Abstract
Rhabdomyosarcoma (RMS) is one of the most common soft-tissue sarcomas in children and adolescents with poor prognosis. Yet, there is lack of effective prognostic biomarkers for RMS. The present study, therefore, aimed to explore potential biomarkers for RMS based on our previous findings using array comparative genomic hybridization. We investigated guanine nucleotide exchange factor, GEFT, at expression level in 45 RMS patients and 36 normal striated muscle controls using immunohistochemistry using tissue microarrays. The expression rate of GEFT in RMS samples (42/45, 93.33%) was significantly higher (P<0.05) than that in normal controls (5/36, 13.89%). Moreover, the overexpression rate of GEFT in RMS (31/45, 68.89%) was also significantly higher (P<0.05) than that in normal controls (0/36, 0.00%). Increased expression of GEFT correlated significantly with advanced disease stages (stages III/IV) (P=0.001), lymph node metastasis (P=0.019), and distant metastasis (P=0.004), respectively, in RMS patients. In addition, RMS patients having overexpressed GEFT experienced worse overall survival (OS) than those having low levels of GEFT (P=0.001). GEFT overexpression was determined to be an independent prognostic factor for poor OS in RMS patients (hazard ratio: 3.491, 95% confidence interval: 1.121-10.871, P=0.004). In conclusion, these observations provide the first evidence of GEFT overexpression in RMS and its correlations with disease aggressiveness and metastasis. These findings suggest that GEFT may serve as a promising biomarker predicting poor prognosis in RMS patients, thus implying its potential as a therapeutic target.
Keywords: Rhabdomyosarcoma, guanine nucleotide exchange factor, overexpression, prognosis
Introduction
Rhabdomyosarcoma (RMS) is one of the most common soft-tissue sarcomas in children and adolescents and is hypothesized to be a malignant tumor derived from skeletal myoblasts or their progenitors. RMS tumors are classified into 3 different histologic subtypes, with embryonic rhabdomyosarcoma (ERMS) being the most prevalent; alveolar rhabdomyosarcoma (ARMS), the most aggressive; and pleomorphic rhabdomyosarcoma (PRMS), a rare adult variant. The clinical outcome of RMS is generally poor with the 5-year survival rate varying significantly depending on distinct clinical features. Approximately 16% of newly diagnosed RMS patients have metastatic disease [1], which tends to be the main cause of death in cases of RMS. Although several biomarkers, such as the PAX3-FOXO1 or PAX7-FOXO1 fusion gene in ARMS, have been reported to be of diagnostic and prognostic values in RMS, discovery of novel biomarkers, especially for ERMS and PRMS, remains an urgent requirement.
The GEFT gene is located on chromosome 12q13.3, a region frequently amplified in sarcomas [2]. Our previous observations via microarray-based comparative genomic hybridization have revealed high copy numbers of GEFT in RMS samples [3], which agrees with a report by Paulson et al [4]. GEFT, also known as p63RhoGEF or ARHGEF25, belongs to the Rho guanine nucleotide exchange factor (GEF) family, which is highly expressed in excitable tissues such as the brain, heart, and muscle [5]. It is the first mammalian GEF reported to modulate the myogenic-versus-adipogenic cell fate decision of progenitor mesenchymal cells, thus regulating muscle regeneration, myogenesis, and adipogenesis [5]. The largest family of Rho GEFs selectively regulates specific functions by targeting the Rho family of small guanosine triphosphatases (Rho GTPases) [6]. Rho GEFs and Rho GTPases are involved in tumorigenesis, development, invasion, and metastasis of various tumors [7-9], including RMS [10]. Like other Rho GEFs, GEFT is capable of activating Rho GTPases, especially RhoA, Rac1, and Cdc42, by catalyzing the exchange of bound guanosine diphosphates (GDP) for guanosine triphosphates (GTP) on Rho proteins, thus inducing their activation and the activity of their downstream targets [11,12].
GEFT has also been reported to participate in lens cell differentiation through a Rac1-dependent mechanism [13]. Mechanistically, GEFT transduces signals and induces the activation of RhoA and its downstream effectors by binding to G alpha q/11 and linking it specifically to G-protein-coupled receptors (GPCRs) [14-16]. Such a signaling cascade is important in multiple physiological processes and has been suggested to be involved in hypertension-associated bladder dysfunction [17]. Moreover, several studies have reported that GEFT upregulates key signaling cascades and transcription factors that are involved in neuroblastoma differentiation [18,19], and breast carcinoma progression and metastasis [20,21]. However, to the best of our knowledge, the effects of GEFT in RMS have not been reported in the literature.
In this study, we have assessed GEFT protein expression in RMS patients and normal controls and analyzed correlations of GEFT expression with patient survival and various clinicopathologic features. More importantly, we have also determined the potential of GEFT as a predictive marker for prognosis of RMS.
Materials and methods
Patients and tissue specimens
Forty-five formalin-fixed paraffin-embedded RMS samples were obtained from the Department of Pathology, the First Affiliated Hospital of Shihezi University School of Medicine, Xinjiang Uygur Autonomous Region People’s Hospital, and the First Affiliated Hospital of Xinjiang Medical University, China. Clinical and demographic data were recorded from patients’ medical charts. The diagnosis of all patients who received surgery was confirmed by histological and immunohistochemical analyses. The presence of PAX37-FOXO1 PAX7-FOXO1 fusion gene was determined by Reverse transcription polymerase chain reaction (RTPCR). Clinical staging was performed according to the National Comprehensive Cancer Network 2012 guidelines for soft tissue tumors. Follow-up surveys were conducted via telephone or by sending a letter. Additionally, 36 normal striated muscle samples were obtained as controls. This study was approved by the institutional ethics committee at the First Affiliated Hospital of Shihezi University School of Medicine and conducted in accordance with the ethical guidelines of the Declaration of Helsinki.
Tissue microarray (TMA) construction
Two representative fields were selected from hematoxylin and eosin slides of each tumor sample. Subsequently, the areas corresponding to those selected fields were located in the paraffin blocks for TMA construction. Each area of interest was reviewed to ensure that at least 70% of tumor cells were present. A tissue arraying instrument (Alphelys, Plaisir, France) was used to create tissue cores from paraffin blocks. The cores were then collected using a hollow needle with an inner diameter of 1.0 mm, held in an X-Y axis precision guide. Serial sections of 4-μm thickness were prepared from the TMA blocks for immunohistochemical staining.
Immunohistochemical (IHC) study
IHC staining was performed using the EnVision system (DAKO, Carpinteria, CA), as previously described [22]. Rabbit anti-GEFT concentrated polyclonal antibody recognizing a region within amino acids 226-446 of human GEFT (Abcam, Cambridge, UK) was used for IHC (1:300 dilution). A 3,3’-diaminobenzidine peroxidase substrate kit (DAKO, Carpinteria, CA) was used to identify positive regions. Negative control sections were incubated with phosphate-buffered saline instead of the primary antibody.
The expression of GEFT was scored semi-quantitatively according to the percentage of positive cells and the nuclear staining intensity. A percentage of positively stained cells of <5% received a score of 0, whereas a score of 1 corresponded to 6-35% positive cells, 2 being 36-65%, and 3 meaning more than 66% positively stained cells. The nuclear staining intensity scoring was as follows: 0, no staining; 1, buff; 2, yellow; and 3, brown. The 2 above-mentioned scores were then multiplied, resulting in a 0-9 final scoring scale. A final score of 0 indicated negative expression (-), whereas a score of 1-3 represented weak positive expression (+). Similarly, a score of 4-6 was considered to signify moderate positive expression (2+), and a score of 7-9 denoted strong positive expression (3+). A score of 4-9 was considered to signify increased positive expression or overexpression (2+/3+). Two pathologists independently reviewed 5 random fields from each sample slide. Cases with discrepant scores were reviewed with the use of a 5-headed microscope and re-assigned a consensus score.
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Science (SPSS) (IBM Corp., Armonk, NY, USA) version 17.0. The χ2 test or Fisher exact test was used to determine the statistical significance of GEFT protein expression in RMS samples versus normal controls, as well as the correlations between GEFT expression and clinicopathologic factors. The Kaplan-Meier method was used to calculate the survival rates, and the log-rank test was used to compare the survival curves. A Cox proportional hazards model was used to calculate univariate and multivariate hazard ratios for the variables. In all statistical analyses, a P-value of less than 0.05 was considered statistically significant.
Results
Clinical and pathological features
Forty-five patients were included in the present study, including 20 cases of ARMS, 23 cases of ERMS, and 2 cases of PRMS. The sex ratio of the study cohort was approximately 1:1 (22 males, 23 females), with a median age at surgery of 16 years, ranging from 8 months to 68 years. Anatomic locations of the tumors included the head and neck region in 19 cases (42.22%), the extremities and trunk in 11 (24.44%), the genitourinary tract in 7 (15.56%), and the thoracic cavity or retroperitoneum in 8 (17.78%).
GEFT expression in RMS samples and normal controls
The rate of GEFT expression in RMS samples was 93.33% (42/45) with 100% (20/20) of ARMS cases, 91.30% (21/23) of ERMS cases, and 50.00% (1/2) of PRMS cases showing GEFT expression. The overall rate of GEFT overexpression in RMS was 68.89% (31/45), with GEFT overexpression observed in 80.00% (16/20) of ARMS cases, 60.87% (14/23) of ERMS cases, and 50.00% (1/2) PRMS cases (Figure 1A). A summary of IHC staining results is shown in Table 1. The trend of GEFT expression and overexpression rates seemed to decrease from ARMS, to ERMS and PRMS cases. On the other hand, the rate of GEFT expression in normal controls was 13.89% (5/36), and that of GEFT overexpression was 0% (0/36). Our results indicated that the rates of GEFT expression and overexpression in RMS was significantly higher than that in normal controls (93.33% vs. 13.89%, P<0.05 and 68.89% vs. 0%, P<0.05, respectively) (Figure 1B). Interestingly, GEFT expression mainly localized in the nuclei of RMS cells, whereas GEFT expression was present in the cytoplasm of normal control cells. Representative images presented in Figure 2.
Figure 1.

A: The rates of GEFT expression and overexpression rates are decreased from ARMS, to ERMS and PRMS cases. B: The rates of GEFT expression in rhabdomyosarcoma were significantly higher than normal muscle tissues (p<0.01).
Table 1.
GEFT expression in rhabdomyosarcoma patients and normal muscle tissue
| Tissue Type | n | GEFT | |||
|---|---|---|---|---|---|
|
| |||||
| - (%) | 1+ (%) | 2+ (%) | 3+ (%) | ||
| RMS | 45 | 3 (6.67) | 11 (24.44) | 16 (35.56) | 15 (33.33) |
| ARMS | 20 | 0 (0.00) | 4 (20.00) | 8 (40.00) | 8 (40.00) |
| ERMS | 23 | 2 (8.71) | 7 (30.43) | 7 (30.43) | 7 (30.43) |
| PRMS | 2 | 1 (50.00) | 0 (0.00) | 1 (50.00) | 0 (0.00) |
| Normal muscle tissue | 36 | 31 (86.11) | 5 (13.89) | 0 (0.00) | 0 (0.00) |
Figure 2.
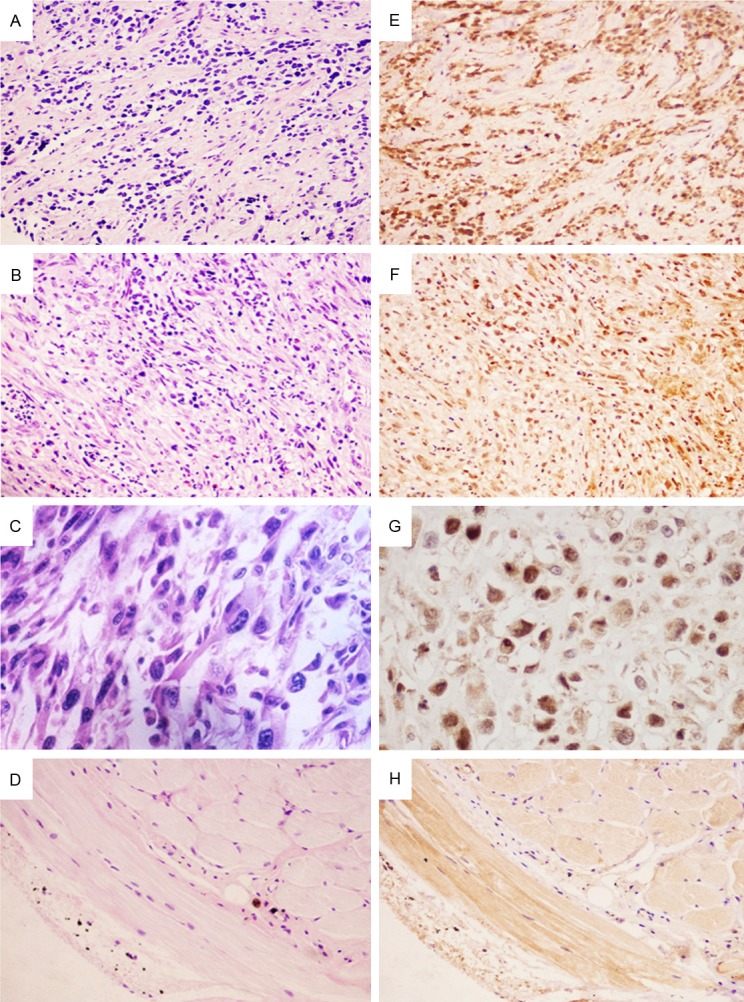
Immunohistochemical staining of GEFT expression in rhabdomyosarcoma and normal muscle tissues. Hematoxylin and eosin staining is shown for (A) ARMS, ×200; (B) ERMS, ×200; (C) PRMS, ×400; and (D) normal muscle tissue , ×200. Immunohistochemical staining for GEFT demonstrated strong nuclear expression in (E) ARMS (×200), (F) ERMS (×200), and (G) PRMS (×400) with less cytoplasmic expression, whereas GEFT staining was present in the cytoplasm in the (H) normal muscle tissue (×200).
Correlations between GEFT protein expression and tumor-node-metastasis (TNM) stage, lymph node metastasis, and distant metastasis in RMS
In our study, GEFT overexpression was significantly associated with advanced clinical stages (P=0.001), lymph node metastasis (P=0.019), and distant metastasis (P=0.004). These data suggest that up-regulated expression of GEFT may be involved in tumor metastasis and aggressiveness. However, no significant correlation was observed between increased GEFT expression and other examined clinicopathologic factors. These results are summarized in Table 2.
Table 2.
Association between GEFT protein expression and clinicopathologic features
| Variables | Cases | GEFT | χ2 value | P value | |
|---|---|---|---|---|---|
|
| |||||
| -/1+ (%) | 2+/3+ (%) | ||||
| Gender | 0.554 | 0.53 | |||
| Male | 22 | 8 (36.36) | 14 (63.64) | ||
| Female | 23 | 6 (26.09) | 17 (73.91) | ||
| Age (yrs) | 0.105 | 0.502 | |||
| ≤5 | 13 | 5 (38.46) | 8 (61.54) | ||
| >5 | 32 | 9 (28.13) | 23 (71.87) | ||
| Ethnicity | 2.074 | 0.202 | |||
| Han | 25 | 10 (40.00) | 15 (60.00) | ||
| #Other minorities | 20 | 4 (20.00) | 16 (80.00) | ||
| Tumor diameter | |||||
| ≤5 cm | 25 | 7 (28.00) | 18 (72.00) | 0.254 | 0.614 |
| >5 cm | 20 | 7 (35.00) | 13 (65.00) | ||
| Histologic type | 2.175 | 0.337 | |||
| ARMS | 20 | 4 (20.00) | 16 (80.00) | ||
| ERMS | 23 | 9 (39.13) | 14 (60.87) | ||
| PRMS | 2 | 1 (50.00) | 1 (50.00) | ||
| Location | - | 0.364 | |||
| head and neck | 19 | 5 (26.32) | 14 (73.68) | ||
| extremities and trunk | 11 | 2 (18.18) | 9 (81.82) | ||
| genitourinary tract | 7 | 4 (57.14) | 3 (42.86) | ||
| thoracic cavity or retroperitoneal | 8 | 3 (37.50) | 5 (62.50) | ||
| TNM Stage | 12.056 | *0.001 | |||
| I and II | 24 | 13 (54.17) | 11 (45.83) | ||
| III and IV | 21 | 1 (4.76) | 20 (95.24) | ||
| Lymph node metastasis | 4.090 | *0.019 | |||
| No | 35 | 14 (40.00) | 21 (60.00) | ||
| Yes | 10 | 0 (0.00) | 10 (100.00) | ||
| Distant metastasis | 6.341 | *0.004 | |||
| No | 32 | 14 (43.75) | 18 (56.25) | ||
| Yes | 13 | 0 (0.00) | 13 (100.00) | ||
P<0.05 indicates a significant association among the variables;
Significant difference.
including Uygur (n=17), Kazak (n=2) and Hui (n=1).
GEFT overexpression predicts poor prognosis in RMS
The endpoint of interest in the present study was overall survival (OS). Clinical follow-up information was available for 38 cases. The end date of follow-up was the date of final contact or the date of death through August 2013. The mean follow-up period was 25 months after surgery (range, 1.5-117 months).
Univariate analysis of all 45 enrolled patients revealed that patients with higher GEFT staining scores had poorer outcomes than those with lower scores. The median survival time of patients with lower GEFT expression was 50.00 months (range, 3-117 months), whereas those with GEFT overexpression only has a median survival of 26.00 months (range, 1.5-67 months). The 1-, 2-, 5-years survival rates of patients with lower GEFT expression was significantly higher than that of patients with GEFT overexpression (91.67% vs. 76.00%, 83.33% vs. 40.00%, 33.33% vs. 4.00%, respectively). As shown in Figure 3A, Kaplan-Meier analysis of patients’ survival indicated that GEFT overexpression was a significant prognostic factor (χ2=11.676, P=0.001). Patients with GEFT overexpression experienced a worse OS and greater risk of death after surgery than those with a weak or negative GEFT expression (Figure 3B).
Figure 3.

Kaplan-Meier survival curves for patients with overexpressed GEFT and those with low GEFT levels. A: RMS patients with overexpressed GEFT (2+/3+) experienced a significantly shorter survival period after surgery than those with low GEFT levels (-/1+) (P=0.001). B: Patients with GEFT overexpression had a greater risk of death than those with lower GEFT levels (P=0.001).
Multivariate analysis was performed for all clinicopathologic factors included in the univariate analysis. GEFT overexpression was determined to be a significant independent prognostic factor for poor OS (P=0.004, Exp(B)=3.491, Table 3) in RMS. Our findings suggest that GEFT protein overexpression could potentially be used as a biomarker for prognosis evaluation in RMS. Moreover, tumor size and TNM stage were also found to be independent prognostic factors for patient survival.
Table 3.
Univariate and multivariate Cox proportional hazard models for overall survival (OS)
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| GEFT protein | ||||
| -/1+ | 1 | 1 | ||
| 2+/3+ | 4.241 (1.749, 10.281) | *0.001 | 3.491 (1.121, 10.871) | *0.004 |
| Gender | ||||
| Male | 1 | 1 | ||
| Female | 1.534 (0.721, 3.266) | 0.267 | 0.363 (0.119, 1.103) | 0.074 |
| Age (yrs) | ||||
| ≤5 | 1 | 1 | ||
| >5 | 1.482 (0.678, 3.237) | 0.324 | 0.586 (0.178, 1.934) | 0.381 |
| Ethnicity | ||||
| Han | 1 | 1 | ||
| #Other minorities | 1.187 (0.590, 2.390) | 0.631 | 1.647 (0.593, 4.577) | 0.338 |
| Tumor diameter | ||||
| ≤5 cm | 1 | 1 | ||
| >5 cm | 0.453 (0.213, 0.963) | *0.040 | 0.200 (0.063, 0.633) | *0.006 |
| Histologic type | ||||
| ARMS | 1 | 1 | ||
| ERMS | 0.346 (0.157, 0.762) | *0.008 | 0.823 (0.247, 2.738) | 0.75 |
| PRMS | 0.000 (-) | 0.986 | 0.000 (-) | 0.993 |
| Location | ||||
| head and neck | 1.318 (0.556, 3.125) | 0.531 | 0.516 (0.118, 2.256) | 0.379 |
| extremities and trunk | 0.745 (0.239, 2.323) | 0.612 | 0.724 (0.176, 2.986) | 0.655 |
| genitourinary tract | 0.000 (0.000, 2.450) | 0.937 | 0.000 (0.000, 4.743) | 0.84 |
| thoracic cavity or retroperitoneal | 1 | 1 | ||
| TNM Stage | ||||
| I and II | 1 | 1 | ||
| III and IV | 2.121 (1.472, 3.056) | *0.000 | 3.198 (1.350, 7.578) | *0.008 |
| Lymph node metastasis | ||||
| No | 1 | 1 | ||
| Yes | 3.584 (1.515, 8.482) | *0.002 | 2.239 (0.398, 12.586) | 0.36 |
| Distant metastasis | ||||
| No | 1 | 1 | ||
| Yes | 9.941 (3.571, 27.671) | *0.000 | 0.161 (0.023, 1.138) | 0.067 |
Note: HR: hazard radio, CI: confidence interval;
Significant difference that 95% CI of HR was not including 1.
including Uyghur (n=17), Kazakh (n=2) and Hui (n=1).
Discussion
GEFT is a guanine nucleotide exchange factor for the Rho family of small GTPases identified in 2003 by Guo et al [2]. GEFT regulates cell processes by catalyzing the GDP/GTP exchange on Rho GTPases. Previous studies have reported on GEFT and its target Rho GTPases. Overexpression of GEFT transforms Rac1 and Cdc42 to their active (GTP-bound) state, inducing cell cytoskeleton reorganization; cell morphology change; and the formation of micro-spike, filopodia, lamellipodia, axons, and dendrites [2,18,19]. Meanwhile, GEFT activates Rac1/Cdc42-mediated transcriptional activities of SRE, Elk1, and SAP1, and induces the activation of c-Jun and AP-1 transcription factors, downstream targets of the JNK mitogen activated signaling pathway [2]. Other studies have also suggested a role of GEFT in angiotensin II-dependent proliferation, contractility, cell elongation, and morphology changes in smooth muscle cells as well as in the regulation of blood pressure and cardiovascular remodeling in humans [23,24]. These cellular functions are related to tumorigenesis, invasion, and metastasis. Moreover, Rho and Rho kinase could induce the change of endothelial cytoskeleton in endothelial cells, prompting sarcoma cells to migrate to the outside vessels through gaps between endothelial cells [25].
However, the role of GEFT in tumors remains elusive. To the best of our knowledge, the present investigation is the first study to report on the expression of GEFT in RMS samples. In this study, the rate of GEFT expression in RMS samples was 93.33% (42/45), while the rate of GEFT expression in normal controls was 13.89% (5/36). The overall rate of GEFT overexpression in RMS was 68.89% (31/45). On the other hand, the rate of GEFT overexpression was 0% (0/36). Our results indicated that the rates of GEFT expression and overexpression in RMS was significantly higher than that in normal controls (93.33% vs. 13.89%, P<0.05 and 68.89% vs. 0%, P<0.05, respectively). We observed that GEFT expression was mostly localized to the nuclei of RMS cells, whereas it was present in the cytoplasm of normal controls. This interesting observation, together with the findings presented above, indicate that GEFT nuclear expression may play an important role in RMS. However, a mechanistic explanation for GEFT subcellular localization in RMS remains unclear. This phenomenon may be associated with the presence of 2 single nucleotide polymorphisms in the coding region of the GEFT protein under certain conditions [2]. The intriguing issue certainly warrants further investigation. Furthermore, a similar situation occurs with Ect2, another Rho GEF. Ect2 subcellular mislocalization is an important mechanism of spatially inappropriate Rho GTPase activation by Ect2, leading to cancer growth [26,27].
Because the metastatic disease, rather than the primary tumor, is the main cause of death for RMS patients, it is important to understand the molecular events implicated in the disease’s invasion and metastasis. In the present study, of the 45 enrolled patients, 10 had lymph node metastasis, and 13 had distant metastasis. Interestingly, all of those were determined to have GEFT overexpression. We also found that GEFT overexpression was significantly associated with clinical stage, lymph node metastasis, and distant metastasis. Such strong associations suggest that GEFT overexpression is involved in tumor progression and may contribute to the increased invasion and metastasis of RMS. Since RMS cells must acquire a highly invasive and motile phenotype to metastasize, GEFT, with its regulatory effects on Rho GTPase activation, may play a fundamental role. Our results are in accordance with many other reports on the involvement of other GEFs in various tumors [28-32]. The largest family of Rho GEFs that selectively regulates specific functions of the target Rho GTPases reflects the very specific role of each Rho GEF in controlling distinct signaling mechanisms involved in tumors. Tang et al. demonstrated that the GEFT-RhoA/Rac1 signaling pathway and Gaq coupling to GPR116 (a GPCR) promoted breast cancer progression and metastasis [20]. Another study has highlighted that GEFT plays a crucial role in serum-induced chemotaxis by limiting lamellipodial protrusion to a certain direction via RhoA activation, which is required for chemotactic migration in breast carcinoma cells [21]. Taken together, overexpression ofGEFT might activate RhoA, Rac1, and Cdc42 signaling pathways and transcription factors in RMS. Further investigation to understand the molecular mechanisms of GEFT-associated signaling pathways and their regulators or effectors in RMS is urgently required.
Since all the above mentioned clinicopathologic variables were prognostic factors in RMS, we further conducted a systematic analysis to confirm the relationship between GEFT protein expression and the outcome of 45 patients with histologically proven RMS. Our results showed that patients with higher GEFT expression in RMS tissue had a worse OS than those with lower GEFT expression. Cox analysis revealed that GEFT overexpression could serve as a prognostic marker to predict the risk of death with a hazard ratio of 3.491 for OS. GEFT overexpression was significantly correlated with advanced TNM stages III/IV, lymph node metastasis, and distant metastasis in RMS patients. Such findings also suggested that GEFT overexpression could be included in the initial diagnosis to help design an optimal, individualized therapeutic strategy, and to discern patients who require closer monitoring after surgery. However, owing to the heterogeneity of our study population, additional research is necessary to confirm our findings.
In summary, we have shown, for the first time, the evidence that GEFT protein is expressed in RMS. Our results indicate that GEFT may predominantly act as a tumor promoter in RMS suggested by its correlations with advanced TNM stages III/IV, LNM, and distant metastasis. Accordingly, overexpression of GEFT may identify patients at high risk of death after surgery. We also place special emphasis on the overexpression of GEFT, which serves as an independent biomarker for poor prognosis of RMS. Although the molecular mechanism(s) of GEFT in RMS are poorly understood, the findings in this study have scratched the surface of the unknown arena warranting further studies in molecular details. Therefore, the analysis of GEFT-mediated signaling cascades in RMS is prioritized in our ongoing investigations. Clear understanding the specific role of the channel members and using targeted intervention might be helpful in determining the efficacy of drug treatment, thereby improving the prognosis of patients with this highly malignant tumor.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (No. 81160322).
Disclosure of conflict of interest
None.
References
- 1.Amankwah EK, Conley AP, Reed DR. Epidemiology and therapies for metastatic sarcoma. Clin Epidemiol. 2013;5:147–162. doi: 10.2147/CLEP.S28390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo X, Stafford LJ, Bryan B, Xia C, Ma W, Wu X, Liu D, Songyang Z, Liu M. A Rac/Cdc42-specific exchange factor, GEFT, induces cell proliferation, transformation, and migration. J Biol Chem. 2003;278:13207–15. doi: 10.1074/jbc.M208896200. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Li D, Hu J, Jiang J, Zhang W, Chen Y, Cui X, Qi Y, Zou H, Zhang W, Li F. Chromosomal and genetic imbalances in Chinese patients with rhabdomyosarcoma detected by high-resolution array comparative genomic hybridization. Int J Clin Exp Pathol. 2014;7:690–698. [PMC free article] [PubMed] [Google Scholar]
- 4.Paulson V, Chandler G, Rakheja D, Galindo RL, Wilson K, Amatruda JF, Cameron S. High-resolution array CGH identifies common mechanisms that drive embryonal rhabdomyosarcoma pathogenesis. Genes Chromosomes Cancer. 2011;50:397–408. doi: 10.1002/gcc.20864. [DOI] [PubMed] [Google Scholar]
- 5.Bryan BA, Mitchell DC, Zhao L, Ma W, Stafford LJ, Teng BB, Liu M. Modulation of muscle regeneration, myogenesis, and adipogenesis by the Rho family guanine nucleotide exchange factor GEFT. Mol Cell Biol. 2005;25:11089–101. doi: 10.1128/MCB.25.24.11089-11101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherfils J, Zeghouf M. Rho, regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93:269–309. doi: 10.1152/physrev.00003.2012. [DOI] [PubMed] [Google Scholar]
- 7.Cook DR, Rossman KL, Der CJ. Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease. Oncogene. 2013:1–15. doi: 10.1038/onc.2013.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrio-Real L, Kazanietz MG. Rho GEFs and cancer: linking gene expression and metastatic dissemination. Sci Signal. 2012;5:pe43. doi: 10.1126/scisignal.2003543. [DOI] [PubMed] [Google Scholar]
- 9.Lazer G, Idelchuk Y, Schapira V, Pikarsky E, Katzav S. The haematopoietic specific signal transducer Vav1 is aberrantly expressed in lung cancer and plays a role in tumourigenesis. J Pathol. 2009;219:25–34. doi: 10.1002/path.2579. [DOI] [PubMed] [Google Scholar]
- 10.Leabu M, Uniyal S, Xie J, Xu YQ, Vladau C, Morris VL, Chan BM. Integrin α2β1 modulates EGF stimulation of Rho GTPase-dependent morphological changes in adherent human rhabdomyosarcoma RD cells. J Cell Physiol. 2005;202:754–66. doi: 10.1002/jcp.20163. [DOI] [PubMed] [Google Scholar]
- 11.Momotani K, Somlyo AV. p63RhoGEF: A New Switch for Gq-Mediated Activation of Smooth Muscle. Trends Cardiovasc Med. 2012;22:122–27. doi: 10.1016/j.tcm.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith TK, Hager HA, Francis R, Kilkenny DM, Lo CW, Bader DM. Bves directly interacts with GEFT, and controls cell shape and movement through regulation of Rac1/Cdc42 activity. Proc Natl Acad Sci U S A. 2008;105:8298–303. doi: 10.1073/pnas.0802345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell DC, Bryan BA, Liu L, Hu XH, Huang XQ, Ji WK, Chen PC, Hu WF, Liu JP, Zhang J, Liu M, Li DW. GEFT, A Rho family guanine nucleotide exchange factor, regulates lens differentiation through a Rac1-mediated mechanism. Curr Mol Med. 2011;11:465–80. doi: 10.2174/156652411796268687. [DOI] [PubMed] [Google Scholar]
- 14.Lutz S, Shankaranarayanan A, Coco C, Ridilla M, Nance MR, Vettel C, Baltus D, Evelyn CR, Neubig RR, Wieland T, Tesmer JJ. Structure of Gaq-p63RhoGEF-RhoA Complex Reveals a Pathway for the Activation of RhoA by GPCRs. Science. 2007;318:1923–27. doi: 10.1126/science.1147554. [DOI] [PubMed] [Google Scholar]
- 15.Swenson-Fields KI, Sandquist JC, Rossol-Allison J, Blat IC, Wennerberg K, Burridge K, Means AR. MLK3 limits activated Gαq signaling to Rho by binding to p63RhoGEF. Mol Cell. 2008;32:43–56. doi: 10.1016/j.molcel.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Momotani K, Artamonov MV, Utepbergenov D, Derewenda U, Derewenda ZS, Somlyo AV. p63RhoGEF Couples Gαq/11-Mediated Signaling to Ca2+ Sensitization of Vascular Smooth Muscle Contractility. Circ Res. 2011;109:993–1002. doi: 10.1161/CIRCRESAHA.111.248898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yono M, Yoshida M, Yamamoto Y, Imanishi A, Fukagawa A, Latifpour J, Eto M. Identification of potential therapeutic targets in hypertension-associated bladder dysfunction. BJU Int. 2010;105:877–83. doi: 10.1111/j.1464-410X.2009.08809.x. [DOI] [PubMed] [Google Scholar]
- 18.Bryan B, Kumar V, Stafford LJ, Cai Y, Wu G, Liu M. GEFT, a Rho family guanine nucleotide exchange factor, regulates neurite outgrowth and dendritic spine formation. J Biol Chem. 2004;279:45824–32. doi: 10.1074/jbc.M406216200. [DOI] [PubMed] [Google Scholar]
- 19.Bryan BA, Cai Y, Liu M. The Rho-family guanine nucleotide exchange factor GEFT enhances retinoic acid- and cAMP-induced neurite outgrowth. J Neurosci Res. 2006;83:1151–9. doi: 10.1002/jnr.20814. [DOI] [PubMed] [Google Scholar]
- 20.Tang X, Jin R, Qu G, Wang X, Li Z, Yuan Z, Zhao C, Siwko S, Shi T, Wang P, Xiao J, Liu M, Luo J. GPR116, an Adhesion G-Protein-Coupled Receptor, Promotes Breast Cancer Metastasis via the G aq-p63RhoGEF-Rho GTPase Pathway. Cancer Res. 2013;73:6206–6218. doi: 10.1158/0008-5472.CAN-13-1049. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi A, Hiatari R, Tsuji T, Ohashi K, Mizuno K. p63RhoGEF-mediated formation of a single polarized lamellipodium is required for chemotactic migration in breast carcinoma cells. FEBS Lett. 2013;587:698–705. doi: 10.1016/j.febslet.2013.01.043. [DOI] [PubMed] [Google Scholar]
- 22.Qi Y, Wang CC, He YL, Zou H, Liu CX, Pang LJ, Hu JM, Jiang JF, Zhang WJ, Li F. The correlation between morphology and the expression of TGF-β signaling pathway proteins and epithelial-mesenchymal transition-related proteins in synovial sarcomas. Int J Clin Exp Pathol. 2013;6:2787–2799. [PMC free article] [PubMed] [Google Scholar]
- 23.Wuertz CM, Lorincz A, Vettel C, Thomas MA, Wieland T, Lutz S. p63RhoGEF—a key mediator of angiotensin II-dependent signaling and processes in vascular smooth muscle cells. FASEB J. 2010;24:4865–76. doi: 10.1096/fj.10-155499. [DOI] [PubMed] [Google Scholar]
- 24.Calò LA, Davis PA, Pessina AC. Does p63RhoGEF, a new key mediator of angiotensin II signalling, play a role in blood pressure regulation and cardiovascular remodelling in humans? J Renin Angiotensin Aldosterone Syst. 2011;12:634–6. doi: 10.1177/1470320311407232. [DOI] [PubMed] [Google Scholar]
- 25.Xin H, Zhen YJ, Han ZG, Wang WZ. Effect of endothelial Rho and Rho kinase in extravasation migration of sarcoma cell. Chinese Journal of Pathophysiology. 2004;20:950–953. [Google Scholar]
- 26.Saito S, Liu XF, Kamijo K, Raziuddin R, Tatsumoto T, Okamoto I, Chen X, Lee CC, Lorenzi MV, Ohara N, Miki T. Deregulation and mislocalization of the cytokinesis regulator ECT2 activate the Rho signaling pathways leading to malignant transformation. J Biol Chem. 2004;279:7169–79. doi: 10.1074/jbc.M306725200. [DOI] [PubMed] [Google Scholar]
- 27.Justilien V, Fields AP. Ect2 links the PKCι-Par6α complex to Rac1 activation and cellular transformation. Oncogene. 2009;28:3597–607. doi: 10.1038/onc.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Citterio C, Menacho-Márquez M, García-Escudero R, Larive RM, Barreiro O, Sánchez-Madrid F, Paramio JM, Bustelo XR. The Rho exchange factors Vav2 and Vav3 control a lung metastasis-specific transcriptional program in breast cancer cells. Sci Signal. 2012;5:ra71. doi: 10.1126/scisignal.2002962. [DOI] [PubMed] [Google Scholar]
- 29.Bennett G, Sadlier D, Doran PP, Macmathuna P, Murray DW. A functional and transcriptomic analysis of NET1 bioactivity in gastric cancer. BMC Cancer. 2011;11:50. doi: 10.1186/1471-2407-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Li Y, Wang Y, Han ZG, Cai B. C9orf100, a new member of the Dbl-family guanine nucleotide exchange factors, promotes cell proliferation and migration in hepatocellular carcinoma. Mol Med Rep. 2012;5:1169–74. doi: 10.3892/mmr.2012.783. [DOI] [PubMed] [Google Scholar]
- 31.Jin H, Li T, Ding Y, Deng Y, Zhang W, Yang H, Zhou J, Liu C, Lin J, Ding Y. Methylation status of T-lymphoma invasion and metastasis 1 promoter and its overexpression in colorectal cancer. Human Pathology. 2011;42:541–51. doi: 10.1016/j.humpath.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Ayala I, Giacchetti G, Caldieri G, Attanasio F, Mariggiò S, Tetè S, Polishchuk R, Castronovo V, Buccione R. Faciogenital dysplasia protein Fgd1 regulates invadopodia biogenesis and extracellular matrix degradation and is up-regulated in prostate and breast cancer. Cancer Res. 2009;69:747–52. doi: 10.1158/0008-5472.CAN-08-1980. [DOI] [PubMed] [Google Scholar]


