Abstract
The effects of acute and subacute toxicity of 1,8-cineole in Kunming mice were studied. After acute oral administration, the LD50 value (95% CL) was 3849 mg/kg (3488.8~4247.1 mg/kg). In the subacute toxicity study, there were no significant differences in body weight and relative organ weight between the control group and 1,8-cineole treatment groups. The histopathological examinations showed that granular degeneration and vacuolar degeneration appeared in liver and kidney tissue after administration of high dose of 1,8-cineole. Under electron microscopy, a series of ultrastructural changes were observed: The electron microscopy assays indicated that the influence of 1,8-cineole on the target organ at the subcellular level were mainly on the mitochondria, endoplasmic reticulum and other membrane type structure of liver and kidney.
Keywords: 1, 8-cineole, toxicity, histopathology, ultrastructural changes, mice
Introduction
1,8-cineole, also named cineole or eucalyptol, is a monoterpene oxide which presents in many plant essential oils, such as eucalyptus oil, Cinnamomum longepaniculatum oil, rosemary, psidium and clary sage oil; especially in eucalyptus oil and Cinnamomum longepaniculatum oil, the content of the 1,8-cineole in these two essential oils are up to 80% [1-4].
1,8-Cineole is traditionally used as a food flavoring agent, while for years it was proved to possess a various pharmacological activities including anti-microbial, anticancer, anti-inflammatory, antioxidation etc. [5-11] and was widely used for treatment of rheumatism, cough, bronchial asthma, and septic-shock-associated pathologies, as well as for pharmaceutical preparations like external applicant, nasal spray, disinfectant, analgesic, or food-flavoring agent [12-14].
Given to the widespread use in the food industry and medicine, more detail information about the toxicity of the 1,8-cineole is required. The present study was performed to evaluate the subacute toxicity of 1,8-cineole according to the NO. 407 of OECD test guideline with slight modifications. The goal of this paper was to reveal the potential target organs for toxicity of 1,8-cineole application to mice.
Materials and methods
Plant material
1,8-cineole was supplied by Yi bin Chuan Hui Perfumery Co. Ltd. (Yi bin, P.R. China) and stored in the pharmaceutical laboratory of College of Veterinary Medicine, Sichuan Agricultural University (Ya’an, P.R. China). All chemicals used in the test were analytical reagent (AR >99%).
Animals and diets
The experiment was carried out following the Regulations of Animal Experimentation of College of Veterinary Medicine, Sichuan Agricultural University, which is based on the Guidelines of the International Committee on Laboratory Animals.
Kunming strain male and female mice (a closed strain coming from Kunming, Yun nan province, P.R. China) were purchased from the Chengdu Dossy Experimental Animals Co. Ltd. [License No. SCXK (Sichuan) 2008-24], weight 18~22 g. Animal rooms were maintained at 22°C, a relative humidity of 40-70%, and a 12 h light/dark cycle. Mice were fed by Laboratory animals-mice and rats formula feeds and water ad libitum. The test animals were quarantined for 13 days before experiment.
Acute toxicity study
50 mice either sex were divided into five groups of ten. 1,8-cineole was emulsified in 2% Tween 80 and administrated mice by oral gavage in a final volume of 10 mL/kg. Mice were exposed to 1,8-cineole with a series of doses of 2969.4, 3374.86, 3847.34, 4385.96 and 5000 mg/kg b.w. The animals were observed for 24 h after administration with test compounds and the mortality were recorded. The LD50 value (95% CL) was calculated using the method of Litchfield and Wilcoxon [15].
Subacute toxicity study
Treatment of animals
Four groups of mice, each group containing 10, were randomly allotted to the control and 1,8-cineole treatment groups. 2% Tween 80 was used in Group I as placebo control. The mice in Group II, III and IV were treated with 1,8-cineole with the concentration of 1/20, 1/60, 1/180 of LD50, respectively. The animal behavior, body weight and mortality were recorded for 30 days.
Chromatin immunoprecipitation
Relative organ weight
The animals were sacrificed on day 31. After fasted overnight, all the mice were euthanized. The organs/tissues were carefully examined macroscopically and gross lesions were recorded. The relative weight (organ to body weight ratios) of liver, spleen and double kidneys were immediately calculated.
Pathological findings
Heart, liver, spleen, lungs, kidney, testis and ovary of 1,8-cineole treatment groups and control group were excised and then fixed in 10% neutral buffered formalin. Following dehydration and embedding, they were sectioned at 5 μm, stained with Hematoxylin and Eosin (H and E) and examined microscopically.
Ultrastructural observation
The organs were excised and prefixed in 2.5% glutaraldehyde solution, diced into 1 mm3, followed by three 15 min rinses with 0.1 M phosphate buffer (pH 7.4). Post-fixation was in cold 1% aqueous osmium tetroxide for 1 h. After rinsing with phosphate buffer again, the specimens were dehydrated in a graded ethanol series of 50~100% and then embedded in Epon 812. Ultra-thin sections were sliced with glass knives on a LKB-V ultramicrotome (Nova, Sweden), and stained with uranyl acetate and lead citrate. The sections were examined under a Hitachi H-600 transmission electron microscope.
Statistical analysis
All results were expressed as mean±standard deviation (x±S.D.) for the indicated number of experiments. The statistical significance of differences between means was calculated using One-way Analysis of Variance (ANOVA) followed by Dunnett’s test for multiple comparisons with the control group.
Results
Acute toxicity
Rapid cyanosis and stupor accompanied with irregular breathing, extreme sensitivity to noise and convulsions was observed in mice which treated with a lethal dose, and then these mice died due to respiratory failure. The mortality in each 1,8-cineole treatment groups were showed in Table 1. The mortalities were positively correlated with the dose of the 1,8-cineole. The LD50 values of 1,8-cineole calculated by the method of Litchfield and Wilcoxon [15] was 3849 mg/kg, which suggested that the toxicity of 1,8-cineole belongs to class 4 or low toxic according to the standard of Hodge and Sterner Scalle [16] and criteria of acute toxic classifications [17].
Table 1.
The result of oral acute toxicity test of 1,8-cineole in mice
| Groups | Dose | Animal number | Number of animal | Mortality | Livability |
|---|---|---|---|---|---|
|
| |||||
| (mg/kg) | (n) | dead/dosed | (p) | (q) | |
| I | 5000.00 | 10 | 9 | 0.9 | 0.1 |
| II | 4385.96 | 10 | 7 | 0.7 | 0.3 |
| III | 3847.34 | 10 | 5 | 0.5 | 0.5 |
| IV | 3374.86 | 10 | 3 | 0.3 | 0.7 |
| V | 2969.40 | 10 | 1 | 0.1 | 0.9 |
The percent mortality was recorded at 24 h post-treated with 1,8-cineole.
Subacute toxicity study
General observation, body and relative organ weight
In this assay, all the mice were survived. The animals in all groups were in good condition. The average body weights in different groups were shown in Figure 1. The body weights of the mice was increasing weekly in 1,8-cineole treated groups and the control group, and there was no significant difference among all the groups (P<0.05). There were also no significant differences in organ weights and relative organ weights between the 1,8-cineole treated groups and the control group after administration for 30 days (Table 2).
Figure 1.
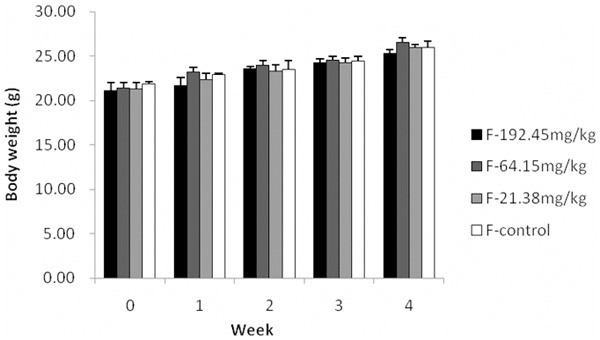
Effects of 1,8-Cineole on body weight increment of mice in 30 day treatment. Data are the mean±S.D. of 20 animals (One-way ANOVA).
Table 2.
The weight and relative organ weight of liver, spleen and kidney in the subacute toxicity study
| Dose | Liver | Spleen | Kidney | |||
|---|---|---|---|---|---|---|
|
| ||||||
| mg/kg | g | % | g | % | g | % |
| Group I: 0 | 0.95±0.03 | 4.15±0.37 | 0.09±0.02 | 0.44±0.14 | 0.25±0.03 | 1.16±0.04 |
| Group II: 21.38 | 0.95±0.07 | 4.59±0.33 | 0.08±0.01 | 0.41±0.02 | 0.25±0.06 | 1.18±0.12 |
| Group III: 64.15 | 1.06±0.11 | 4.55±0.52 | 0.08±0.02 | 0.37±0.11 | 0.28±0.09 | 1.22±0.36 |
| Group IV: 192.45 | 0.95±0.08 | 4.39±0.26 | 0.08±0.01 | 0.39±0.04 | 0.28±0.05 | 1.29±0.22 |
Values are mean±S.D.; n=20. Values are absolute wet weight of organ (g) and relative organ weight (%, per body weight). No significant difference from the control group at P<0.05 (using one-way ANOVA followed by Dunnett’s test for multiple comparisons with the control group).
Pathological findings
After 30 days of treatment with different dose of 1,8-cineole, the obvious pathological changes appeared in the liver and kidney of mice. The normal appearance of hepatic artery (HA), portal vein (PV), bile duct (BD) and sinusoids (Si) of liver of in Group I was presented in Figure 2A. Central venous congestion of liver lobule and granular degeneration of hepatocytes were appeared in Group II and III (Figure 2B, 2C), while in Group IV, more serious pathological changes involving central venous congestion, granular degeneration, vacuolar degeneration and hepatic necrosis appeared (Figure 2D). Normal appearances of kidney were appeared in Group I and II, in which glomerulus and renal tubule structure was clearly conserved (Figure 3A, 3B). Group III and Group IV showed pathological changes: capillary of glomerulus and interstitial angiectasis hyperemia, renal tubular epithelial cells swelling, granular degeneration and partially separating from basement membrane; amount of eosinophilic protein exudation existing in tubular lumen (Figure 3C, 3D). There were no distinct histopathological changes in the other organs examined in this test.
Figure 2.
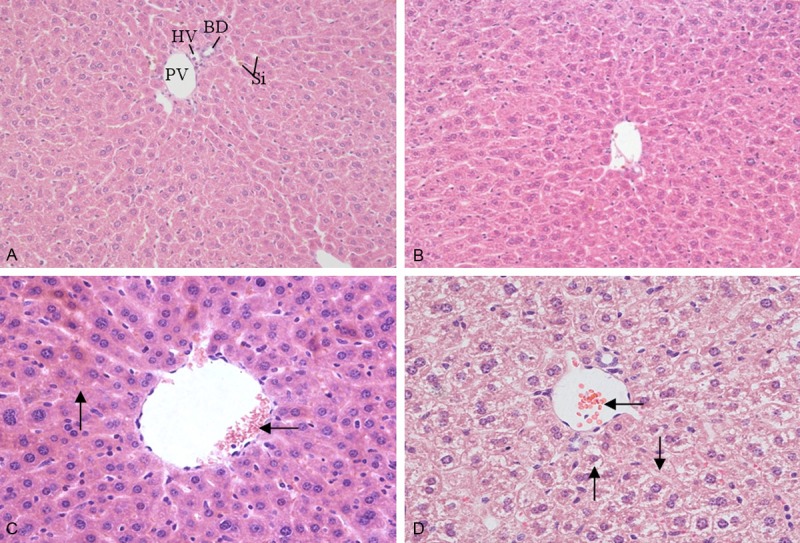
Effect of 1,8-cineole on the microstructures of liver in mice after administration for 30 days. A: Group I (0 mg/kg, HE 200×), control group showed hepatic artery (HA), portal vein (PV), bile duct (BD), sinusoids (Si); B: Group II (21.38 mg/kg, HE 200×), showed the normal characteristics of hepatic lobule; C: Group III (64.15 mg/kg, HE 400×), showed central venous congestion of liver lobule (←) and granular degeneration of hepatocytes (↑); D: Group IV (192.45 mg/kg, HE 400×), showed central venous congestion (←), granular degeneration and vacuolar degeneration (↑) and necrosis (↓) in hepatic cells.
Figure 3.
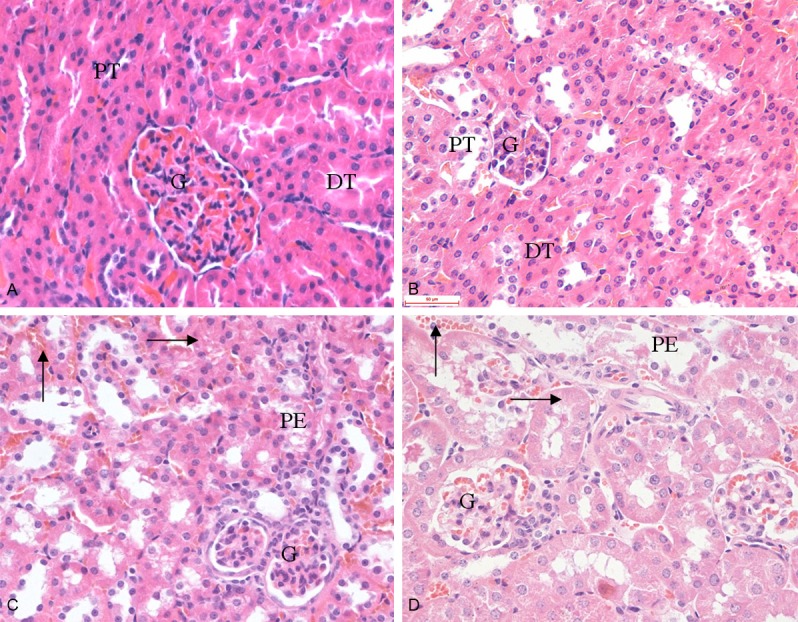
Effect of 1,8-cineole on the microstructures of kidneys in mice after administration for 30 days. A: Group I (0 mg/kg, HE 400×); B: Group II (21.38 mg/kg, HE 400×); C: Group III (64.15 mg/kg, HE 400×); D: Group IV (192.45 mg/kg, HE 400×). A and B: Showed the normal appearance of kidney, glomerulus (G), proximal tubule (PT) and distal tubule (DT). C and D: Showed a dose-related haemorrhagia (↑), granular degeneration (→), renal tubular epithelial cells swelling and separated from basement membrane. The renal tubal lumen containing eosinophilic protein exudation (PE).
Ultrastructural observation
Electron microscope has revealed specific ultrastructural changes in hepatic cells and renal tubular epithelial cells under experimental conditions. Details of a normal hepatic cell and its organelles are illustrated in Figure 4A. After 30 days of administrated with 1,8-cineole (192.45 mg/kg), the endoplasmic reticulum was distorted and fractured (Figure 4B). The ribosomal fell off from the rough endoplasmic reticulum membrane and scattered into the cytoplasm. The mitochondria were found to be swollen and the cristae within the mitochondria were disorganized.
Figure 4.
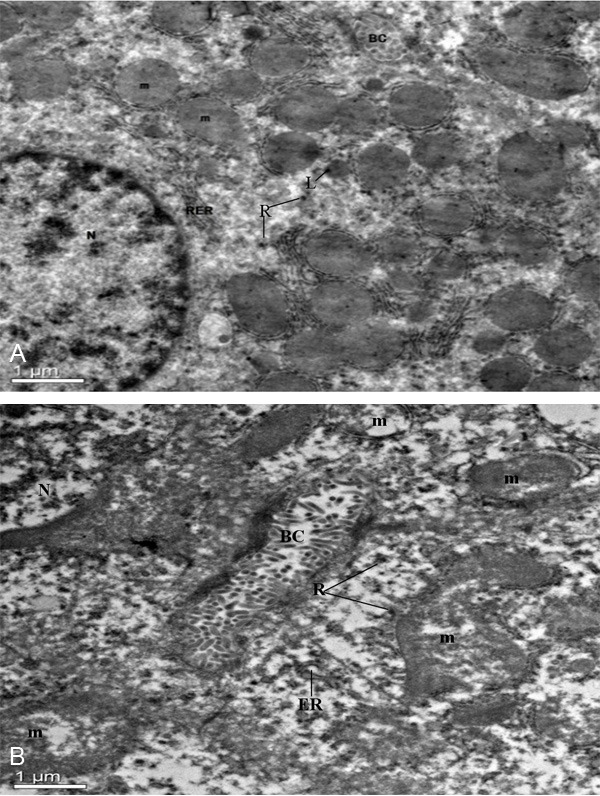
The ultrastructural changes of hepatic cells treated with or without 1,8-cineole. A: Normal hepatic cell of mice, ×2,550. Nucleus (N), mitochondria (m), bile canaliculus (BC), lysosomes (L), ribosome (R), rough endoplasmic reticulum (RER); B: Hepatic cell of 1,8-cineole (192.45 mg/kg) treated mice, ×2,550. Nucleus (N), bile canaliculus (BC), mitochondrion (m), endoplasmic reticulum (ER), ribosome (R).
The characterizations of intraluminal brush border of a normal proximal convoluted tubular cell are presented in Figure 5A. After 30 days of administrated with 1,8-cineole (192.45 mg/kg), some proximal convoluted tubule epithelial cells was interrupted and escape from the cytoplasm to the lumen of the tubule (Figure 5B). The usually elongated mitochondria in the basilar portion of the distal convoluted tubule epithelial cells became more spherical, with marked disorganization and swelling of the cristae. However, the basement membrane of mitochondria was intact (Figure 5C). No significant alteration was observed in the glomeruli. The well preserved fine structure of these areas was illustrated in Figure 5D.
Figure 5.
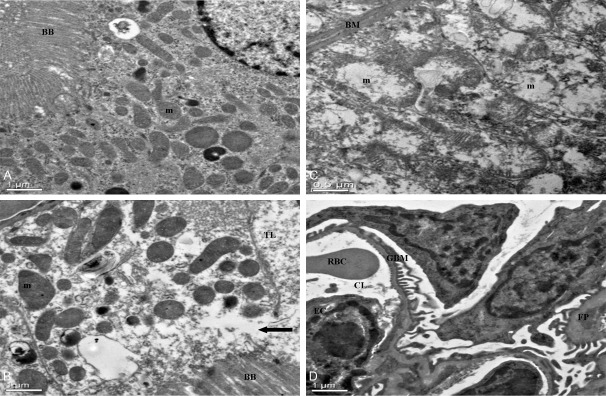
The ultrastructural changes of kidney cells treated with or without 1,8-cineole. A: Normal mice kidney, ×2,550. Brush border (BB), mitochondria (m), Nucleus (N); B: Proximal tubular cell of kidney, 30 days of administrated by 1,8-cineole (192.45 mg/kg). Note swelling and disorganization of mitochondria, and discontinuity of brush border at arrow, with escape of cytoplasm into tubular lumen, ×2,550. Brush border (BB), mitochondrion (m), tubular lumen (TL). C: Distal tubule of kidney, ×6,000. 30 days of administrated by 1,8-cineole (192.45 mg/kg). Note swelling and disorganization of mitochondria (m), the fingerlike projections of the basement membrane (BM) of distal convoluted tubule epithelial cells appeared distorted by the mitochondrial swelling, marked disorganization of the cristae and stippling in the matrix. D: Glomerulus, ×2,550. 30 days of administrated by 1,8-cineole (192.45 mg/kg). The fine structure appears well preserved. Glomerular Basement Membrane (GBM), foot process (FP), endothelial cells (EC), capillary lumen (CL), red blood cell (RBC).
Discussion
The aim of the present study was to evaluate the safety of 1,8-cineole when given as a single oral high dose or as a subacute administration of relatively low doses in long term exposure. The acute toxic potency of 1,8-cineole was relatively low (LD50 value = 3849 mg/kg). Mice which died in acute toxicity study were associated with hypoactivity and irregular breathing. These results were similar to the research reports of accidental intoxications caused by eucalyptus oil [18,19].
Our research of pathological and ultrastructural examinations demonstrated that the target organs of 1,8-cineole subacute toxicity were the liver and kidneys. The pathological findings showed that 21.38 and 64.15 mg/kg/day doses of 1,8-cineole had no or mild damages on liver and kidney, while 192.45 mg/kg/day dose of 1,8-cineole had serious damages on liver and kidney, with main lesions of granular and vacuolar degeneration and vascular congestion.
Subacute 1,8-cineole intoxication caused pathological and function damages in liver and kidney of mice. The damages of the sensitive organelles of somatic cells caused by xenobiotics can be captured by electron microscopy [20]. By method of transmission electron microscopy, the ultrastructure changes of hepatic cells and renal tubular epithelial cells were revealed for the first time. The main ultrastructure changes were mitochondrion swelling, vacuolar degeneration, cristae separating, rough endoplasmic reticulum fracture and degranulation. The results indicated that the influence of 1,8-cineole on the target organ at the subcellular level are mainly on the mitochondria, endoplasmic reticulum and other membrane type structure of liver and kidney.
In conclusion, The effect of 1,8-cineole on the liver and kidneys showed a good dose-dependent relationship that it caused no or slight damages on organs in the dose range of 0-64.15 mg/kg/day. The pathological and Ultrastructural observations indicated that the target organs of 1,8-cineole toxicity were the liver and kidney, but the toxicity mechanism of 1,8-cineole on liver and kidney needed further studied.
Acknowledgements
This study was supported by Foundation for the Author of Excellent Doctoral Dissertation of Sichuan agriculture university (YB201304); National Natural Science Foundation of China (Grant No. 31272612, 31372477); The Sichuan Youth Science and Technology Innovation Research Team for Resources Utilization of Cinnamomum longepaniculatum (2011JTD0035); The Sichuan Youth Science and Technology Innovation Research Team for waterfowl disease prevention and control (2013TD0015) and Sichuan provincial achievement transformation cultivation project (11ZZ022).
Disclosure of conflict of interest
None.
References
- 1.Kovar KA, Gropper B, Friess D, Ammon HP. Blood levels of 1,8-cineole and locomotor activity of mice after inhalation and oral administration of rosemary oil. Planta Med. 1987;53:315–318. doi: 10.1055/s-2006-962725. [DOI] [PubMed] [Google Scholar]
- 2.Manoel AN, José WA, Adriano NC, Edilberto RS, Terezinha GB. Volatile constituents of Psidium pohlianum and Psidium guyanensis Pers. J Essent Oil Res. 1994;6:299–300. [Google Scholar]
- 3.Farhat GN, Affara NI, Gali-Muhtasib HU. Seasonal changes in the composition of the essential oil extract of East Mediterranean sage (Salvia libanotica) and its toxicity in mice. Toxicon. 2001;39:1601–1605. doi: 10.1016/s0041-0101(01)00143-x. [DOI] [PubMed] [Google Scholar]
- 4.Cha JD, Kim YH, Kim JY. Essential oil and 1,8-cineole from artemisia lavandulaefolia Induces apoptosis in KB cells via mitochondrial stress and caspase activation. Food Sci Biotechnol. 2010;19:185–191. [Google Scholar]
- 5.Cermelli C, Fabio A, Fabio G, Quaglio P. Effect of eucalyptus essential oil on respiratory bacteria and viruses. Curr Microbiol. 2008;56:89–92. doi: 10.1007/s00284-007-9045-0. [DOI] [PubMed] [Google Scholar]
- 6.Santos FA, Rao VSN. Mast cell involvement in the rat paw oedema response to 1,8-cineole, the main constituent of eucalyptus and rosemary oils. Eur J Pharmacol. 1997;331:253–258. doi: 10.1016/s0014-2999(97)01013-3. [DOI] [PubMed] [Google Scholar]
- 7.Santos FA, Rao VSN. Inflammatory edema induced by 1,8-cineole in the hindpaw of rats: a model for screening antiallergic and anti-inflammatory compounds. Phytomedicine. 1998;5:115–119. doi: 10.1016/S0944-7113(98)80007-X. [DOI] [PubMed] [Google Scholar]
- 8.Santos FA, Silva RM, Campos AR, De Araújo RP, Lima Júnior RC, Rao VS. 1,8-cineole (eucalyptol), a monoterpene oxide attenuates the colonic damage in rats on acute TNBS-colitis. Food Chem Toxicol. 2004;42:579–584. doi: 10.1016/j.fct.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Juergens UR, Dethlefsen U, Steinkamp G, Gillissen A, Repges R, Vetter H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respir Med. 2003;3:250–256. doi: 10.1053/rmed.2003.1432. [DOI] [PubMed] [Google Scholar]
- 10.Juergens UR, Engelen T, Racké K, Stöber M, Gillissen A, Vetter H. Inhibitory activity of 1,8-cineol (eucalyptol) on cytokine production in cultured human lymphocytes and monocytes. Pulm Pharmacol Ther. 2004;17:281–7. doi: 10.1016/j.pupt.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Santos FA, Rao VS. 1,8-Cineole, a food flavoring agent, prevents ethanol-induced gastric injury in rats. Dig Dis Sci. 2001;46:331–337. doi: 10.1023/a:1005604932760. [DOI] [PubMed] [Google Scholar]
- 12.Juergens UR, Stöber M, Schmidt-Schilling L, Kleuver T, Vetter H. Antiinflammatory effects of eucalyptol (1,8-cineole) in bronchial asthma: inhibition of arachidonic acid metabolism in human blood monocytes ex vivo. Eur J Med Res. 1998;17:407–12. [PubMed] [Google Scholar]
- 13.Laude EA, Morice AH, Grattan TJ. The antitussive effects of menthol, camphor and cineole in conscious guineapigs. Pulm Pharmacol. 1994;7:179–84. doi: 10.1006/pulp.1994.1021. [DOI] [PubMed] [Google Scholar]
- 14.Levison KK, Takayama K, Isowa K, Okabe K, Nagai T. Formulation optimization of indomethacin gels containing a combination of three kinds of cyclic monoterpenes as percutaneous penetration enhancers. J Pharm Sci. 1994;83:1367–72. doi: 10.1002/jps.2600830932. [DOI] [PubMed] [Google Scholar]
- 15.Litchfield JT, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- 16.Hodge HC, Sterner JH. Tabulation of toxicity classes. Am Ind Hyg Assoc Q. 1949;10:93–96. doi: 10.1080/00968204909344159. [DOI] [PubMed] [Google Scholar]
- 17.Technical standards for testing and assessment of health food. Beijing: Ministry of Health P. R. China; 2003. pp. 203–204. [Google Scholar]
- 18.Webb NJA, Pitt WR. Eucalyptus oil poisoning in childhood: 41 cases in south-east Queensland. J Paediatr Child Health. 1993;29:368–371. doi: 10.1111/j.1440-1754.1993.tb00537.x. [DOI] [PubMed] [Google Scholar]
- 19.Darben T, Cominos B, Lee CT. Topical eucalyptus oil poisoning. Australas J Dermatol. 1998;39:265–267. doi: 10.1111/j.1440-0960.1998.tb01488.x. [DOI] [PubMed] [Google Scholar]
- 20.Wu ZB. Ultra pathological diagnosis. Shanghai: Shanghai Scientific and Technical Publishers; 2003. pp. 1–5. [Google Scholar]


