Abstract
Objective: To evaluate the therapeutic efficacy of uncultured bone marrow mononuclear cells (BMMCs) and bone mesenchymal stem cells in an osteoarthritis (OA) model of sheep. Methods: Induction of sheep OA was performed surgically through anterior cruciate ligament transection and medial meniscectomy. After 12 weeks, concentrated BMMCs obtained from autologous bone marrow harvested from anterior iliac crest or a single dose of 10 million autologous bone mesenchymal stem cells (BMSCs) suspended in phosphate-buffered saline (PBS) was delivered to the injured knee via direct intra-articular injection. Animals of the PBS group received vehicle alone. The contra-lateral joints were selected randomly as the control group. Knees of the four groups were compared macroscopically and histologically, and glycosaminoglycan (GAG) contents normalized to cartilage wet weight were measured at lesions of cartilage from medial condyle of the femur head. Gene expression levels of type II collagen (Col2A1), Aggrecan and matrix metalloproteinase-13 (MMP-13) in cartilage were measured based on RT-PCR and prostaglandin E2 (PGE2), Tumor Necrosis Factor-α (TNF-α) and Transforming Growth Factor beta (TGF-β) concentrations in synovial fluid were determined with ELISA assays at 8 weeks after injection. Results: At 8 weeks post cell transplantation, partial cartilage repair was observed in the cell therapy, but not the PBS group (P<0.05). The BMSCs group showed higher regeneration of cartilage and lower proteoglycan loss than the BMMCs group (P<0.05). Concentrated BMMCs injection led to a weaker treatment effect, but also inhibited PGE2, TNF-α and TGF-β levels in synovial fluid and promoted higher levels of Aggrecan and Col2A1 and downregulation of MMP-13 in sheep chondrocytes in a similar manner to BMSCs, compared with the PBS group. Conclusions: Bone marrow cells showed therapeutic efficacy in a sheep model of OA. Despite similar therapeutic potential, the easier and faster process of collection and isolation of BMMCs supports their utility as an effective alternative for OA treatment in the clinic.
Keywords: Mesenchymal stem cells, bone marrow mononuclear cells, OA, treatment, sheep
Introduction
Osteoarthritis (OA) is the most common joint disease in middle-aged and older people [1]. To date, no drugs have been successfully developed to structurally modify OA processes or prevent disease progression [2]. Various therapeutic interventions, surgical or pharmacological, can be performed to relieve the symptoms. However, none of the current OA treatments leads to satisfactory outcomes and often end in arthroplasty.
Mesenchymal stem cells (MSCs) can be easily isolated from various mesenchymal tissues, including bone marrow, fat or synovial membrane, and massively expanded in culture in an undifferentiated state for therapeutic use [3]. Their intrinsic self-renewal ability and differentiation potential into chondrocytes, adipocytes and osteocytes are well documented [4]. Moreover, MSCs protect cartilage from further tissue destruction and facilitate the regeneration of progenitor cells via secretion of various bioactive soluble factors [5,6]. OA is characterized by a catabolic and inflammatory joint environment. Thus, the key to effective OA treatment is to promote cartilage regeneration and reduce local inflammation [7]. Owing to the above characteristics, MSCs have significant therapeutic potential, and several recent studies have confirmed the utility of MSCs implantation as an alternative treatment for osteoarthritis [8-11].
The majority of studies on cell therapy to date have used BMSCs instead of BMMCs. To maximize the therapeutic effect of BMSCs, expansion in the laboratory to obtain adequate numbers and highly pure cells for implantation is essential. Notably, BMMCs, enriched with BMSCs, have also been shown to be beneficial. Earlier clinical trials have demonstrated therapeutic effects of BMMCs in ischemic brain injury [12], myocardial infarction [13], spinal cord injury [14], chronic liver damage [15] and osteonecrosis of femoral head [16]. BMMCs and BMSCs appear to share similar potential in promoting tissue protection and functional recovery. Although BMMCs have been increasingly applied to treat bone and joint disorders and other diseases, studies on their application in OA therapy are lacking.
In the present study, we prepared concentrated autologous BMMCs and culture-expanded BMSCs from bone marrow harvested from the anterior iliac crest of sheep. Subsequently, collected autologous BMMCs and BMSCs were injected into knees which had subjected to anterior cruciate ligament (ACL) transection and medial meniscectomy to induce OA model, respectively. In this study, we wish to address the following questions: 1) whether intra-articular injection of autologous BMMCs delays OA progression, and 2) whether BMMCs are an effective alternative to BMSCs in OA treatment.
Materials and methods
Study design
Eighteen sheep (Small Tail Han, Inner Mongolia, China) approximately 32 months old with an average weight of 80 kg were used in the study. Animals were randomly divided into three treatment groups: PBS (n=6), PBS + BMSCs (n=6), and PBS + BMMCs (n=6). Contralateral joints were selected at random as the control group (n=6). We observed no differences between the groups with respect to age and weight. OA was induced in the right knee joint of all donor animals via complete ACL transection and medial meniscectomy. The PBS + BMSCs group underwent bone marrow aspiration for BMSCs preparation 9 weeks after surgery. Animals in different treatment groups received a single intra-articular injection of autologous BMSCs or BMMCs as a suspension in PBS or PBS alone at 12 weeks after surgery.
Osteoarthritis induction in sheep
The study was approved by the Nanjing Medical University Animal Ethical Committee. Animals were treated with 3 mg/kg ketamine and 5 mg atropine via intramuscular injection for induction of anesthesia, and maintained with intravenous injection of 3% pentobarbital. ACL excision and medial meniscectomy were performed using the methods described by Murphy et al.[8], with modifications. A lateral parapatellar skin incision was made beginning at a level 2 cm proximal to the patella and extending to the tibial plateau level. Subcutaneous tissue was incised, the lateral aspect of the vastus lateralis and joint capsule incised, and the patella luxated medially to expose the trochlear groove and medial and lateral condyles of the distal femur.
ACL removal was performed by initially excising its attachment on the medial aspect of the lateral femoral condyle. The proximal attachment was brought forward and the entire ligament excised from its tibial attachment. A drawer test was performed to ensure that the entire cruciate ligament had been excised.
Meniscus was removed via sharp excision. The caudal horn of the meniscus was grasped with a hemostat and its lateral attachment excised from its tibial attachment. Working from caudal to lateral and then cranial, the meniscus was excised from its attachments until complete removal was achieved.
Postoperatively, sheep received an intramuscular injection of 2.4 million units of penicillin to prevent infection.
After a recovery period of 2 weeks, all animals were made to run on a hard surface for a distance of 2 km once a week for 10 weeks. At other times, all sheep were allowed free movement.
Isolation of BMMCs and expansion of BMSCs
Bone marrow of donor sheep was collected under general anesthesia from the iliac crest. An aliquot of bone marrow (30 ml) was aspirated with a 50 ml heparinized sterile syringe. As the bone marrow components are stratified in accordance with density, BMMCs were isolated via density gradient centrifugation with Cellgro (Mediatech), according to the manufacturer’s instructions. After centrifugations, some red blood cells (non-nucleated cells) and plasma were isolated and removed. BMMCs were collected and washed with PBS. Following cell counting, BMMCs were resuspended in PBS to a final concentration of 9×107 cells/ml. Finally, a yield of 5 ml concentrated BMMCs was obtained and placed in syringes for injection. For BMSCs preparation, the aspirate was centrifuged at 1500 rpm for 20 min. Precipitated cells were suspended in DMEM/F12 (Ham’s F12:high glucose DMEM 1:1; Hyclone) supplemented with 10% fetal bovine serum (FBS; Hyclone), 1× antibiotic (Hyclone), plated in a 75 cm2 plastic culture flask and maintained at 37°C with 5% CO2 in the same medium. After 7 days, red blood cells were washed with PBS, and fresh medium added. On day 10, cells reached confluence and were detached with 0.25% Trypsin/0.1% EDTA (GIBCO) and recultured as the first passage with the same culture medium.
Intra-articular injection of BMSCs and BMMCs
At 12 weeks after surgery, cells cultured for 3 weeks (Passage 2) were trypsinized, washed and suspended in PBS at a density of 2×106 cells/ml. The PBS + BMSCs group received a single dose of 1×107 cells (5 ml) via intra-articular injection into the osteoarthritic knee joint using an 18-gauge needle after anesthesia. Animals of the PBS + BMMCs group underwent bone marrow collection and preparation of BMMCs as described above, and an intra-articular injection of 4.5×108 cells suspended in 5 ml PBS. The PBS group received an injection of the same volume of PBS alone. Following injection, the joint was repeatedly flexed and extended to disperse the suspension throughout the articular space.
ELISA assays for synovial fluid analyses
Synovial fluid was collected from the knees of animals from all four groups at eight weeks after injection. Concentrations of prostaglandin E2 (PGE2), Tumor Necrosis Factor-α (TNF-α) and Transforming Growth Factor beta (TGF-β) in synovial fluid samples were measured using the Sheep prostaglandin E2 (PGE2) ELISA kit, Sheep Tumor Necrosis Factor α (TNF-α) ELISA Kit and Sheep Transforming growth factor beta (TGFB) ELISA kit (Cusabio), respectively. Assays were performed according to the manufacturer’s instructions. Reactions were read at 450 nm, and the concentrations calculated.
Macroscopic examination
At eight weeks post-injection, animals were sacrificed. Knees of experimental sheep were examined macroscopically by two independent (blinded) examiners. Macroscopic findings were classified and scored in accordance with the International Cartilage Repair Society (ICRS) cartilage repair assessment system [17]. Overall, repair assessment of the articular cartilage surface of the femoral condyle was scored from normal (0 point) to severely abnormal (4 points). Finally, scores assigned by the two examiners were averaged to obtain the overall score.
Histological examination
After macroscopic measurements, the distal head of the femur was dissected, fixed in 10% formalin, decalcified and processed for histologic analysis. Sections were embedded in paraffin, cut into 5 μm slices, and stained with haematoxylin and eosin (H&E) and safranin-O using standard methods. We assessed the severity of knee OA using modified Mankin criteria described by Armstrong and colleagues [18]. All sections were graded by two independent assessors blinded to the treatment group, and the mean scores used for statistical analysis.
Proteoglycan assay
Full-thickness plugs of cartilage (5 mm in diameter) were removed from lesions of medial condyle of the femur head. Each sample was weighed, dried at 50°C for 12 h, digested in 2 ml papain solution overnight at 65°C, and stored at 4°C. The total proteoglycan concentration in cartilage was determined using a standard glycosaminoglycan (GAG) assay based on the dimethylmethylene blue method [19]. GAG concentrations were normalized to cartilage wet weight (μg/mg), and the results presented as GAG content (n=6 per group).
RNA extraction and RT-PCR analysis of cartilage
Total RNA was isolated from regenerated cartilage using TRIzol (Invitrogen) and chloroform reagents, according to the manufacturer’s instructions. RNA concentrations were measured using a spectrophotometer, and samples with values of 1.7-2.0 were used. Complementary DNA (cDNA) was synthesized from RNA using a PrimeScript RT reagent kit (TaKaRa, Japan). Real-time PCR was carried out to determine the gene expression levels of type II collagen (Col2A1), Aggrecan, matrix metalloproteinase-13 (MMP-13) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) of sheep chondrocytes. The generation of specific PCR products was confirmed with melting-curve analysis, and data presented as target gene expression normalized to GAPDH. The primers used are shown in Table 1.
Table 1.
Sequences of primers for the real-time RT-PCR analysis
| Gene | Primer 5’ to 3’ | Fragment (bp) |
|---|---|---|
| Col2A | F: GTGTCTGTGACACTGGGACT | 238 |
| XM_004006408.1 | R: CTGGGTCCTTGTTCACCTGC | |
| Aggrecan | F: GCCATCTGCTACACAGGTGA | 218 |
| AF019758.1 | R: AAAGGCTCCTCAGGTTCTGG | |
| MMP-13 | F: GAGGTGACTGGCAGACTTGATG | 222 |
| NM_001166179.1 | R: CAGAGGTGTCACATCAGACCAC | |
| GAPDH | F: AGTTCCACGGCACAGTCAAG | 230 |
| NM_001190390.1 | R: AAACATGGGAGCGTCAGCAG |
Statistical analysis
All quantitative datasets are expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to assess statistically significant differences between the groups. In cases where differences were statistically significant, the SNK-q test was performed for pairwise comparisons. Date were considered statistically significant at P<0.05.
Results
Clinical observations
Animals recovered 1-2 h after surgery. There was no evidence of infection in any of the animals during the experiments. All animals bore weight on the operated joint well after surgery. We observed no inflammation, immobilization, or unloading of the joint resulting from cell treatment.
Macroscopic findings
Macroscopically, the articular cartilage surface of medial femoral condyle in the PBS group varied from complete absence of regeneration to the presence of craters eight weeks after injection (Figure 1B). The joints receiving BMMCs showed smaller lesions of the femoral condyle, compared to the PBS group (Figure 1C). In the BMSCs group, the surface of the medial femoral condyle was relatively smooth (Figure 1D). No evidence of cartilage destruction was observed in any of the contra-lateral joints (Figure 1A).
Figure 1.
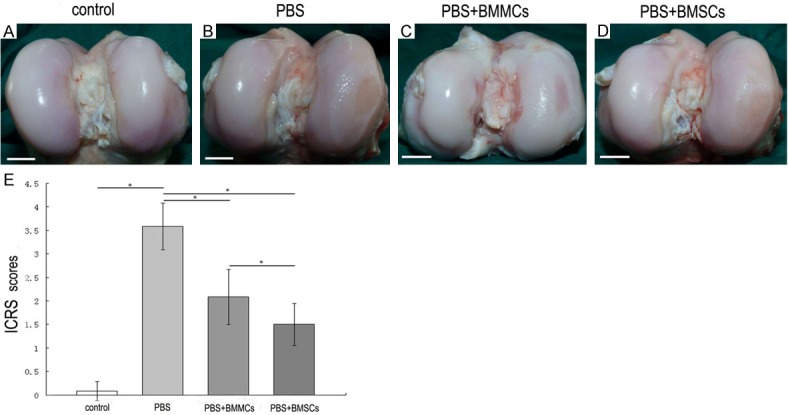
Macroscopic evaluation of the articular surface of the femoral condyle 8 weeks after cell injection. The PBS group displayed severe OA lesion without any evidence of repair (B). BMMCs group showed some evidence of repair of the cartilage surface (C). BMSCs group demonstrated a smaller lesion with smooth edges (D). Normal contra-lateral knee joint showed smooth edges with no sign of OA (A). Bar=1 cm. ICRS Sores of the joints (E). Data are mean ± standard deviation of 6 sheep in each group. The PBS group scored 3.4 ± 0.38 points, the BMMCs group scored 0.8 ± 0.35 points and the BMSCs group scored 1.22 ± 0.89 points. Scores were compared by analysis of variance. *P<0.05.
According to the ICRS grading system, a lesion score of 3.58 ± 0.49 was assigned to the PBS group, while animals of the BMMCs and BMSCs groups received lesion scores of 2.08 ± 0.59 and 1.5 ± 0.45, respectively. Improved recovery was observed in both BMMCs and BMSCs transplantation groups, compared with the non cell-injected PBS group. These differences were statistically significant (P<0.05). Comparison of the ICRS scores between the cellular therapy groups clearly indicated enhanced improvement with BMSCs relative to BMMCs (P<0.05) (Figure 1E).
Histological observations
Joints of animals in the PBS group exhibited substantial fibrillation of the articular surface with loss of extracellular matrix (Figure 2B, 2F), compared with the contralateral joints (Figure 2A, 2E). Despite some damage to the superficial and middle layers of cartilage, the BMMCs group displayed a degree of cartilage regeneration (Figure 2C, 2G). The BMSCs group displayed large numbers of chondrocytes with substantial extracellular matrix (ECM) staining of the articular cartilage comparable to normal contra-lateral knee joint, which showed no evidence of cartilage destruction (Figure 2A, 2D, 2E and 2H).
Figure 2.
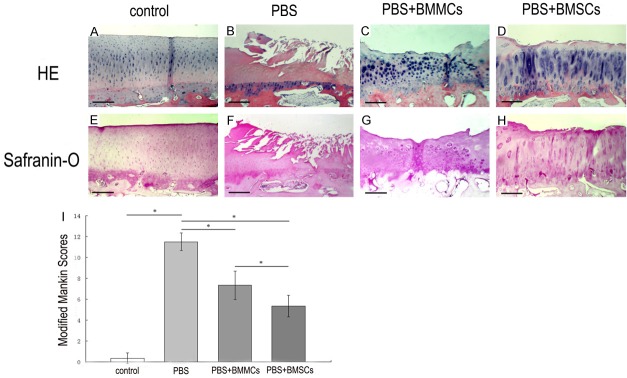
Histological analysis by H&E and Safranin-O staining of the medial femoral condyle. Photomicrographs showing serial sections 5 μm thick stained with H&E (A-D) and Safranin-O (E-H). Bar=250 μm. PBS group exhibited substantial fibrillation and absence of the articular surface almost exposing the bony layer with loss of extracellular matrix (B, F) as compared with the contra-lateral joints (A, E). BMMCs group displayed some degree of regeneration of cartilage, but there existed some damage to the superficial and middle layer of the cartilage (C, G). Changes to the cartilage and bone were much less severe in the BMSCs group. Mild surface roughening and reasonable extracellular matrix staining in the surface zone were evident (D, H). Histological grade of knee osteoarthritis assessed by modified Mankin criteria 8 weeks after injection (I). Data are mean ± standard deviation of 6 sheep in each group. *P<0.05.
At 8 weeks post-transplantation, the PBS group displayed a statistically significant higher OA score, compared with the control group (P<0.05). Significant differences in OA scores were observed between the PBS and cell therapy groups, implying that cell therapy leads to reasonable regeneration of cartilage (P<0.05). Moreover, the BMSCs group displayed a lower OA score, compared to the BMMCs group, which was statistically significant (P<0.05) (Figure 2I).
GAG content of lesions of cartilage
Despite variations in data, the same trend was essentially observed across all specimens. The proteoglycan content of cartilage was significantly decreased in the PBS group, compared with the control group (P<0.05). However, the GAG content in cartilage was markedly higher in the cellular therapy groups, compared to the PBS group (P<0.05). Analysis of the BMMCs and BMSCs groups revealed a higher GAG protection effect with injection of BMSCs after 8 weeks of treatment, causing a significant increase in the proteoglycan concentration (P<0.05) (Figure 3).
Figure 3.
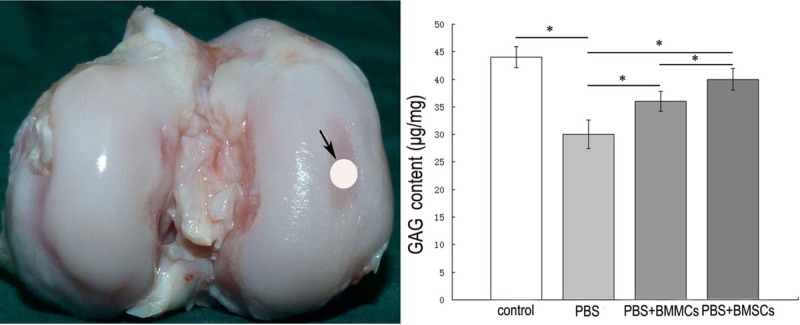
Glycosaminoglycan content in the cartilage from lesions of medial condyle of the femur head. The view of a distal head of specimen and the view of the location used for proteoglycan assay (left). Columns and error bars show means and standard deviations based on analysis of 6 specimens (right). *P<0.05.
RT-PCR analysis
Quantitative real-time PCR was performed to analyze Col2A1, Aggrecan and MMP-13 gene expression in chondrocytes from different groups. At eight weeks post-injection, we observed a significant decrease in Col2A1 and Aggrecan and an increase in MMP-13 gene expression in the PBS group, compared with the control group (P<0.05). Cellular therapy led to increased Col2A1 and Aggrecan and decreased MMP-13 levels (P<0.05). Compared to the BMMCs group, chondrocytes from injured knees receiving BMSCs treatment showed higher Aggrecan and lower MMP-13 gene expression (P<0.05). Additionally, the BMSCs group showed increased Col2A1 gene expression compared with the BMMCs group, but this difference was not statistically significant (Figure 4).
Figure 4.
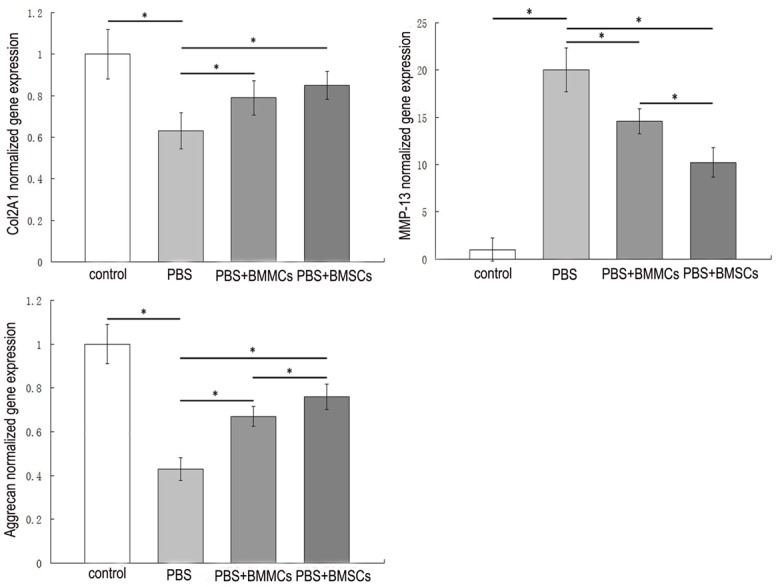
Effects of cell therapy on expression of genes (Col2a1, Aggrecan, MMP-13) in sheep cartilage. Values are mean ± standard deviation of the relative quantities normalized to sheep-specific GAPDH; n=6 for each group. *P<0.05.
Cytokine concentrations in synovial fluid
Synovial PGE2 concentrations were significantly increased with induction of OA (5.13 ± 0.21 pg/mL; P<0.05), compared to the control group (1.52 ± 0.13 pg/ml). The BMSCs group (4.27 ± 0.17 pg/ml) displayed a significant (P<0.05) decrease in synovial PGE2 concentration, compared with the PBS group. No significant treatment effects were evident in the BMMCs group.
TNF-α concentrations in synovial fluid were markedly increased with induction of OA (P<0.05). OA-affected joints contained increased TNF-α levels (3.56 ± 0.26 pg/mL), compared to the control group (0.87 ± 0.07). A significant decrease in synovial TNF-α concentration was evident in sheep treated with BMSCs (2.97 ± 0.22 pg/mL; P<0.05), compared to those administered PBS. BMMCs injection inhibited the TNF-α level in synovial fluid induced by OA (3.42 ± 0.19 pg/mL), but not to a significant extent.
Synovial fluid TGF-β concentrations were significantly increased with induction of OA (19.38 ± 1.45 ng/mL; P<0.05), compared with the control group (2.66 ± 0.17 ng/mL). Cellular therapy groups displayed a considerable decrease in synovial fluid TGF-β concentrations (P<0.05), compared with the PBS group. A more significant (P<0.05) decrease in synovial TGF-β concentrations was evident in BMSCs-treated (13.5 ± 1.29 ng/mL) than BMMCs-treated sheep (17.26 ± 1.36 ng/mL) (Figure 5).
Figure 5.
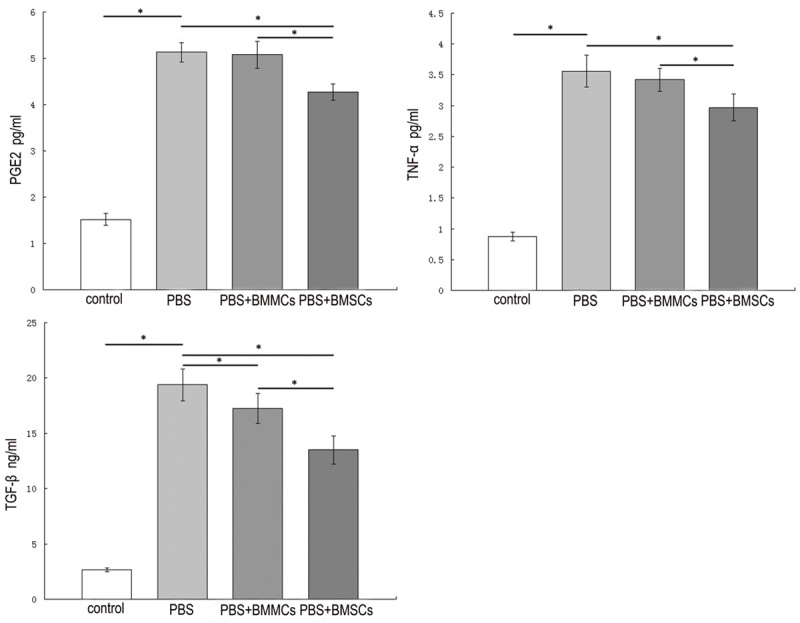
Cytokines concentrations in synovial fluid of different groups by ELISA assays. Values are mean ± standard deviation of 6 sheep in each group. *P<0.05.
Discussion
Bone marrow hosts multipotent stem cells that are easily purified by adhesion to plastic surfaces and share numerous properties. MSCs from bone marrow are widely used for cartilage tissue regeneration due to their self-renewal and differentiating properties into chondrogenic lineages [6]. Since Murphy et al. showed that local delivery of adult MSCs to injured joints stimulates regeneration of meniscal tissue and retards progressive cartilage destruction in a sheep OA model [8], stem cell therapy has displayed extensive potential in tissue regeneration and function recovery for a variety of pathologies, including rheumatoid arthritis [20], osteonecrosis [21], stroke [22], myocardial infarct [23], and meniscus injury [24].
As reported previously, injection of BMSCs enhanced the regeneration of cartilage in surgically induced OA. The ability of MSCs to differentiate into diverse phenotypes makes them excellent candidates as therapeutic cells for the repair of damaged tissues. Additionally, MSCs migrate to injured tissue and act as a local “factory” of molecules promoting angiogenesis, neuroprotection, modulation of inflammatory response, and reduction of scar formation [22,25,26]. These “trophic” effects are distinct from direct differentiation of MSCs into repair tissue [27]. Occasionally, marked therapeutic effects along with a very low number of MSCs expressing differentiated markers signify a contribution of the trophic effect of MSCs to positive therapeutic effects. Consistent with the above results, upregulation of Col2A1 and Aggrecan, downregulation of MMP-13, and significant increase in GAG content in the cell therapy groups in our study imply that bone marrow cells promote cartilage matrix synthesis and reduced inflammation of the microenvironment of chondrocytes.
Here, we showed that bone marrow cell injection regulates the proinflammatory cytokine levels in synovial fluid. We obtained similar results to Frisbie et al. [28] who reported that treatment with BMSCs significantly decreases synovial fluid PGE2 levels in the OA-affected limb in a horse model. Additionally, the decrease in TNF-α and TGF-β following treatment with autologous bone marrow cells further clarified that the therapeutic effect of bone marrow cells is partially attributed to regulation of the joint microenvironment. Cytokines, such as TNF-α, produced by activated synoviocytes, mononuclear cells or articular cartilage itself, significantly upregulate metalloproteinases (MMPs) gene expression. TNF-α induces joint articular cells, such as chondrocytes and synovial cells, to stimulate proteases and prostaglandin PGE2 production [29]. Historically, an increase in PGE2 is positively correlated with increase in pain. MSCs are immunosuppressive and anti-inflammatory cells that inhibit production of TNF-α by activated macrophages in vitro [30]. TGF-β is traditionally considered a central anabolic or reparative mediator. However, TGF-β-mediated signaling through the activin receptor-like kinase 5 (ALK5) pathway causes transition of chondrocytes to a fibrogenic phenotype, resulting in many of the destructive processes of OA [31]. High TGF-β levels in synovial fluid in the OA-affected joint and significant decrease in TGF-β upon injection of bone marrow cells indicates that MSCs alter TGF-β secretion and participate in the anti-inflammatory effect. Although the precise mechanism is yet to be clarified, changes in the cytokine concentrations in synovial fluid were consistent with other findings.
Of special interest was the finding that transplantation of BMMCs also retards the progress of OA, albeit not to the same extent as BMSCs. A number of clinical researchers have validated the potential of BMMCs in the treatment of osteoarthritis and partial or full-thickness articular cartilage defects. In 2006, Centeno and co-workers published a case report describing partial articular surface regeneration in a Figure 5. Cytokines concentrations in synovial fluid of different groups by ELISA assays. Values are mean ± standard deviation of 6 sheep in each group. *P<0.05. severely degenerated hip after autologous nucleated cell BM injections in a patient [32]. Slynarski et al. [33] combined periosteum with fresh BM in toto implantation for the treatment of a full-thickness cartilage defect in 14 patients, with satisfactory results.
The BMMCs preparation consists of a heterogenous population of cells, including MSCs, hematopoietic stem cells (HSCs), hematopoietic progenitor cells (HPCs), endothelial progenitor cells (EPCs), adipocytes, macrophages, monocytes, neutrophils, and platelets [34,35]. BMSCs migrate to target tissues and participate in repairing injured tissues [8,36]. Freitas and colleagues showed that mouse MSCs in vivo fluorescence tracking with GFP transplanted into mouse spinal cord migrates towards the injury site [14]. In our study, a single dose of BMSCs was isolated and expanded in vitro from the same volume of bone marrow used to concentrate BMMCs, with the aim of comparing the results of different treatment groups. However, MSCs are a relatively rare population of cells within bone marrow. Only a small percentage (0.01%-0.001%) of the total mononuclear cells represents MSCs, and their amounts increase by 100-10,000 fold over several weeks under culture conditions [37-39]. Transplantation of BMMCs led to a weaker, yet significant therapeutic effect, compared with BMSCs. Thus, it is inappropriate to conclude that the therapeutic efficiency of bone marrow cells is completely attributable to its MSCs component.
The group of Wise reported that fresh uncultured BMMCs exhibit a similar degree of osteogenesis as culture-expanded MSCs when cultured in collagen-chitosan microbeads [40]. As mentioned above, BMMCs consist of two major types of components, specifically, BMSCs and HSCs along with other hematopoietic lineage cells. The latter secretes a variety of cytokines and growth factors, and may act in concert through paracrine signaling to enhance the survival and proliferation of BMSCs [34]. There is a synergistic effect between the non-MSCs component of the BMMCs preparation and BMSCs. Thus, we propose that even with a lower proportion of MSCs, BMMCs exert a reasonable regeneration effect on cartilage, and the non-MSCs component may play an important role in this process.
Despite the reported favorable outcomes, the issue of whether BMMCs are a suitable alternative to BMSCs in OA treatment remains a subject of concern. Culture-expanded MSCs are typically used in stem cell-based therapy due to well-established culture methods that allow plastic-adherent stem cells to be easily manipulated and expanded to produce large quantities for proposed clinical applications. However, the major disadvantages of in vitro culture expansion of MSCs include lengthy times, large costs, risk of contamination, and requirement of a Good Manufacturing Practice (GMP) facility [40]. Furthermore, two-dimensional (2D) culture-expanded MSCs in vitro have been shown to exhibit altered antigenic and gene expression [41-45] and loss of multipotential differentiation capacity [46-48], compared with fresh uncultured MSCs. Considering that BMSCs isolated from the elderly may have decreased proliferation and differentiation potential and that OA often occurs in the middle-aged and older generation [49,50], is it clinically as effective as we applied in animal experimental studies? Therefore, it will be a long period before MSCs therapy can be successfully applied in the clinic. However, the method for transplanting BMMCs permits direct processing of cells in the operating room without cultivation and immediate autologous administration, thus allowing transplantation to be performed in “one step”, leading to reduced costs, risk of contamination and no requirement for a GMP facility [51]. In view of these advantages and their reasonable regenerative effects on injured cartilage in OA, BMMCs appear to present a suitable potential alternative to BMSCs prior to clinical application by the GMP facility.
The present study has a number of limitations that it involved animal experiments, the cohort was relatively small, and we did not examine the long-term efficacy of bone marrow cell therapy. Prospective randomized controlled clinical studies with large sample sizes are necessary to confirm the therapeutic effects of intra-articular injection of autologous BMMCs. However, while the therapeutic effects of BMMCs are not as satisfactory as BMSCs, they clearly present a more cost-effective and faster alternative for OA treatment. We conclude that BMMCs have reasonable potential as an alternative therapeutic option for OA, although their long-term effects require further evaluation.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81272033 and No. 81271986) and Jiangsu Provincial Special Program of Medical Science (BL2013033).
Disclosure of conflict of interest
The authors declare that they have no competing interests.
References
- 1.Schaap LA, Peeters GM, Dennison EM, Zambon S, Nikolaus T, Sanchez-Martinez M, Musacchio E, van Schoor NM, Deeg DJ. European Project on OSteoArthritis (EPOSA): methodological challenges in harmonization of existing data from five European population-based cohorts on aging. BMC Musculoskelet Disord. 2011;12:272. doi: 10.1186/1471-2474-12-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvey WF, Hunter DJ. The role of analgesics and intra-articular injections in disease management. Rheum Dis Clin North Am. 2008;34:777–788. doi: 10.1016/j.rdc.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Qi Y, Feng G, Yan W. Mesenchymal stem cell-based treatment for cartilage defects in osteoarthritis. Mol Biol Rep. 2012;39:5683–5689. doi: 10.1007/s11033-011-1376-z. [DOI] [PubMed] [Google Scholar]
- 4.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 5.Agung M, Ochi M, Yanada S, Adachi N, Izuta Y, Yamasaki T, Toda K. Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg Sports Traumatol Arthrosc. 2006;14:1307–1314. doi: 10.1007/s00167-006-0124-8. [DOI] [PubMed] [Google Scholar]
- 6.Koelling S, Miosge N. Stem cell therapy for cartilage regeneration in osteoarthritis. Expert Opin Biol Ther. 2009;9:1399–1405. doi: 10.1517/14712590903246370. [DOI] [PubMed] [Google Scholar]
- 7.Attur M, Samuels J, Krasnokutsky S, Abramson SB. Targeting the synovial tissue for treating osteoarthritis (OA): where is the evidence? Best Pract Res Clin Rheumatol. 2010;24:71–79. doi: 10.1016/j.berh.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 9.Maumus M, Guerit D, Toupet K, Jorgensen C, Noel D. Mesenchymal stem cell-based therapies in regenerative medicine: applications in rheumatology. Stem Cell Res Ther. 2011;2:14. doi: 10.1186/scrt55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11:343–353. [PubMed] [Google Scholar]
- 11.Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 12.Cardoso MM, Franco EC, de Souza CC, Da SM, Gouveia A, Gomes-Leal W. Minocycline treatment and bone marrow mononuclear cell transplantation after endothelin-1 induced striatal ischemia. Inflammation. 2013;36:197–205. doi: 10.1007/s10753-012-9535-5. [DOI] [PubMed] [Google Scholar]
- 13.Baikova YP, Fatkhudinov T, Bol’Shakova GB, Bukharova TB, Slashcheva GA, Khokhlova OV, Murashev AN, Gol’Dshtein DV. Reparation of the myocardium after transplantation of mononuclear bone marrow cells. Bull Exp Biol Med. 2011;150:522–529. doi: 10.1007/s10517-011-1182-6. [DOI] [PubMed] [Google Scholar]
- 14.Ozdemir M, Attar A, Kuzu I, Ayten M, Ozgencil E, Bozkurt M, Dalva K, Uckan D, Kilic E, Sancak T, Kanpolat Y, Beksac M. Stem cell therapy in spinal cord injury: in vivo and postmortem tracking of bone marrow mononuclear or mesenchymal stem cells. Stem Cell Rev. 2012;8:953–962. doi: 10.1007/s12015-012-9376-5. [DOI] [PubMed] [Google Scholar]
- 15.Takami T, Terai S, Sakaida I. Advanced therapies using autologous bone marrow cells for chronic liver disease. Discov Med. 2012;14:7–12. [PubMed] [Google Scholar]
- 16.Wang BL, Sun W, Shi ZC, Zhang NF, Yue DB, Guo WS, Xu SQ, Lou JN, Li ZR. Treatment of nontraumatic osteonecrosis of the femoral head with the implantation of core decompression and concentrated autologous bone marrow containing mononuclear cells. Arch Orthop Trauma Surg. 2010;130:859–865. doi: 10.1007/s00402-009-0939-0. [DOI] [PubMed] [Google Scholar]
- 17.Williams IR, Brophy RH. Cartilage repair procedures: clinical approach and decision making. Instr Course Lect. 2008;57:553–561. [PubMed] [Google Scholar]
- 18.Armstrong S, Read R, Ghosh P. The effects of intraarticular hyaluronan on cartilage and subchondral bone changes in an ovine model of early osteoarthritis. J Rheumatol. 1994;21:680–688. [PubMed] [Google Scholar]
- 19.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 20.Greish S, Abogresha N, Abdel-Hady Z, Zakaria E, Ghaly M, Hefny M. Human umbilical cord mesenchymal stem cells as treatment of adjuvant rheumatoid arthritis in a rat model. World J Stem Cells. 2012;4:101–109. doi: 10.4252/wjsc.v4.i10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feitosa ML, Fadel L, Beltrao-Braga PC, Wenceslau CV, Kerkis I, Kerkis A, Birgel JE, Martins JF, Martins DS, Miglino MA, Ambrosio CE. Successful transplant of mesenchymal stem cells in induced osteonecrosis of the ovine femoral head: preliminary results. Acta Cir Bras. 2010;25:416–422. doi: 10.1590/s0102-86502010000500006. [DOI] [PubMed] [Google Scholar]
- 22.Vasconcelos-dos-Santos A, Rosado-de-Castro PH, Lopes DSS, Da CSJ, Ramos AB, Rodriguez DFG, Barbosa DFL, Gutfilen B, Mendez-Otero R. Intravenous and intra-arterial administration of bone marrow mononuclear cells after focal cerebral ischemia: Is there a difference in biodistribution and efficacy? Stem Cell Res. 2012;9:1–8. doi: 10.1016/j.scr.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Jiang W, Ma A, Wang T, Han K, Liu Y, Zhang Y, Dong A, Du Y, Huang X, Wang J, Lei X, Zheng X. Homing and differentiation of mesenchymal stem cells delivered intravenously to ischemic myocardium in vivo: a time-series study. Pflugers Arch. 2006;453:43–52. doi: 10.1007/s00424-006-0117-y. [DOI] [PubMed] [Google Scholar]
- 24.Horie M, Choi H, Lee RH, Reger RL, Ylostalo J, Muneta T, Sekiya I, Prockop DJ. Intra-articular injection of human mesenchymal stem cells (MSCs) promote rat meniscal regeneration by being activated to express Indian hedgehog that enhances expression of type II collagen. Osteoarthritis Cartilage. 2012;20:1197–1207. doi: 10.1016/j.joca.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maltman DJ, Hardy SA, Przyborski SA. Role of mesenchymal stem cells in neurogenesis and nervous system repair. Neurochem Int. 2011;59:347–356. doi: 10.1016/j.neuint.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, Matsuzaki M, Hamano K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol. 2006;291:H886–H893. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- 27.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 28.Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 2009;27:1675–1680. doi: 10.1002/jor.20933. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–246. [PubMed] [Google Scholar]
- 30.Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther. 2008;10:223. doi: 10.1186/ar2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plaas A, Velasco J, Gorski DJ, Li J, Cole A, Christopherson K, Sandy JD. The relationship between fibrogenic TGFbeta1 signaling in the joint and cartilage degradation in post-injury osteoarthritis. Osteoarthritis Cartilage. 2011;19:1081–1090. doi: 10.1016/j.joca.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Centeno CJ, Kisiday J, Freeman M, Schultz JR. Partial regeneration of the human hip via autologous bone marrow nucleated cell transfer: A case study. Pain Physician. 2006;9:253–256. [PubMed] [Google Scholar]
- 33.Slynarski K, Deszczynski J, Karpinski J. Fresh bone marrow and periosteum transplantation for cartilage defects of the knee. Transplant Proc. 2006;38:318–319. doi: 10.1016/j.transproceed.2005.12.075. [DOI] [PubMed] [Google Scholar]
- 34.Soltan M, Smiler D, Choi JH. Bone marrow: orchestrated cells, cytokines, and growth factors for bone regeneration. Implant Dent. 2009;18:132–141. doi: 10.1097/ID.0b013e3181990e75. [DOI] [PubMed] [Google Scholar]
- 35.Lei SH, Guo L, Yue HY, Zhao DC, Zhang CJ, Du WJ, Huang LZ, Wang J, Dang YX, Liu JS, Hao JL, Wang YL. Marrow stromal stem cell autologous transplantation in denervated fracture healing: an experimental study in rats. Orthop Surg. 2013;5:280–288. doi: 10.1111/os.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mokbel AN, El TO, Shamaa AA, Rashed LA, Sabry D, El SA. Homing and reparative effect of intra-articular injection of autologus mesenchymal stem cells in osteoarthritic animal model. BMC Musculoskelet Disord. 2011;12:259. doi: 10.1186/1471-2474-12-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veronesi F, Giavaresi G, Tschon M, Borsari V, Nicoli AN, Fini M. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev. 2013;22:181–192. doi: 10.1089/scd.2012.0373. [DOI] [PubMed] [Google Scholar]
- 38.Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 39.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 40.Wise JK, Alford AI, Goldstein SA, Stegemann JP. Comparison of Uncultured Marrow Mononuclear Cells and Culture-Expanded Mesenchymal Stem Cells in 3D Collagen-Chitosan Microbeads for Orthopedic Tissue Engineering. Tissue Eng Part A. 2014;20:210–24. doi: 10.1089/ten.tea.2013.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boquest AC, Shahdadfar A, Fronsdal K, Sigurjonsson O, Tunheim SH, Collas P, Brinchmann JE. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Mol Biol Cell. 2005;16:1131–1141. doi: 10.1091/mbc.E04-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boiret N, Rapatel C, Veyrat-Masson R, Guillouard L, Guerin JJ, Pigeon P, Descamps S, Boisgard S, Berger MG. Characterization of nonexpanded mesenchymal progenitor cells from normal adult human bone marrow. Exp Hematol. 2005;33:219–225. doi: 10.1016/j.exphem.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Boos AM, Loew JS, Deschler G, Arkudas A, Bleiziffer O, Gulle H, Dragu A, Kneser U, Horch RE, Beier JP. Directly auto-transplanted mesenchymal stem cells induce bone formation in a ceramic bone substitute in an ectopic sheep model. J Cell Mol Med. 2011;15:1364–1378. doi: 10.1111/j.1582-4934.2010.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Modder UI, Roforth MM, Nicks KM, Peterson JM, McCready LK, Monroe DG, Khosla S. Characterization of mesenchymal progenitor cells isolated from human bone marrow by negative selection. Bone. 2012;50:804–810. doi: 10.1016/j.bone.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian H, Le Blanc K, Sigvardsson M. Primary mesenchymal stem and progenitor cells from bone marrow lack expression of CD44 protein. J Biol Chem. 2012;287:25795–25807. doi: 10.1074/jbc.M112.339622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000;28:707–715. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 47.Mendes SC, Tibbe JM, Veenhof M, Bakker K, Both S, Platenburg PP, Oner FC, de Bruijn JD, van Blitterswijk CA. Bone tissue-engineered implants using human bone marrow stromal cells: effect of culture conditions and donor age. Tissue Eng. 2002;8:911–920. doi: 10.1089/107632702320934010. [DOI] [PubMed] [Google Scholar]
- 48.Alves H, Munoz-Najar U, De Wit J, Renard AJ, Hoeijmakers JH, Sedivy JM, Van Blitterswijk C, De Boer J. A link between the accumulation of DNA damage and loss of multi-potency of human mesenchymal stromal cells. J Cell Mol Med. 2010;14:2729–2738. doi: 10.1111/j.1582-4934.2009.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veronesi F, Torricelli P, Borsari V, Tschon M, Rimondini L, Fini M. Mesenchymal stem cells in the aging and osteoporotic population. Crit Rev Eukaryot Gene Expr. 2011;21:363–377. doi: 10.1615/critreveukargeneexpr.v21.i4.60. [DOI] [PubMed] [Google Scholar]
- 50.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B. One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res. 2009;467:3307–3320. doi: 10.1007/s11999-009-0885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


