ERK1/2), Phospho-ERK1/2, V-Akt Murine Thymoma Viral Oncogene Homolog 1 (AKT), Phospho-AKT (Cell Signaling Technology, Beverly, MA) EGFR, Phospho-EGFR (Epitomics, California, US) and GAPDH (Proteintech, US) were used. After incubating with the IRDye 680 anti-mouse (LI-COR, Lincoln, NE) or IRDye 800 anti-rabbit (LI-COR, Lincoln, NE) secondary antibodies for 1 hour at room temperature, the bands were detected by an Odyssey infrared imaging system (LI-COR, Lincoln, NE).
siRNA transfection
SiRNA duplexes targeted at SERPINA3 and scramble control siRNA duplex were obtained from GenePharma (Shanghai, China). Small interfering RNAs duplexes for SERPINA3 were as follows: siRNA1 sense, 5’-CCUGACAGAGAUUCUCAAATT-3’, anti-sense, 5’-UUUGAGAAUCUCUGUCAGGTT-3’; siRNA2 sense, 5’-GCAGCUGAGUAUGGGAAAUTT-3’, anti-sense, 5’-AUUUCCCAUACUCAGCUGCTT-3’; siRNA3 sense, 5’-GCCCAUGAGUUUGCAUTT-3’, anti-sense, 5’-AUGCAAACUCAUCAUGGGCTT-3’. The scramble control siRNA duplex were as sence, 5’-UUCUCCGAACGUGUCACGUTT-3’, anti-sense, 5’-ACGUGACACGUUCGGAGAATT-3’. Transfection was performed by using the Lipofectamine RNAiMAX Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instruction.
Cell viability assay
Cells were seeded into a 96-well plate at 3×103 cells per well with 100 μl culture medium supplemented with 10% FBS and cultured at 37°C. 10 μl Cell Counting Kit-8 (CCK-8, WST-8, Dojindo, Japan) was added to each well after 24 h, 48 h and 72 h, respectively. In viable cells, WST-8 was metabolized to produce a colorimetric dye that can be detected at 450 nm using a microplate reader (SpectraMax M5, Molecular device). The experiment was performed in triplicate and repeated twice.
Cell cycle assay
For cell cycle analysis, 2×105 cells were plated in a 6-well culture plate and grown for 24 h. Cells were then incubated with 1 mM thymidine (Sigma-Aldrich) for 24 h to synchronize cells at the G1/S boundary. The cells were then treated with serum-deprived culture medium for another 24 h. Next, the cells were trypsinized, washed twice with cold PBS and fixed with cold 70% ethanol at -20°C overnight. The cells were then washed twice with PBS and incubated with 10 mg/ml RNase A and 400 mg/ml propidium iodide (PI) in PBS at room temperature for 30 mins. Cells were subsequently analyzed by flow cytometry (Becton, Dickinson and Company).
Fluorescence activated cell sorter (FACS) apoptosis assay
5×105 cells per well were cultured in 12-well plates for 48 hours at 37°C in a 5% CO2 atmosphere. Adherent cells were detached with 0.25% trypsin in 1×PBS. Detached and suspended cells were harvested in culture medium supplemented with 10% FBS and centrifuged at 1000 rpm for 5 minutes. Each of the cells were washed with 1×PBS and stained with 100 μl binding buffer containing 3.5 μl Annexin V and 3.5 μl propidium iodide (PI). Cells were incubated at room temperature for 15 minutes and analyzed by flow cytometry.
Statistical analysis
Data were presented as the means ± standard error of the mean (SEM). Statistical analyses were done using SPSS 16.0 for windows (IBM). The chi-square test, or student’s t-test were used for comparison between groups. Values of P<0.05 were considered statistically significant.
Results
SERPINA3 expression is elevated in endometrial cancer tissues
By analyzing two independent datasets from the GEO database, we found that the mRNA levels of SERPINA3 were upregulated in the EC tissues compared to endometrium tissues (P=0.0386 in GSE3013 and P=0.164 in GSE17025). In order to reveal the relevance of SERPINA3 with endometrial cancer, we first detected SERPINA3 expression in 20 cases of ECs and proliferative phase of endometria by quantitative qRT-PCR. The results showed that the expression of SERPINA3 increased 5.3 times in EC samples compared with normal endometrial tissues (Figure 1A). We further detected SERPINA3 expression in a tissue microarray (TMA) containing 157 EC samples and 60 normal endometrial samples by immunohistochemical staining. The clinicopathological characteristics of EC patients are summarized in Table 1. Analysis showed that SERPINA3 was significantly upregulated in EC samples compared with normal endometrial samples (Figure 1B), with high expression in 49.04% (77/157) of EC samples and only in 20% (12/60) of normal endometrial samples (Table 2). Notably, SERPINA3 was localized predominantly in the cytoplasm of EC cells as well as in extra-cellular space (Figure 1B).
Figure 1.

SERPINA3 was significantly up-regulated in endometrial cancers. (A) SERPINA3 expression was analyzed in 20 matched pairs of EC tissues and normal endometrial tissues by quantitative RT-PCR. Relative expression was shown by means ± SD of SERPINA3/18S ratio [2-ΔCt(SERPINA3-18S)] from triplicate experiments. (B) Representative images of SERPINA3 immunohistochemical staining in a tissue microarray that contains 60 normal endometrial tissue samples (30 each in the secretory and proliferative phases) and 157 EC tissue samples, Scale bar: 50 μm.
Table 2.
Association of SERPINA3 expression level with clinical parameters
| Variable | Expression Level of SERPINA3 | Cases | Χ2-value | P-value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| High | Low | |||||
| Tissue Group | Normal | 12 | 48 | 60 | 15.137 | <.0001 |
| Cancer | 77 | 80 | 157 | |||
| Stage | I | 30 | 64 | 94 | 37.291 | <.0001 |
| II | 14 | 13 | 27 | |||
| III-IV | 33 | 3 | 36 | |||
| Histology Type | G1 | 13 | 33 | 46 | 22.434 | <.0001 |
| G2 | 27 | 35 | 62 | |||
| G3+UPSC+CC | 37 | 12 | 49 | |||
| Menopause | Y | 60 | 57 | 117 | 0.920 | 0.337 |
| N | 17 | 23 | 40 | |||
| Estrogen Receptor α (ERα) | (+) | 38 | 61 | 99 | 12.187 | <.0001 |
| (-) | 39 | 19 | 58 | |||
| Progesterone Receptor (PR) | (+) | 57 | 67 | 124 | 2.235 | 0.135 |
| (-) | 20 | 13 | 33 | |||
| Depth of Myometrial Invasion | ≤50% | 38 | 48 | 86 | 1.796 | 0.180 |
| >50% | 39 | 32 | 71 | |||
| Lymph node status | (+) | 16 | 0 | 16 | <.0001# | |
| (-) | 61 | 80 | 141 | |||
| Vascular thrombosis | (+) | 55 | 77 | 126 | 7.429 | 0.006 |
| (-) | 22 | 9 | 31 | |||
Fisher’s exact test; χ2 tests were applied for all other analysis.
SERPINA3 is associated with adverse clinical characteristics by immunohistochemical analysis in tissue microarray
In order to investigate the relevance of SERPINA3 with EC development and progression, we analyzed SERPINA3 expression status with respect to various pathological parameters of EC samples in the TMA. We found that highly positive of SERPINA3 was significantly associated with adverse clinicopathological parameters of EC, including low differentiation, high stage, and positive lymph node and vascular thrombosis status of endometrial carcinomas (Table 2). Moreover, immunohistochemical observation indicated that the high SERPINA3 expression was significantly related with negative ER expression (Table 2), a proven independent risk factor of EC, indicating a poor prognosis.
Knockdown of SERPINA3 inhibited EC cells viability in vitro
SERPINA3 expression was detected in six endometrial cancer cell lines as KLE, Ishikawa, HEC-1-A, AN3CA, RL95-2 and ECC-1 by qRT-PCR (Figure 2A). SERPINA3 highly expressed cell lines HEC-1A and KLE were transfected with small interfering RNA (siRNA) to knockdown SERPINA3 expression. The change in the SERPINA3 expression level was analyzed 48 hours after transfection. As shown in Figure 2B and 2C, both mRNA and protein levels of SERPINA3 were successfully decreased after siRNA transfection. We chose siRNA1 and siRNA2 that exhibited better interference efficacy to perform further experiments. By a CCK-8 assay, we found that cells viabilities were inhibited by SERPINA3 silencing both in HEC-1A and KLE cells (Figure 3).
Figure 2.
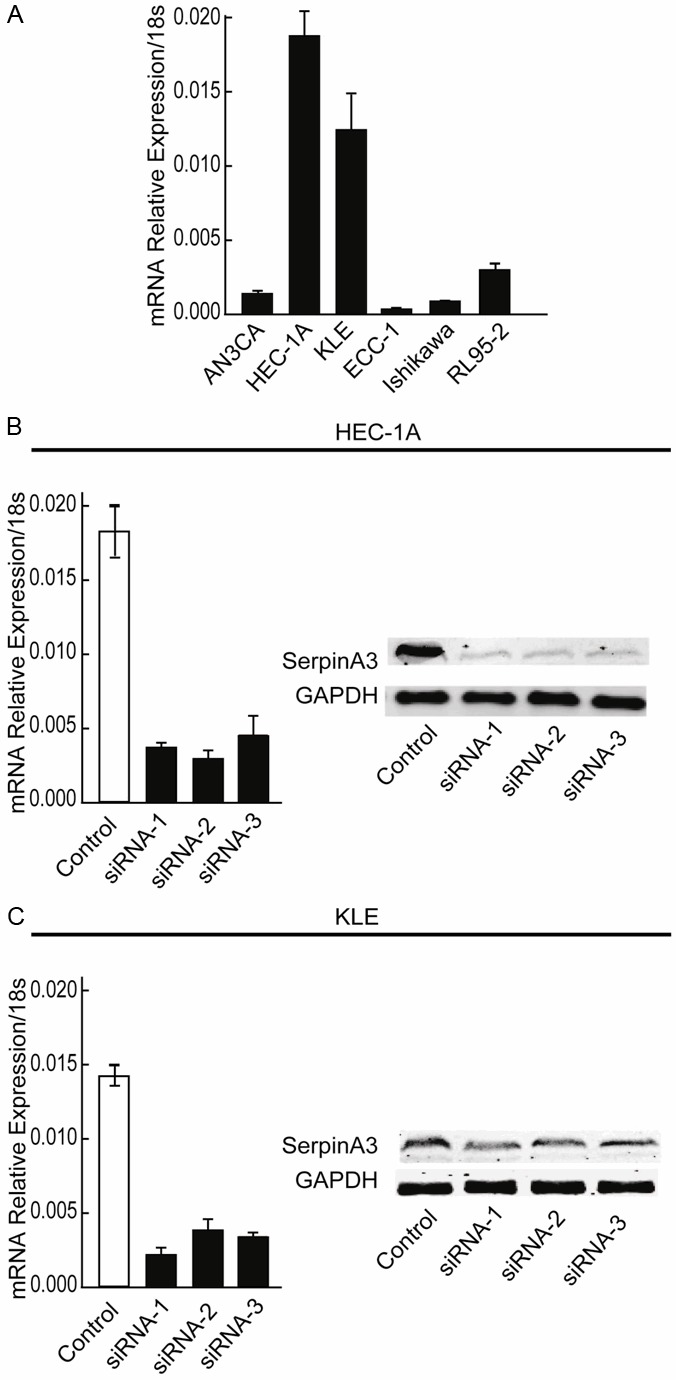
The expression level of SERPINA3 in different EC cells lines and validation of siRNA interference efficiency. (A) The expression level of SERPINA3 was detected by real-time PCR in different EC cell lines. (B and C) The expression level of SERPINA3 was detected by real-time PCR and western-bolting in SERPINA3 knockdown HEC-1A (B) and KLE (C) cells.
Figure 3.
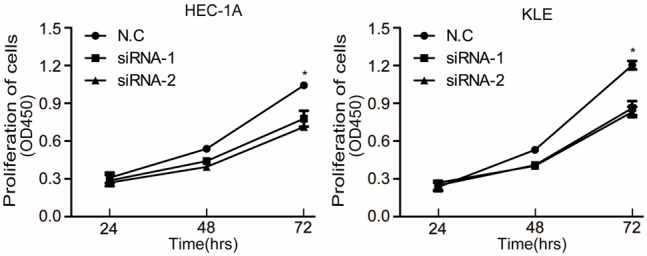
Knockdown of SERPINA3 significantly inhibited endometrial carcinoma cells proliferation. Effect of SERPINA3 knock-down on proliferation of HEC-1A and KLE cells, analyzed by the CCK-8 assay. Data are representative of three independent experiments. *, P<0.05.
Knockdown of SERPINA3 expression arrested cell cycle at G2/M phase
To further elucidate the effect of SERPINA3 on EC cells viability, we analyzed the cell cycle distributions of SERPINA3 knockdown cells by flow cytometry analysis (Figure 4). Following treatment with 1 mM thymidine for 24 h to synchronize cells at the G1/S border, the cells were cultured in serum-free medium for 24 h. As shown in Figure 4, HEC-1A and KLE cells infected with SERPINA3 siRNA contained more cells ratio at the G2 or M (G2/M) phase compared with control cells (P<0.05). These data indicated that knockdown of SERPINA3 expression inhibited the proliferation of EC cells by blocking cell cycle progression at the G2/M phase.
Figure 4.
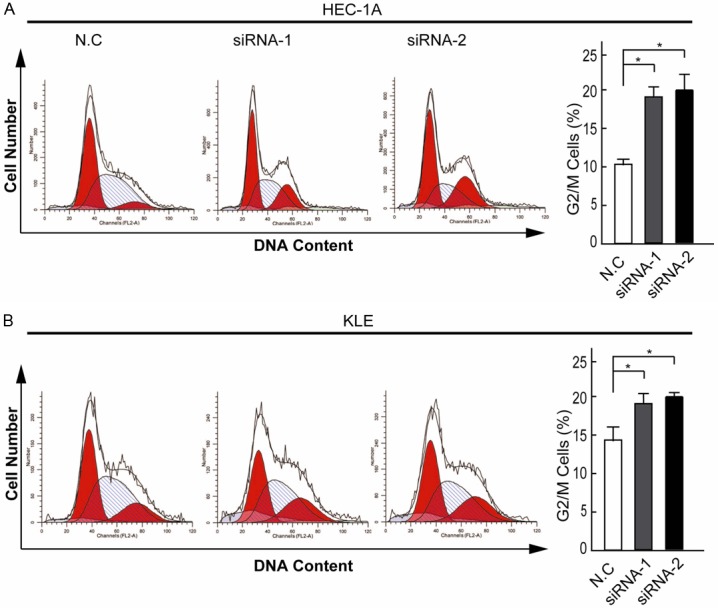
Knockdown of SERPINA3 arrested endometrial carcinoma cells at G2/M. The cell cycle distribution of HEC-1A (A) and KLE (B) cells detected by flow cytometric analysis. A statistical graph of the cell cycle distribution was shown left. The columns indicate mean of cell percentage from triplicate samples. Data are representative of three independent experiments. *, P<0.05.
Knockdown of SERPINA3 induced endometrial cancer cells apoptosis
Previous studies have found that SERPINA3 overexpression protect human choriocarcinoma JEG-3 cells from apoptosis [24]. So we further used the Annexin V/PI double staining method and flow cytometry analysis to assess whether interference of SERPINA3 expression induces apoptosis. Our results revealed that SERPINA3 knockdown significantly increased the percentage of apoptotic cells from (4.28±0.47)% in control groups to (12.45 ±1.35)% in siRNA-1 groups and (13.81± 2.42)% in siRNA-2 groups of KLE cells cultured in serum-free medium for 48 h. And the apoptotic rates of HEC-1A cells were similarly increased by SERPINA3 knockdown (Figure 5). Taken together, these results above indicated that SERPINA3 promoted EC cell growth both by regulating G2/M cell cycle checkpoint and apoptosis.
Figure 5.
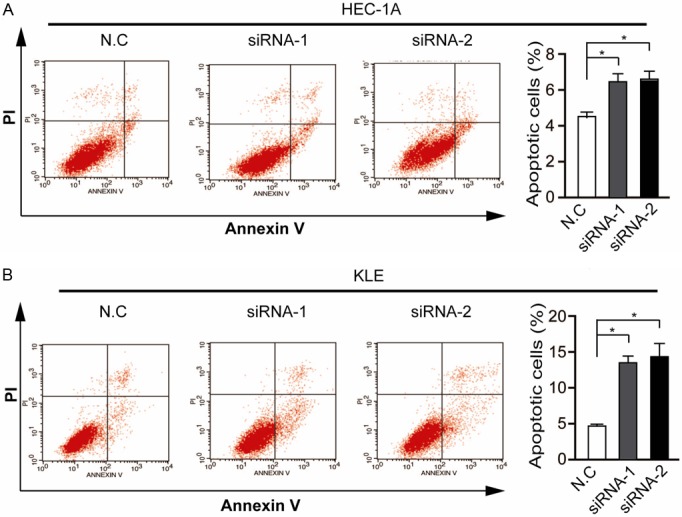
Silencing of SERPINA3 promotes endometrial carcinoma cells apoptosis induced by serum deprivation. Apoptosis of SERPINA3 knockdown HEC-1A (A) and KLE (B) cells and control cells were analyzed by Annexin V and PI staining and flow cytometric analysis. Statistic data shown right are means ± SD of apoptotic cell rates from triplicate samples. Data are representative of three independent experiments. *, P<0.05.
AKT and ERK1/2 were inactivated by SERPINA3 knockdown in EC cells
To investigate the intracellular signaling pathways that mediated SERPINA3 function on EC cells, we examined the activation of several signaling pathways known to play important roles in regulating cell cycle and apoptosis after SERPINA3 knockdown, including AKT, ERK1/2, EGFR, SRC, and FAK [25,26]. As shown in Figure 6, SERPINA3 knockdown significantly reduced phosphorylation-dependent activation of signaling components such as AKT, ERK1/2, whereas the phosphorylation levels of EGFR, and SRC, FAK were not changed. Our results indicated that SERPINA3 probably promote EC cell viability through activating AKT and ERK1/2 pathways.
Figure 6.
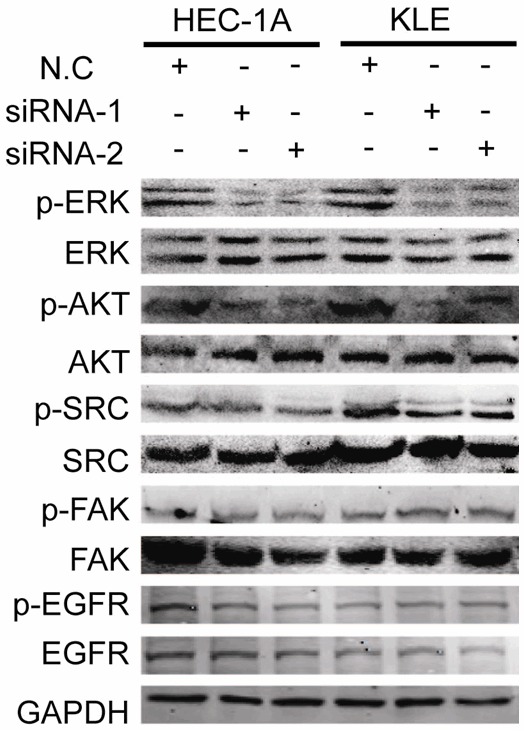
Knockdown of SERPINA3 inhibits activation of ERK1/2 and AKT. Analysis of activation of ERK1/2, AKT, Src, EGFR and FAK in SERPINA3 knockdown HEC-1A and KLE cells, using GAPDH as a loading control.
Discussion
Endometrial cancer is the most common gynecological malignancy in the western world [27] and the sixth most common cancer worldwide among females [3]. An increased incidence and a younger age of patients are also predicted to occur, therefore elucidation of the pathological mechanisms is very important. The identification of proteins differentially expressed in endometrial carcinoma versus normal endometrium seems crucial for understanding of the progression of this pathology.
Matricellular proteins are often dysregulated in various tumors including ECs, where they actively contribute to tumor progression and metastasis [28]. For example, The ubiquitous expression of placenta-specific protein 1 (PLAC1) in endometrial tumors is significantly related to the higher stage, more aggressive carcinoma [29]. The Epidermal growth factor–containing fibulin-like extracellular matrix protein 1 (EFEMP1) is silenced by promoter hypermethylation, which could inhibit EC growth and invasion both in vitro and in vivo [30].
In previous studies, matricellular protein SERPINA3 was identified as one of acute phase response genes, which were upregulated during inflammatory processes [31]. SERPINA3 was also found to be upregulated in different types of carcinoma and correlated with poor prognosis [15-18]. SERPINA3 was upregulated in recurrent ovarian cancer and possibly related with ovarian cancer progression and chemoresistance [23]. In the cervical carcinoma, SERPINA3 expression correlated significantly with tumor size and HPV status and with poor overall survival of HLA-positive cervical carcinoma [31]. However, comprehensive investigations on detailed function and mechanism of SERPINA3 on tumorigenesis are lacking.
In our study, we found that SERPINA3 was highly expressed in ECs and closely associated with adverse clinicopathological parameters including low differentiation, high stage, and positive lymph node and vascular thrombosis status of endometrial carcinomas, suggesting that SERPINA3 might be a potential indicator of poor prognosis. Silence of SERPINA3 expression significantly inhibited EC cell growth. Arguably the most fundamental trait of cancer cells involves their ability to sustain chronic proliferation, which is resulted from the cell cycle deregulation [32]. And targeting to mediate critical cell cycle processes and inhibit tumor cells proliferation is an emerging strategy for cancer treatment [33]. To further elucidate the effect of SERPINA3 on EC cells growth we determined the cell cycle distributions of two EC cell lines (HEC-1A and KLE), and found that SERPINA3 silenced cells were arrested in G2/M phase. The G2/M checkpoint allows the cell to repair DNA damage before entering mitosis [34] and is the most conspicuous target for many anticancer drugs [35,36], which would cause cell death through the induction of apoptosis. Our data implies that high SERPINA3 expression can facilitate EC cells proliferate continuously and evade from apoptosis which may be associated with chemotherapy resistance. However, more in-depth study will be required to reveal the clinical value of SERPINA3 to predict EC patient survival and the effects of SERPINA3 on in vivo progression of EC deserve further study.
The MAPK/ERK1/2 and PI3K/AKT signaling pathways are known to play crucial roles in the promotion of cell survival and the inhibition of apoptosis [25,26,37]. The cross-talk between the MAPK/ERK1/2 and the PI3K/AKT pathways has also been demonstrated in cancer cells [38,39]. Our investigations revealed that AKT and ERK1/2 activation were inhibited by SERPINA3 knockdown in EC cells. However, whether the functions of SERPINA3 on EC cell proliferation and apoptosis are mediated by MAPK/ERK1/2 or PI3K/AKT pathways remains unconfirmed and need future verification.
In conclusion, this study provides a clear expression pattern of one matricellular protein SERPINA3 in EC clinical samples and its close correlation with EC malignancy. We also demonstrate SERPINA3a promotes EC cell growth by facilitating cell cycle and preventing apoptosis, which is probably mediated by AKT and ERK1/2 pathway. These findings above suggest that SERPINA3 plays an important role in endometrial carcinoma growth and metastasis, indicating a potential therapeutic strategy for personalized medicine by targeting SEEERPINA3 straightly towards cancer cells in type II EC patients.
Acknowledgements
We appreciate Drs Yun Li, Ya-Hui Wang and Yan-Li Zhang for assistance in experiments and comments on the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, Sessa C ESMO Guidelines Working Group. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi33–38. doi: 10.1093/annonc/mdt353. [DOI] [PubMed] [Google Scholar]
- 2.Colas E, Pedrola N, Devis L, Ertekin T, Campoy I, Martinez E, Llaurado M, Rigau M, Olivan M, Garcia M, Cabrera S, Gil-Moreno A, Xercavins J, Castellvi J, Garcia A, Ramon y Cajal S, Moreno-Bueno G, Dolcet X, Alameda F, Palacios J, Prat J, Doll A, Matias-Guiu X, Abal M, Reventos J. The EMT signaling pathways in endometrial carcinoma. Clin Transl Oncol. 2012;14:715–720. doi: 10.1007/s12094-012-0866-3. [DOI] [PubMed] [Google Scholar]
- 3.Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, Sessa C ESMO Guidelines Working Group. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi35–39. doi: 10.1093/annonc/mdr374. [DOI] [PubMed] [Google Scholar]
- 4.Delmonte A, Sessa C. Molecule-targeted agents in endometrial cancer. Curr Opin Oncol. 2008;20:554–559. doi: 10.1097/CCO.0b013e32830b0deb. [DOI] [PubMed] [Google Scholar]
- 5.Fujimoto T, Nanjyo H, Fukuda J, Nakamura A, Mizunuma H, Yaegashi N, Sugiyama T, Kurachi H, Sato A, Tanaka T. Endometrioid uterine cancer: histopathological risk factors of local and distant recurrence. Gynecol Oncol. 2009;112:342–347. doi: 10.1016/j.ygyno.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Lax SF, Pizer ES, Ronnett BM, Kurman RJ. Comparison of estrogen and progesterone receptor, Ki-67, and p53 immunoreactivity in uterine endometrioid carcinoma and endometrioid carcinoma with squamous, mucinous, secretory, and ciliated cell differentiation. Hum Pathol. 1998;29:924–931. doi: 10.1016/s0046-8177(98)90197-6. [DOI] [PubMed] [Google Scholar]
- 7.Voss MA, Ganesan R, Ludeman L, McCarthy K, Gornall R, Schaller G, Wei W, Sundar S. Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer-a clinical and pathological evaluation. Gynecol Oncol. 2012;124:15–20. doi: 10.1016/j.ygyno.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 8.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 9.Thigpen JT, Brady MF, Homesley HD, Malfetano J, DuBeshter B, Burger RA, Liao S. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a gynecologic oncology group study. J. Clin. Oncol. 2004;22:3902–3908. doi: 10.1200/JCO.2004.02.088. [DOI] [PubMed] [Google Scholar]
- 10.Thigpen T, Brady MF, Homesley HD, Soper JT, Bell J. Tamoxifen in the treatment of advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. J. Clin. Oncol. 2001;19:364–367. doi: 10.1200/JCO.2001.19.2.364. [DOI] [PubMed] [Google Scholar]
- 11.Felix AS, Weissfeld J, Edwards R, Linkov F. Future directions in the field of endometrial cancer research: the need to investigate the tumor microenvironment. Eur J Gynaecol Oncol. 2010;31:139–144. [PMC free article] [PubMed] [Google Scholar]
- 12.Abal M, Llaurado M, Doll A, Monge M, Colas E, Gonzalez M, Rigau M, Alazzouzi H, Demajo S, Castellvi J, Garcia A, Ramon y Cajal S, Xercavins J, Vazquez-Levin MH, Alameda F, Gil-Moreno A, Reventos J. Molecular determinants of invasion in endometrial cancer. Clin Transl Oncol. 2007;9:272–277. doi: 10.1007/s12094-007-0054-z. [DOI] [PubMed] [Google Scholar]
- 13.Baker C, Belbin O, Kalsheker N, Morgan K. SERPINA3 (aka alpha-1-antichymotrypsin) Front Biosci. 2007;12:2821–2835. doi: 10.2741/2275. [DOI] [PubMed] [Google Scholar]
- 14.Laursen I, Lykkesfeldt AE. Purification and characterization of an alpha 1-antichymotrypsin-like 66 kDa protein from the human breast cancer cell line, MCF-7. Biochim Biophys Acta. 1992;1121:119–129. doi: 10.1016/0167-4838(92)90345-e. [DOI] [PubMed] [Google Scholar]
- 15.Ordonez NG, Manning JT Jr. Comparison of alpha-1-antitrypsin and alpha-1-antichymotrypsin in hepatocellular carcinoma: an immunoperoxidase study. Am J Gastroenterol. 1984;79:959–963. [PubMed] [Google Scholar]
- 16.Bjork T, Bjartell A, Abrahamsson PA, Hulkko S, di Sant’Agnese A, Lilja H. Alpha 1-antichymotrypsin production in PSA-producing cells is common in prostate cancer but rare in benign prostatic hyperplasia. Urology. 1994;43:427–434. doi: 10.1016/0090-4295(94)90225-9. [DOI] [PubMed] [Google Scholar]
- 17.Higashiyama M, Doi O, Yokouchi H, Kodama K, Nakamori S, Tateishi R. Alpha-1-antichymotrypsin expression in lung adenocarcinoma and its possible association with tumor progression. Cancer. 1995;76:1368–1376. doi: 10.1002/1097-0142(19951015)76:8<1368::aid-cncr2820760812>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Katsunuma T, Tsuda M, Kusumi T, Ohkubo T, Mitomi T, Nakasaki H, Tajima T, Yokoyama S, Kamiguchi H, Kobayashi K, Shinoda H. Purification of a serum DNA binding protein (64DP) with a molecular weight of 64,000 and its diagnostic significance in malignant diseases. Biochem Biophys Res Commun. 1980;93:552–557. doi: 10.1016/0006-291x(80)91112-2. [DOI] [PubMed] [Google Scholar]
- 19.Takada S, Tsuda M, Fujinami S, Yamamura M, Mitomi T, Katsunuma T. Incorporation of alpha-1-antichymotrypsin into carcinoma cell nuclei of human stomach adenocarcinoma transplanted into nude mice. Cancer Res. 1986;46:3688–3691. [PubMed] [Google Scholar]
- 20.Tahara E, Ito H, Taniyama K, Yokozaki H, Hata J. Alpha 1-antitrypsin, alpha 1-antichymotrypsin, and alpha 2-macroglobulin in human gastric carcinomas: a retrospective immunohistochemical study. Hum Pathol. 1984;15:957–964. doi: 10.1016/s0046-8177(84)80125-2. [DOI] [PubMed] [Google Scholar]
- 21.Rubin H, Wang ZM, Nickbarg EB, McLarney S, Naidoo N, Schoenberger OL, Johnson JL, Cooperman BS. Cloning, expression, purification, and biological activity of recombinant native and variant human alpha 1-antichymotrypsins. J Biol Chem. 1990;265:1199–1207. [PubMed] [Google Scholar]
- 22.Schechter NM, Jordan LM, James AM, Cooperman BS, Wang ZM, Rubin H. Reaction of human chymase with reactive site variants of alpha 1-antichymotrypsin. Modulation of inhibitor versus substrate properties. J Biol Chem. 1993;268:23626–23633. [PubMed] [Google Scholar]
- 23.Jinawath N, Vasoontara C, Jinawath A, Fang X, Zhao K, Yap KL, Guo T, Lee CS, Wang W, Balgley BM, Davidson B, Wang TL, Shih Ie M. Oncoproteomic analysis reveals co-upregulation of RELA and STAT5 in carboplatin resistant ovarian carcinoma. PLoS One. 2010;5:e11198. doi: 10.1371/journal.pone.0011198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chelbi ST, Wilson ML, Veillard AC, Ingles SA, Zhang J, Mondon F, Gascoin-Lachambre G, Doridot L, Mignot TM, Rebourcet R, Carbonne B, Concordet JP, Barbaux S, Vaiman D. Genetic and epigenetic mechanisms collaborate to control SERPINA3 expression and its association with placental diseases. Hum Mol Genet. 2012;21:1968–1978. doi: 10.1093/hmg/dds006. [DOI] [PubMed] [Google Scholar]
- 25.Arafa el SA, Zhu Q, Barakat BM, Wani G, Zhao Q, El-Mahdy MA, Wani AA. Tangeretin sensitizes cisplatin-resistant human ovarian cancer cells through downregulation of phosphoinositide 3-kinase/Akt signaling pathway. Cancer Res. 2009;69:8910–8917. doi: 10.1158/0008-5472.CAN-09-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brzezianska E, Pastuszak-Lewandoska D. A minireview: the role of MAPK/ERK and PI3K/Akt pathways in thyroid follicular cell-derived neoplasm. Front Biosci (Landmark Ed) 2011;16:422–439. doi: 10.2741/3696. [DOI] [PubMed] [Google Scholar]
- 27.Llaurado M, Ruiz A, Majem B, Ertekin T, Colas E, Pedrola N, Devis L, Rigau M, Sequeiros T, Montes M, Garcia M, Cabrera S, Gil-Moreno A, Xercavins J, Castellvi J, Garcia A, Ramon y Cajal S, Moreno G, Alameda F, Vazquez-Levin M, Palacios J, Prat J, Doll A, Matias-Guiu X, Abal M, Reventos J. Molecular bases of endometrial cancer: new roles for new actors in the diagnosis and the therapy of the disease. Mol Cell Endocrinol. 2012;358:244–255. doi: 10.1016/j.mce.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Chiodoni C, Colombo MP, Sangaletti S. Matricellular proteins: from homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2010;29:295–307. doi: 10.1007/s10555-010-9221-8. [DOI] [PubMed] [Google Scholar]
- 29.Devor EJ, Leslie KK. The oncoplacental gene placenta-specific protein 1 is highly expressed in endometrial tumors and cell lines. Obstet Gynecol Int. 2013;2013:807849. doi: 10.1155/2013/807849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang T, Qiu H, Bao W, Li B, Lu C, Du G, Luo X, Wang L, Wan X. Epigenetic Inactivation of EFEMP1 Is Associated with Tumor Suppressive Function in Endometrial Carcinoma. PLoS One. 2013;8:e67458. doi: 10.1371/journal.pone.0067458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kloth JN, Gorter A, Fleuren GJ, Oosting J, Uljee S, ter Haar N, Dreef EJ, Kenter GG, Jordanova ES. Elevated expression of SerpinA1 and SerpinA3 in HLA-positive cervical carcinoma. J Pathol. 2008;215:222–230. doi: 10.1002/path.2347. [DOI] [PubMed] [Google Scholar]
- 32.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Zhivotovsky B, Orrenius S. Cell cycle and cell death in disease: past, present and future. J Intern Med. 2010;268:395–409. doi: 10.1111/j.1365-2796.2010.02282.x. [DOI] [PubMed] [Google Scholar]
- 34.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 35.Huang WW, Ko SW, Tsai HY, Chung JG, Chiang JH, Chen KT, Chen YC, Chen HY, Chen YF, Yang JS. Cantharidin induces G2/M phase arrest and apoptosis in human colorectal cancer colo 205 cells through inhibition of CDK1 activity and caspase-dependent signaling pathways. Int J Oncol. 2011;38:1067–1073. doi: 10.3892/ijo.2011.922. [DOI] [PubMed] [Google Scholar]
- 36.Visanji JM, Thompson DG, Padfield PJ. Induction of G2/M phase cell cycle arrest by carnosol and carnosic acid is associated with alteration of cyclin A and cyclin B1 levels. Cancer Lett. 2006;237:130–136. doi: 10.1016/j.canlet.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 37.Roskoski R Jr. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012;66:105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Byun HJ, Hong IK, Kim E, Jin YJ, Jeoung DI, Hahn JH, Kim YM, Park SH, Lee H. A splice variant of CD99 increases motility and MMP-9 expression of human breast cancer cells through the AKT-, ERK-, and JNK-dependent AP-1 activation signaling pathways. J Biol Chem. 2006;281:34833–34847. doi: 10.1074/jbc.M605483200. [DOI] [PubMed] [Google Scholar]
- 39.Dai R, Chen R, Li H. Cross-talk between PI3K/Akt and MEK/ERK pathways mediates endoplasmic reticulum stress-induced cell cycle progression and cell death in human hepatocellular carcinoma cells. Int J Oncol. 2009;34:1749–1757. doi: 10.3892/ijo_00000306. [DOI] [PubMed] [Google Scholar]


