Abstract
T-box 1 (Tbx1) gene is closely involved in embryonic kidney development. To explore the role of Tbx1 in acute kidney injury (AKI) and the underlying mechanism, we detected the expression of Tbx1 and components of transforming growth factor-beta (TGF-β) signaling pathways including TGF-β, phosphorylated Smad2/3 (p-Smad2/3) and phosphorylated Smad1/5/8 (p-Smad1/5/8) in kidney tissues derived from a rat model for AKI induced by gentamicin (GM). Apoptosis of renal cells was assessed by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL), along with the expression of two essential genes involved in apoptosis, caspase-3 and Bcl-2. Correlation between Tbx1 expression and the number of TUNEL-positive cells was analyzed by a Spearman test. Expression of TGF-β, p-Smad2/3 and p-Smad1/5/8 in Tbx1-knockdown NRK cells was also analyzed by real-time RT-PCR and Western blotting. Markedly increased Tbx1 expression was found in the injured kidney tissues, which has activated the TGFβ-Smad2/3 pathway whilst suppressed Smad1/5/8 expression. Conversely, decreased TGF-β and p-Smad2/3 levels, and elevated p-Smad1/5/8 levels were detected in Tbx1-knockdown NRK cells. More apoptotic cells were detected in the injured kidneys, which has well correlated with the expression of Tbx1. Expression of caspase-3 was markedly increased, while Bcl-2 was decreased in the injured kidney tissues. Above findings suggested that activation of Tbx1 is involved in AKI through the TGFβ-Smad2/3 pathway. Tbx1 expression may therefore serve as a marker for AKI, and Tbx1-blocking therapies may provide an option for treating GM-induced nephropathy.
Keywords: Acute kidney injury, gentamicin, Smad1/5/8, Smad2/3, Tbx1, TGF-β
Introduction
Gentamicin (GM) is a commonly used aminoglycoside antibiotic which is effective for most gram-negative microorganisms. However, therapeutic doses of GM can also induce kidney injury, that has occurred in 10~20% of patients undergoing the therapeutic regimen. GM-induced nephrotoxicity is among the most common causes of acute kidney injury (AKI), which can affect renal blood flow and urinary concentrating ability, and eventually lead to renal insufficiency [1].
Various transcription factors are expressed during kidney injury and recovery, which include transforming growth factor-beta (TGF-β), hypoxia-inducible factors, α-smooth muscle actin, monocyte chemotactic protein-1, and U-STAT3. Notably, all such factors have also been shown to play a role in kidney development [2-6].
T-box genes are an evolutionary conserved family of transcription factors involved in various developmental processes and signaling pathways. They play a key role in molecular mechanisms underlying various processes of tissue differentiation [7]. T-box 1 (Tbx1) gene plays an important role in embryogenesis. Its human homologue, TBX1, lies within a 1.5 Mb del22q11.2 region which is associated with a wide spectrum of birth defects (DiGeorge syndrome). In mice, mutations in Tbx1 can cause a DiGeorge syndrome-like phenotype in which development of aortic arch, thymus, parathyroid gland, skull, face, ears, kidneys, and teeth can be affected [8]. However, the role of TBX1 in the development of genitourinary system has not yet been explored. A substantial proportion of 22q11 deletion carriers have genitourinary malformations [7,9-12]. Studies have suggested that TBX1 is associated with at least five phenotypes of 22q11 deletion including those in the genitourinary system [13]. Fu et al. have demonstrated that Tbx1 can alter the TGF-β/bone morphogenetic protein (BMP) signaling pathway through interacting with HOXD10 during kidney development, which has shed light on the mechanism of TBX1 mutations that lead to renal malformations found in patients carrying a 22q11 deletion [14].
TGF-β and downstream Smad signaling have been shown to play an essential role in kidney injury and diseases. The TGF-β superfamily signaling comprises two branches: a TGF-β/nodal/activin branch that signals through activation of Smad2/3, and a BMP/growth and differentiation factor branch that signals through Smad1/ 5/8 [15,16]. These phosphorylated (p)-Smad proteins can then bind with Smad4 protein to regulate the transcription of target genes in a pathway-specific manner [17].
The present study was aimed to explore the potential role of the Tbx1 gene during AKI induced by GM and the underlying molecular mechanism.
Materials and methods
Animal care and treatment
Seventy 6-week-old male Wistar rats weighing 190 ± 10 g each were obtained from the Laboratory Animal Center of China Medical University. The rats were housed in plastic cages under standard conditions and allowed free access to water and a standard diet. The rats were divided into two groups (35 each) based on average weight and received daily intramuscular injections of GM sulfate (Xiangfan Pharmaceutical Factory, Hubei, China) in saline at 0 mg/kg (saline only, designated as the CTRL group) or 150 mg/kg for 1, 2, or 3 consecutive days (designated as the GM group) starting at day 0 [18]. Three animals from each group were sacrificed on day 0 (designated as the GM-0d group). Eight animals from each group were sacrificed on day 3 (designated as the GM-3d group) at 24 h following the third injection. Subsequently, eight animals from each group were sacrificed on days 7, 10, and 21 (designated as GM-7d, GM-10d and GM-21d groups, respectively).
16 h before the sacrifice, the rats were placed in metabolic cages on a refrigerated collection rack for urine collection and measurement of urinary protein. They were then anaesthetized with sodium pentobarbital, with blood collected via the femoral artery for renal function test. Rat kidneys were quickly removed, longitudinally sectioned, and placed in 10% buffered formalin (pH 7.4) for histological analysis. The remaining tissues were stored at -80°C for RNA and total protein isolation. The study protocol was approved by the Animal-Humane Ethics Committee of China Medical University.
Serum and urine analyses of kidney injury
Serum creatinine (Cr) and blood urea nitrogen (BUN) were determined with a KEYSYS biochemical Analyzer (Boehringer, Mannheim, Germany). Urinary β2 microglobulin (β2-MG) and albumin (Alb) were measured on an electrochemiluminescence platform (Meso Scale Discovery, Gaithersburg, MD, USA).
RNA isolation, reverse transcription and real-time PCR
RNA was extracted from kidney tissues or NRK cells (a rat renal tubular cell line) using TRIzol reagent (Invitrogen, Shanghai, China) following the manufacturer’s protocol. cDNA was synthesized using a RNA PCR kit (TaKaRa, Dalian, China). Using five pairs of primers (Table 1), real-time RT-PCR was carried out on an ABI 7500 system (Applied Biosystems, Foster City, CA, USA). Relative concentrations of mRNA were calculated based on Ct values and normalized to the level of β-actin mRNA of each sample. All reactions were performed with appropriate negative (template-free) controls. Four replicate wells were prepared for each sample. All reactions were repeated twice to ensure the reproducibility of the results.
Table 1.
Primer sequences for real-time PCR
| Target/control gene | Primer sequences | Amplicon size (bp) |
|---|---|---|
| Tbx1 | F: 5’-TAACCTGCTGGACGACAA-3’ | 121 |
| R: 5’-GAAGTTCTCCTCTGCGTATT-3’ | ||
| TGF-β | F: 5’-GCAACAACGCAATCTATGA-3’ | 201 |
| R: 5’-CAAGGTAACGCCAGGAAT-3’ | ||
| Caspase-3 | F: 5’-CAAGTCGATGGACTCTGGAA-3’ | 129 |
| R: 5’-GTACCATTGCGAGCTGACAT-3’ | ||
| Bcl-2 | F: 5’-CCGGGAGAACAGGGTATGATAA-3’ | 81 |
| R: 5’-CCCACTCGTAGCCCCTCTG-3’ | ||
| β-actin | F: 5’-CCCATCTATGAGGGTTACGC-3’ | 150 |
| R: 5’-TTTAATGTCACGCACGATTTC-3’ |
Western blotting
Frozen kidney tissues or cultured NRK cells were lysed in buffer. The protein concentration of each lysate was determined with a bicinchoninic acid kit (Keygen Biotech Co Ltd., Nanjing, China) following the manufacturer’s instructions. Total protein (30 μg) was applied to a 12% SDS-polyacrylamide gel. After electrophoresis and transfer to polyvinylidene fluoride membranes, the membranes were washed in Tris-buffered saline containing 0.1% Tween-20, and incubated with a primary antibody (goat anti-Tbx1, goat anti-phosho-Smad2/3, or goat anti-phosho-Smad1/5/8 diluted at 1:200; Santa Cruz Biotechnology, CA, USA). Membranes were then incubated with a secondary antibody (1:4000), and immunostained bands were detected with a ProtoBlot II AP System and a stabilized substrate (Promega, Madison, WI, USA). β-actin was used as an internal control.
Histopathology, immunohistochemistry and immunofluorescence assays
A formalin-preserved and paraffin-embedded kidney from each animal was sectioned longitudinally at a thickness of 4 μm and stained with hematoxylin/eosin (HE). Histological sections were examined under a light microscope to evaluate changes of renal tubular pathology. An expert pathologist blinded to the treatment would use 4~6 sections to evaluate the degree of tubular injury based on the Klausner classification [19]. The injuries were semiquantitatively scored as minimal (score 1, < 5% of tubules involved), mild (score 2, 5%~25% of tubules involved), moderate (score 3, 25%~75% of tubules involved), and severe (score 4, > 75% of tubules involved).
For immunohistochemistry, the sections were incubated with an anti-Tbx1 monoclonal antibody (Santa Cruz Biotechnology, CA, USA) at 1:100 dilutions. Normal mouse serum (Santa Cruz Biotechnology, CA, USA) was used as the negative control. After staining, images were acquired under an Axiophot microscope equipped with a high sensibility color camera (Axiocam, Carl Zeiss, Germany). A set of images was analyzed to assess the percentage of positive tubular cells using KS400 software (Kontron System; Zeiss Vision, Oberkochen, Germany).
For immunofluorescence, frozen kidney sections were routinely prepared and incubated in normal goat serum (Sigma-Aldrich G9023, USA) to block non-specific binding. The sections were then incubated with a goat anti-Tbx1 antibody (Santa Cruz Biotechnology, CA, USA) at 1:50 dilution overnight, followed by a donkey anti-goat secondary antibody (Santa Cruz Biotechnology, CA, USA) at 1:100 dilution for 2 h. For the control, the primary antibody was omitted and no staining was detected (data not shown).
Detection of apoptotic tubular cells in tissue sections
A terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay was used to identify double-stranded DNA fragmentation, which is characteristic of DNA degradation due to apoptosis. Briefly, tissue sections were deparaffinized and treated with proteinase K (20 μg/ml) for 20 min at room temperature. The sections were then quenched in 2% hydrogen peroxide. After rinsing in phosphate-buffered saline (pH 7.4), the sections were incubated in 1× equilibration buffer for 10~15 s. The sections were then incubated with terminal deoxynucleotidyl transferase (rTdT) for 1 h at 37°C, blocked with a stop/washing buffer, followed by incubation with a peroxidase-conjugated anti-digoxigenin antibody for 30 min at room temperature. Finally, the sections were developed with diaminobenzidine (Promega, Madison, WI, USA). Counterstaining was performed with hematoxylin, and commercially available rat lymph node sections were used as positive controls. Negative controls for the TUNEL assay was set by staining rat kidney tissue in the same manner but without incubation with the rTdT enzyme. Nuclei were viewed and counted by two persons blinded to the experiment.
Cell culture and Tbx1-short interfering (si) RNA transfection
NRK cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum. After the cells have grown to confluence, they were placed in a quiescent medium (0.5% fetal bovine serum) for 16 h. To silence Tbx1 gene expression, siRNA transfection was carried out using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). A short interfering RNA targeting rat Tbx1 (Tbx1-siRNA) was designed and synthesized by GenePharma, Shanghai, China. The sequences of Tbx1-siRNA were as follows: 5’-GGCGGUGUAGAUACAUGUAGA-3’ and 5’-UACAUGUAUCUACACCGCCCG-3’. siRNA transfection was performed according to the manufacturer’s instruction (Targeting Systems, San Diego, CA, USA). Tbx1-siRNA or scramble siRNA (negative control) was transfected into NRK cells at a final concentration of 100 nmol/L. The efficacy of Tbx1 knockdown was assessed by real-time RT-PCR (48 h after transfection) and Western blotting (72 h after transfection).
Statistics
All values were presented as mean ± standard deviation. One-way analysis of variance or t-tests were used to compare experimental groups and matched controls. For nonparametric data, the Mann-Whitney rank-sum test was used [20]. For correlation analyses of the number of TUNEL-positive cells, the Spearman test was used. P < 0.05 was considered to be statistically significant. Data were analyzed using SPSS software (version 13.0; SPSS Inc, Chicago, IL, USA).
Results
Increased BUN, Cr, urinary β2-MG and Alb in GM-induced AKI
As shown in Table 2, urine and serum changes in the GM groups exhibited a trend with obvious magnitude and statistical differences. BUN was significantly elevated on days 3 and 7, and Cr was significantly elevated on days 7 and 10. Significant increases of urinary β2-MG and Alb levels were observed as early as 3 days post-injection, which has peaked on day 7 and showed a decreasing trend in the following days. By day 21, all had resolved to control levels.
Table 2.
Group means for serum and urine biomarker assays
| D | Group | n | Serum | Urine | ||
|---|---|---|---|---|---|---|
|
| ||||||
| BUN (mmol/L) | Cr (μmol/L) | β2-MG (μg·mmol-1·Cr-1) | Alb (mg·mmol-1·Cr-1) | |||
| 0 | CTRL | 3 | 7.39 ± 1.36 | 40.48 ± 0.09 | 13.25 ± 6.36 | 0.65 ± 0.10 |
| GM | 3 | 7.98 ± 1.56 | 41.46 ± 0.11 | 12.26 ± 7.39 | 0.69 ± 0.11 | |
| 3 | CTRL | 8 | 7.12 ± 2.07 | 39.55 ± 0.09 | 13.57 ± 9.73 | 0.91 ± 0.52 |
| GM | 8 | 29.21 ± 5.66a | 85.69 ± 0.13a | 139.27 ± 99.35a | 3.66 ± 0.36a | |
| 7 | CTRL | 8 | 8.01 ± 2.12 | 40.58 ± 0.05 | 12.28 ± 8.37 | 0.86 ± 0.12 |
| GM | 8 | 38.06 ± 6.72b | 122.58 ± 0.26b | 253.68 ± 90.36b | 9.25 ± 0.36b | |
| 10 | CTRL | 8 | 7.99 ± 1.28 | 41.70 ± 0.08 | 13.77 ± 7.33 | 0.78 ± 0.15 |
| GM | 8 | 22.46 ± 3.55c | 103.28 ± 0.32c | 152.20 ± 58.26c | 6.33 ± 0.51c | |
| 21 | CTRL | 8 | 7.32 ± 1.27 | 39.33 ± 0.06 | 12.12 ± 7.31 | 0.98 ± 0.57 |
| GM | 8 | 8.05 ± 1.28d,e | 42.35 ± 0.07d,e | 15.46 ± 8.66d,e | 1.23 ± 0.55d,e | |
Abbreviations: D: days from initial dose; n: number of rats in the group; β2-MG in μg/mmol urine creatinine (uCr); Alb in mg/mmol uCr.
P < 0.01 vs. CTRL and GM-0d groups;
P < 0.01 vs. CTRL, GM-0d and GM-3d groups;
P < 0.01 vs. CTRL, GM-0d and GM-7d groups;
P > 0.05 vs. CTRL and GM-0d groups;
P < 0.01 vs. GM-7d and GM-10d groups.
Histological changes in GM-induced AKI
On days 3, 7, and 10, there were significant changes in the histopathology of the GM group compared with the CTRL group. Kidney tissues from the CTRL group showed a normal glomerular and renal tubular structure. No significant degeneration or necrosis was observed in proximal and distal tubules. The tubular epithelium appeared to be normal, and there was no interstitial infiltration by mononuclear cells (Figure 1A). In the GM group, there was a mild to severe degree of tubular damage including degeneration and necrosis of renal tubules at each time point. A significant increase of necrosis was detected in the GM group on day 3, which has peaked on day 7 (Figure 1B and 1C), and attenuated on day 10. However, on day 21, there were no detectable differences in histopathology between the GM and CTRL groups. For a semi-quantitative analysis of tubulo-interstitial injury by Klausner’s method, mean values for GM-0d, -3d, -7d, -10d and -21d groups were 0, 1.50 ± 0.25, 2.90 ± 0.25, 1.75 ± 0.25 and 0, respectively. The scores were significantly higher in the GM-7d group than those in GM-0d, -3d, -10d and -21d groups (P < 0.01).
Figure 1.
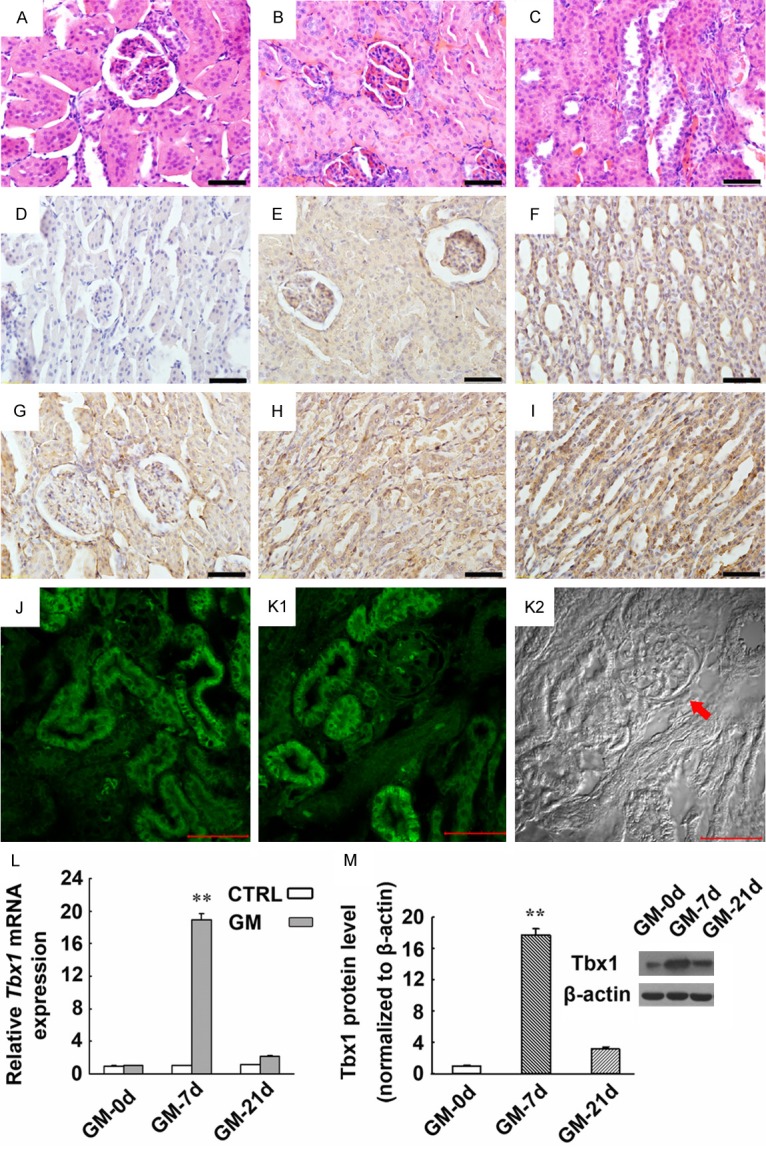
Expression of Tbx1 mRNA and protein in kidney tissues derived from a rat model for AKI induced by GM. (A-C) CTRL rats have shown normal tubular epithelial cells (A). Swollen renal tubular epithelial cells, poorly defined cell boundaries, dissolved nuclei, disappearance of lumen structure (B), and shedding renal tubular epithelial cells were detected in the injured kidneys of the GM-7d group (C) (HE staining). (D-I) Immunohistochemical staining showed the expression and localization of Tbx1 in the kidneys of the GM-7d and CTRL groups. Tbx1 was predominantly expressed in the cytoplasm of renal tubular epithelial cells and collecting duct cells in the cortex and medulla (E-I), which was mild in the kidneys of the CTRL group (E, F), but markedly increased in the GM-7d group (G-I). Magnification: ×400; scale = 50 μm. A negative control is also shown (D). (J-K2) Immunofluorescence staining demonstrated that Tbx1 expression was located predominantly in the cytoplasm of tubular epithelial cells of GM-7d rats (J, K1). The same image in K1 was also captured using DIC microscopy (K2). The arrowhead indicates the glomerulus that is not shown in K1. Magnification: ×400; scale = 50 μm. (L) Tbx1 mRNA expression was significantly up-regulated in the kidneys of the GM-7d group compared with the CTRL, GM-0d and GM-21d groups. (M) Tbx1 protein as detected by Western blotting has increased accordingly compared with the CTRL, GM-0d and GM-21d groups. **, P < 0.01 vs. CTRL, GM-0d and GM-21d groups. No significant changes were found between the GM-0d and GM-21d groups.
Elevated Tbx1 expression in GM-induced AKI
Expression of Tbx1 mRNA has significantly increased on days 3, 7 and 10 compared with the CTRL and GM-21d groups, which has peaked on day 7 and persisted until day 10. However, by day 21, the expression has returned to that of the CTRL group (Figure 1L). Western blotting also confirmed Tbx1 protein expression to be significantly up-regulated on days 3, 7 and 10, and peaked on day 7 (Figure 1M). Immunohistochemistry indicated that Tbx1 was mildly expressed in renal tubular cells in adult rats (Figure 1E and 1F). At 7 days after GM injection, a strong expression of Tbx1 was detected in renal tubular cells (Figure 1G-I). Immunofluorescence staining also demonstrated that expression of Tbx1 was located predominantly in the cytoplasm of tubular epithelial cells in the GM-7d group (Figure 1J and 1K1).
Activation of the TGFβ-Smad2/3 pathway and inhibition of Smad1/5/8 in the injured kidney following GM administration
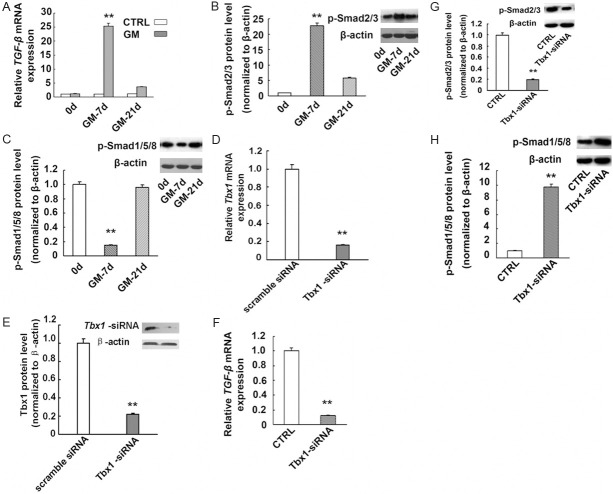
The Tbx1 mRNA level has been up-regulated following the kidney injury. The levels of TGF-β and its target proteins, p-Smad2/3, were increased accordingly (Figure 2A and 2B). The level of Tbx1 mRNA appeared to be strongly correlated with that of TGF-β mRNA and p-Smad2/3 (r = 0.982 and 0.967, respectively, P < 0.01). However, the p-Smad1/5/8 protein level was decreased while the expression of Tbx1 was up-regulated on day 7 (Figure 2C). We also found a negative correlation between the expression of Tbx1 and that of p-Smad1/5/8 protein (r = -0.995, P < 0.01).
Figure 2.
Altered expression of the TGFβ-Smad2/3 signaling pathway in GM-injured kidneys as well as Tbx1-knockdown NRK cells. TGF-β mRNA expression was markedly increased in the kidneys of the GM-7d group (A). **, P < 0.01, vs. GM-0d, -21d and CTRL groups. Representative Western blot and quantitative data for p-Smad2/3 (B) and p-Smad1/5/8 (C) in rat kidney tissues. Increased p-Smad2/3 levels and reduced p-Smad1/5/8 levels were detected in the GM-7d group.**, P < 0.01 vs. GM-0d and GM-21d groups. Results are representative of at least three independent experiments. (D-F) NRK cells transiently transfected with Tbx1-specific siRNA (Tbx1-siRNA) or scramble siRNA. Tbx1 mRNA expression was determined by real-time PCR (D), and Tbx1 protein expression was detected by Western blotting (E). As detected by real-time RT-PCR and Western blotting, Tbx1 gene knockdown has resulted in decreased expression of TGF-β mRNA (F) and p-Smad2/3 protein (G), and increased Smad1/5/8 protein expression (H). β-actin was used as a control. **, P < 0.01 vs. CTRL group. TGF-β, transforming growth factor-beta gene.
Elevated Smad1/5/8 levels and down-regulated TGFβ-Smad2/3 expression in Tbx1-knockdown NRK cells
A reduction in the Tbx1 mRNA level was detected at 48 h by RT-PCR and at 72 h by Western blotting after transfection (Figure 2D and 2E). Furthermore, as determined by real-time RT-PCR or Western blotting, the expression of p-Smad1/5/8 increased after transfection, while TGF-β and p-Smad2/3 expression decreased compared with that in the controls (P < 0.01; Figure 2F-H).
Increased renal tubular cell apoptosis in GM-induced AKI and its correlation with Tbx1 expression
Compared with the CTRL group, the number of TUNEL-positive renal tubular epithelial cells has significantly increased in the kidneys of the GM-7d group (Figure 3A-F). More such cells were detected in the cortex than medulla of the GM-7d group (Figure 3G). There were significantly more TUNEL-positive tubular cells/HPF (high powered field, HPF) in the kidneys of the GM-7d group compared with the CTRL, GM-0d and GM-21d groups (66.89 ± 5.36 vs. 8.79 ± 1.26, 9.36 ± 1.57 and 8.39 ± 1.69, P < 0.01, respectively; Figure 3H), and no such difference was found among GM-0d, GM-21d and CTRL groups (P > 0.05; Figure 3H). By statistical analysis, the number of TUNEL-positive apoptotic tubular cells/HPF appeared to be strongly correlated with the mRNA level of Tbx1 (r = 0.97, P < 0.01; Figure 3K).
Figure 3.
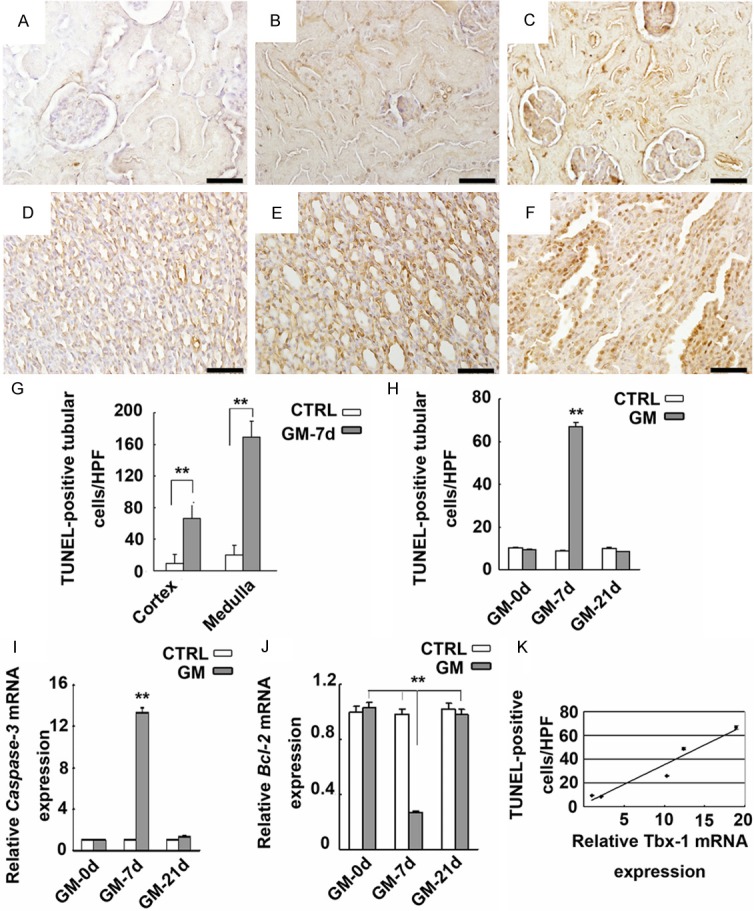
Apoptosis of kidney cells and its correlation with Tbx1 expression. (A-F) TUNEL staining (×400). Detection of TUNEL-positive renal tubular cells in the cortex and medulla of CTRL (A, D) and GM-7d (B, C, E, F) groups. Quantification of TUNEL-positive cells/HPF of the cortex and medulla in GM-7d rats (G). **, P < 0.01 vs. CTRL group. More TUNEL-positive tubular cells/HPF was detected in the cortex of kidneys in the GM-7d group (H). **, P < 0.01 vs. GM-0d and GM-21d groups. Expression of caspase-3 and Bcl-2 genes, as determined by real-time RT-PCR, in the kidney tissues of GM-0d, GM-7d, GM-21d and CTRL groups (I, J). **, P < 0.01, vs. GM-0d, GM-21d and CTRL groups. There was a correlation between TUNEL-positive tubular cells in the cortex and mRNA expression of Tbx1 (K). (r = 0.97, P < 0.01).
As shown in Figure 3I, caspase-3 expression was stronger in the kidneys of the GM-7d group compared with the GM-0d, GM-21d and CTRL groups (P < 0.01). In addition, Bcl-2 expression in the kidneys of the GM-7d group was weaker compared with the GM-0d, GM-21d and CTRL groups (P < 0.01; Figure 3J). No significant differences were found between the GM-0d, GM-21d and CTRL groups (P > 0.05; Figure 3J).
Discussion
As a critical gene involved in DiGeorge syndrome, TBX1 has been studied primarily for its relationship with heart malformations and craniofacial anomalies [13,21]. The role of TBX1 in kidney development has not yet been explored, despite that abundant expression of TBX1 has been found in the kidneys [14]. The role of Tbx1 in development is exquisitely dosage sensitive. Progressive reduction of Tbx1 mRNA has been associated with non-linear increases in phenotypic severity, whereas over-expression of Tbx1 may lead to structural heart and thymic defects [22,23]. During mouse kidney development, expression of Tbx1 has peaked at embryonic day 0.5, reduces gradually, and then maintains at a low level throughout adulthood [14]. In the present study, mild expression of Tbx1 was detected in the renal tubules of normal adult rats. Tbx1 expression was up-regulated as early as on day 3 post-injury, peaked at day 7, and then gradually decreased. By day 21, the expression of Tbx1 has resolved to that of the control group, which has suggested that Tbx1 probably plays a role in renal tubular injury induced by GM.
The TGF-β superfamily comprises more than 30 structurally related dimeric cytokines including TGF-β, BMP/growth and differentiation factors, and activins. Activation of the TGF-β pathway plays a key role in the pathogenesis of renal injury by promoting apoptosis, epithelial-mesenchymal transition, matrix protein synthesis, and other pro-fibrotic events leading to disruption of renal structure and function [3,24]. TGF-β exerts its biological functions largely via complex downstream signaling molecules including p-Smad proteins. The critical role of the TGFβ-Smad2/3 signaling pathway in the pathogenesis of renal injury and fibrosis is well recognized [3,15,24]. Smad2/3 is strongly activated in animal and human kidney diseases including diabetic nephropathy, obstructive kidney diseases, 5/6 nephrectomy, and hypertensive nephropathy [25]. In the present study, elevated TGF-β and p-Smad2/3 levels were found in AKI induced by GM. Expression of TGF-β was strongly correlated with that of Tbx1. Similarly, knockdown of Tbx1 in NRK cells has led to decreased TGF-β and Smad2/3 levels, which also suggested an association between Tbx1, TGF-β and Smad2/3. Tbx1 may therefore participate in kidney injury via the TGFβ-Smad2/3 pathway.
It has been shown that Tbx1 can alter TGF-β/BMP signaling by interacting with Hoxd10 during kidney development [14]. As demonstrated by Fulcoli et al., Tbx1 can down-regulate the BMP/Smad1 signaling pathway through binding with Smad1 and suppressing BMP-4/Smad1 signaling [26]. In the context of kidney injury, Smad1/5/8 is protective and plays an important role in recovery following renal injury in addition to the renal protective effect of BMP-7 [17,27]. Expression of Smad1/5/8, target proteins of BMP, was reduced in the injured kidneys along with increased Tbx1 expression. Knockdown of Tbx1 can result in increased Smad1/5/8 levels and a reduced TGF-β level in Tbx1-silenced NRK cells. Therefore, Tbx1 expression probably has a suppressive effect on the Smad1/5/8 pathway in injured kidneys.
Of note, more TUNEL-positive tubular cells and altered expression of caspase-3 and Bcl-2 were detected in GM-induced AKI. Expression of Tbx1 and TGF-β seemed to be strongly correlated with the number of TUNEL-positive tubular cells. Evidence has shown that apoptosis is a prominent and characteristic feature of kidney injury caused by nephrotoxic medications. Excessive apoptosis contributes to atrophy and promotes fibrosis and organ dysfunction. Activation of the TGF-β-Smad2/3 pathway may accelerate kidney injury by inducing renal tubular cell apoptosis [24]. An antibody against TGF-β can ameliorate tubular apoptosis in unilateral ureteral obstruction [17,28,29]. Above observations appear to support that over-expression of TGF-β, p-Smad2/3 and caspase-3, and down-regulated expression of Bcl-2 in GM-induced kidney injury. A plausible explanation for this may be that activation of Tbx1 following GM administration can induce renal tubular cell apoptosis through the TGFβ-Smad2/3 pathway in AKI.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of China (No. 81300130, 81070131), Liaoning Higher Education Project (No. L2012271). Liaoning Province Scientific Research Start Fund for Doctorates (No. 20081049), and the Support Program from the Department of Science and Technology of Sichuan Province (No. 2012SZ0027).
Disclosure of conflict of interest
None.
References
- 1.Quiros Y, Vicente-Vicente L, Morales AI, Lopez-Novoa JM, Lopez-Hernandez FJ. An integrative overview on the mechanisms underlying the renal tubular cytotoxicity of gentamicin. Toxicol Sci. 2011;119:245–256. doi: 10.1093/toxsci/kfq267. [DOI] [PubMed] [Google Scholar]
- 2.Arany I, Reed DK, Grifoni SC, Chandrashekar K, Booz GW, Juncos LA. A novel U-STAT3-dependent mechanism mediates the deleterious effects of chronic nicotine exposure on renal injury. Am J Physiol Renal Physiol. 2012;302:F722–729. doi: 10.1152/ajprenal.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bottinger EP. TGF-beta in renal injury and disease. Semin Nephrol. 2007;27:309–320. doi: 10.1016/j.semnephrol.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Schodel J, Klanke B, Weidemann A, Buchholz B, Bernhardt W, Bertog M, Amann K, Korbmacher C, Wiesener M, Warnecke C, Kurtz A, Eckardt KU, Willam C. HIF-prolyl hydroxylases in the rat kidney: physiologic expression patterns and regulation in acute kidney injury. Am J Pathol. 2009;174:1663–1674. doi: 10.2353/ajpath.2009.080687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu CP, Lee IT, Sheu WH, Lee WJ, Liang KW, Lee WL, Lin SY. The levels of circulating and urinary monocyte chemoattractant protein-1 are associated with chronic renal injury in obese men. Clin Chim Acta. 2012;413:1647–1651. doi: 10.1016/j.cca.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Wang HY, Wang YJ, Cui MJ, Gu CM, Yang LZ, Zhao Y, Chen Y, Zhao D, Li TS, Chi BR. Hepatocyte growth factor-induced amelioration in renal interstitial fibrosis is associated with reduced expression of alpha-smooth muscle actin and transforming growth factor-beta1. Indian J Biochem Biophys. 2011;48:308–315. [PubMed] [Google Scholar]
- 7.Wilson V, Conlon FL. The T-box family. Genome Biol. 2002;3:REVIEWS3008. doi: 10.1186/gb-2002-3-6-reviews3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldini A. Dissecting contiguous gene defects: TBX1. Curr Opin Genet Dev. 2005;15:279–284. doi: 10.1016/j.gde.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Kobrynski LJ, Sullivan KE. Velocardiofacial syndrome, DiGeorge syndrome: the chromosome 22q11.2 deletion syndromes. Lancet. 2007;370:1443–1452. doi: 10.1016/S0140-6736(07)61601-8. [DOI] [PubMed] [Google Scholar]
- 10.Kujat A, Schulz MD, Strenge S, Froster UG. Renal malformations in deletion 22q11.2 patients. Am J Med Genet A. 2006;140:1601–1602. doi: 10.1002/ajmg.a.31289. [DOI] [PubMed] [Google Scholar]
- 11.Uddin RK, Zhang Y, Siu VM, Fan YS, O’Reilly RL, Rao J, Singh SM. Breakpoint Associated with a novel 2.3 Mb deletion in the VCFS region of 22q11 and the role of Alu (SINE) in recurring microdeletions. BMC Med Genet. 2006;7:18. doi: 10.1186/1471-2350-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Zhong T, Wang Y, Jiang Q, Song H, Gui Y. TBX1, a DiGeorge syndrome candidate gene, is inhibited by retinoic acid. Int J Dev Biol. 2006;50:55–61. doi: 10.1387/ijdb.052036lz. [DOI] [PubMed] [Google Scholar]
- 13.Yagi H, Furutani Y, Hamada H, Sasaki T, Asakawa S, Minoshima S, Ichida F, Joo K, Kimura M, Imamura S, Kamatani N, Momma K, Takao A, Nakazawa M, Shimizu N, Matsuoka R. Role of TBX1 in human del22q11.2 syndrome. Lancet. 2003;362:1366–1373. doi: 10.1016/s0140-6736(03)14632-6. [DOI] [PubMed] [Google Scholar]
- 14.Fu Y, Li F, Zhao DY, Zhang JS, Lv Y, Li-Ling J. Interaction between Tbx1 and HoxD10 and connection with TGFbeta-BMP signal pathway during kidney development. Gene. 2014;536:197–202. doi: 10.1016/j.gene.2012.06.069. [DOI] [PubMed] [Google Scholar]
- 15.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 16.Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol. 2001;187:265–276. doi: 10.1002/jcp.1080. [DOI] [PubMed] [Google Scholar]
- 17.Manson SR, Niederhoff RA, Hruska KA, Austin PF. The BMP-7-Smad1/5/8 pathway promotes kidney repair after obstruction induced renal injury. J Urol. 2011;185:2523–2530. doi: 10.1016/j.juro.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouse RL, Zhang J, Stewart SR, Rosenzweig BA, Espandiari P, Sadrieh NK. Comparative profile of commercially available urinary biomarkers in preclinical drug-induced kidney injury and recovery in rats. Kidney Int. 2011;79:1186–97. doi: 10.1038/ki.2010.463. [DOI] [PubMed] [Google Scholar]
- 19.Klausner JM, Paterson IS, Goldman G, Kobzik L, Rodzen C, Lawrence R, Valeri CR, Shepro D, Hechtman HB. Postischemic renal injury is mediated by neutrophils and leukotrienes. Am J Physiol. 1989;256:F794–802. doi: 10.1152/ajprenal.1989.256.5.F794. [DOI] [PubMed] [Google Scholar]
- 20.Berry KJ, Mielke PW Jr. Exact and Monte carlo resampling procedures for the Wilcoxon-Mann-Whitney and Kruskal-Wallis tests. Percept Mot Skills. 2000;91:749–754. doi: 10.2466/pms.2000.91.3.749. [DOI] [PubMed] [Google Scholar]
- 21.Lindsay EA, Vitelli F, Su H, Morishima M, Huynh T, Pramparo T, Jurecic V, Ogunrinu G, Sutherland HF, Scambler PJ, Bradley A, Baldini A. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410:97–101. doi: 10.1038/35065105. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Baldini A. In vivo response to high-resolution variation of Tbx1 mRNA dosage. Hum Mol Genet. 2008;17:150–157. doi: 10.1093/hmg/ddm291. [DOI] [PubMed] [Google Scholar]
- 23.Portnoi MF. Microduplication 22q11.2: a new chromosomal syndrome. Eur J Med Genet. 2009;52:88–93. doi: 10.1016/j.ejmg.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Casalena G, Daehn I, Bottinger E. Transforming growth factor-beta, bioenergetics, and mitochondria in renal disease. Semin Nephrol. 2012;32:295–303. doi: 10.1016/j.semnephrol.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lan HY, Chung AC. TGF-beta/Smad signaling in kidney disease. Semin Nephrol. 2012;32:236–243. doi: 10.1016/j.semnephrol.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Fulcoli FG, Huynh T, Scambler PJ, Baldini A. Tbx1 regulates the BMP-Smad1 pathway in a transcription independent manner. PLoS One. 2009;4:e6049. doi: 10.1371/journal.pone.0006049. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Leeuwis JW, Nguyen TQ, Chuva de Sousa Lopes SM, van der Giezen DM, van der Ven K, Rouw PJ, Offerhaus GJ, Mummery CL, Goldschmeding R. Direct visualization of Smad1/ 5/8-mediated transcriptional activity identifies podocytes and collecting ducts as major targets of BMP signalling in healthy and diseased kidneys. J Pathol. 2011;224:121–132. doi: 10.1002/path.2844. [DOI] [PubMed] [Google Scholar]
- 28.Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS. Matrix rigidity regulates a switch between TGF-beta1-induced apoptosis and epithelial-mesenchymal transition. Mol Biol Cell. 2012;23:781–791. doi: 10.1091/mbc.E11-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyajima A, Chen J, Lawrence C, Ledbetter S, Soslow RA, Stern J, Jha S, Pigato J, Lemer ML, Poppas DP, Vaughan ED, Felsen D. Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int. 2000;58:2301–2313. doi: 10.1046/j.1523-1755.2000.00414.x. [DOI] [PubMed] [Google Scholar]