Abstract
We have reported Metadherin (MTDH) was proven to be overexpression and involved in malignance of chronic lymphocytic leukemia (CLL) via Wnt signaling pathway. In this study, we further investigate the role of MTDH in regulation of BCR signaling pathway in CLL. Six CLL samples whose cells were proliferation after BCR activation were chosen from patients with unmutated IgVH. CCK-8 method used to evaluate the proliferation rate. MTDH expression was measured by quantitative PCR and Western blot. After BCR activation, there exist upregulation of MTDH expression in mRNA and protein level in all six CLL patients (P<0.05). In cell line MEC-1, we observed the same pro-proliferation effect accompanying with elevated MTDH expression. The proliferation effects of BCR activation to MEC-1 can be inhibited by MTDH interference. The results of this study indicate that MTDH involved in the pro-proliferation effect of BCR activation in CLL. And the results imply that MTDH can be a potential therapy target of CLL.
Keywords: Chronic lymphocytic leukemia, B cell receptor, Metadherin
Introduction
Chronic lymphocytic leukemia (CLL) is characterize by the accumulation of abnormal monoclonal, mature B cells in peripheral blood, bone marrow, lymph node, spleen, liver and other organs [1,2]. Interactions between malignant B lymphocytes by its surface molecules and their tissue microenvironment play a major role in the pathogenesis of CLL [3,4]. In CLL, this cross talk leads to survival and expansion of the CLL clone and protects CLL cells from conventional cytotoxic drugs [5]. Inhibiting these pathways represents an alternative therapeutic strategy to more conventional chemo-immunotherapy program [4,6]. BCR is one of the most important signaling components in CLL. The mutational status of immunoglobulin genes is a strong prognostic predictor of CLL and BCR signaling has been postulated to have a role in CLL trafficking and interacts with the stromal microenvironment [7]. Understanding the function of B-cell receptor in CLL is important in gaining insights into the pathogenesis of the disease [8]. The relevant clinical advances of inhibitors targeting the BCR-associated kinases such as SYK (fostamatinib), BTK (ibrutinib), and PI3Kδ (idelalisib) highlight the importance of BCR signaling [4,6,9-12].
MTDH is a factor found in 2002 for its activation in human fetal astrocyte cells after treatment with TNF-α or HIV-1 [13]. In human fetal astrocyte, MTDH was proven to be activated by Ras through PI3K/AKT pathway via lead to c-Myc binding to key E-box element of MTDH promoter region [14]. Recently, MTDH was treated as a tumor oncogene and therapeutic target for its role in proliferation, migration and chemoresistance of tumor cells such as hepatocellular cancer, gastric cancer and diffuse large-B-cell lymphoma (DLBCL) [15-17]. In our previous studies, MTDH was proven to be an oncogene which overexpression in CLL primary cells and CLL cell line MEC-1. MTDH expression is associated with apoptosis-resistance and proliferation effects of CLL cells partially through activation of Wnt signaling pathway.
In this article, we try to investigate the role of MTDH in BCR signaling pathway in CLL. The correlated result may further improve our understanding of the pathogenesis of CLL.
Materials and methods
Patients samples and cell line
Venous blood was collected by EDTA anticoagulant tube. Before samples acquired, all patients were signed informed consent and approved by the Shandong Provincial Hospital ethics committee. Peripheral blood mononuclear cells were separated by Ficoll density gradient centrifugation. Then isolated using CD19+ magnetic selection kits (Miltenyi Biotec). The purity of selection were labeled by CD19-PE (eBioscience) antibody and detected by flow cytometry.
Human CLL cell line MEC-1 was cultured in Iscove’s Modified Dulbecco’s Medium (IMDM; Hyclone, Logan, UT, USA) with 10% fetal calf serum (FBS, Hyclone, Logan, UT, USA) maintained at 37°C in 5% carbon dioxide.
BCR activation assay
For BCR activation experiments, isolated B cells were cultured in IMDM supplemented with 10% FBS containing 10 ug/ml F(ab’)2 fragment goat and incubated at 37°C in a 5% CO2 humidified atmosphere for 48 hours.
Quantitative PCR
Total RNA was extracted from cells using TRNzol (TaKaRa, Dalian, China). RNA quality and concentration was assessed on an Agilent Bioanalyzer (Agilent Technologies) and the OD values of all samples were between 1.8 and 2.0. The reverse transcription system was made up as instructions of TaKaRa reverse transcription reagents with gDNA Eraser (TaKaRa, Dalian, China) and 1 ug of RNA was added to each 20 ul reaction system. Amplification was performed with SYBR Premix Ex Taq (TaKaRa, Dalian, China) in a total volume of 20 ul which contains SYBR Premix Ex TaqTM 10 μl, 0.4 μl of each primer (10 μM), 2 μl of 1:10 diluted cDNA and dH2O 7.2 μl. Standard curves and PCR results were analyzed using Light Cycler480 Gene Scanning Software_V1.5 (Roche Diagnostics, Dubai, UAE). Primers in this study we adopted from previous articles [16,18], and correspondence sequence were as follows: MTDH, F: 5’-TTACCACCGAGCAACTTACAAC-3’, R-5’-ATTCCAGCCTCCTCCATTGAC-3’; β-actin F: 5’-TGACGTGGACATCCGCAAAG-3’, R: 5’-CTGGAAGGTGGACAGCGAGG-3’. Data were analyzed using the 2-ΔゔCT method.
Western blot
Total protein was extracted from PBMCs of CLL using RIPA and 1% PMSF (Shenergy Biocolor, Shanghai, China). The concentration of extracted protein was measured by the BCA assay (Shenergy Biocolor) following the instructions. Electrophoresis was performed on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred on polyvinylidene difluoride (PVDF) membranes. After blocking by 5% milk for 1 hour in room temperature, membranes were covered with primary antibodies at 4°C overnight. Then Wash membranes by TBST for 10 min by three times, immunodetection was done with enhanced-chemiluminescence detection kit (Millipore, Massachusetts, USA) after one hour incubation with anti-rabbit IgG horseradish peroxidase (HRP)-conjugated or anti-mouse IgG HRP-conjugated antibodies (Zhongshan Goldenbridge, Beijing, China). Protein bands were measured by laser densitometry and analyzed by Multi Gauge Ver.4.0 software (Fujifilm Life Science, Japan). The concentration of antibodies used in this study were described below: anti-MTDH 1:250 (Invitrogen), anti-β-actin 1:2000 (Zhongshan Goldenbridge, Beijing, China).
Small interference RNAs
siRNAs targeting MTDH and a control scrambled siRNA were purchased from Shanghai Genechem Co. The Sequencing was designed following as other reports [17,19]. And siRNA transfection experiment method was established in our previous studies [16]. Methods were described briefly as followed: 104 MEC-1 cells were plated into 96-well plates and coculture with lentivirus with sequences of siRNA interfering MTDH gene or negative control for 10 hours. The multiplicity of MOI we used in this study is 150. Interfering efficiency in MEC-1 was determined in our previous studies by fluorescence microscope and flow cytometry. The transfection efficiency is about 60%.
Proliferation assays
5×103 cell were plated in 96 well plate. Before proliferation detected, 10 ul of CCK8 (Beyotime Institute of Biotechnology) added to the 100 ul cultured cell. After cocultured for 4 h, the absorbance was detected at a wavelength of 450 nm and adjusted at a wavelength of 690 nm.
Statistical methods
All statistical analyses were performed by using the SPSS18.0 software. The numerical data were statistically analyzed by 2-tailed Student’s t test, P<0.05.
Results
BCR activation promotes the proliferation of CLL cells
BCR can be activated by cross-linking the BCR with anti-IgM antibody [20,21]. Researchers have proven that cells of CLL patients with clinical characteristics such as um-IgVH, CD38 positive or ZAP70 positive are prone to proliferation after stimulation by anti-IgM antibody, and the most reliable predict factor is um-IgVH expression [9]. Considering the discrepancy responsive characteristic of two genotypic CLL patients with UM-IgVH or M-IgVH, In this study, we chose six CD19+ B cells samples whose cells were proliferation after F(ab’)2 fragment goat anti-human IgM antibody stimulation [22]. After stimulation by anti-IgM antibody for 48H coculture, the average proliferation rate is about 2.5 fold measured by CCK-8 methods (Figure 1).
Figure 1.

The proliferation rate of six CLL patients after activation of BCR. *p<0.05.
BCR activation accompanied by up-regulation of MTDH expression
To further interrogate the relationship between BCR activation and MTDH expression, MTDH expression was measured by quantity RT-PCR and Western blot to evaluate the expression of MTDH in cells of these 6 CLL patients. Results showed obvious overexpression of MTDH in mRNA and protein levels after BCR activation (Figure 2A and 2B) (P<0.05). This indicated that MTDH is involved in BCR signaling transduction.
Figure 2.

MTDH expression after BCR activation in CLL. A. mRNA expression of six CLL patients before and after BCR activation. B. The change of protein expression in six CLL patients.
MTDH involved in BCR activation of MEC-1
In our previous studies, MTDH expression was interfered successfully in CLL cell line MEC-1. The transfection efficacy is about 60% with thirty percent downregulation of MTDH protein. MEC-1 is a cell line of CLL with um-IgVH. To further investigate the role of MTDH in mediating BCR signaling pathway, we first observed the proliferation change of MEC-1 after stimulate by F(ab’)2 fragment goat anti-human IgM antibody. Results showed that cell proliferation rate was increased about 1.3 fold. To evaluate the role of MTDH in BCR signaling pathway, the proliferation changes of MTDH interference cell (MTDHi), control negative sequencing samples (NCi) and normal control (con) of MEC-1 cell line were measured by CCK-8 method (Figure 3) (P<0.05). The results indicated that proliferation effect of BCR activation can be blocked by MTDH interference in MEC-1. This result illustrated that BCR signaling transduction is dependent on MTDH expression.
Figure 3.
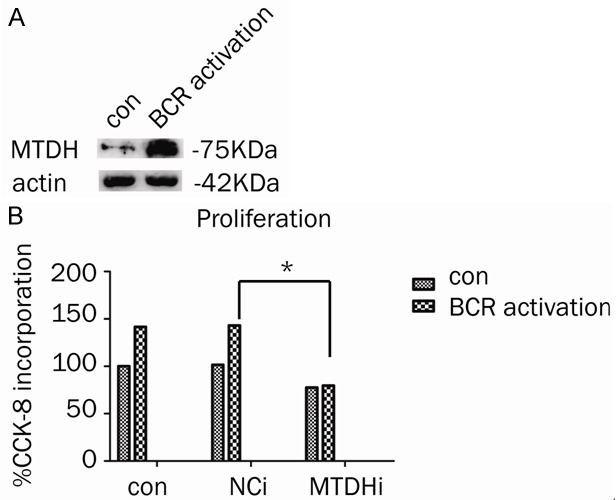
The role of MTDH in MEC-1 with BCR activation. A. MTDH expression in MEC-1 with or without BCR activation. B. The proliferation changes of MTDH in MEC-1 cell line with interference cell (MTDHi), control negative sequencing samples (NCi) and normal control (con).
Discussions
In the present study, we investigated the role of MTDH in BCR signaling pathway in CLL. The results showed that MTDH involved in BCR signaling transduction. Interference expression of MTDH can block the pro-proliferation effect of BCR activation.
The pathogenesis of CLL was proven to be associated with aberration regulation of apoptosis. In CLL, cells undergo apoptosis quickly under cultivating condition in vitro. But culture CLL cells in a condition monitoring microenvironment in vivo portrayed that cells viability are prone to increase [3]. CLL is a disease characterized by an extremely heterogeneous clinical courses, some patients may live for many years without requiring any treatment, whereas others, despite therapy, experience a rapid progression and in some cases an unfavorable fate. CLL patients with um-IgVH can be used to predict response to BCR stimulation which indicates a poor prognostic [23]. At the same time, in patients who maintain mutated VH gene, surface immunoglobulin M ligation failed to induce receptor translocation to rafts or to prolong cell survival [24].
In normal B cells, after stimulating by antigen, Syk and Lyn recruit and activate the downstream adapter proteins and molecules such as BLNK, Btk, PLCγ2, PI3K/AKT and NF-κB [25,26] (Figure 4). CLL intracellular signaling largely resembles that of healthy B cells. In CLL, BCR signaling associated kinases and their downstream factors were proven to be overexpressed in CLL, such as Syk, Lyn and PLCr2 [11,27-32]. Targeting these kinases molecules or their downstream targets leads to apoptosis of CLL cells [4]. One interesting phenomenon is that CLL cells with um-IgVH stimulated with anti-IgM are prone to proliferation [23].
Figure 4.

Schema for BCR mediated signaling in B cell.
In other tumors, the pro-tumor effect of MTDH is associated with PI3K/AKT pathway, MAPK pathway, Wnt or NOTCH signaling pathway [17,33,34]. In CLL, we had been proven that MTDH is associated with activation of Wnt signaling pathway by induce overexpression of LEF-1. In this study, we tried to investigate the role of MTDH in mediate BCR signaling pathway in six CLL patients chosen from patients with um-IgVH for their cells were proliferation at 48 hours after BCR stimulation. This pro-proliferation effect in CLL cells accompanying with MTDH up-regulation. This indicated that MTDH is involved in signaling transduction of BCR signaling.
To investigate the role of MTDH in proliferation which is induced by BCR activation, we verified the proliferation effect of MEC-1 characterized with UM-IgVH after BCR activation. After interfering MTDH expression, the proliferation effects of BCR activation can be inhibited evidently. This may implied that MTDH is a key component during the transduction of BCR signaling. This study indicate MTDH may be another effective therapeutic target in CLL. Together with our former research results, we propose that MTDH may be a key component of multiple signaling pathways and it may be a potential therapeutic target in CLL. The therapeutic role of MTDH is not only via inhibition of Wnt signaling but also via inhibiting of BCR signaling.
There remains many questions regarding the relationship between MTDH expression and BCR signaling pathway in CLL. Our further studies will focus on discussing the concise downstream pathway of BCR signaling of which MTDH regulating and investigating the difference role of MTDH in um-IgVH and m-IgVH.
Acknowledgements
This work was supported by National Natural Science Foundation (No. 81270598), Natural Science Foundations of Shandong Province (No. Y2007C053, No. 2009ZRB14176 and No. ZR2012HZ003), Technology Development Projects of Shandong Province (No. 2007GG10, and No. 2010GSF10250), National Public Health Grand Research Foundation (No. 201202017), Program of Shandong Medical Leading Talent and Taishan Scholar Foundation of Shandong Province.
Disclosure of conflict of interest
None.
References
- 1.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 2.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, Kipps TJ. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han TT, Fan L, Li JY, Xu W. Role of chemokines and their receptors in chronic lymphocytic leukemia: Function in microenvironment and targeted therapy. Cancer Biol Ther. 2014;15:3–9. doi: 10.4161/cbt.26607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Weerdt I, Eldering E, van Oers MH, Kater AP. The biological rationale and clinical efficacy of inhibition of signaling kinases in chronic lymphocytic leukemia. Leuk Res. 2013;37:838–847. doi: 10.1016/j.leukres.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Packham G, Stevenson F. The role of the B-cell receptor in the pathogenesis of chronic lymphocytic leukaemia. Semin Cancer Biol. 2010;20:391–399. doi: 10.1016/j.semcancer.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Burger JA, Montserrat E. Coming full circle: 70 years of chronic lymphocytic leukemia cell redistribution, from glucocorticoids to inhibitors of B-cell receptor signaling. Blood. 2013;121:1501–1509. doi: 10.1182/blood-2012-08-452607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quiroga MP, Balakrishnan K, Kurtova AV, Sivina M, Keating MJ, Wierda WG, Gandhi V, Burger JA. B-cell antigen receptor signaling enhances chronic lymphocytic leukemia cell migration and survival: specific targeting with a novel spleen tyrosine kinase inhibitor, R406. Blood. 2009;114:1029–1037. doi: 10.1182/blood-2009-03-212837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, Wang X. The role of TP53 network in the pathogenesis of chronic lymphocytic leukemia. Int J Clin Exp Pathol. 2013;6:1223–1229. [PMC free article] [PubMed] [Google Scholar]
- 9.Lanham S, Hamblin T, Oscier D, Ibbotson R, Stevenson F, Packham G. Differential signaling via surface IgM is associated with VH gene mutational status and CD38 expression in chronic lymphocytic leukemia. Blood. 2003;101:1087–1093. doi: 10.1182/blood-2002-06-1822. [DOI] [PubMed] [Google Scholar]
- 10.Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, Buggy JJ. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, Flynn J, Jones J, Blum KA, Buggy JJ, Hamdy A, Johnson AJ, Byrd JC. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li PP, Wang X. Role of signaling pathways and miRNAs in chronic lymphocytic leukemia. Chin Med J (Engl) 2013;126:4175–4182. [PubMed] [Google Scholar]
- 13.Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao W, Volsky DJ, Fisher PB. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene. 2002;21:3592–3602. doi: 10.1038/sj.onc.1205445. [DOI] [PubMed] [Google Scholar]
- 14.Lee SG, Su ZZ, Emdad L, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proc Natl Acad Sci U S A. 2006;103:17390–17395. doi: 10.1073/pnas.0608386103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu JB, Wu H, He YL, Zhang CH, Zhang LJ, Cai SR, Zhan WH. Astrocyte-elevated gene-1 overexpression is associated with poor prognosis in gastric cancer. Med Oncol. 2011;28:455–462. doi: 10.1007/s12032-010-9475-6. [DOI] [PubMed] [Google Scholar]
- 16.Ge X, Lv X, Feng L, Liu X, Gao J, Chen N, Wang X. Metadherin contributes to the pathogenesis of diffuse large B-cell lymphoma. PLoS One. 2012;7:e39449. doi: 10.1371/journal.pone.0039449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoo BK, Emdad L, Su ZZ, Villanueva A, Chiang DY, Mukhopadhyay ND, Mills AS, Waxman S, Fisher RA, Llovet JM, Fisher PB, Sarkar D. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. J Clin Invest. 2009;119:465–477. doi: 10.1172/JCI36460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutierrez A, Tschumper RC, Wu X, Shanafelt TD, Eckel-Passow J, Huddleston PM, Slager SL, Kay NE, Jelinek DF. LEF-1 is a prosurvival factor in chronic lymphocytic leukemia and is expressed in the preleukemic state of monoclonal B-cell lymphocytosis. Blood. 2010;116:2975–2983. doi: 10.1182/blood-2010-02-269878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene. 2008;27:1114–1121. doi: 10.1038/sj.onc.1210713. [DOI] [PubMed] [Google Scholar]
- 20.Bernal A, Pastore RD, Asgary Z, Keller SA, Cesarman E, Liou HC, Schattner EJ. Survival of leukemic B cells promoted by engagement of the antigen receptor. Blood. 2001;98:3050–3057. doi: 10.1182/blood.v98.10.3050. [DOI] [PubMed] [Google Scholar]
- 21.Nédellec S, Renaudineau Y, Bordron A, Berthou C, Porakishvili N, Lydyard PM, Pers JO, Youinou P. B cell response to surface IgM cross-linking identifies different prognostic groups of B-chronic lymphocytic leukemia patients. J Immunol. 2005;174:3749–3756. doi: 10.4049/jimmunol.174.6.3749. [DOI] [PubMed] [Google Scholar]
- 22.Guarini A, Chiaretti S, Tavolaro S, Maggio R, Peragine N, Citarella F, Ricciardi MR, Santangelo S, Marinelli M, De Propris MS, Messina M, Mauro FR, Del Giudice I, Foa R. BCR ligation induced by IgM stimulation results in gene expression and functional changes only in IgV H unmutated chronic lymphocytic leukemia (CLL) cells. Blood. 2008;112:782–792. doi: 10.1182/blood-2007-12-127688. [DOI] [PubMed] [Google Scholar]
- 23.Mockridge CI, Potter KN, Wheatley I, Neville LA, Packham G, Stevenson FK. Reversible anergy of sIgM-mediated signaling in the two subsets of CLL defined by VH-gene mutational status. Blood. 2007;109:4424–4431. doi: 10.1182/blood-2006-11-056648. [DOI] [PubMed] [Google Scholar]
- 24.Allsup DJ, Kamiguti AS, Lin K, Sherrington PD, Matrai Z, Slupsky JR, Cawley JC, Zuzel M. B-cell receptor translocation to lipid rafts and associated signaling differ between prognostically important subgroups of chronic lymphocytic leukemia. Cancer Res. 2005;65:7328–7337. doi: 10.1158/0008-5472.CAN-03-1563. [DOI] [PubMed] [Google Scholar]
- 25.Pogue SL, Kurosaki T, Bolen J, Herbst R. B cell antigen receptor-induced activation of Akt promotes B cell survival and is dependent on Syk kinase. J Immunol. 2000;165:1300–1306. doi: 10.4049/jimmunol.165.3.1300. [DOI] [PubMed] [Google Scholar]
- 26.Choi MY, Kipps TJ. Inhibitors of B-cell receptor signaling for patients with B-cell malignancies. Cancer J. 2012;18:404–410. doi: 10.1097/PPO.0b013e31826c5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contri A, Brunati AM, Trentin L, Cabrelle A, Miorin M, Cesaro L, Pinna LA, Zambello R, Semenzato G, Donella-Deana A. Chronic lymphocytic leukemia B cells contain anomalous Lyn tyrosine kinase, a putative contribution to defective apoptosis. J Clin Invest. 2005;115:369–378. doi: 10.1172/JCI22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gobessi S, Laurenti L, Longo PG, Carsetti L, Berno V, Sica S, Leone G, Efremov DG. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia. 2009;23:686–697. doi: 10.1038/leu.2008.346. [DOI] [PubMed] [Google Scholar]
- 29.Buchner M, Fuchs S, Prinz G, Pfeifer D, Bartholomé K, Burger M, Chevalier N, Vallat L, Timmer J, Gribben JG, Jumaa H, Veelken H, Dierks C, Zirlik K. Spleen tyrosine kinase is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukemia. Cancer Res. 2009;69:5424–5432. doi: 10.1158/0008-5472.CAN-08-4252. [DOI] [PubMed] [Google Scholar]
- 30.Ringshausen I, Schneller F, Bogner C, Hipp S, Duyster J, Peschel C, Decker T. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase Cdelta. Blood. 2002;100:3741–3748. doi: 10.1182/blood-2002-02-0539. [DOI] [PubMed] [Google Scholar]
- 31.Wang YH, Fan L, Wang L, Zhang R, Zou ZJ, Fang C, Zhang LN, Li JY, Xu W. Expression levels of Lyn, Syk, PLCgamma2 and ERK in patients with chronic lymphocytic leukemia, and higher levels of Lyn are associated with a shorter treatment-free survival. Leuk Lymphoma. 2013;54:1165–1170. doi: 10.3109/10428194.2012.736983. [DOI] [PubMed] [Google Scholar]
- 32.Buchner M, Fuchs S, Prinz G, Pfeifer D, Bartholome K, Burger M, Chevalier N, Vallat L, Timmer J, Gribben JG, Jumaa H, Veelken H, Dierks C, Zirlik K. Spleen tyrosine kinase is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukemia. Cancer Res. 2009;69:5424–5432. doi: 10.1158/0008-5472.CAN-08-4252. [DOI] [PubMed] [Google Scholar]
- 33.Hu G, Wei Y, Kang Y. The multifaceted role of MTDH/AEG-1 in cancer progression. Clin Cancer Res. 2009;15:5615–5620. doi: 10.1158/1078-0432.CCR-09-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C, Yang Q. Astrocyte elevated gene-1 and breast cancer (Review) Oncol Lett. 2011;2:399–405. doi: 10.3892/ol.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]


