Abstract
In recent years, the study of microRNAs associated with neoplastic processes has increased. Patterns of microRNA expression in different cell lines and different kinds of tumors have been identified; however, little is known about the alterations in regulatory pathways and genes involved in aberrant set of microRNAs. The identification of these altered microRNAs in several cervical cancer cells and potentially deregulated pathways involved constitute the principal goals of the present study. In the present work, the expression profiles of cellular microRNAs in Cervical Cancer tissues and cell lines were explored using microRNA microarray, Affymetrix. The most over-expressed was miR-196a, which was evaluated by real time PCR, and HOXC8 protein as potential target by immunohistochemistry assay. One hundred and twenty three human microRNAs differentially expressed in the cell tumor, 64 (52%) over-expressed and 59 (48%) under-expressed were observed. Among the microRNAs over-expressed, we focused on miR-196a; at present this microRNA is poorly studied in CC. The expression of this microRNA was evaluated by qRT-PCR, and HOXC8 by immunohistochemistry assay. There is not a specific microRNA expression profile in the CC cells, neither a microRNA related to HPV presence. Furthermore, the miR-196a was over-expressed, while an absence of HOXC8 expression was observed. We suggest that miR-196a could be played as oncomiR in CC.
Keywords: Cervical cancer, microRNAs, miR-196a, HOXC8
Introduction
microRNAs are small non-coding RNA of 18-25 nucleotides (nt) found in the genome and are transcribed and processed by microRNA biogenesis machinery to generate the mature microRNA which is carried to its target (mRNA) [1-4]. These tiny regulators of gene expression have the ability to bind totally or partially to 3’ UTR regions of mRNA [5]. In mammals, there are partial complementary between microRNA and mRNA, consequently the translation of mRNA target is repressed [1,4-6].
At present, microRNA expression patterns have been determined in different tumors, leading to elucidation of its role in several cellular processes in cancer research [7-10]. In addition, new approaches for molecular signature by high throughput have given coverage to the management of oncology patients in terms of diagnosis, identification of cancer of unknown primary origin, prognosis, and therapy.
Cervical cancer (CC) is one of most common cancer in women worldwide, including Mexico, with 529,409 new cases and 274,883 deaths per year, representing a major public health problem [11]. The most important factor associated to CC development is the persistent Human Papillomavirus Virus (HPV) infection. The oncogenic role of HPV oncogenes that interact with pRb and p53 tumor suppressor proteins is widely known; however, HPV is not sufficient for CC development [12].
Currently, reports on microRNAs in CC cells have shown substantial differences with contradictory results [13-19]. For instance, under-expressed of miR-145 has been reported in many studies of cancer including CC [13,16-18], different from an increased in miR-145 expression reported in CC by another author [14]. These differences could arise by microRNAs variability expression, technological platforms used, the number of microRNAs screened, and characteristics of sample study. In addition, the knowledge of altered regulatory pathway in CC cells has been poorly studied. In the present work, the expression profile of cellular microRNAs in CC cells was explored by means of Affymetrix GeneChip miRNA Array. The most significantly over-expressed was the miR-196a, which was evaluated by real time PCR, and HOXC8 protein was evaluated by immunohistochemistry assay.
Material and methods
Cervical cell culture
The human cervical cancer cell lines included were as follows: CaSki and SiHa (HPV16); HeLa, CaLo, INBL, Rova, ViPa, C4-I, and MS-751 (HPV18); C33A, ViBo and keratinocyte cell line HaCaT (HPV-negative). These cell lines were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Life Technologies, Carlsbad, CA USA) supplemented with 10% Fetal bovine serum (FBS) (Gibco) and 1% Penicillin-Streptomycin (Gibco) at 37°C and 5% CO2. All cells were cultured at 70-80% of confluence. In addition, cells were cultured in slide chamber with 50-60% of confluence for immunocytochemistry.
Tissues samples
All tissues used in this study were obtained from Gynecology and Oncology Department of the General Hospital of Mexico, SS. Each tissue specimen obtained Ethical Committee approval and informed consent was obtained from every patient. We used healthy cervical tissue obtained from patients subjected to hysterectomy by uterine myomatosis. Inclusion criteria were as follows: no previous cervical surgery (such as the loop electrosurgical excision procedure or cone biopsy); no HPV infection; no hormonal treatment, and last three negative Pap smears. In addition, 10 Low-grade Squamous Intraepithelial Lesions (LSIL), 10 High-grade Squamous Intraepithelial Lesions (HSIL) and 25 cervical carcinomas were collected and classified according FIGO nomenclature. The biopsies obtained were divided into two fragments immediately after surgery. One fragment was stored in RNAlater (Qiagen, Valencia, CA, USA) at -70°C until nucleic acids isolation. The remaining fragment was fixed in 4% formaldehyde for two days and then was paraffin-embedded. The paraffin blocks were sliced and stained with Hematoxylin-Eosin (H&E) for histological examination and construction of the tissue array.
HPV typing and sequencing
Genomic DNA was isolated from cell line and tissue samples using the Promega Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA), according to the manufacturer’s instructions. DNA quantity and purity was measured with a Nanodrop ND-1000 Spectophotometer. DNA integrity was checked by 1% agarose gel electrophoresis. Each DNA sample was subjected to HPV typing by PCR using GP5+/GP6+ primers [20]. 100 ng of DNA was subjected to 40 amplification cycles with the following steps: 5 min at 94°C for pre-denaturation, 1 min at 94°C, 1 min at 44°C, 1 min at 72°C, and 5 min at 72°C as final extension step. The PCR products were purified in Wizard SV gel and the PCR Clean-up System Kit (Promega). Sequencing was performed by Big Dye Terminator Kit v3.1 (Applied Biosystems, Life Technologies, Carlsbad, CA, USA) and analysed in a 3100 ABI PRISM Genetic Analyzer (Applied Biosystems). The sequences were aligned and compared with National Center for Biotechnology Information databases.
RNA isolation and quality control
The tissue samples were disrupted with TissueLyser (Qiagen) from 1 to 3 min. Total RNA was obtained with the miRVana Isolation Kit (Ambion, Life Technologies, CA, USA) according to the manufacturer’s instructions. RNA quantity and purity was measured using Nanodrop ND-1000 Spectrophotometer and RNA quality was analysed with Agilent RNA 6000 Nano in Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). In this study, we used only total RNA with RNA Integrated Number (RIN) >8.
Affymetrix miRNA microarray assay
We used the GeneChip miRNA Array (Affymetrix, Santa Clara, CA, USA). This chip contains miRNAs and pre-miRNAs of the Sanger miRNA database v11 of 71 organisms, such as: human, mouse, rat, dog, and monkey, among others. Each array contains 45,930 probes comprising of 7,788 miRNAs probe sets as quadruplicates and remaining probes as internal controls. The assay was started with 1 μg of total RNA. Total RNA samples were first extended with the Poly (A)-Tailing Reaction followed by the Biotin Labelling Reaction employing FlashTag Biotin HSR RNA Labelling Kit (Genisphere, Hatfield, PA, USA). The labelling process was checked with Enzyme Linked Oligosorbent Assay QC (ELOSA). Biotin-labelled total RNA samples were hybridized to the arrays, washed, and stained according to the manufacturer’s instructions. Microarrays were scanned using GeneChip Scanner 3000 7G (Affymetrix). Median fluorescent intensity was obtained with Affymetrix GeneChip Command Console software through Command Console Library File miRNA-1_0_2Xgain.
miRNA microarray analysis and target predictor analysis of miRNA
miRNA microarray data analysis was performed in three stages. First, the data were analysed for quality control purposes using free miRNA QC Tool software (Affymetrix). In this analysis, the background correction was adjusted by means of Robust Multi-chip Average (RMA). Subsequently, the data were log2-transformed, summarized, and normalized with Median-polish and Quantile, respectively. In the second stage, microarray analysis was achieved by means of CEL files of Partek Genomics Suite v6.6 software. Probe set were summarized by means of Median Polish, Quantile normalization. Background noises correction was performed by means of RMA, finally the data were transformed by Log2. The next step was inspection of miRNA expression by means of Principal Component Analysis (PCA). The final stage consisted of identifying differential microRNA expression in the samples by mean of Analysis of Variance (ANOVA) with the healthy cervical tissues as baseline. Moreover, CC cell lines, and CC tumors were compared also against healthy cervical tissue by geometric least squares model. The significant microRNA expressed were selected by means of cut-off based on fold change >2, <-2, and a False Discovery Ration (FDR) <0.05 (Table 1). Finally, the hierarchical cluster of significant expressed microRNAs was obtained by means of Euclidian method (dissimilarity of samples).
Table 1.
Differential microRNAs among CC tissues and CC cell lines
| Comparison | Number of Probe set | Number of hsa-microRNAs | Number of microRNAsOver-expressed | Number of microRNAs Under-expressed |
|---|---|---|---|---|
| Tumor vs. Healthy | 512 | 60 | 32 | 28 |
| Cell Lines vs. Healthy | 1247 | 174 | 98 | 76 |
| Tumor and Cell Lines vs. Healthy | 942 | 123 | 64 | 59 |
For target predictor analysis of miR-196a we used different database as Diana-microT v5.0, TarBase v6.0, MirTarget2 v2, TargetScan v6.2, microRNA.org, PicTar and miRecords for predicted miRNA-196a potential target. In addition, we used GeneGo MetaCore (Thompson Reuters) to build the microRNA network to known predicted targets and regulator genes of miR-196a. We employed 50 interactions to generate a network; thus we were able to identify genes upstream and downstream of microRNA to know the features and network of specific microRNA and target microRNA. For a more detailed GeneGo MetaCore analysis, go to http://www.genego.com/.
miRNA quantitive RT-PCR
mRNA was subject to quantitative PCR using the TaqMan miRNA Reverse Transcription Kit and miRNA Assay (Applied Biosystems) according to the manufacturer’s instructions. TaqMan microRNA assay hsa-miR-196 and small nucleoli RNU6B was used as small housekeeping RNA. These housekeeping genes not showed variations. In brief, the 7.5-mL master mix contained 10 ng of total RNA, 3.0 mL 5X RT primer, 1.5 mL 10X Reverse transcription buffer, 0.15 mL of 100 mM dNTP 1.0 mL, MultiScribe Reverse Transcriptase 50 U/mL, 0.19 mL RNAase Inhibitor 20 U/mL, and free RNase water. The mix reaction was incubated for 30 min at 16°C, 30 min at 42°C, and for 5 min at 85°C. A 10-mL mix reaction of real-time PCR contained 1.33 mL RT product, 0.5 mL TaqMan Small RNA Assay (20X), 5.0 mL TaqMan Universal Master Mix II with UNG and 3.17 mL free RNase water. The mix reaction was incubated for 2 min at 50°C, 10 min at 95°C, and 40 amplification cycles for 15 sec at 95°C and for 60 sec at 60°C. Relative quantification was calculated by the 2 e(-DDCt) method, where DCt = Ct(miR-196a) - Ct(RNU6B) and DDCt = DCt(Cell line) - DCt(Average Normal) [21].
Immunodetection of HOXC8 in cervical cancer cells
Detection of HOXC8 was performed in CC tissues and in cervical cell lines. For detection of cervical cancer tissues, a tissue microarray was constructed with 24 CC cases and normal cervical tissues by using a tissue microarrayer (Chemicon Co., Billerica, MA, USA). The immunostaining steps comprised antigen exposure with Trilogy solution, which was incubated for 45 min at 96°C. After blocking of endogenous peroxidase with 3% H2O2, the slide was incubated with Bovine Serum Albumin (BSA) for 30 min at room temperature. Next, the slides were incubated with polyclonal anti-HOXC8 antibody (ab86236; Abcam, Cambridge, MA, USA) overnight at 4°C (1:200 dilution in 1% BSA). Later, the slides were treated with Streptavidin-Biotin Complex Peroxidase Mouse/Rabbit Method (Dako, Glostup, Denmark) for 1 h at room temperature. Signal development was obtained with 0.05% 3,3-diaminobenzidine tetrahydrochloride, 0.01% H2O2, and counterstained with haematoxylin. HOXC8 expression was evaluated with 10X and 20X microscope as negative and positive staining. Healthy kidney or healthy bladder tissue were used as positive and negative controls, respectively.
Results
Differential expression of cellular microRNAs in cervical cancer cell lines
microRNA expression was determined from 7,788 microRNAs in four healthy cervical tissues, four cervical cancer tissues and twelve cervical cancer cell lines using GeneChip miRNA microarray, Affymetrix. Selection of tissue for microarray study was performed using a systematic workflow as described in the methods. The overall average number of probe sets detected in the samples was 31.3% (2,439 of 7,788 for each chip) for healthy cervical tissue, 34% (2,720 of 7,788) for cervical tumor and 28.4% (2,213 of 7,788) for the 12 cell lines. Pearson correlation of 0.90-0.98 for quality controls by QC Tool was obtained. Therefore, the microarray data of healthy, tumor and cell lines were comparable for expression analysis.
The data clearly showed overall clustering of microRNA expression among the three studies group, indicating differences in microRNA expression by tumor tissues and CC cell lines compared with healthy cervical tissues (Figure 1A). Identification of altered microRNA in CC was carried out using one-way ANOVA, comparing tumor, CC cell lines and healthy tissues. The highest number of differentially expressed probe sets obtained was from CC cell lines with 1,247, followed by tumor tissues & CC cell lines with 942, and tumor with 512 (Table 1). For instance, 942 differential expression sequences were obtained out of which 518 were over-expressed and 424 under-expressed. The heat map displayed microRNA expression in the samples, where the tumor tissues showed a transition of microRNAs expression between CC cell lines and healthy tissue (Figure 1B). From initial list (815 microRNA sequences of 942 in total), only 123 sequences correspond to human sequences, representing 14.5% of altered expression of human microRNAs in CC cell lines. Most of these microRNA sequences are commonly found in different mammalian species such as rat, mouse, chimpanzee, gorilla, and dog, suggesting that microRNA group is highly conserved among species. Table 2 depicts some microRNAs that were identified in more than five species, including human, in addition to their expression levels and other reports on CC.
Figure 1.
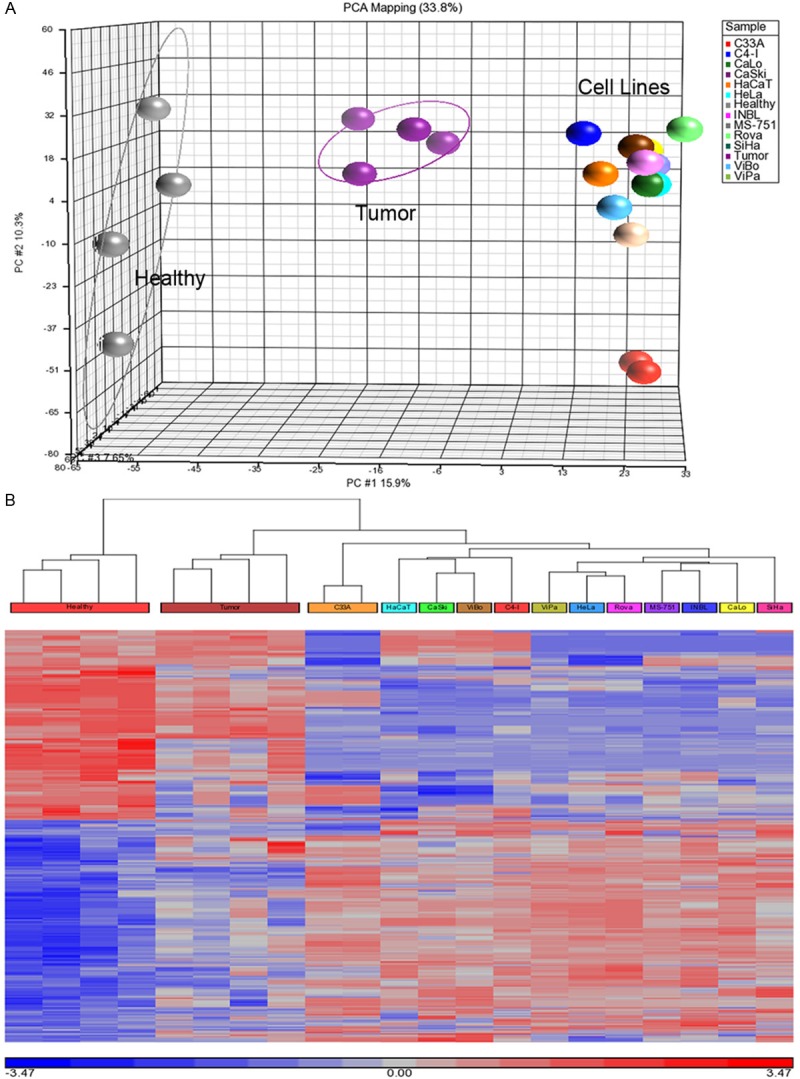
Differential microRNA expressed in cervical cancer cells. A. Principal Component Analysis (PCA) of microRNAs (gray balls: healthy cervical tissues; purple balls: tumor cervical tissues; the remaining balls represent each cervical cancer cell line). MicroRNAs expression patterns suggest a differential microRNAs expression in tumor cervical tissues and cancer cell lines compared with healthy cervical tissues; B. microRNAs expression heat map depicting microRNAs differentially in cervical cancer cells with fold change >2 and <-2, FDR 0.05. Red color represents over-expression and blue color under-expression.
Table 2.
miRNAs reported as over-expressed and under-expressed in cervical cells
| microRNA | Expression in the present study | Expression in other studies |
|---|---|---|
| miR-196a | Over-expressed | Over*,+,ºº |
| miR-18b | Over-expressed | -- |
| miR-183 | Over-expressed | Over*,+ |
| miR-500 | Over-expressed | -- |
| miR-18a | Over-expressed | Over*,+ |
| miR-25 | Over-expressed | Over*,+,º |
| miR-182 | Over-expressed | Over*,+, Under** |
| miR-20b | Over-expressed | Under* |
| miR-106a | Over-expressed | Over*,+, Underºº |
| miR-20a | Over-expressed | Over*,++ |
| miR-125b | Under-expressed | Under+, Over*,++ |
| miR-10b | Under-expressed | Under*,+ |
| miR-143 | Under-expressed | Under*,+,++,ºº |
| miR-337-5p | Under-expressed | -- |
| miR-199a-5p | Under-expressed | Under+,++,ºº, Overº |
| miR-199b-3p | Under-expressed | Under+ |
| miR-127-3p | Under-expressed | Overº |
| miR-214 | Under-expressed | Under+ Overº |
| miR-379 | Under-expressed | -- |
| miR-145 | Under-expressed | Under+,++,ºº Overº |
Lui et al. 2007;
Muralidhar et al. 2007;
Martínez et al. 2008;
Wang et al. 2008,
Lee et al. 2008,
Pereira et al. 2010.
Among the ten microRNAs, the most significantly over-expressed were: miR-196a, miR-18b, miR-183, miR-500, miR-18a, miR-25, miR-182, miR-20b, miR-106a, and miR-20a. On the other hand, among significantly under-expressed were miR-145, miR-379, miR-214, miR-127-3p miR-199b miR-199a, miR-337-5p, miR-143, miR-10b, and miR-125b. All cell lines were cultured under the same growth conditions to avoid positive false in microRNA expression. In addition, a technical replica of C33A cell line in Principal Component Analysis (PCA) and heat map had similar result as those of the original analysis, revealing high reproducibility of results.
Increased expression of miR-196a in cervical cancer cell lines and cervical tissues
One the most expressed microRNAs in tumor tissues and CC cells was miR-196a in microarray assay (p=4.75E-04, p=1.32E-07, respectively). To demonstrate over-expression of miR-196a, total RNA was subjected to quantitative RT-PCR assay to examine the relative expression in LSIL (Low-grade Squamous Intraepithelial Lesion, n=10), HSIL (High-grade Squamous Intraepithelial Lesion, n=10), tumor tissues (n=15) and CC cell lines (n=12) compared with healthy cervical tissues (Figure 2). As expected, expression of miR-196a in LSIL and HSIL showed a tiny variation with respect to healthy tissues, whereas tumor tissues showed a slight increase in expression but not comparable to CC cell lines. Increase expression in all CC tissues was not statistically significant, whereas in CC cell lines, the increased expression of miR-196a was statistically significant (p<0.0001) in comparison with healthy tissues.
Figure 2.

miR-196a increased expression in cervical cells. Tiny expression change of miR-196a in LSIL and HSIL with respect to healthy tissues, while a tendency of increased expression of miR-196 in cervical carcinomas was shown. As expected, a significant over-expression of miR-196a in CC cell lines was observed. X-axis corresponds to the samples, Y-axis to the fold change of miR-196a in arbitrary units. (*) indicates that the expression of miR-196a level was significantly higher in CC cell lines.
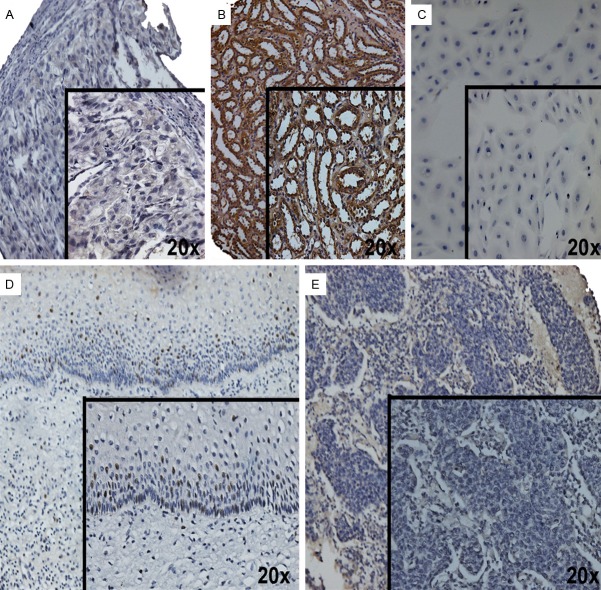
In order to find putative target genes of miR-196a, we performed bioinformatics analysis. First analysis was performed to identify miR-196a network interaction using GeneGo Metacore Suite. This analysis showed downstream and upstream interaction genes such as: embryonic development (HOX genes) and cellular remodelling (Actin/Keratin) (Figure 3). We identified indirect interaction genes such as p53 and SMAD that are associated with cell death [22]. Second, we identified miR-196a target based on experimental data and/or bioinformatics prediction such as: reporter gene assay, qPCR, western blot, sequencing, among others (Table 3). Target prediction analysis showed to HOXC8 as the best target of miR-196a. Finally, in search of probable relationship in HOXC8 expression in CC, healthy cervical tissues and cervical carcinoma tissues on tissue microarray were subjected to immunodetection with healthy bladder (negative tissue) and healthy kidney (positive tissue) as controls (Figure 4A, 4B), as well as CC cell lines (Figure 4C). Positive immunostaining was observed in nucleus of cervical epithelium cells (Figure 4D). In contrast, a negative immunoreaction was observed in the CC tissue and cell lines (Figure 4C and 4E).
Figure 3.
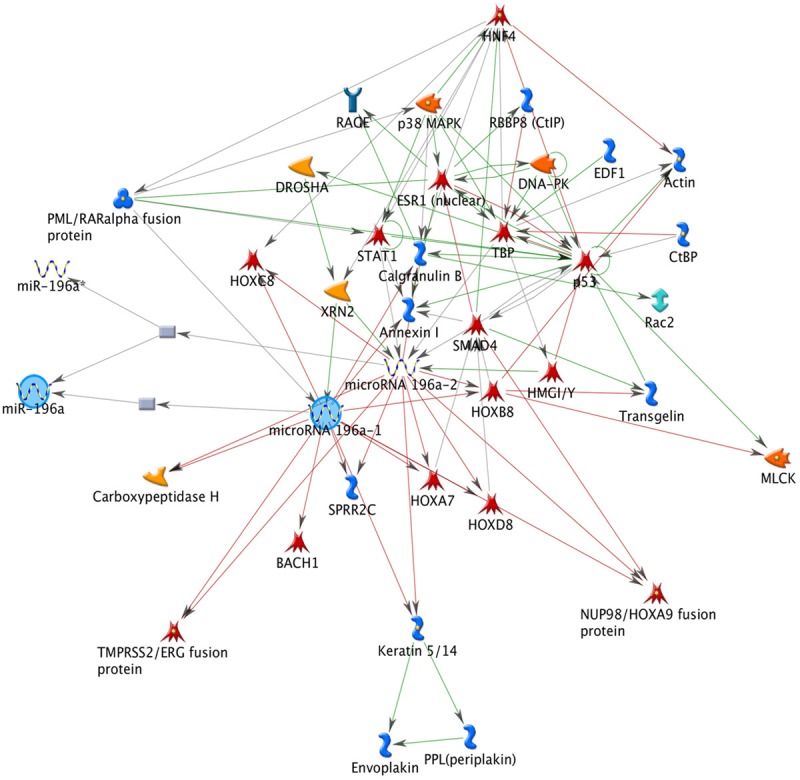
Upstream (regulators) and downstream (targets) genes related to miRNA-196a. HOX gene as HOXC8, HOXA7, HOXD8 such as predicted target of miR-196a, using fifty interactions in GeneGo MetaCore Software, p<0.005.
Table 3.
Putative target of miR-196a in different database
| Database | Target (10 top rank) | Scorea |
|---|---|---|
| DIANA-microT v5.0 | HOXC8 | 1 |
| HOXA7 | 1 | |
| HOXA5 | 0.999927 | |
| SLC9A6 | 0.999925 | |
| ERI2 | 0.999813 | |
| HAND1 | 0.999774 | |
| LRP1B | 0.999064 | |
| HOXA9 | 0.998916 | |
| HOXB7d | 0.998827 | |
| GATA6 | 0.998732 | |
| TarBase v6.0b | HOXC8c | 1.000 |
| HOXA7d | 1.000 | |
| HAND1e | 1.000 | |
| CCDC47e | 0.999 | |
| IGF2BP1e | 0.990 | |
| ARHGAP28e | 0.986 | |
| LIN28Be | 0.961 | |
| TSPAN12e | 0.930 | |
| RP5-862P8.2e | 0.909 | |
| DFFAe | 0.818 | |
| MirTarget2 v2 | HOXA7 | 100 |
| HOXC8 | 99 | |
| SLC9A6 | 98 | |
| ZMYND11 | 97 | |
| CCDC47 | 95 | |
| RG9MTD2 | 94 | |
| TMX1 | 88 | |
| ERI2 | 86 | |
| PRTG | 85 | |
| DIP2A | 84 | |
| TargetScan v6.2 | HOXC8 | -1.43 |
| HOXA7 | -1.24 | |
| SLC9A6 | -0.88 | |
| HOXA9 | -0.64 | |
| KLHL23 | -0.55 | |
| PHOSPHO2-KLHL23 | -0.55 | |
| ZMYND11 | -0.51 | |
| PACRGL | -0.50 | |
| HOXB8 | -0.49 | |
| MAP3K1 | -0-48 | |
| MicroRNA.org | HOXA7 | -3.65 |
| HOXC8 | -3.59 | |
| SMC3 | -2.66 | |
| SLC9A6 | -2.46 | |
| HOXA9 | -2.35 | |
| HMGA2 | -2.31 | |
| CTBS | -2.18 | |
| ZMYND11 | -2.10 | |
| C1orf88 | -2.07 | |
| C3orf88 | -2.06 | |
| PicTar | HOXC8 | 12.74 |
| ZMYND11 | 6.56 | |
| CCNJ | 5.08 | |
| MLR2 | 4.77 | |
| KIAA0685 | 4.64 | |
| HMGA2 | 4.32 | |
| PPP1R15B | 4.27 | |
| FLJ46247 | 4.20 | |
| SLC9A6 | 4.18 | |
| DDX19 | 4.08 | |
| miRecords | HOXC8 | - |
| GAN | - | |
| RBM26 | - | |
| CDKN1B | - | |
| EPHA7 | - | |
| ERG | - | |
| HOXB7 | - | |
| CDYL | - | |
| ZNF385B | - | |
| RSPO2 | - |
score values were according each database.
Database with curated Collection of experimentally supported microRNAs targets.
Validated by Report Gene Assay, qPCR, Sequencing; Western Blot and Other techniques.
Validated by other techniques.
Validated by sequencing.
Figure 4.
HOXC8 Immunodetection in CC. A. Negative control of HOXC8 expression (bladder tissue); B. Positive control of HOXC8 expression (kidney tissue); C. Absence HOXC8 expression in cervical cell lines (HeLa); D. HOXC8 expression in the nucleus of healthy cervical epithelium cells; E. HOXC8 expression was absence in tumor cervical tissue.
Discussion
At present, CC is an important health problem worldwide. Despite the wide acceptance of CC screening programs in Mexico, more than 12,000 women are diagnosed with CC, and among these, 50% will die annually. It is accepted that CC is related to persistent HPV infection; however, the knowledge on cervical carcinogenesis is scarce. Most of the studies on CC are focused on HPV infection, gene expression and currently on proteomic studies, all of which shade light on multiple hits presented in transformed cell [23]. During the last decade, microRNA studies in cancer research were carried out define specific expression patterns and those associated with diagnosis and prognosis [24].
In the present study, >100 human microRNAs were differentially expressed in CC tissues and CC cell lines in comparison with healthy cervices. Among the microRNAs over-expressed were miR-25, -106b, -17-92, and -106a clusters that have been previously reported altered in several neoplasms [25-29]. These clusters are related to growth, and proliferation via Tumor Growth Factor-beta (TGF-ß), and inhibition of differentiation [30,31]. We also observed an over-expression of miRNA-15b. Similar results have been previously reported in colorectal, lung, and pancreatic cancer [32-34], correlating this over-expression with prognosis. These data support the fact that these microRNA clusters are commonly altered in human cancer and that they are probably associated with the process of carcinogenesis. Moreover, under-expressed has also been reported in miR-143/145 cluster in several cancer types including CC [15,35]. This cluster is associated with angiogenesis and metastasis pathways [35-37]. The agreement of our results with previous data suggests that miRNA-145 could act as microRNA suppressor regulator in cancer.
To know whether all tumor tissues and cell lines exhibited similar behaviour in terms of microRNA expression, the PCA and differential expression analysis of the cells were carried out. As expected, the samples were grouped based on miRNA expression. In addition, heat map showed a transition of microRNAs expression to tumor tissues between healthy tissue and cell lines. Interestingly, all cell lines were clustered in PCA, except C33A cell line. It is known that C33A presents mutated pRb and p53 genes. These molecular events could explain our PCA results.
In order to know whether the presence of HPV could influences cellular microRNA expression, the analysis was performed in positive HPV and negative HPV cell lines without finding any differential expression of microRNAs with regard to HPV infection. Furthermore, two of the three negative HPV cell lines (ViBo, and HaCaT cells) were clustered positive HPV. These data suggest that the presence of specific HPV do not influence cellular microRNA expression in cell lines. In a recent study of E5 HPV16-transfected HaCaT cells, it was found that the presence of E5 viral gene does not affect changes in microRNA expression [38]. This finding could support partially the present results.
Furthermore, the most significantly over-expressed microRNA in the tumor tissues and CC cell lines was miR-196a, this microRNA is a redundant sequence in the genome. MIR196A1 and MIR196A2 genes are transcribed and processed in microRNA biogenesis pathway to generate mature miR-196a, which are located on 17q21.32 (-) (in the RP11 gene) and 12q13.13 (+) (intron 1 of the HOXC5 and HOXC6 genes) (see Figure 5). Recent findings of our group in comparative genomic hybridization analysis showed a gain of copy number of RP11 gene in CC samples (In preparation). In this situation, we hypothesize that over-expression of miR-196a could derive in part from MIR196A1 gene amplification.
Figure 5.

Diagram of HOXC8 in Cervical Cancer. miR-196a is a redundant sequence in the genome. MIR196A1 and MIR196A2 genes are transcribed and processed in miR-196a by microRNA biogenesis. According to the results in silico, HOX genes were candidate targets predictive for miR-196a; HOXC8 had a high score on all databases (Table 3), and it was also involved in relevant Networks. Some of the other HOX gene targets of miRNA196a have been investigated in cervical cells such as HOXA9, HOXB1, HOXB6, HOXB7 and HOXB8, and they showed no difference in mRNA expression [46,47]; black squares indicate high expression of mRNA and empty squares indicate no expression. Finally, experimentally some of these HOX genes like HOXC8, HOXB7, HOXB8 and HOXD8 have been validated mechanistically in other models of cancer [41,43,45], which is indicated by the word “Validated”.
At present, there is scarce knowledge on expression of this microRNA in cancer, however several studies have reported its over-expression in melanoma, oesophageal, lung, and pancreatic carcinomas [39-42]. We found statistically significant over-expression of miR-196a in CC cell lines in compared healthy tissues, whereas miR-196 expression in tumor tissues showed a slight increase but not comparable to CC cell lines, probably the low expression in CC tumors could be by cellular heterogeneity while cell lines are clonal cells, as well as lesion precancerous cervix (LSIL and HSIL).
In order to acquire some additional information on potential target, first analysis consisted in a network regulation of miR-196a based on GeneGo Metacore which showed that the potential targets of miR-196a were genes involved in embryonic development and cellular remodelling such as: HOXs (HOXC8, HOXA7, HOXD8, HOXA9, HOXB8) and Actin/Keratin. Second analysis, seven targets predictor databases were used in the first analysis with all of them depicting HOXC8 as the best target of miR-196a (Table 3), bioinformatics approaches were used and this revealed a potential regulator target of miR-196a to HOXC8. Moreover, HOXC8 has been validated as target of miR-196a in different studies [41,43-45].
Recent works showed that the absence of HOXC8 expression in some tumors is related with progression and metastasis [43-45]. In CC, several member of the family of HOX genes have been evaluated, however did not show change in the level of expression of HOXA9, HOXB1, HOXB6, HOXB7 and HOXB8 [46,47]. In additional, two studies has reported a controversial mRNA expression of HOXC8 [48,49]. According to our bioinformatics finding and report Huang et al. HOXC8 immunodetection assay was performed in healthy cervical epithelium, CC tissues, and CC cell lines. The result of the assay indicated absence of HOXC8 protein in CC samples, suggesting that HOXC8 could be a gene involved in cervical cancer process. This finding is in agreement with previous study by Alami et al., [48]. We suggest that miR-196a could be an oncogenic element and an important factor in CC cells.
In summary, our present data is showing a heterogeneity microRNAs expression in CC cells, suggesting that development of cancer in cervical cells is an extremely complex event due to high complexity of microRNA expression among other factors. We could not provide a specific microRNA expression profile related with the HPV infection, which could suggest the presence of fine molecular events making to the CC as more complex disease. Finally, we also suggest that miR-196a could be another oncogenic element in cervical cancer cells. However, we suggest that more studies be carried out to ascertain the results of the present study.
Acknowledgements
This work was supported in part by grant Fondos Sectoriales 69719 and 87244 from CONACYT Mexico. This paper constitutes a partial fulfilment of the Graduate Program in PhD Biological Sciences of National Autonomous University of México (UNAM) for Villegas-Ruiz V. She acknowledges the scholarship and financial support provided by CONACYT, UNAM and IMSS. We thanks to MD J. Ortiz and H. Serna for providing samples; Miss R. Coronel for helpful ELOSA microarrays standardization. Dra P. Piña for technical help for tissue microarrays, and PhD L Padilla for his critical review of this manuscript.
Disclosure of conflict of interest
The authors declare no interest conflicts.
References
- 1.Gregory RI, Shiekhattar R. MicroRNA biogenesis and cancer. Cancer Res. 2005;65:3509–3512. doi: 10.1158/0008-5472.CAN-05-0298. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer PS. Cancer genomics: small RNAs with big impacts. Nature. 2005;435:745–746. doi: 10.1038/435745a. [DOI] [PubMed] [Google Scholar]
- 3.Mendell JT. MicroRNAs: critical regulators of development, cellular physiology and malignancy. Cell Cycle. 2005;4:1179–1184. doi: 10.4161/cc.4.9.2032. [DOI] [PubMed] [Google Scholar]
- 4.Fabbri M, Croce CM, Calin GA. MicroRNAs. Cancer J. 2008;14:1–6. doi: 10.1097/PPO.0b013e318164145e. [DOI] [PubMed] [Google Scholar]
- 5.Lytle JR, Yario TA, Steitz JA. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5’ UTR as in the 3’ UTR. Proc Natl Acad Sci U S A. 2007;104:9667–9672. doi: 10.1073/pnas.0703820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouellet DL, Perron MP, Gobeil LA, Plante P, Provost P. MicroRNAs in gene regulation: when the smallest governs it all. J Biomed Biotechnol. 2006;2006:69616. doi: 10.1155/JBB/2006/69616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 8.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 9.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 10.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. http://www.who.int/mediacentre/factsheets/fs380/en/
- 12.Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10:550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 13.Hu X, Schwarz JK, Lewis JS Jr, Huettner PC, Rader JS, Deasy JO, Grigsby PW, Wang X. A microRNA expression signature for cervical cancer prognosis. Cancer Res. 2010;70:1441–1448. doi: 10.1158/0008-5472.CAN-09-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JW, Choi CH, Choi JJ, Park YA, Kim SJ, Hwang SY, Kim WY, Kim TJ, Lee JH, Kim BG, Bae DS. Altered MicroRNA expression in cervical carcinomas. Clin Cancer Res. 2008;14:2535–2542. doi: 10.1158/1078-0432.CCR-07-1231. [DOI] [PubMed] [Google Scholar]
- 15.Lui WO, Pourmand N, Patterson BK, Fire A. Patterns of known and novel small RNAs in human cervical cancer. Cancer Res. 2007;67:6031–6043. doi: 10.1158/0008-5472.CAN-06-0561. [DOI] [PubMed] [Google Scholar]
- 16.Martinez I, Gardiner AS, Board KF, Monzon FA, Edwards RP, Khan SA. Human papillomavirus type 16 reduces the expression of microRNA-218 in cervical carcinoma cells. Oncogene. 2008;27:2575–2582. doi: 10.1038/sj.onc.1210919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muralidhar B, Goldstein LD, Ng G, Winder DM, Palmer RD, Gooding EL, Barbosa-Morais NL, Mukherjee G, Thorne NP, Roberts I, Pett MR, Coleman N. Global microRNA profiles in cervical squamous cell carcinoma depend on Drosha expression levels. J Pathol. 2007;212:368–377. doi: 10.1002/path.2179. [DOI] [PubMed] [Google Scholar]
- 18.Pereira PM, Marques JP, Soares AR, Carreto L, Santos MA. MicroRNA expression variability in human cervical tissues. PLoS One. 2010;5:e11780. doi: 10.1371/journal.pone.0011780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Tang S, Le SY, Lu R, Rader JS, Meyers C, Zheng ZM. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS One. 2008;3:e2557. doi: 10.1371/journal.pone.0002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitt M, Dondog B, Waterboer T, Pawlita M. Homogeneous amplification of genital human alpha papillomaviruses by PCR using novel broad-spectrum GP5+ and GP6+ primers. J Clin Microbiol. 2008;46:1050–1059. doi: 10.1128/JCM.02227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Atfi A, Baron R. p53 brings a new twist to the Smad signaling network. Sci Signal. 2008;1:pe33. doi: 10.1126/scisignal.126pe33. [DOI] [PubMed] [Google Scholar]
- 23.Higareda-Almaraz JC, Enriquez-Gasca Mdel R, Hernandez-Ortiz M, Resendis-Antonio O, Encarnacion-Guevara S. Proteomic patterns of cervical cancer cell lines, a network perspective. BMC Syst Biol. 2011;5:96. doi: 10.1186/1752-0509-5-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schoof CR, Botelho EL, Izzotti A, Vasques Ldos R. MicroRNAs in cancer treatment and prognosis. Am J Cancer Res. 2012;2:414–433. [PMC free article] [PubMed] [Google Scholar]
- 25.Ambs S, Prueitt RL, Yi M, Hudson RS, Howe TM, Petrocca F, Wallace TA, Liu CG, Volinia S, Calin GA, Yfantis HG, Stephens RM, Croce CM. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 2008;68:6162–6170. doi: 10.1158/0008-5472.CAN-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diosdado B, van de Wiel MA, Terhaar Sive Droste JS, Mongera S, Postma C, Meijerink WJ, Carvalho B, Meijer GA. MiR-17-92 cluster is associated with 13q gain and c-myc expression during colorectal adenoma to adenocarcinoma progression. Br J Cancer. 2009;101:707–714. doi: 10.1038/sj.bjc.6605037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim YK, Yu J, Han TS, Park SY, Namkoong B, Kim DH, Hur K, Yoo MW, Lee HJ, Yang HK, Kim VN. Functional links between clustered microRNAs: suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res. 2009;37:1672–1681. doi: 10.1093/nar/gkp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motoyama K, Inoue H, Takatsuno Y, Tanaka F, Mimori K, Uetake H, Sugihara K, Mori M. Over- and under-expressed microRNAs in human colorectal cancer. Int J Oncol. 2009;34:1069–1075. doi: 10.3892/ijo_00000233. [DOI] [PubMed] [Google Scholar]
- 29.Pichiorri F, Suh SS, Ladetto M, Kuehl M, Palumbo T, Drandi D, Taccioli C, Zanesi N, Alder H, Hagan JP, Munker R, Volinia S, Boccadoro M, Garzon R, Palumbo A, Aqeilan RI, Croce CM. MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. Proc Natl Acad Sci U S A. 2008;105:12885–12890. doi: 10.1073/pnas.0806202105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jevnaker AM, Khuu C, Kjole E, Bryne M, Osmundsen H. Expression of members of the miRNA17-92 cluster during development and in carcinogenesis. J Cell Physiol. 2011;226:2257–2266. doi: 10.1002/jcp.22562. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Shi JY, Zhu GQ, Shi B. MiR-17-92 cluster regulates cell proliferation and collagen synthesis by targeting TGFB pathway in mouse palatal mesenchymal cells. J Cell Biochem. 2012;113:1235–1244. doi: 10.1002/jcb.23457. [DOI] [PubMed] [Google Scholar]
- 32.Miko E, Czimmerer Z, Csanky E, Boros G, Buslig J, Dezso B, Scholtz B. Differentially expressed microRNAs in small cell lung cancer. Exp Lung Res. 2009;35:646–664. doi: 10.3109/01902140902822312. [DOI] [PubMed] [Google Scholar]
- 33.Xi Y, Formentini A, Chien M, Weir DB, Russo JJ, Ju J, Kornmann M, Ju J. Prognostic Values of microRNAs in Colorectal Cancer. Biomark Insights. 2006;2:113–121. [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Li M, Wang H, Fisher WE, Lin PH, Yao Q, Chen C. Profiling of 95 microRNAs in pancreatic cancer cell lines and surgical specimens by real-time PCR analysis. World J Surg. 2009;33:698–709. doi: 10.1007/s00268-008-9833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akao Y, Nakagawa Y, Naoe T. MicroRNAs 143 and 145 are possible common onco-microRNAs in human cancers. Oncol Rep. 2006;16:845–850. [PubMed] [Google Scholar]
- 36.Takagi T, Iio A, Nakagawa Y, Naoe T, Tanigawa N, Akao Y. Decreased expression of microRNA-143 and -145 in human gastric cancers. Oncology. 2009;77:12–21. doi: 10.1159/000218166. [DOI] [PubMed] [Google Scholar]
- 37.Wang CJ, Zhou ZG, Wang L, Yang L, Zhou B, Gu J, Chen HY, Sun XF. Clinicopathological significance of microRNA-31, -143 and -145 expression in colorectal cancer. Dis Markers. 2009;26:27–34. doi: 10.3233/DMA-2009-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greco D, Kivi N, Qian K, Leivonen SK, Auvinen P, Auvinen E. Human papillomavirus 16 E5 modulates the expression of host microRNAs. PLoS One. 2011;6:e21646. doi: 10.1371/journal.pone.0021646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braig S, Mueller DW, Rothhammer T, Bosserhoff AK. MicroRNA miR-196a is a central regulator of HOX-B7 and BMP4 expression in malignant melanoma. Cell Mol Life Sci. 2010;67:3535–3548. doi: 10.1007/s00018-010-0394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luthra R, Singh RR, Luthra MG, Li YX, Hannah C, Romans AM, Barkoh BA, Chen SS, Ensor J, Maru DM, Broaddus RR, Rashid A, Albarracin CT. MicroRNA-196a targets annexin A1: a microRNA-mediated mechanism of annexin A1 downregulation in cancers. Oncogene. 2008;27:6667–6678. doi: 10.1038/onc.2008.256. [DOI] [PubMed] [Google Scholar]
- 41.Schimanski CC, Frerichs K, Rahman F, Berger M, Lang H, Galle PR, Moehler M, Gockel I. High miR-196a levels promote the oncogenic phenotype of colorectal cancer cells. World J Gastroenterol. 2009;15:2089–2096. doi: 10.3748/wjg.15.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu XH, Lu KH, Wang KM, Sun M, Zhang EB, Yang JS, Yin DD, Liu ZL, Zhou J, Liu ZJ, De W, Wang ZX. MicroRNA-196a promotes non-small cell lung cancer cell proliferation and invasion through targeting HOXA5. BMC Cancer. 2012;12:348. doi: 10.1186/1471-2407-12-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Zhang M, Chen H, Dong Z, Ganapathy V, Thangaraju M, Huang S. Ratio of miR-196s to HOXC8 messenger RNA correlates with breast cancer cell migration and metastasis. Cancer Res. 2010;70:7894–7904. doi: 10.1158/0008-5472.CAN-10-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mueller DW, Bosserhoff AK. MicroRNA miR-196a controls melanoma-associated genes by regulating HOX-C8 expression. Int J Cancer. 2011;129:1064–1074. doi: 10.1002/ijc.25768. [DOI] [PubMed] [Google Scholar]
- 45.Adwan H, Zhivkova-Galunska M, Georges R, Eyol E, Kleeff J, Giese NA, Friess H, Bergmann F, Berger MR. Expression of HOXC8 is inversely related to the progression and metastasis of pancreatic ductal adenocarcinoma. Br J Cancer. 2011;105:288–295. doi: 10.1038/bjc.2011.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez R, Garrido E, Pina P, Hidalgo A, Lazos M, Ochoa R, Salcedo M. HOXB homeobox gene expression in cervical carcinoma. Int J Gynecol Cancer. 2006;16:329–335. doi: 10.1111/j.1525-1438.2006.00350.x. [DOI] [PubMed] [Google Scholar]
- 47.Lopez R, Garrido E, Vazquez G, Pina P, Perez C, Alvarado I, Salcedo M. A subgroup of HOX Abd-B gene is differentially expressed in cervical cancer. Int J Gynecol Cancer. 2006;16:1289–1296. doi: 10.1111/j.1525-1438.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 48.Alami Y, Castronovo V, Belotti D, Flagiello D, Clausse N. HOXC5 and HOXC8 expression are selectively turned on in human cervical cancer cells compared to normal keratinocytes. Biochem Biophys Res Commun. 1999;257:738–745. doi: 10.1006/bbrc.1999.0516. [DOI] [PubMed] [Google Scholar]
- 49.Hung YC, Ueda M, Terai Y, Kamagai K, Ueki K, Kanda K, Yamaguchi H, Akise D, Ueki M. Homeobox gene expression and mutation in cervical carcinoma cells. Cancer Sci. 2003;94:437–441. doi: 10.1111/j.1349-7006.2003.tb01461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]