Abstract
Skin-derived precursors (SKPs), which are located at skin’s dermis, display multi-lineage potential and can produce both neural and mesodermal progeny in vitro. SKPs are considered to take part in dermal reconstruction and may be an important source of fibroblast during wound repairing. To explore the possibility of differentiation of SKPs into fibroblasts, the 3rd passage SKPs were treated with 0, 20, 40, 100, or 500 ng/ml human recombinant connective tissue growth factor (CTGF) for 48 h or treated with 100 ng/ml CTGF for 0, 24, 48, 72, or 96 h. Subsequently, a series of methods were to be used to observe cells immunocytochemistry changes under fluorescence microscope, to validate the mRNA expression change of collagen I, collagen III, fibroblast-specific protein 1 (FSP-1) and alpha smooth muscle actin (α-SMA) by quantitative real-time reverse transcriptase polymerase chain reaction (QRT-PCR), to analyze the expression of collagen I and collagen III protein by Enzyme-linked immunosorbent assay (ELISA), to semiquantitatively measure the expression of FSP-1 and α-SMA by western-blot. After differentiation, cells showed that positively staining for collagen I, collagen III, α-SMA, and FSP-1, which are markers for fibroblasts, but negative expression for neural precursors. The effects of CTGF on collagen I, collagen III, FSP-1 and α-SMA in SKPs were detected both on the transcriptional and posttranscriptional levels. These findings indicate that SKPs can be induced to differentiate into fibroblast-like cells with CTGF treatment that may be a key source of fibroblast in wound healing.
Keywords: Skin-derived precursor (SKP), differentiation, fibroblast-like cell, connective tissue growth factor (CTGF)
Introduction
Skin is a large, complex, and highly regenerative organ, and it is also prone to injury when exposed to harmful environments. Fibroblasts play critical roles in normal wound healing, especially in the late inflammatory phase and during epithelialization [1]. The exact origin of the fibroblasts involved in wound healing is a matter of debate. For the most part, these fibroblasts are recruited from the dermis and tissues around the wound site. Another potential source of these fibroblasts is from epithelial cells that alter their phenotype via a process called epithelial-mesenchymal transition (EMT) [2].
Many different precursor and stem cell populations exist in the skin. Such stem cell populations include both follicular and interfollicular epidermal stem cells, dermal mesenchymal stem cells, various hair follicle stem cells, endothelial and hematopoietic stem cells, as well as precursors of specific neural crest derivatives such as melanocytes [3]. Therefore, it is possible that such stem/precursor cells also give rise to fibroblasts. Our previous study substantiated that dermal papilla cells, a population of stem cells located at the bottom of hair follicles, can be induced to differentiate into fibroblasts by transforming growth factor-beta 1 (TGF-β1) [4]. In recent research, multipotent skin-derived precursors (SKPs), which are isolated from the skin’s dermis, are deemed to have a precursor (or stem cell) role. SKPs display multi-lineage potential and can produce both neural and mesodermal progeny in vitro [5,6], thus showing properties distinct from other known precursors within the skin. A previous study showed that after being transplanted into adult mice, SKPs were morphologically similar to endogenous fibroblasts and expressed the dermal fibroblast markers PDGFRα, type I collagen, vimentin, fibronectin, and fibroblast-specific antigens [7]. Additionally, in vivo, SKPs were recruited to the fibrotic lesion in response to bleomycin and differentiated into myofibroblasts in response to serum. These results suggest the intriguing possibility that SKPs contribute to skin fibrosis [8]. On the basis of these findings, we hypothesize that skin contains precursor cells (SKPs) that have the potential to differentiate into fibroblasts. Therefore, this study aimed to investigate the possibility that SKP differentiation into fibroblasts provides some of the fibroblasts used in wound regeneration and skin development.
Materials and methods
Reagents
Major reagents included the following: Dulbe- cco’s modified Eagle’s medium (DMEM)/F12 (Invitrogen Corporation, Carlsbad, CA); B27 (2%, Invitrogen); epidermal growth factor (EGF; 20 ng/ml, Peprotech EC LTD, London, UK); basic fibroblast growth factor (bFGF; 40 ng/ml, Peprotech EC LTD); Hank’s balanced salt solution (HBSS; Invitrogen); trypsin (Gibco BRL, Rockville, MD); recombinant human CTGF (Gibco BRL); collagen I antibody (1:200) and collagen III antibody (1:400) (Sigma Aldrich, St. Louis, MO); α-SMA antibody (1:400, Sigma Aldrich); FSP-1 antibody (1:500, Millipore, Billerica, CA); nestin antibody (1:400, BD Biosciences, San Jose, CA); stem cell antigen-1 (Sca-1) antibody (1:100, BD Biosciences); the Hoechst 33258 stain (5 μg/ml Sigma, Oakville, Ontario, Canada); anti-rabbit IgG-FITC (1:200); and IgG-CY3 antibodies (1:400) (Sigma).
Cultivation of SKPs
SKPs were isolated as previously reported [9,10]. Briefly, dorsal or facial skin from juvenile mice (3-21 days old) were obtained, and skin samples were cut into 2-3-mm2 pieces, then digested with 0.1% trypsin at 37°C for 40 min. Digestion was terminated by DMEM/F12. The skin pieces were mechanically dissociated and filtered through a 40-μm cell strainer (Falcon, BD Biosciences, San Diego, CA). The filtrate was centrifuged and resuspended in 10 ml DMEM/F12 supplemented with 2% B27, 20 ng/ml EGF, and 40 ng/ml bFGF. The cells were cultured in 25 cm2 tissue culture flasks (Corning Inc, Acton, MA) in a 37°C, 5% CO2 tissue-culture incubator. To passage the floating clusters of cells, the pellet was mechanically dissociated reseeded in fresh medium containing B-27 and the above-mentioned growth factors. The cells were passaged every 6-7 d. In the present study, SKPs at passage 3 were used for all experiments.
For differentiation, spheres were centrifuged, the growth factor–containing supernatant was removed, and cells dissociated from spheres were plated onto 4-well Nunclon culture slides coated with poly-D-lysine/laminin for 1 h. Then they were treated with 0, 20, 40, 100, or 500 ng/ml human recombinant CTGF for 48 h or treated with 100 ng/ml CTGF for 0, 24, 48, 72, or 96 h. All these experimental conditions were found to be appropriate in a previous study on fibroblasts [11].
Immunocytochemistry
Immunocytochemical analysis was performed on cells plated on chamber slides (Nalge Nunc) as previously described [9]. After treating cells with 100 ng/ml CTGF for 96 h, they were washed twice by HBSS and fixed with 4% paraformaldehyde for 5 min and then rinsed with HBSS. The cells were blocked by 3% goat serum containing 0.1% Triton-X100 (Sigma) and dialysed for 20 min. Cells were then incubated with primary antibodies diluted in blocking solution overnight at 4°C. After rinsing with HBSS, anti-rabbit IgG-FITC and IgG-CY3 antibodies were added to the cells, which were then cultured at 37°C for 20 min and washed with HBSS. Hochest 33258 was added to stain the nucleus before washing with HBSS, and then the slides were sealed. All the above procedures were carried out in a darkroom, and HBSS was used as a negative control instead of the primary antibody. The samples were observed and imaged under a fluorescence microscope (Leica DMI6000, Solms, Germany).
QRT-PCR analysis
Total RNA of stimulated cells was extracted using TRIzol®. Culture medium was removed and TRIzol was added directly onto the cells. The cells were then scraped and the resulting solution was collected in sterile 2-ml tubes. RNA extraction was then carried out according to the manufacturer’s instructions. The yield of total RNA was quantified by optical density (OD) readings at 260 nm, and the purity was estimated by the ratio of 260 nm to 280 nm. Reverse transcription of RNA to cDNA was carried out in a final volume of 20 μl using a PrimeScript RT Reagent Kit (TaKaRa, Japan) following the manufacturer’s instructions. QRT-PCR was carried out with the SYBR Premix Ex Taq kit (TaKaRa, Japan) according to the manufacturer’s instructions, and it was performed using the 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Primer sequences used for QRT-PCR are given as follows. β-actin: sense 5’-GCT TCT GGG TTC CGA TGA TA-3’, antisense 5’-CCT GGC ACA CCA TCA TCT TG-3’; collagen I: sense 5’-GAG CCC TCG CTT CCG TAC TC-3’, antisense 5’-TGT TCC CTA CTC AGC CGT CTG T-3’; collagen III: sense 5’-CTT CTC ACC CTT CTT CAT CCC-3’, antisense 5’-AGC CTG TTC AAA TCG GTA CC-3’; FSP-1: sense 5’-CTG GGG AAA AGG ACA GAT GA-3’, antisense 5’-TGC AGG ACA GGA AGA CAC AG-3’; α-SMA: sense 5’-CTG ACA GAG GCA CCA CTG AA-3’, antisense 5’-CAT CTC CAG AGT CCA GCA CA-3’. QRT-PCR was performed according to the manufacturer’s instruction with each cycle in an Eppendorf Thermal Cycler (Takara, Tokyo, Japan) using the appropriate cycle profile. After the reaction, aliquots of the product were run on a 1% agarose gel and stained with ethidium bromide. Amplification efficiencies (performed in triplicate) were validated and normalized against mouse β-actin using the comparative CT method.
ELISA of collagen I and III
Cell culture medium from different groups was collected and centrifuged at 3000 rpm for 15 min. Collagen I and collagen III were analyzed with ELISA (R&D Systems, Minneapolis, MN). The ELISA kits for collagen I and collagen III were purchased from Takara Bio Inc. (Otsu, Shiga, Japan). The levels of collagen I and collagen III secreted from treated cells were quantified according to the manufacturer’s recommended protocols. Measurements were repeated at least three times with independent cell batches.
Western blot analysis of FSP-1 and α-SMA
Western blot analysis was used to assess the protein expression of FSP-1 and α-SMA as previously described [12]. Briefly, cells were lysed using a 1% protease inhibitor mixture, 1% phenylmethylsulfonyl fluoride, and 1% sodium orthovanadate in RIPA lysis buffer (Santa Cruz Biotechnology, CA). The samples were scraped, collected, and centrifuged at 2500 rpm for 10 min. The supernatant was collected, and protein concentrations were determined by the Bradford assay. Electrophoresis was carried out and the separated proteins were then transferred to a nitrocellulose membrane (Amersham Biosciences). The membrane was blocked with Tris-buffered saline (TBS) containing 5% nonfat powdered milk for 1 h and then incubated with the FSP-1 and α-SMA antibodies at 4°C overnight. Expression of GAPDH was analyzed as a control to ensure equal loading (anti-GAPDH, 1:1000 diluted in TBS). Proteins were visualized using enhanced chemiluminescence (Amersham Biosciences) according to the manufacturer’s instructions, and the density of each band was determined using NIH image analysis software and expressed as a percentage of GAPDH density.
Statistical analysis
The statistical significance between the experimental groups and the control samples were determined by the Student’s t-test or analysis of variance (ANOVA) followed by Newman-Keuls multiple comparison test using GraphPad Prism5 (San Diego, CA). Graphs show mean ± s.d., and P<0.05 was considered statistically significant.
Results
Morphological and immunocytochemical changes of cells
Before they were treated with CTGF, SKPs grew as spheres in suspension (Figure 1A). After cells had been treated for 12 h, the cell spheres began to stick to the slides of the culture flasks. By 24 h, cells showed triangular or polygonal morphologies and started spreading out radially from the cell spheres (Figure 1B). At subsequent times, some of the cells began to proliferate and divide. After cells were treated with CTGF for 48 h, spindle-shaped cells appeared around the spheres, which had long spindle-shaped morphologies and a whorled arrangement. These cells lost their ability to aggregate after they were treated for 96 h (Figure 1C).
Figure 1.
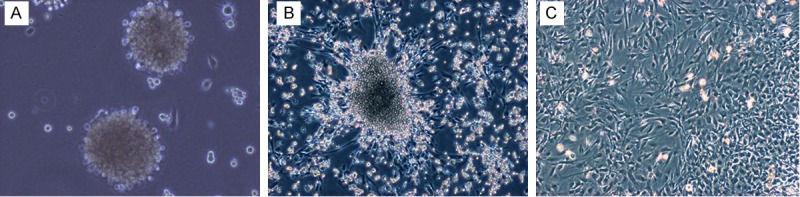
Morphological changes of SKPs after treated with CTGF. A: The appearance and growth of SKPs at passage three. B: The appearance of differentiated cells for 24 h after treated with CTGF with 100 ng/ml CTGF. C: The appearance of differentiated cells for 96 h after treated with 100 ng/ml CTGF. A-C: Magnification is ×100. Abbreviations: SKPs, skin-derived precursors; CTGF, connective tissue growth factor.
To evaluate the differentiation of SKPs into fibroblast-like cells, we first assessed several markers of cell differentiation using immunocytochemistry. Markers for fibroblasts, such as collagen I (Figure 2A), collagen III (Figure 2B), α-SMA (Figure 2D), and FSP-1 (Figure 2E) [13], were positively expressed. At the same time, these cells were negative for both nestin (Figure 2C) and Sca-1 (Figure 2F), which are markers for neural precursors [14,15]. All results were consistent with immunophenotypic markers of fibroblasts.
Figure 2.
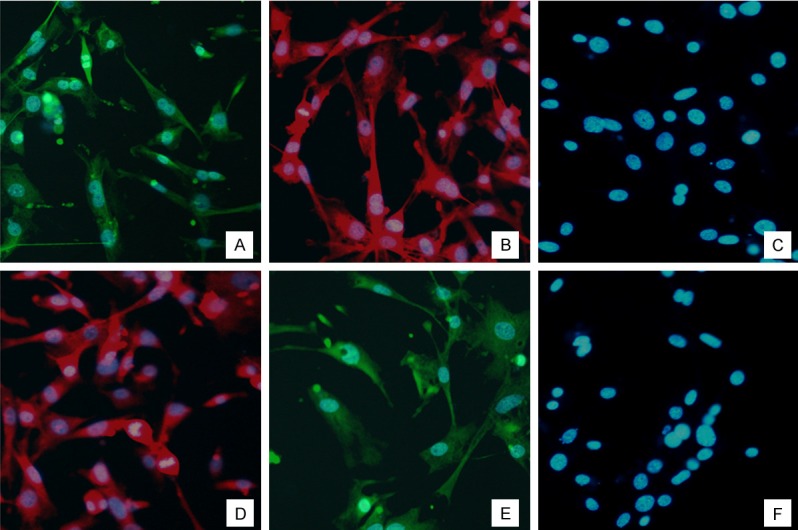
Immunofluorescence staining of differentiated cells for 96 h after treated with 100 ng/ml CTGF. A: Immunofluorescence staining of Collagen I in the treated group. B: Immunofluorescence staining of Collagen III in the treated group. C: Immunofluorescence staining of Nestin in the treated group. D: Immunofluorescence staining of FSP 1 in the treated group. E: Immunofluorescence staining of α-SMA in the treated group. F: Immunofluorescence staining of Sca-1 in the treated group. A-F: Magnification is ×200. Abbreviations: CTGF, connective tissue growth factor.
QRT-PCR analysis of gene expression of fibroblast
To assess whether CTGF altered gene expression relevant to fibroblasts, SKPs were treated with various doses of CTGF as described above, and then mRNA levels for collagen I, collagen III, α-SMA, and FSP-1 in the treated cells were measured using QRT-PCR. The results showed that 40-500 ng/ml CTGF led to a significant increase in mRNA levels for collagen I, collagen III, FSP-1, and α-SMA. On the other hand, only significant changes in collagen III mRNA levels were observed in the group given 20 ng/ml CTGF (Figure 3A).
Figure 3.
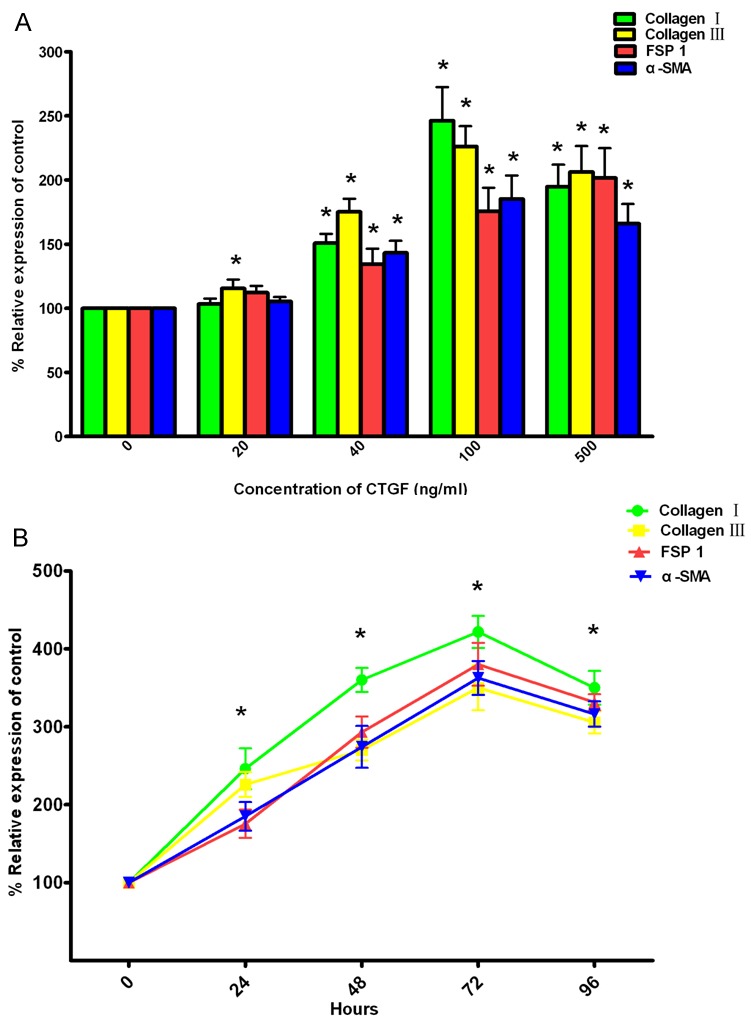
Effect of CTGF on mRNA levels for Collagen I, Collagen III, FSP-1 and α-SMA in SKPs. A: Dose response of CTGF in SKPs. SKPs were treated with 20-500 ng/ml CTGF for 48 hours, and then mRNA levels for Collagen I, Collagen III, FSP 1 and α-SMA were assessed by quantitative real-time reverse transcriptase polymerase chain reaction (QRT-PCR). B: The kinetics of CTGF responsiveness in SKPs. SKPs were treated with 100 ng/ml CTGF for 0-96 hours, and then mRNA levels for Collagen I, Collagen III, FSP-1 and α-SMA were assessed by QRT-PCR. All values indicates by an * are significantly different from control values (p<0.05). Abbreviations: SKPs, skin-derived precursors; CTGF, connective tissue growth factor; FSP-1, fibroblast-specific protein 1; α-SMA, alpha smooth muscle actin.
To assess how the timing of CTGF administration affected mRNA expression of fibroblasts, SKPs were treated with 100 ng/ml CTGF for various times, and then mRNA levels were calculated. Relative to control groups, significant increases in mRNA levels for the aforementioned genes were observed starting at 24 h, which subsequently peaked at 72 h. Then the mRNA levels decreased, but values remained significantly higher than the control values (Figure 3B).
ELISA of collagen I and collagen III
To evaluate the influence of CTGF on levels of collagen I and III, SKPs were treated with 20-500 ng/ml CTGF. Then the secreted levels of these proteins were assessed by ELISA. The results showed that 40-500 ng/ml CTGF led to a significant increase in protein levels of collagen I and III. The highest protein levels of collagen I and III were observed with 100 ng/ml CTGF; they decreased in the presence of 500 ng/ml CTGF, but these values remained significantly above the control value (Figure 4A).
Figure 4.
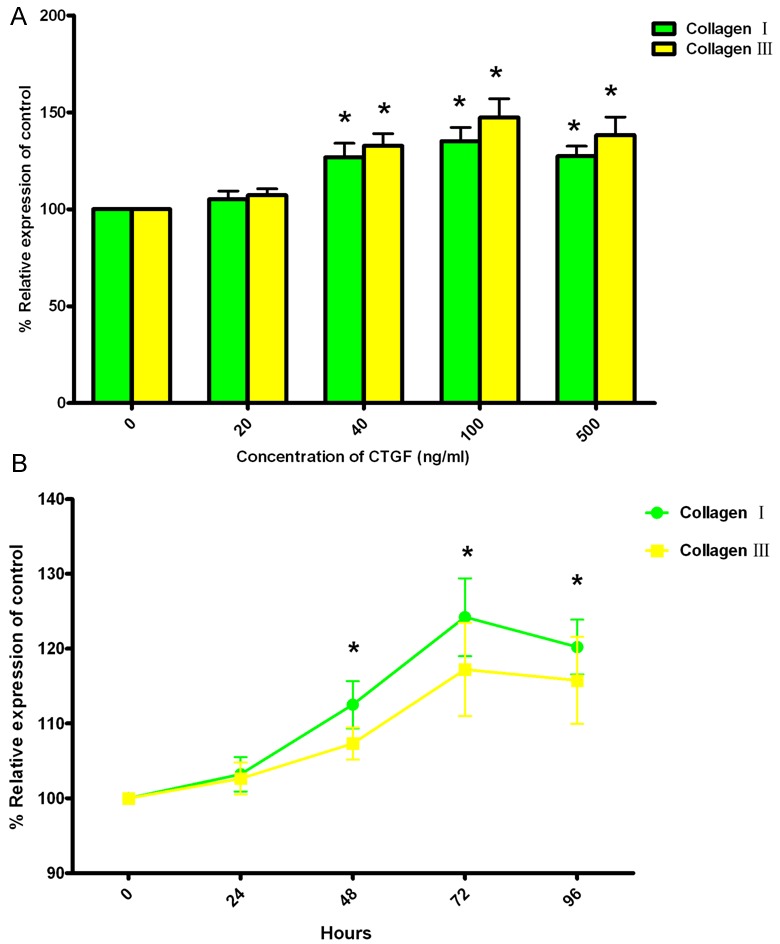
Effect of CTGF on protein levels for Collagen I and Collagen III in SKPs. A: Dose response of CTGF in SKPs. SKPs were treated with 20-500 ng/ml CTGF for 48 hours, and then protein levels for Collagen I and Collagen III were assessed by enzyme-linked immunosorbent assay (ELISA). B: The kinetics of CTGF responsiveness in differentiated cells. SKPs were treated with 100 ng/ml CTGF for 0-96 hours, and then protein levels for Collagen I and Collagen III were assessed by ELISA. All values indicates by an * are significantly different from control values (p<0.05). Abbreviations: SKPs, skin-derived precursors; CTGF, connective tissue growth factor.
The time-course experiment showed that protein levels for both collagen I and III were significantly elevated after 48 h of treatment, and highest levels were observed by 72 h. At 96 h, protein levels for both collagen I and III decreased, but these values were significantly higher than the control value (Figure 4B).
Western blot analysis of FSP-1 and α-SMA
To estimate how CTGF affected protein levels of FSP-1 and α-SMA, SKPs were treated with 20-500 ng/ml CTGF, and then the secreted protein levels were assessed by western blot analysis. The results showed that the protein levels of FSP-1 and α-SMA were significantly increased after treatment with 100 ng/ml CTGF, and they reached maximal levels with 500 ng/ml CTGF (Figure 5A).
Figure 5.
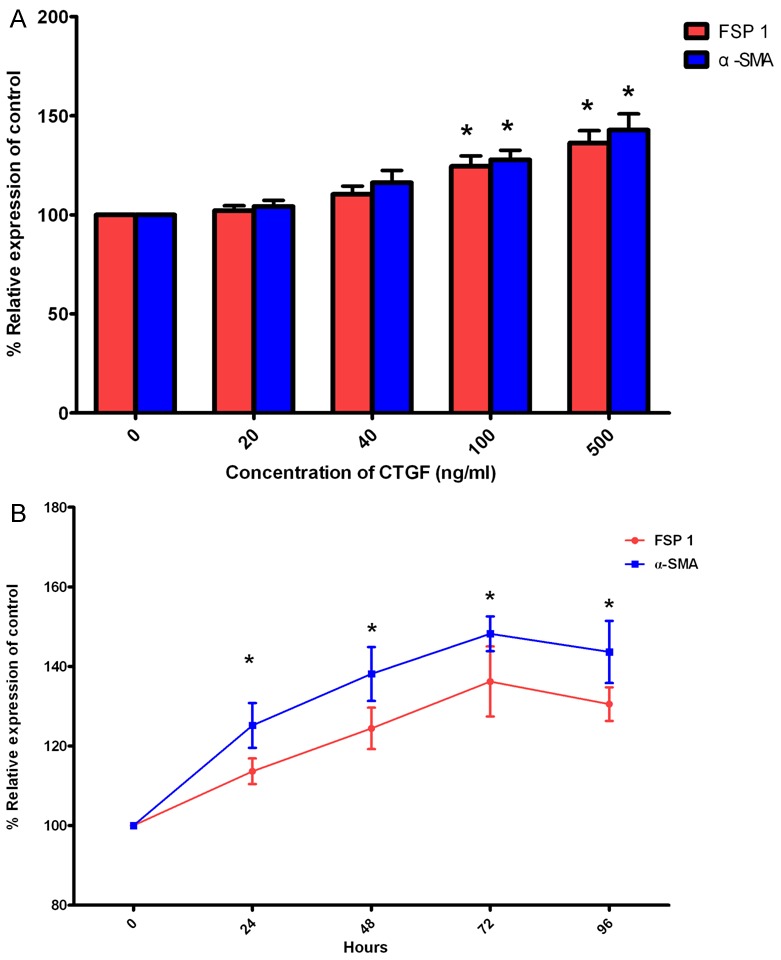
Effect of CTGF on protein levels for FSP-1 and α-SMA in SKPs. A: Dose response of CTGF in SKPs. SKPs were treated with 20-500 ng/ml CTGF for 48 hours, and then protein levels for FSP 1 and α-SMA were assessed by Western Blot. B: The kinetics of CTGF responsiveness in differentiated cells. SKPs were treated with 100 ng/ml CTGF for 0-96 hours, and then protein levels for FSP-1 and α-SMA were assessed by Western Blot. All values indicates by an * are significantly different from control values (p<0.05). Abbreviations: SKPs, skin-derived precursors; CTGF, connective tissue growth factor; FSP-1, fibroblast-specific protein 1; α-SMA, alpha smooth muscle actin.
To determine whether the increase in FSP-1 and α-SMA protein levels after being exposed to CTGF was a time-dependent response, SKPs were treated with 100 ng/ml CTGF for various times, as mentioned above. As shown in Figure 5B, treatment for 24 h led to a significant increase in protein levels for FSP-1 and α-SMA, and maximal levels were observed by 72 h. The protein levels for both FSP-1 and α-SMA decreased at 96 h, but these values remained significantly higher than the control value.
Discussion
The origin of differentiated fibroblasts in skin is poorly understood. Recent evidence suggests that resident and circulating stem cells may provide progenitors of tissue fibroblasts [16,17]. Although SKPs are widely accepted as pluripotent cells that can differentiate into different lineages [18-20], the differentiation of SKPs into fibroblasts is still unconfirmed.
Though SKPs are probably not responsible for skin reinnervation, as sensory neurons reside in dorsal root ganglia, they have enormous potential to take part in dermal reconstruction. These in vitro experiments demonstrate that SKPs can secrete collagen I and collagen III, which are important components of dermal ECM, after exposure to CTGF. In addition, we found that differentiated cells undergo morphologic changes toward a fibroblastic lineage. After induction with CTGF, the cells stained positively for collagen I, collagen III, FSP-1, and α-SMA, and they did not contain neural precursor markers (nestin and Sca-1). Overall, we found that administration of exogenous CTGF significantly induced the differentiation of fibroblast-like cells from murine SKPs, which did not show characteristics of neural precursors.
CTGF, a member of the CCN (CTGF, Cyr61/Cef10, Nov) protein family, is highly expressed in multiple fibrotic pathologies and induces fibroblast adhesion, extracellular matrix production, and proliferation [21,22]. During cutaneous wound repair, CTGF has physiological roles in regulating fibroblast proliferation and phenotype/differentiation as well as in regulating vascular cell populations [23]. In a previous study, administering CTGF in vivo promoted the generation of orderly healing fibers at the burn site and increased the proliferative response [24]. Recently, some studies have indicated that stem/progenitor cells are capable of differentiating into fibroblasts after exposing them to CTGF [25,26].
In our study, we explored using CTGF to induce differentiation of SKPs into fibroblasts in vitro. Our results suggest that genes expressed in fibroblasts, namely collagen I, collagen III, FSP-1, and α-SMA, are highly expressed during SKP differentiation with a time- and dose-dependent response. Moreover, the protein levels of collagen I and III as well as FSP-1 and α-SMA were elevated after cells were exposed to CTGF. Such findings are consistent with those indicating that CTGF can regulate the production of the extracellular matrix (ECM) [27]. These observations coincide with other results showing that subcutaneously injecting SKPs into the dorsal skin of adult NOD/SCID mice results in SKPs that integrate into the interfollicular dermis and express dermal fibroblast markers [7].
Some of the molecules with which CTGF has been reported to interact are cytokines and growth factors such as TGF-β. Increased levels of TGF-β, which is also implicated in fibrotic repair, precede CTGF induction after a wound occurs, suggesting that CTGF acts downstream of TGF-β signaling [28]. In theory, SKPs might also exhibit the characteristics of fibroblast differentiation under TGF-β treatment. However, previous studies have shown that TGF-β facilitates SKP growth, but not fibroblast differentiation [29]. We therefore hypothesize that the reliance on CTGF for fibroblast differentiation in vivo arises because CTGF signaling is required for the expression of fibroblast mitogens other than TGF-β. Taken together, the present findings may support the notion that CTGF-dependent TGF-β signaling promotes fibroblast differentiation and the formation of ECM during wound healing. Additional experiments that monitor the activity of TGF-β are necessary to confirm how CTGF induces fibroblast differen- tiation.
In summary, our study demonstrates that cells with the morphologic and functional features of fibroblasts can be reliably differentiated from murine SKPs by treating them with CTGF. Therefore, this study indicates that SKPs may participate in wound healing and may represent a key source of cutaneous fibroblasts. Moreover, the method we have developed provides a means to define the mechanisms that regulate fibroblast differentiation, which can be used in subsequent studies. In our future studies, we will investigate the role and mechanism of SKPs during the course of wound healing in vivo. A more complete understanding of how SKPs contribute to and differentiate into cells that regulate normal and impaired wound healing is vital for developing treatment strategies to improve wound healing.
Acknowledgements
This study was supported by Grants 81272153 and 81372062 from National Natural Science Foundation of China and should be attributed to department of burns in the First Affiliated Hospital of Sun Yat-sen University.
Disclosure of conflict of interest
None.
References
- 1.Forrest L. Current concepts in soft connective tissue wound healing. Br J Surg. 1983;70:133–140. doi: 10.1002/bjs.1800700302. [DOI] [PubMed] [Google Scholar]
- 2.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunt DP, Jahoda C, Chandran S. Multipotent skin-derived precursors: from biology to clinical translation. Curr Opin Biotechnol. 2009;20:522–530. doi: 10.1016/j.copbio.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Hou-Dong L, Bin S, Ying-Bin X, Yan S, Shao-Hai Q, Tian-Zeng L, Xu-Sheng L, Jin-Ming T, Ju-Lin X. Differentiation of rat dermal papilla cells into fibroblast-like cells induced by transforming growth factor β1. J Cutan Med Surg. 2012;16:400–406. doi: 10.1177/120347541201600608. [DOI] [PubMed] [Google Scholar]
- 5.Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–737. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes KJ, Toma JG, Miller FD. Multipotent skin-derived precursors: adult neural crest-related precursors with therapeutic potential. Philos Trans R Soc Lond B Biol Sci. 2008;363:185–198. doi: 10.1098/rstb.2006.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biernaskie J, Paris M, Morozova O, Fagan BM, Marra M, Pevny L, Miller FD. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell. 2009;5:610–623. doi: 10.1016/j.stem.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S, Herault Y, Pavlovic G, Leask A. Skin progenitor cells contribute to bleomycin-induced skin fibrosis. Arthritis Rheumatol. 2014;66:707–13. doi: 10.1002/art.38276. [DOI] [PubMed] [Google Scholar]
- 9.Toma JG, Akhavan M, Fernandes KJ, Barnabé-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabé-Heider F, Biernaskie J, Junek A, Kobayashi NR, Toma JG, Kaplan DR, Labosky PA, Rafuse V, Hui CC, Miller FD. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6:1082–1093. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- 11.Wang JF, Olson ME, Ball DK, Brigstock DR, Hart DA. Recombinant connective tissue growth factor modulates porcine skin fibroblast gene expression. Wound Repair Regen. 2003;11:220–229. doi: 10.1046/j.1524-475x.2003.11311.x. [DOI] [PubMed] [Google Scholar]
- 12.Bin S, Li HD, Xu YB, Qi SH, Li TZ, Liu XS, Tang JM, Xie JL. BMP-7 attenuates TGF-β1-induced fibroblast-like differentiation of rat dermal papilla cells. Wound Repair Regen. 2013;21:275–281. doi: 10.1111/wrr.12015. [DOI] [PubMed] [Google Scholar]
- 13.Ibrini J, Fadel S, Chana RS, Brunskill N, Wagner B, Johnson TS, El Nahas AM. Albumin-induced epithelial mesenchymal transformation. Nephron Exp Nephrol. 2012;120:e91–102. doi: 10.1159/000336822. [DOI] [PubMed] [Google Scholar]
- 14.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 15.McKenzie IA, Biernaskie J, Toma JG, Midha R, Miller FD. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J Neurosci. 2006;26:6651–6660. doi: 10.1523/JNEUROSCI.1007-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lama VN, Phan SH. The extrapulmonary origin of fibroblasts: stem/progenitor cells and beyond. Proc Am Thorac Soc. 2006;3:373–376. doi: 10.1513/pats.200512-133TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu Z, Miao C, Li J, Lei X, Liu S, Guo W, Cao Y, Duan EK. Skeletal myogenic potential of mouse skin-derived precursors. Stem Cells Dev. 2010;19:259–268. doi: 10.1089/scd.2009.0058. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Pradhan S, Liu C, Le LQ. Skin-derived precursors as a source of progenitors for cutaneous nerve regeneration. Stem Cells. 2012;30:2261–2270. doi: 10.1002/stem.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinbach SK, El-Mounayri O, DaCosta RS, Frontini MJ, Nong Z, Maeda A, Pickering JG, Miller FD, Husain M. Directed differentiation of skin-derived precursors into functional vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2011;31:2938–2948. doi: 10.1161/ATVBAHA.111.232975. [DOI] [PubMed] [Google Scholar]
- 21.Leask A, Parapuram SK, Shi-Wen X, Abraham DJ. Connective tissue growth factor (CTGF, CCN2) gene regulation: a potent clinical bio-marker of fibroproliferative disease? J Cell Commun Signal. 2009;3:89–94. doi: 10.1007/s12079-009-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oemar BS, Lüscher TF. Connective tissue growth factor. Friend or foe? Arterioscler Thromb Vasc Biol. 1997;17:1483–1489. doi: 10.1161/01.atv.17.8.1483. [DOI] [PubMed] [Google Scholar]
- 23.Alfaro MP, Deskins DL, Wallus M, DasGupta J, Davidson JM, Nanney LB, A Guney M, Gannon M, Young PP. A physiological role for connective tissue growth factor in early wound healing. Lab Invest. 2013;93:81–95. doi: 10.1038/labinvest.2012.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu LD, Shi HJ, Jiang L, Wang LC, Ma SH, Dong CH, Wang JJ, Zhao HL, Liao Y, Li QH. The repairing effect of a recombinant human connective-tissue growth factor in a burn-wounded rhesus-monkey (Macaca mulatta) model. Biotechnol Appl Biochem. 2007;47:105–112. doi: 10.1042/BA20060114. [DOI] [PubMed] [Google Scholar]
- 25.Lee CH, Shah B, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest. 2010;120:3340–3349. doi: 10.1172/JCI43230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Mao JJ, Chen L. Epithelial-mesenchymal interactions as a working concept for oral mucosa regeneration. Tissue Eng Part B Rev. 2011;17:25–31. doi: 10.1089/ten.teb.2010.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frazier K, Williams S, Kothapalli D, Klapper H, Grotendorst GR. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol. 1996;107:404–411. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- 28.Ujike K, Shinji T, Hirasaki S, Shiraha H, Nakamura M, Tsuji T, Koide N. Kinetics of expression of connective tissue growth factor gene during liver regeneration after partial hepatectomy and D-galactosamine-induced liver injury in rats. Biochem Biophys Res Commun. 2000;277:448–454. doi: 10.1006/bbrc.2000.3693. [DOI] [PubMed] [Google Scholar]
- 29.Kawase Y, Yanagi Y, Takato T, Fujimoto M, Okochi H. Characterization of multipotent adult stem cells from the skin: transforming growth factor-beta (TGF-beta) facilitates cell growth. Exp Cell Res. 2004;295:194–203. doi: 10.1016/j.yexcr.2003.12.027. [DOI] [PubMed] [Google Scholar]


