Abstract
Neural cell adhesion molecule (NCAM) and fibroblast growth factor receptor (FGFR) have a role in epithelial-mesenchymal transformation during tumor genesis. Interplay between both molecules activates FGFR signaling and it could be responsible for tumor development. Renal epithelial tumors were analyzed for FGFR1 and NCAM coexpression by immunohistochemistry and for colocalization of these molecules on the particular tumor cells by triple immunofluorescence. Detection of NCAM isoforms in renal tumors was evaluated by RT-PCR. Applying immunohistochemistry we revealed that the majority of analyzed renal neoplasms, including renal cell carcinoma (RCC) and oncocytoma coexpressed NCAM and FGFR1. Triple immunofluorescent technique confirmed that both markers are commonly colocalized on the same tumor cells. Interestingly, it seemed that different position of NCAM and FGFR1 expression on renal tumor cells is related to renal tumor type or grade: exclusively membranous FGFR1/NCAM expression occurred in low grade clear cell RCC (cRCC); cytoplasmatic and membranous expression was present in high grade cRCC and other RCC types; oncocytoma showed only cytoplasmatic staining of both markers. NCAM-140 and NCAM-120 were detected in almost all analyzed renal neoplasms. Expression of both molecules on different cell compartments in various kidney tumors indicated that NCAM/FGFR1 interaction could play distinct roles in renal tumor genesis.
Keywords: NCAM, FGFR1, coexpression, colocalization, renal tumors
Introduction
Kidney cancers represent about 2% of all cancers. The most common form of renal neoplasms, with highest mortality rate of the genitourinary cancers in adults is renal cell carcinoma (RCC) [1]. RCC is comprised of several histological cell types; each type has differences in origin, genetics, morphology and behavior [2]. A better understanding of tumor molecular pathways that lead to tumor appearance and growth may help in the development of new strategies for the early detection and treatment of renal carcinoma.
Neural cell adhesion molecule (NCAM, CD56), a member of a large family of cell-surface glycoproteins, plays a major role during development and controls various functions in the nervous system [3]. NCAM has been expressed in various tumors of neuroendocrine and neuroectodermal differentiation [4]. In kidney, NCAM is widely expressed during development [5], also on rare interstitial cells in adults [6] and on renal tumors [7-10]. Thus, in kidney NCAM has a multiple role in mesenchymal-epithelial transformation, migration, proliferation and epithelial-mesenchymal transformation, but the precise molecular signaling mechanisms have not been defined yet.
Fibroblast growth factor receptors (FGFRs) are a family of four tyrosine kinase receptors and can be activated by their cognate ligands [11] and by cell adhesion molecules such as NCAM [12]. FGFR play multifunctional roles in cell proliferation and migration, differentiation, apoptosis, survival, epithelial-mesenchymal transformation and tumorigenesis [13-16].
Interaction of these two molecules has been shown in neural [17], non-neural [18] and some tumor cells [19]. In non-neural cells, such as fibroblast and epithelial cells, FGFR1 and NCAM interaction induced specific set of biochemical events, responsible for cell migration [20]. NCAM localized in membrane compartments outside lipid rafts binds and stimulates FGFR through its fibronectin type III (F3) domains and activates MAPK signaling pathways [21,22].
In tumors, as it has been recently published, interaction between FGFR and NCAM promotes development of ovarian cancer [22], while in pancreatic tumors NCAM modulates tumor-cell adhesion to matrix through FGFR signaling [19].
Independent studies of NCAM and of FGFR revealed that both molecules are involved in cell processes such as cell migration, proliferation, epithelial-mesenchymal transformation, events that transform normal epithelial cell to mesenchymal tumor cell [15,22]. It has been also shown that NCAM and FGFR together could promote invasive potential of epithelial tumors [22]. Separate expression of NCAM [7,10] and of FGFR1 [23] has been already described in renal tumors. So far, there has been no study that compares expression of both markers on human kidney tumor tissues. Assuming that similar NCAM/FGFR1 interaction could promote development of renal tumors, in the present study for the first time coexpression of NCAM and FGFR1 in human renal neoplasms was evaluated. Furthermore, applying triple immunofluorescent labeling, colocalization of NCAM and FGFR1 on same tumor cell in tumor tissue has been investigated. In addition, presence of NCAM isoforms 120 and 140 kDa was investigated in renal tumor tissues using RT-PCR analyses.
Material and methods
Renal tissues and antibodies
Renal cell carcinoma (RCC) tissue was obtained from 49 patients undergoing nephrectomy, after informed consent of the patients. Normal renal tissue was obtained from 4 different healthy kidney donors that were not transplanted. The local ethic committee approved this study (reference number: 29/VI-7). One piece of each sample was put in 4% buffered formalin for routine histopathology diagnostic and immunohistochemistry staining. The second part of the tissue was put into cell culture medium RPMI 1640 (PAA Laboratories, GmbH, Austria) immediately after removal, snap-frozen in liquid nitrogen, while third part was conserved in RNAlater (Qiagen Ltd., Germany), a RNA stabilization reagent, for subsequent efficient RT-PCR analysis, both samples were stored at -80°C until further analysis.
Monoclonal mouse antibodies: NCAM, clone 123C3.D5 (Thermo Scientific, USA), specific for fibronectin type 3 (F3) domain (4) and FGFR1, clone M19B2 (Abcam, USA) which recognizes both α and β isoforms of FGFR1 protein, were used for immunoperoxidase staining. For triple immunofluorescent labeling, rabbit monoclonal antibody NCAM, clone EP2567Y, (Epitomics, USA) corresponding to residues near the C-terminus of human NCAM together with FGFR1 antibody was applied.
Immunoperoxidase staining
Tumor sections (5 μm thick) were deparaffinized and dehydrated. For antigen retrieval citrate buffer (pH 6.0, 20 minutes in microwave) was used. Primary antibodies NCAM/ 123C3.D5 (ready to use) and FGFR1 (dilution 1:100) were incubated for 1 hour. Sections were then treated with EnVisionTM Detection System (DAKO, Germany), using 3,3’-diaminobenzidine or 3-amino-9-ethylcarbazole as substrate and counterstained with hematoxylin. Negative controls were performed by omitting the first antibody and stained by the EnVisionTM method. Two pathologists (J.M.L. and J.V.) reviewed all slides of presence (+) or absence (-) of FGFR1 and NCAM expression on tumor samples. The slides were evaluated using the light microscope BX53 with DP12-CCD camera (Olympus, Germany).
Triple immunofluorescence labeling
Five μm-thick cryostat sections were treated as is was previously described [24]. In brief: after fixation the slides were incubated for 1 hour at room temperature with rabbit monoclonal antibody against NCAM (diluted 1:500), followed by Cy3-conjugated goat anti-rabbit antibody (diluted 1:2000; Dianova). Then, the mouse monoclonal antibody against FGFR1 (diluted 1:100) was added followed by goat anti-mouse IgG-Alexa 488 (diluted 1:1000, Invitrogen). The cell nuclei were identified by counterstaining with 4,6-diamino-2-phenylindolyl-dihydrochloride (DAPI; 1 μg/ml). Negative controls were performed in all experiments by omitting the first antibodies. Sections were mounted with Fluoro Preserve Reagent (Calboichem). All slides were analyzed on epifluorescence microscopy with F-View-CCD camera (Olympus, Germany). Digital pictures from every fluorescence channel were taken and superimposed for the specific antibody staining, using the software AnalySIS from Soft Imaging Systems (Olympus). Fluorescence green signal represents FGFR1 expression and red signal NCAM expression; while yellow to orange signal on merge revealed colocalization on the single cell.
Colocalization of FGFR1 and NCAM on same tumor cell was assessed by number of positive cells with respect to the total quantity of cells assessed by DAPI nuclear staining, expressed on four-value discrete scale (scores 0, 1, 2 and 3): 0 - no FGFR1+/NCAM+ tumor cells; 1 - less than 30% FGFR1+/NCAM+ cells; 2 - 30 to 60% FGFR1+/NCAM+ cells; 3 - more than 60% FGFR1+/NCAM+ cells. Coloca-lization was quantified by the consensus of two observers (J.M.L. and S.C.).
Statistical analysis
Results evaluated by immunohistochemistry (presence or absence of NCAM and FGFR1 expression) were statistically correlated with different clinic-pathological parameters (sex, tumor size, histological type etc., listed in Table 1) using standard Fisher’s exact test in cases of low expected frequencies. A p-value<0.05 was considered to indicate statistical significance. All analyses were conducted in SPSS 15 (IBM).
Table 1.
Summary of the clinicopathological features, FGFR1 and NCAM expression in RCC
| Case no. | Age | Sex | Tumor size (cm)# | Nuclear Grade | Stage | FGFR1 | NCAM | FGFR1/NCAM coexpression |
|---|---|---|---|---|---|---|---|---|
| Clear cell RCC | ||||||||
| 1 | 61 | F | 6 | 1 | pT2NxMx | + | - | no |
| 2 | 39 | M | 3.5 | 1 | pT2NxMx | + | + | yes |
| 3 | 58 | M | 4 | 1 | pT1aNxMx | + | + | yes |
| 4 | 87 | F | 2.5 | 1 | pT3aNxMx | - | - | no |
| 5 | 71 | M | 5.5 | 1 | pT3aNxMx | + | + | yes |
| 6 | 54 | M | 10 | 2 | pT3bNoMx | + | + | yes |
| 7 | 64 | M | 9 | 2 | pT3aNxMx | + | + | yes |
| 8 | 46 | F | 12 | 2 | pT3aNxMx | - | - | no |
| 9 | 58 | M | 6 | 3 | pT1bNxMx | + | + | yes |
| 10 | 74 | F | 5 | 3 | pT3aNxMx | + | + | yes |
| 11 | 44 | M | 7 | 3 | pT1bNxMx | + | + | yes |
| 12 | 36 | M | 9 | 3 | pT4N2M1 | + | - | no |
| 13 | 60 | F | 13.5 | 4 | pT3aNoMx | + | + | yes |
| 14 | 75 | F | 8 | 4 | pT3aNxMx | + | + | yes |
| 15 | 57 | F | 8 | 4 | pT3N2Mx | + | + | yes |
| 16 | 64 | M | 8 | 4 | pT3aNxMx | + | + | yes |
| Multilocular cystic RCC | ||||||||
| 1 | 47 | M | 4 | 1 | pT1aNxMx | - | - | no |
| 2 | 34 | M | 4.5 | 1 | pT1bNxMx | - | - | no |
| 3 | 44 | F | 6 | 2 | pT1bNxMx | + | + | yes |
| Papillary RCC | ||||||||
| 1 | 62 | M | 4.2 | 1, type I | pT3aNxMx | + | + | yes |
| 2 | 59 | M | 3 | 1, type II | pT3aNxMx | + | + | yes |
| 3 | 59 | M | 6 | 1, type II | pT3NxMx | - | - | no |
| 4 | 55 | M | 4.5 | 2, type II | pT1bNxMx | + | + | yes |
| 5 | 59 | M | 3.5 | 2, type II | pT1aNxMx | + | + | yes |
| 6 | 47 | M | 4 | 2, type II | pT1aNxMx | - | - | no |
| 7 | 59 | M | 3.5 | 2, type II | pT1aNxMx | + | + | yes |
| 8 | 50 | M | 1.5 | 2, type II | pT1aNxMx | + | + | yes |
| 9 | 55 | M | 4.5 | 3, type II | pT3aNxMx | + | + | yes |
| 10 | 65 | M | NK | 3, type I | pT3bNxMx | + | + | yes |
| 11 | 56 | M | 10 | 3, type II | pT3aNxMx | + | + | yes |
| 12 | 48 | M | 12 | 4, type II | pT3aNxMx | + | + | yes |
| Chromophobe RCC | ||||||||
| 1 | 43 | M | NK | 1 | NK | + | - | no |
| 2 | 39 | F | 5 | 2 | pT1bNxMx | + | - | no |
| 3 | 60 | M | 2 | 2 | pT1aNxMx | + | + | yes |
| 4 | 70 | F | 1.5 | 2 | pT1aNxMx | + | + | yes |
| 5 | 61 | M | 7 | 2 | pT1bNxMx | + | + | yes |
the biggest tumor diameter, RCC - renal cell carcinoma, FGFR1 - fibroblast growth factor receptor 1, NCAM - neural cell adhesion molecule, NK - not known.
Isolation of RNA and RT-PCR
Qiagen RNeasy-Kit (Qiagen) was used for total RNA isolation. After DNA digestion with DNaseI for 20 minutes at 25°C, 1 μg of total RNA was reverse-transcribed with 0.5 μg of oligo (dT) 12-18 using SuperScript III First-Strand kit (Invitrogen). Specially designed primers for RT-PCR amplification of NCAM-120 (forward: 5’-GAACCTGATCAAGCAGGATGACGG-3’, reverse: 5’-CTAACAGAGCAAAAGAAGAGTC-3’, NCBI accession number NM_001076682.2) and NCAM-140 (forward: 5’-GTCCTGCTCCTGGTGGTTGTG-3’, reverse: 5’-CCTTCTCGGGCTCCGTCAGT-3’, NCBI accession number NM_000615.5) were used. The β-actin (forward: 5’-TCAGAA-GGATTCCTATGTGGGC-3’, reverse: 5’-CCATCACGATGCCAGTGGTA-3’, NCBI accession number NM_001101.3) was amplified as the internal control. Amplification product was run on a 2% agarose gel and stained with ethidium bromide to monitor for specificity.
Results
Analysis of the expression of FGFR1 and NCAM was performed using specimens from 49 renal tumor patients (29 males, 20 females), the mean age was 53.9 years (range 34-87). In the present study there were analyzed: 16 clear cell RCC (cRCC), 12 papillary RCC (pRCC), 5 chromophobe RCC (chRCC) and 3 multilocular cystic RCC (mcRCC), as well as 9 oncocytoma, 2 collecting duct carcinoma, one cortical fibroma, one metanephric adenoma. Detailed clinical and pathological information was available for all RCC cases, included histological subtypes, tumor size, nuclear grade (NG) and TNM staging (Table 1).
Control normal kidney tissue staining
In all analyzed normal tissue cryostat samples stained with monoclonal rabbit antibodies clone EP2567Y revealed exclusively strong staining on rare interstitial cells and on some glomerular cells, while paraffinе sections stained with monoclonal mouse antibody clone 123C3.D5 was detected weakly on some tubular cells in addition to rare interstitial cells. In paraffin and cryostat samples FGFR1 was present on some tubular epithelial cells and smooth muscles of blood vessel.
Coexpression of FGFR1 and NCAM in RCC obtained by immunohistochemistry
Total number of RCC cases analyzed in our study was 36. From all analyzed RCC cases 30 (83.3%) had expression of FGFR1, whereas the expression of NCAM was observed in 26 (72.2%) cases. Coexpression of FGFR1 and NCAM was detected in 26 (72.2%) cases. All NCAM positive cases were usually FGFR1 positive. Lack of both molecules was found in 6 (16.6%) cases. Immunohistochemistry showed that staining pattern could be membranous and/or cytoplasmatic of NCAM and FGFR1, thus percentage of positive cells with coexpression of both molecules was evaluated only by triple immunofluorescent technique. In positive RCC cases expression of FGFR1 and NCAM has been detected in the majority of tumor cells. Intensity of staining was variable from case to case and it was not assessed.
Sixteen analyzed cRCC cases revealed FGFR1 expression in 14 (87.5%) and NCAM expression in 12 (75%) cases. Coexpression was present in 12 (75%) cases, since all NCAM positive cases were also FGFR1 positive (Table 1). Interestingly, FGFR1 and NCAM expression in cRCC with lower NG (I and II) was usually membranous and uniformly spread on the cell surface (Figure 1A, 1B, 1E and 1F), while higher NG (III and IV) had predominantly cytoplasmic in addition to membranous expression of these markers (Figure 1C, 1D, 1G and 1H). Three cases of mcRCC were also studied. Only one case of mcRCC had uniform membranous staining of FGFR1 and NCAM, whereas the absence of FGFR1 and NCAM was noticed in 2 samples (Table 1).
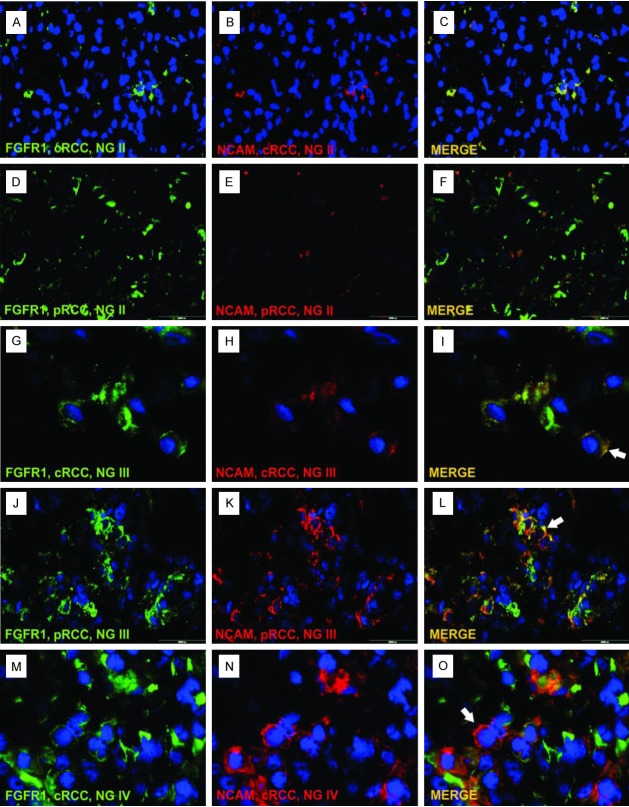
Figure 1.
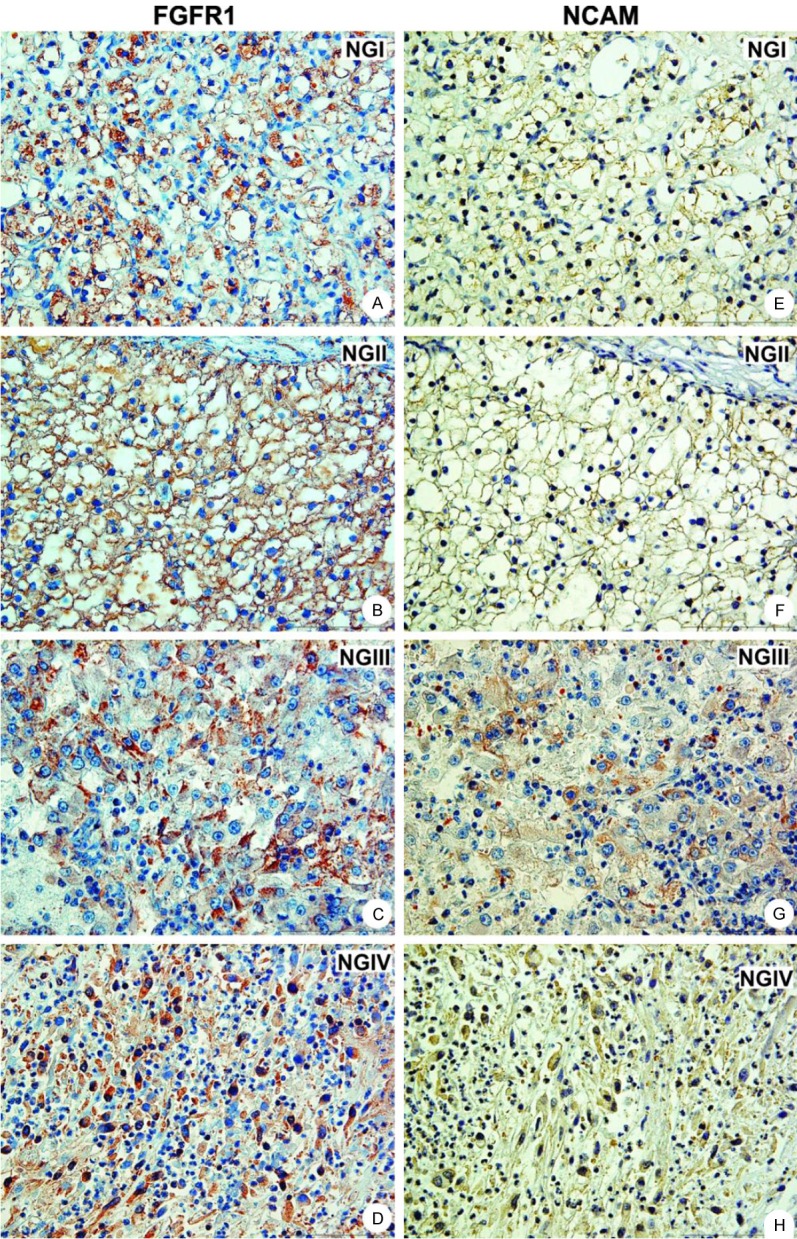
Coexpression of FGFR1 and NCAM in clear cell RCC. Lower NG had exclusively membranous expression of FGFR1 (A, B) and NCAM (E, F). In contrast, higher NG showed cytoplasmatic expression of FGFR1 (C, D) and NCAM (G, H) in addition to membranous; magnification 400x. Abbreviations: NG - nuclear grade.
In 12 pRCC cases, 10 (83.3%) had coexpression of FGFR1 and NCAM. Two pRCC, with NG I and II lacked both molecules (Table 1). In contrast to cRCC, expression of FGFR1 and NCAM in pRCC appeared to be mainly cytoplasmatic regardless to NG (Figure 2). All 5 chRCC cases expressed FGFR1, while weak NCAM expression was observed in 3 cases (Figure 3A and 3B) with predominantly cytoplasmatic staining.
Figure 2.
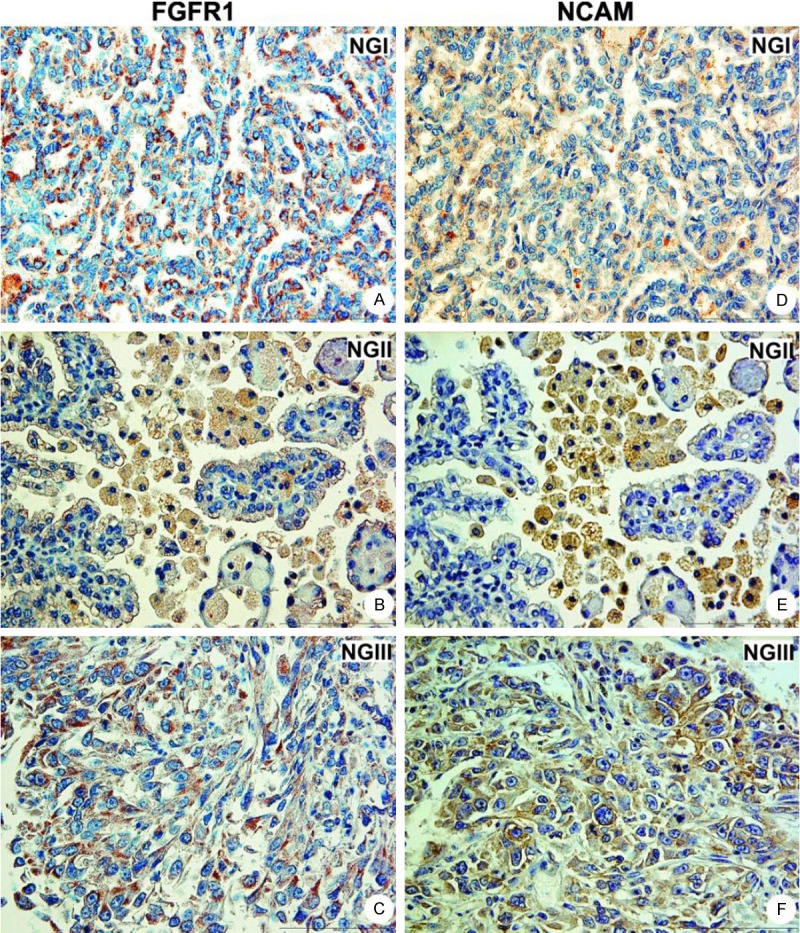
Coexpression of FGFR1 and NCAM in papillary RCC. FGFR1 (A-C) and NCAM (D-F) showed dominant cytoplasmatic expression in addition to membranous in all nuclear grades; magnification 400x.
Figure 3.
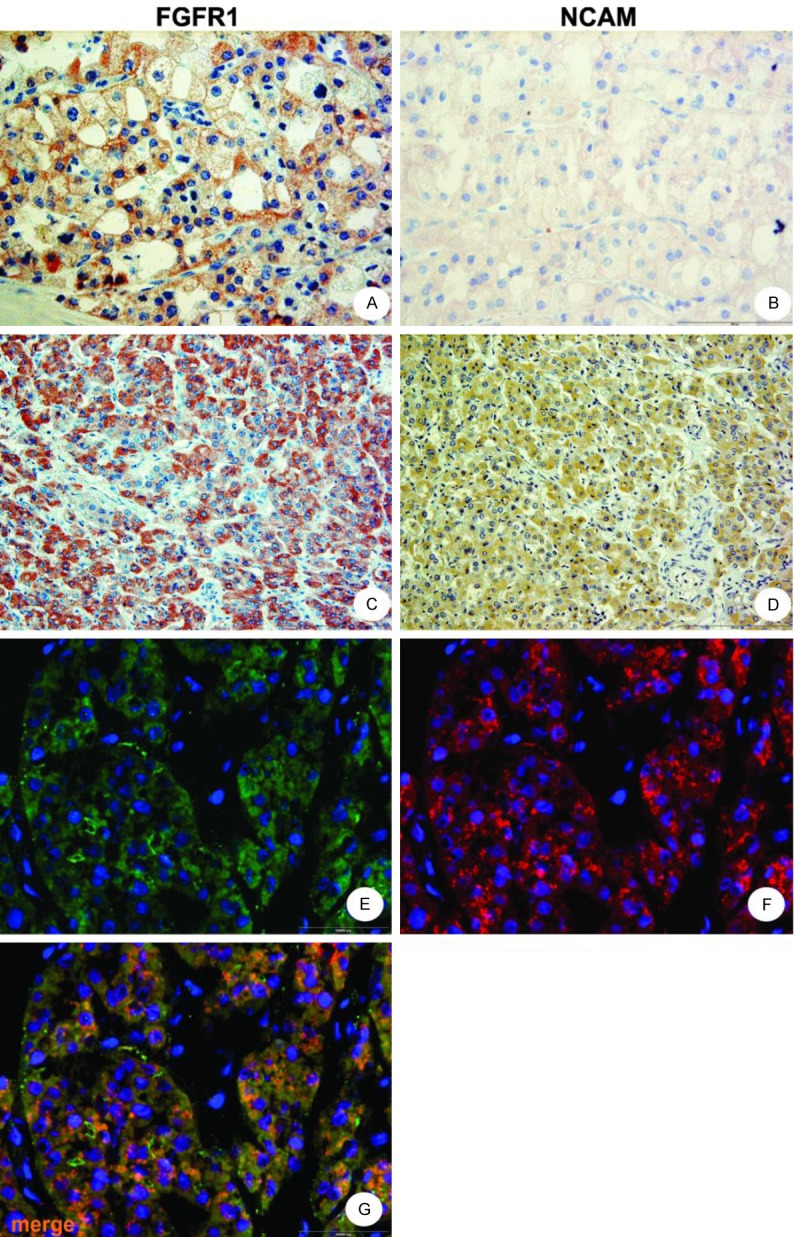
Immunohistochemistry and immunofluorescent staining pattern in chromophobe RCC and in oncocytoma. Chromophobe RCC showed mainly cytoplasmatic and membranous FGFR1 expression (A), while NCAM (B) had weak predominantly cytoplasmatic pattern in these RCC. In oncocytoma both methods revealed clearly cytoplasmatic expression of FGFR1 (C, E) and NCAM (D, F). Colocalization of FGFR1 and NCAM in oncocytoma, yellow to orange merge, (G) Magnification 400x, cell nuclei blue, stained with DAPI.
Statistical analysis of the data obtained by immunohistochemistry showed that coexpression of FGFR1 and NCAM in RCC was present regardless of histological type and all other clinicopathological data listed in Table 1 (p<0.05).
FGFR1/NCAM colocalization in RCC detected by triple immunofluorescence
Results obtained by triple immunofluorescent staining clearly showed colocalization of FGFR1 and NCAM, i.e. expression of both markers on single renal tumor cell (Figure 4, merged photos). By triple immunofluorescent technique, NCAM positive cells better corresponded to FGFR1 positive cells in comparison to immunohistochemical staining where FGFR1 was usually stronger than NCAM (compare Figures 1, 2 and 3 to Figure 4). Using this technique, it was much easier to see that cancer cells of RCC with higher NG, in addition to cytoplasmatic, had aggregated membranous FGFR/NCAM colocalization (Figure 4I, 4L and 4O, white arrows). The semi-quantitative approach showed that number of FGFR1+/NCAM+ RCC cells was increasing with nuclear grade in cRCC and pRCC, since the majority of cases with lower NG (I-II) had colocalization score 0-2 (Figure 4A-F), while in high NG RCC (III-IV) colocalization score was 3, meaning that more than 60% of tumor cells had FGFR1 and NCAM expressed on the same tumor cell (Figure 4G-O).
Figure 4.
Colocalization of FGFR1 and NCAM in clear cell and papillary RCC. Lower NG tumors (I-II) had colocalization score between 0 and 2 (C, F). In tumors with higher NG (III-IV) colocalization score was 3 (I, L, O). Legend: cRCC - clear cell RCC, pRCC - papillary RCC, NG - nuclear grade, green - FGFR1, red - NCAM, yellow to orange - merge of FGFR1 and NCAM, blue - nuclei stained with DAPI.
FGFR1 and NCAM expression in oncocytoma and other renal neoplasms
Both techniques - immunohistochemistry (Figure 3C and 3D) and immunofluorescence (Figure 3E-G) - clearly revealed exclusive cytoplasmatic expression of FGFR1 and NCAM in 9 oncocytoma cases (Table 2).
Table 2.
Summary of the clinicopathological features, FGFR1 and NCAM expression in oncocytoma
| Case no. | Age | Sex | Tumor size (cm)# | FGFR1 cytoplasmatic | NCAM cytoplasmatic | FGFR1/NCAM coexpression |
|---|---|---|---|---|---|---|
| 1 | 61 | F | 2.0 | + | + | Yes |
| 2 | 43 | F | 2.3 | + | + | Yes |
| 3 | 51 | F | 2.5 | + | + | Yes |
| 4 | 60 | F | 3.5 | + | + | Yes |
| 5 | 53 | M | 4.0 | + | + | Yes |
| 6 | 57 | F | 6.0 | + | + | Yes |
| 7 | 59 | F | 6.5 | + | + | Yes |
| 8 | 81 | F | 8.0 | + | + | Yes |
| 9 | 69 | M | 9.5 | + | + | Yes |
the biggest tumor diameter, FGFR1 - fibroblast growth factor receptor 1, NCAM - neural cell adhesion molecule.
Absence of FGFR1 and NCAM expression was found in one case of cortical fibroma and in two samples of collecting duct carcinoma, while single case of metanephric adenoma showed expression of FGFR1 and NCAM.
Presence of NCAM 120 and 140 isoforms in renal neoplasms
Five different tumor types, which had FGFR1 and NCAM expression by immunomorphology, were studied for NCAM 120 and 140 mRNA. All analyzed RCC cases: cRCC, pRCC, chRCC, as well as two benign tumors, oncocytoma and metanephric adenoma, revealed presence of NCAM 140 (Figure 5A). NCAM 120 was present only in chRCC and in both benign renal neoplasms (Figure 5B). Two RCC cases, cRCC and pRCC, were negative for NCAM 120.
Figure 5.
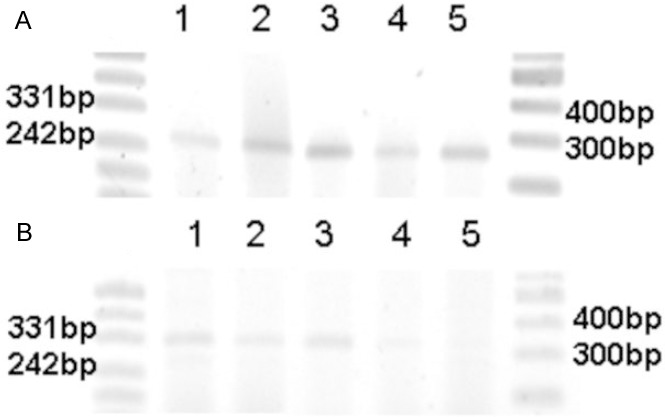
RT-PCR expression of NCAM 140 (A) and 120 (B) isoforms in various renal neoplasms. Legend: line 1 - metanephric adenoma, line 2 - oncocytoma, line 3 - chromophobe RCC, line 4 - papillary RCC, line 5 - clear cell RCC.
Discussion
In the present study, we described for the first time coexpression of FGFR1 and NCAM in different renal tumors. Coexpression of these two markers was analyzed by immunohistochemistry in cRCC, pRCC and chRCC, in collecting duct carcinoma, oncocytomas and few other malignant and benign renal neoplasms. Coexpression of both molecules was detected in the majority of studied renal tumors and moreover, colocalization of these molecules on the same renal tumor cells was revealed by triple immunofluorescent technique.
First functional interaction between FGFR1 and NCAM has been reported in neurons [17]. Thereafter, interplay between these two molecules in different non-neural cells was reported indicating that interaction of NCAM with FGFR results in stimulation of FGFR signaling in various cell types [18,19]. It has been shown that NCAM-mediated activation of FGFR leads to different cellular responses [21]. Recent publication on experimental cell cultures showed that NCAM-dependent FGFR signaling promotes cell migration and invasion in epithelial ovarian carcinoma [22]. The mechanism of FGFR1 activation by NCAM is not well clarified. In particular, FGFR membrane-proximal Ig3 module is known to bind to NCAM membrane-proximal F3(2) module [12]. Also further analyses showed that FGFR Ig2 module is involved in direct binding to the F3(1-2) domains of NCAM and that Ig2 module has two binding sites for NCAM [20]. Several speculative models for the molecular mechanisms of NCAM interplay with FGFR1 were formulated based on this data [12,20].
We used triple labeled immunofluorescent technique to determine whether NCAM and FGFR1 were present on same tumor cells. Thus, colocalization of NCAM and FGFR1 on the same renal tumor cells detected by triple staining could indicate that functional interplay between NCAM and FGFR1 could occur in renal tumor tissues. Nevertheless, semi-quantitative analysis of triple immunofluorescence showed that number of FGFR1+/NCAM+ cells was increased with nuclear grade in cRCC and pRCC suggesting that NCAM/FGFR1 interaction may be related and lead to aggressive behavior of these tumors.
In our study, staining pattern of NCAM expression was usually similar to the FGFR1 in different RCC types. Lower grade RCC (I-II) had uniformly membranous expression of NCAM and FGFR1. Thus, we could say that membranous expression of both molecules in lower grade RCCs could fit to “Kochoyan’s model” [20] suggesting that when NCAM is not involved in cell adhesion between two neighboring cells, molecules are uniformly spread on cell membrane, and FGFR molecules do not bind significantly to NCAM. In these cases FGFR and NCAM molecules do not interact with each other, suggesting that this model could be the key for low aggressive potential of these RCC. Our results are somehow in concordance with recently obtained data based on human ovarian carcinoma cell lines. In this study, ovarian carcinoma cell line which prominently express FGFRs were transfected with NCAM without F3(2) module (NCAM-ΔF2). In vitro experiments showed that cells with NCAM-ΔF2 failed to promote cell migration and invasion. Also, same cell line in mice formed tumors with smooth margins within the ovary, indicating that absence of NCAM/FGFR interaction is responsible for poorly invasive potential of developed tumor [22].
However, cRCC with higher NG (III-IV) and all pRCC (NG I-IV) in addition to membranous had cytoplasmatic expression of both markers, while in chRCC membranous and cytoplasmatic expression of FGFR1 was followed by weak mainly cytoplasmatic NCAM expression. Interestingly, triple immunofluorescent technique in RCC with high nuclear grade showed aggregated colocalized expression on cell membrane of both molecules (Figure 4G-O). Thus, we found that expression of FGFR1 and NCAM in tumor cells with higher nuclear grade leads to accumulation of both molecules in cytoplasm and on membrane. Previous data show that when NCAM is involved in cell-cell adhesion, NCAMs may aggregate/accumulate themselves forming cis-dimers. These dimers via trans-homophilic bindings mediated cell-cell adhesion making so-called “zipper” formations [25], presumed aggregation of NCAMs lead to subsequent aggregation of FGFR molecules [20]. Thus, Kochoyan et al proposed a second model of NCAM/FGFR interaction: FGFR is expected to bind to and becomes activated by NCAMs only when NCAM is clustered through a trans-homophilic binding mechanism [20]. It might be that this model could be applied to RCCs with high nuclear grade, suggesting that aggregations of NCAM and FGFR1 on cell membrane could activate NCAM/FGFR1 interplay which is then responsible for migration and invasive potential of these RCCs. Study on ovarian carcinoma mentioned above [22], also pointed to crucial role of NCAM/FGFR interaction in aggressive tumor behavior. Same cell lines were now transfected with full-length NCAM. Thus, cells which now had F3(2) domain which is necessary for FGFR binding to NCAM showed migrations and invasion of tumor cells in vitro and in vivo experiments [22], conforming the role of NCAM/FGFR interaction in aggressive tumor behavior.
Our results considering oncocytoma and metanephric adenoma, showed exclusively cytoplasmatic colocalization of FGFR1 and NCAM. Since in both benign renal tumors no membranous staining was detected, it seems that membranous expression is crucial event for NCAM and FGFR1 interplay leading to tumor invasiveness while the lack of NCAM/FGFR1 membranous expression is characteristic of renal benign tumors. In the present study NCAM expression was detected in less number of RCC in comparison to FGFR1. Expression of NCAM in renal tumors was also evaluated previously [7,10]. Daniel et al detected membranous NCAM expression in cRCC only, and no staining in pRCC and chRCC. They claim that NCAM expressing tumors behave aggressively and metastasize preferentially to NCAM-expressing organs like CNS and adrenal gland [10]. Other, recently published data on NCAM expression in RCC, revealed cytoplasmatic NCAM staining not only in cRCC but also in pRCC and chRCC, and they did not find any association between NCAM expression and histological RCC type, nuclear grade or stage [7]. It remains unclear, why cited studies [7,10] had presented different staining pattern knowing that in both studies monoclonal antibody for NCAM (CD56, Novocastra) which recognized external domain of NCAM molecule had been used. On the other hand, in our study we revealed both staining patterns, membranous and cytoplasmatic, using monoclonal NCAM antibody specific for external (F3) domain. Lower grade tumors (I and II) cRCC and mcRCC had membranous, while high grade cRCC, all pRCC, chRCC in addition to membranous had also cytoplasmatic pattern. Thus, switch from NCAM negative to NCAM positive RCC could be a crucial event for tumor aggressiveness and invasiveness, as it was shown in the experiments by Lehembre et al [21], which clarified the importance of NCAM expression for epithelial mesenchymal transition in the development and progression of epithelial tumors.
In our study, NCAM expression was detected by RT-PCR and immunostaining. NCAM-140 was present in all analyzed malignant and benign renal neoplasms. NCAM-140 plays role in growth cones and axon shafts of developing neurons and modulates cell adhesion, neuron growth and cell motility [26]. Recent study showed that NCAM-140, which has transmembrane domain, was expressed on various human malignancies, inducing antiapoptotic/proliferative pathways and specifically phosphorylated calcium-dependent kinases that are relevant for tumorigenesis [7]. However, NCAM-120, which does not have transmembrane domain, was present only in chRCC, oncocytoma and metanephric adenoma. Functions of these two isoforms still have not been cleared up in renal neoplasms and remain to be further investigated.
FGFR1 was present in great number of all analyzed renal neoplasms. Actually, FGFR1 was present in high percentage of RCC, corresponding to recently published data which showed expression of FGFR1 in 98% of primary RCC [23]. Interestingly, FGFR1 regulates different processes in invasive and non-invasive urothelial carcinomas in cell culture experiments. FGFR1 expression in non-invasive urothelial carcinoma promotes proliferation and survival, while in invasive urothelial carcinoma FGFR1 mediates invasion [27]. We clearly revealed by immunohistochemistry and by precise immunofluorescent technique on cryostat sections variable FGFR1 expressions on different cell compartments: membranous in lower grade mcRCC and cRCC (NG I-II); cytoplasmatic in addition to membranous in higher grade cRCC (NG III-IV), and in all pRCC and chRCC; cytoplasmatic in all oncocytomas. Thus, variable FGFR1 expression on various renal tumors could indicate different role of FGFR1 signaling in these tumors.
This study for the first time showed that most of analyzed renal tumors coexpressed NCAM and FGFR1 and moreover colocalization of both markers on same tumor cell. Coexpression of NCAM and FGFR1 is not related to histological type, origin, size, stage and nuclear grade. Localization of NCAM and FGFR1 expression in different cell compartments in various renal tumors suggest that NCAM/FGFR1 interaction possibly has multipurpose and different function in tumor oncogenesis in kidney.
Acknowledgements
This work was supported by: Alexander von Humboldt-Foundation, DAAD and Ministry of Education, Science and Technological Development, project number 175047, Government of the Republic of Serbia.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 2.Morris MR, Maher ER. Epigenetics of renal cell carcinoma: the path towards new diagnostics and therapeutics. Genome Med. 2010;2:59. doi: 10.1186/gm180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cambon K, Hansen SM, Venero C, Herrero AI, Skibo G, Berezin V, Bock E, Sandi C. A synthetic neural cell adhesion molecule mimetic peptide promotes synaptogenesis, enhances presynaptic function, and facilitates memory consolidation. J Neurosci. 2004 Apr 28;24:4197–4204. doi: 10.1523/JNEUROSCI.0436-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gattenlöhner S, Stühmer T, Leich E, Reinhard M, Etschmann B, Völker HU, Rosenwald A, Serfling E, Bargou RC, Ertl G, Einsele H, Müller-Hermelink HK. Specific detection of CD56 (NCAM) isoforms for the identification of aggressive malignant neoplasms with progressive development. Am J Pathol. 2009;174:1160–1171. doi: 10.2353/ajpath.2009.080647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metsuyanim S, Harari-Steinberg O, Buzhor E, Omer D, Pode-Shakked N, Ben-Hur H, Halperin R, Schneider D, Dekel B. Expression of stem cell markers in the human fetal kidney. PLoS One. 2009;4:e6709. doi: 10.1371/journal.pone.0006709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marković-Lipkovski J, Müller CA, Klein G, Flad T, Klatt T, Blaschke S, Wessels JT, Müller GA. Neural cell adhesion molecule expression on renal interstitial cells. Nephrol Dial Transplant. 2007;22:1558–1566. doi: 10.1093/ndt/gfm006. [DOI] [PubMed] [Google Scholar]
- 7.Ronkainen H, Soini Y, Vaarala MH, Kauppila S, Hirvikoski P. Evaluation of neuroendocrine markers in renal cell carcinoma. Diagn Pathol. 2010;5:28. doi: 10.1186/1746-1596-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garin-Chesa P, Fellinger EJ, Huvos AG, Beresford HR, Melamed MR, Triche TJ, Rettig WJ. Immunohistochemical analysis of neural cell adhesion molecules. Differential expression in small round cell tumors of childhood and adolescence. Am J Pathol. 1991;139:275–286. [PMC free article] [PubMed] [Google Scholar]
- 9.Daniel L, Lechevallier E, Bouvier C, Coulange C, Pellissier JF. Adult mesoblastic nephroma. Pathol Res Pract. 2000;196:135–139. doi: 10.1016/S0344-0338(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 10.Daniel L, Bouvier C, Chetaille B, Gouvernet J, Luccioni A, Rossi D, Lechevallier E, Muracciole X, Coulange C, Figarella-Branger D. Neural cell adhesion molecule expression in renal cell carcinomas: relation to metastatic behavior. Hum Pathol. 2003;34:528–532. doi: 10.1016/s0046-8177(03)00178-3. [DOI] [PubMed] [Google Scholar]
- 11.Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Kiselyov VV, Skladchikova G, Hinsby AM, Jensen PH, Kulahin N, Soroka V, Pedersen N, Tsetlin V, Poulsen FM, Berezin V, Bock E. Structural basis for a direct interaction between FGFR1 and NCAM and evidence for a regulatory role of ATP. Structure. 2003;11:691–701. doi: 10.1016/s0969-2126(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 13.Bade LK, Goldberg JE, Dehut HA, Hall MK, Schwertfeger KL. Mammary tumorigenesis induced by fibroblast growth factor receptor 1 requires activation of the epidermal growth factor receptor. J Cell Sci. 2011;124:3106–3117. doi: 10.1242/jcs.082651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Xian W, Schwertfeger KL, Rosen JM. Distinct roles of fibroblast growth factor receptor 1 and 2 in regulating cell survival and epithelial-mesenchymal transition. Mol Endocrinol. 2007;21:987–1000. doi: 10.1210/me.2006-0518. [DOI] [PubMed] [Google Scholar]
- 16.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Williams EJ, Furness J, Walsh FS, Doherty P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron. 1994;13:583–594. doi: 10.1016/0896-6273(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 18.Francavilla C, Cattaneo P, Berezin V, Bock E, Ami D, de Marco A, Christofori G, Cavallaro U. The binding of NCAM to FGFR1 induces a specific cellular response mediated by receptor trafficking. J Cell Biol. 2009;187:1101–1116. doi: 10.1083/jcb.200903030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavallaro U, Niedermeyer J, Fuxa M, Christofori G. N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat Cell Biol. 2001;3:650–657. doi: 10.1038/35083041. [DOI] [PubMed] [Google Scholar]
- 20.Kochoyan A, Poulsen FM, Berezin V, Bock E, Kiselyov VV. Structural basis for the activation of FGFR by NCAM. Protein Sci. 2008;17:1698–1705. doi: 10.1110/ps.035964.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehembre F, Yilmaz M, Wicki A, Schomber T, Strittmatter K, Ziegler D, Kren A, Went P, Derksen PW, Berns A, Jonkers J, Christofori G. NCAM-induced focal adhesion assembly: a functional switch upon loss of E-cadherin. EMBO J. 2008;27:2603–2615. doi: 10.1038/emboj.2008.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zecchini S, Bombardelli L, Decio A, Bianchi M, Mazzarol G, Sanguineti F, Aletti G, Maddaluno L, Berezin V, Bock E, Casadio C, Viale G, Colombo N, Giavazzi R, Cavallaro U. The adhesion molecule NCAM promotes ovarian cancer progression via FGFR signalling. EMBO Mol Med. 2011;3:480–94. doi: 10.1002/emmm.201100152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsimafeyeu I, Demidov L, Stepanova E, Wynn N, Ta H. Overexpression of fibroblast growth factor receptors FGFR1 and FGFR2 in renal cell carcinoma. Scand J Urol Nephrol. 2011;45:190–195. doi: 10.3109/00365599.2011.552436. [DOI] [PubMed] [Google Scholar]
- 24.Müller CA, Markovic-Lipkovski J, Klatt T, Gamper J, Schwarz G, Beck H, Deeg M, Kalbacher H, Widmann S, Wessels JT, Becker V, Müller GA, Flad T. Human-Defensins HNPs-1, -2, and -3 in Renal Cell Carcinoma: Influences on Tumor Cell Proliferation. Am J Pathol. 2002;160:1311–24. doi: 10.1016/s0002-9440(10)62558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soroka V, Kolkova K, Kastrup JS, Diederichs K, Breed J, Kiselyov VV, Poulsen FM, Larsen IK, Welte W, Berezin V, Bock E, Kasper C. Structure and interactions of NCAM Ig1-2-3 suggest a novel zipper mechanism for homophilic adhesion. Structure. 2003;11:1291–1301. doi: 10.1016/j.str.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Prag S, Lepekhin EA, Kolkova K, Hartmann-Petersen R, Kawa A, Walmod PS, Belman V, Gallagher HC, Berezin V, Bock E, Pedersen N. NCAM regulates cell motility. J Cell Sci. 2002;115:283–292. doi: 10.1242/jcs.115.2.283. [DOI] [PubMed] [Google Scholar]
- 27.Tomlinson DC, Baxter EW, Loadman PM, Hull MA, Knowles MA. FGFR1-induced epithelial to mesenchymal transition through MAPK/PLCγ/COX-2-mediated mechanisms. PLoS One. 2012;7:e38972. doi: 10.1371/journal.pone.0038972. [DOI] [PMC free article] [PubMed] [Google Scholar]