Abstract
Objective. Uterine sarcomas are rare gynecological malignancies with poor prognosis and high mortality. We provides clinical information of uterine sarcoma patients at Changhai Hospital of Secondary Military Medical University in Shanghai, China, over a 20-year period. Design and Methods. Satisfied the criteria for the study, a total of 80 female patients with uterine sarcomas were retrospectively evaluated. Overall survival was analyzed by Kaplan-Meier method. Main outcome measures. The following information was extracted from our medical records: age, presentations, blood types, stages, ultrasonographic results, therapies and follow-up. Results. Of the 80 patients, the mean age of onset was 57.3±2.03 years, and the highest frequency occurred in 51-60 age group. Endometrial stromal sarcoma was the most common histological type (47.5%). Even population of these patients presented was with early stage (I&II) and advanced stages (III&IV). Among 79 patients underwent primary surgery, 74 cases was hysterectomy and bilateral salping-ooophorectomy. Equal to disease-specific survival, overall survival rates at 1-, 3- and 5-year were 81.3%, 62.5% and 40% respectively. Age, menopausal status, blood type, stage, and pathologic types were all proved to be correlated with the survival. Conclusion. Our retrospective data in part reflect clinical characteristics of uterine sarcoma in China, and form the basis for further concerning researches.
Keywords: Carcinosarcoma, leiomyosarcoma, endometrial stromal sarcoma, blood type, prognosis, China
Introduction
Uterine sarcomas constitute a group of rare aggressive tumors of the female genital tract worldwide, accounting for 2-6% of uterine malignancies and less than 1% of all female genital tract cancers, with an annual incidence as 1.7 per 100,000 women [1]. Uterine sarcomas are characterized by histopathological diversity, absent prognostic factors, rapid invasion, high rates of recurrence and distant metastasis [2]. For decades, a simplified histological classification of uterine sarcoma has been established including three main pathological categories: carcinosarcoma (CS), leiomyosarcoma (LMS), and endometrial stromal sarcoma (ESS) [3]. The remaining consists of a heterogeneous group of vascular, lymphatic and heterologic sarcomas [4], which were categorized as undiferentiated sarcomas in Changhai Hospital. Due to their rarity and pathological diversity, so far no standard treatment modality is widely accepted in handling uterine sarcomas. Management of uterine sarcomas has traditionally followed that of total abdominal hysterectomy with bilateral salpingo oophorectomy. Current knowledge related to uterine sarcomas is largely based on the experience of authors who have reported large series of cases in other countries [5-7]. There is the need to determine characteristics of uterine sarcoma, like the frequency, clinical presentations, histological variants, prognosis and so on in China. In present study, we present here our accumulated experience of 20 years of treating 80 patients with uterine sarcoma in Changhai Hospital, Shanghai. It aims at providing retrospective evidence of clinical characteristics of uterine sarcoma in China and may serve as a baseline for further research in the future.
Materials and methods
The head of the Department of Gynecology and Obstetrics at Changhai Hospital approved the study design as a retrospective analysis. As this was a retrospective analysis, and not all patients live in Shanghai, the ethics committee waived the need for written informed consent from participants. Verbal consent was obtained from each surviving patient and the family of patients who were deceased by the phone call following-up. The study procedure was approved by the ethics committee of Changhai Hospital, affiliated to the Second Military Medical University, China.
Changhai hospital records for histopathology consultation of 80 patients in whom a diagnosis of uterine sarcomas has been made were reviewed covering from January 1st 1988 to December 31st 2007 for the study. The diagnoses of all of these patients are confirmed and ascertained the histological type by retrieving previously corresponding histological slides. These patients were all treated in the Gynecology and Obstetrics Department of Changhai Hospital. Each patient’s medical case records were reviewed and the variables considered include patient characteristics (age, menopausal status, blood type, etc.), medical history (symptoms, surgical procedure, etc.), tumor characteristics (histological type, size, etc.) and follow-up. Histopathological classification of uterine sarcomas was according to WHO classification as mentioned above. Patients were restaged by the 2009 International Figure 1. Frequency distribution and overall survival of 80 uterine sarcoma cases. A. Blood types and frequency distribution; B. Pathologic types and frequency distribution; C. Kaplan-Meier analysis of overall survival as regards all uterine sarcomas. Federation of Gynecology and Obstetrics (FIGO) staging classification: stage I (sarcoma confined to the uterine corpus), II (sarcoma confined to corpus and cervix), III (sarcoma confined to the pelvis) and IV (extra pelvic sarcoma) [8]. Overall survival (OS) was calculated from the date of diagnosis to either the date of death or the date of last follow-up. The cut-off point for the survival study was December 31st, 2007.
Figure 1.
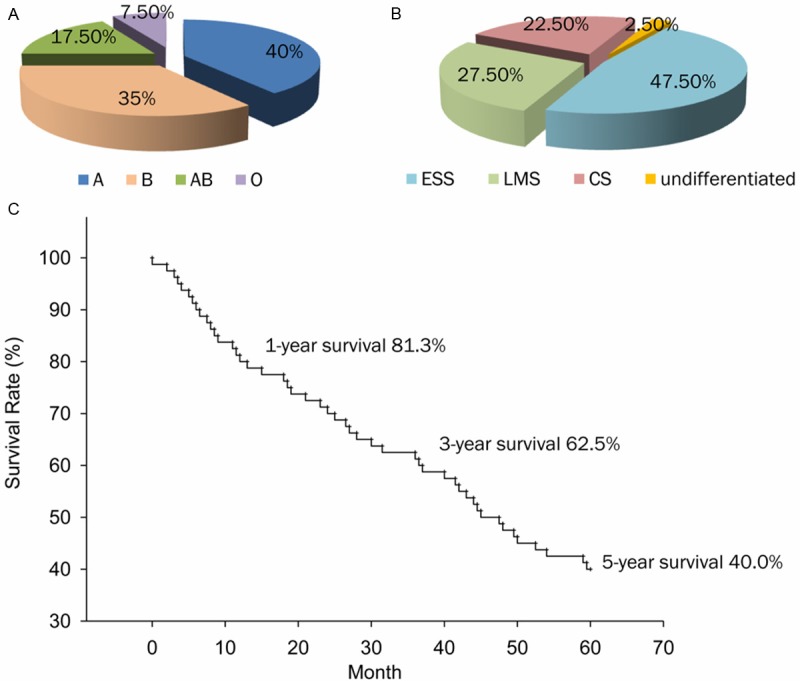
Frequency distribution and overall survival of 80 uterine sarcoma cases. A. Blood types and frequency distribution; B. Pathologic types and frequency distribution; C. Kaplan-Meier analysis of overall survival as regards all uterine sarcomas.
Data were analyzed using SPSS (IBM, Armonk, NY, USA) version 20.0 (Windows 7.0 program) and SigmaPlot 10.0 (Systat Software, Erkrath, German). Mean data with 95% confidence intervals are given. The survival rates were calculated by the Kaplan-Meier method and those variables were evaluated by univariate analysis. The multivariate analysis used to identify correlations between our findings and all potential parameters involved using a logistic regression model. Comparison of survival curves between groups was performed using the log-rank test. P value less than 0.05 was considered significant for all tests.
Results
A total of 80 uterine sarcoma cases were confirmed for the histological diagnosis and retrieved for this retrospective analysis. None of patients had a history of previous malignancy. 32 patients (40%) were type A blood, 28 patients (35%) type B, 14 patients (17.5%) type O and 6 patients (7.5%) type AB (Figure 1A). Histological subtypes were as follows: the majority was ESS (n=38, 47.5%), followed in decreasing order of LMS (n=22, 27.5%), CS (n=18, 22.5%), and undiferentiated sarcoma (n=2, 2.5%) (Figure 1B). The majority of the tumors (n=36, 45.0%) presented with abnormal vaginal bleeding at early stage (Supplementary Table 1). Other commone presenting symptoms contained abdomino-pelvic abdominal mass, abdominal menses and lower abdominal pain or discomfort. Rare clinical manifestations included fever, urinary symptoms, infertility and so on. So far, a large number of these patients showed to have multi-symptoms in their presentation.
Table 1 depicts many clinical characteristics and the frequency distribution of uterine sarcomas grouped by histological subtype seen in the 80 patients. The mean age was 57.3±2.03 years (range from 36 to 87), but the age distribution is variable for different histological subtypes. Mean age of CS group (66.3 years) was higher than those of other groups (ESS: 54.2; LMS: 55.8; undifferentiated: 52.5). The highest frequency occurred in the age group 51-60 years (48.75%). 24 (30.0%) patients were pre-menopausal and 56 (70.0%) post-menopausal. The longest time of post-menopausal was 34 years. Rate of post-menopausal status of ESS group (89.5%, 34/38) was extremely higher than that of LMS (45.5%, 10/22) and CS (55.6%, 10/18). According to FIGO staging, patient rates were evenly split between early stages (I: 42.5%; II: 7.5%) and advanced stages (III: 27.5% and IV: 22.5%). The patients with ESS and LMS were mostly stage I disease (52.6% and 54.5%), whereas CS group were mostly in stage III-IV (66.6%).
Table 1.
Clinical characteristics of 80 uterine sarcoma patients in Changhai Hospital as regards different types of uterine sarcoma
| Clinical Characteristics | All Uterine Sarcoma (n=80) | Histological Type | |||
|---|---|---|---|---|---|
|
| |||||
| ESS n=38, 47.5% | LMS n=22, 27.5% | CS n=18, 22.5% | Undifferentiated Sarcoma n=2, 2.5% | ||
| Age, years [mean (range)] | 57.3 (36-87) | 54.2 (36-72) | 55.8 (44-62) | 66.3 (48-87) | 52.5 (50-55) |
| Age Range (years) | |||||
| 40 and below | 2 (2.5%) | 2 | 0 | 0 | 0 |
| 41-50 | 14 (17.5%) | 7 | 6 | 1 | 0 |
| 51-60 | 39 (48.75%) | 21 | 11 | 5 | 2 |
| 61-70 | 21 (26.25%) | 7 | 5 | 9 | 0 |
| 71-80 | 3 (3.75%) | 1 | 0 | 2 | 0 |
| 81 and above | 1 (1.25%) | 0 | 0 | 1 | 0 |
| Menopause, n (%) | |||||
| Pre-menopausal | 24 (30.0%) | 4 (10.5%) | 12 (54.5%) | 8 (44.4%) | 0 (0%) |
| Post-menopausal | 56 (70.0%) | 34 (89.5%) | 10 (45.5%) | 10 (55.6%) | 2 (100%) |
| Clinical Stages | |||||
| I | 34 (42.5%) | 20 (52.6%) | 12 (54.5%) | 2 (11.1%) | 0 (0%) |
| II | 6 (7.5%) | 0 (0%) | 2 (9.1%) | 4 (22.2%) | 0 (0%) |
| III | 22 (27.5%) | 14 (36.8%) | 0 (0%) | 6 (33.3%) | 2 (100%) |
| IV | 18 (22.5%) | 4 (10.5%) | 8 (36.4%) | 6 (33.3%) | 0 (0%) |
| Total | 80 | 38 | 22 | 18 | 2 |
The ultrasound characteristics before operation are summarized as Table 2. The size of uterus was enlarged in all cases, partly with irregular form and unclear capsular boundary. 45% tumor envelope was clear with the boundary of muscular layer. In half the cases, the mass is intermural, part of other 40 cases appear intramural or subserosal. The internal of the mass can be showed as unevenness, or liquid dark area, or low heterogeneous echo. Mean size of tumor was 8.23 cm in diameter (ranging from 3.0 cm to 16.0 cm), diameter greater than 5 cm accounted for 52.5%, and multiple mass lesions accounted for 62.5%. Moreover, uterine sarcoma had abundant blood stream, as well as neovascularization and blood flow signal can be found especially in the peripheral of the tumor.
Table 2.
The sonography results of 80 uterine sarcoma patients in Changhai Hospital
| Pathologic Types | Number | Capsule | Position | Internal of tumors | Size (cm) | Blood flow signal | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| clear | unclear | intrauterine | subserosal | intermural | without vortex | unevenness | liquid dark area | ≤5 | >5 | outside | inside | |||
| ESS | 38 | 14 | 24 | 16 | 8 | 14 | 6 | 28 | 10 | 24 | 14 | 38 | 14 | |
| LMS | 22 | 16 | 6 | 4 | 2 | 16 | 6 | 18 | 4 | 4 | 18 | 22 | 6 | |
| CS | 18 | 6 | 12 | 8 | 2 | 8 | - | 12 | 6 | 8 | 10 | 18 | 2 | |
| undifferentiated | 2 | - | 2 | - | - | 2 | - | - | 2 | 2 | - | 2 | - | |
| Total | n | 80 | 36 | 44 | 28 | 12 | 40 | 12 | 58 | 22 | 38 | 42 | 80 | 22 |
| % | 45.0 | 55.0 | 35.0 | 15.0 | 50.0 | 15.0 | 72.5 | 27.5 | 47.5 | 52.5 | 100.0 | 27.5 | ||
The surgery is the mainstay treatment for uterine sarcoma. One patient aged 87, with CS in endometrial biopsy samples were not operated upon in the first instance. Pelvic MR imaging (MRI) showed her stage IV disease. The patient received one chemotherapy cycle, but she died 2 months after diagnosis. Common surgical procedures of 79 patients are listed in Table 3. Pure hysterectomy was carried out in 79 patients. 92.5% underwent hysterectomy and bilateral/unilateral salpingo-oophorectomy surgeries. Pelvic lymph node status was clarified in 16 patients. No significant difference in surgical procedures among patients with various histological types were evident. Given that uterine sarcoma has early hematogenous metastasis, all cases were underwent 2 to 8 courses of adjunctive chemotherapy post-surgery to delay the recurrence. Most commonly used chemotherapy combinations were AC, ACD, VAC, VAD and VC. 47.5% cases were recurrent: typical site for distant metastases was lungs (n=11).
Table 3.
Surgical management of different histological types in Changhai Hospital
| Surgical Management | All Uterine Sarcoma (n=80) | ESS (n=38) | LMS (n=22) | CS (n=18) | Undifferentiated Sarcoma (n=2) |
|---|---|---|---|---|---|
| AH | 79 | 37 | 22 | 18 | 2 |
| LH/VH | 1 | 1 | 0 | 0 | 0 |
| BSO | 74 | 36 | 19 | 16 | 2 |
| PLND | 16 | 32 | 13 | 16 | 2 |
| OE | 30 | 35 | 19 | 4 | 1 |
AH=Abdominal Hysterectomy, LH=Laparoscopic Hysterectomy, VH=Vaginal Hysterectomy, BSO=Bilateral Salping-Oophorectomy, PLND=Pelvic Lymphadenectomy, OE=Omentectomy.
As shown in Figure 1C, the 1-, 3- and 5- overall survival rates were 81.3%, 62.5% and 40.0%, and disease-specific survival rates were the same. The mean time was 27±4 months. Survival rates naturally decline with age (Figure 2A). Significant difference between menopausal status and survival rates was shown in Figure 2B. While the 3-year and 5-year survival rates of pre-menopausal patients were all 70.8%, the rates of post-menopausal patients were only 58.9% and 26.8% (Table 4). The prognosis of uterine sarcoma patients with blood type A and B are dramatically poorer than that of other blood types (Figure 2C). Univariate analysis of survival rates showed that advanced FİGO stages were significantly associated with poor survival rates (Figure 3A). In our study, pathologic types were also significantly associated with survival rate. 5-years survival of ESS was better than those of LMS and CS (Figure 3B, left panel). Additionally, survival rate of high malignant ESS showed to be extremely lower than that of low malignant one (Figure 3B, right panel). As shown in Table 4, age, stage and pathologic type were found to have independent influences on OS.
Figure 2.
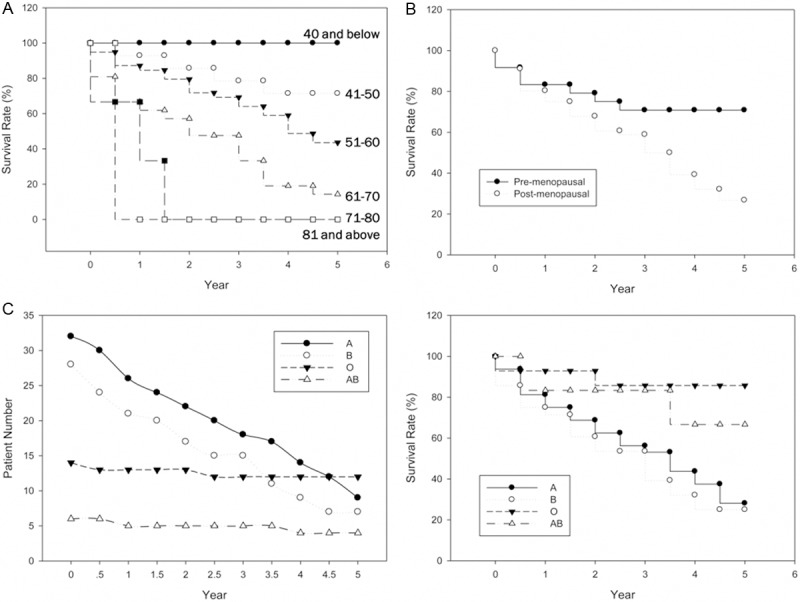
Disease-specific survival as regards the uterine sarcoma with age, menopausal status and blood types. A. The survival rate with six age range (40 and below, 41-50, 51-60, 61-70, 71-80, 81 and above); B. The survival rate with pre- and post-menopausal patients; C. Compare the patient number with four blood types (A, B, O, AB) biannually for five years (left); The survival rate with four blood types (right).
Table 4.
The relationship between clinical characteristics and 1-, 3- or 5-year survival rates in 80 uterine sarcoma patients in Changhai Hospital
| Prognostic Factors | Number | P value | 1-year | 3-year | 5-year | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| n | % | n | % | n | % | ||||
| Age Range (years) | <0.001 | ||||||||
| 40 and below | 2 | 2 | 100 | 2 | 100 | 2 | 100 | ||
| 41-50 | 14 | 13 | 92.9 | 11 | 78.6 | 10 | 71.4 | ||
| 51-60 | 39 | 34 | 87.2 | 27 | 69.2 | 17 | 43.6 | ||
| 61-70 | 21 | 14 | 66.7 | 10 | 47.6 | 3 | 14.3 | ||
| 71-80 | 3 | 2 | 66.7 | 0 | 0 | 0 | 0 | ||
| 81 and above | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Blood Type | 0.009 | ||||||||
| A | 32 | 26 | 81.3 | 18 | 56.3 | 9 | 28.1 | ||
| B | 28 | 21 | 75.0 | 15 | 53.6 | 7 | 25.0 | ||
| O | 14 | 13 | 92.9 | 12 | 85.7 | 12 | 85.7 | ||
| AB | 6 | 5 | 83.3 | 5 | 83.3 | 4 | 66.7 | ||
| Menopausal Status | 0.001 | ||||||||
| Pre-menopausal | 24 | 20 | 83.3 | 17 | 70.8 | 17 | 70.8 | ||
| Post-menopausal | 56 | 45 | 80.4 | 33 | 58.9 | 15 | 26.8 | ||
| Clinical Stages | 0.0036 | ||||||||
| I | 34 | 34 | 100.0 | 33 | 97.1 | 25 | 73.5 | ||
| II | 6 | 6 | 100.0 | 4 | 66.7 | 3 | 50.0 | ||
| III | 22 | 17 | 77.3 | 9 | 40.9 | 4 | 18.2 | ||
| IV | 18 | 8 | 44.4 | 4 | 22.2 | 0 | 0 | ||
| Pathologic Types | <0.001 | ||||||||
| ESS | low malignancy | 24 | 24 | 100.0 | 24 | 100.0 | 20 | 83.3 | |
| high malignancy | 14 | 11 | 78.6 | 8 | 57.1 | 5 | 35.7 | ||
| Total | 38 | 35 | 92.1 | 32 | 84.2 | 25 | 65.8 | ||
| LMS | 22 | 18 | 81.8 | 11 | 50.0 | 5 | 22.7 | ||
| CS | 18 | 12 | 66.7 | 7 | 38.9 | 2 | 11.1 | ||
| undifferentiated | 2 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Surgery Scope | 0.256 | ||||||||
| AH | 79 | 65 | 82.3 | 50 | 63.3 | 31 | 39.2 | ||
| AH+BSO | 58 | 48 | 82.8 | 36 | 62.1 | 19 | 32.8 | ||
| AH+BSO+PLND | 16 | 13 | 81.3 | 11 | 68.8 | 5 | 31.3 | ||
| AH+BSO+OE | 30 | 25 | 83.3 | 20 | 66.7 | 8 | 26.7 | ||
| Total | 80 | 65 | 81.3 | 50 | 62.5 | 32 | 40.0 | ||
Figure 3.
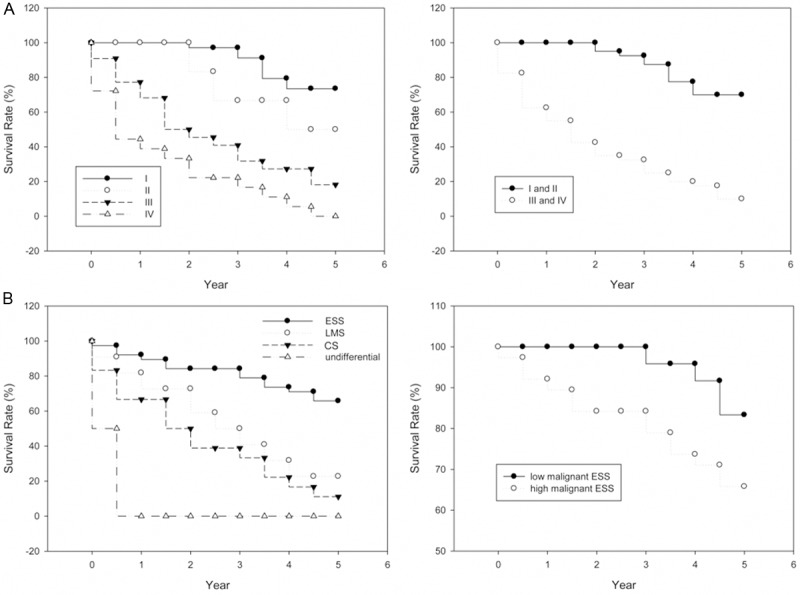
Disease-specific survival as regards the uterine sarcoma with clinical stages and pathologic types. A. The survival rate with four clinical stages (stage I, II, III and IV) (left); The survival rate with early (I and II) and advanced stages (III and IV) (right). B. The survival rate with four pathologic types (left); The survival rate of uterine sarcoma patients with low and high malignant ESS (right).
Discussion
Uterine sarcomas are rare female malignancies worldwide with unknown etiology, and characterized by aggressive progression and poor prognosis, though they only account for 1-3% of female genital tract malignancies and 2-6% of uterine malignancies [9]. However, there is few studies concerning clinical characteristics of uterine sarcoma performed in Chinese. Our results are firstly reported to reflect the scenario in China and may generate interest among the readers for further researches.
Most of our data, but not all, were agreed fairly well with those reported in literature. Uterine sarcoma in postmenopausal women usually Figure 2. Disease-specific survival as regards the uterine sarcoma with age, menopausal status and blood types. A. The survival rate with six age range (40 and below, 41-50, 51-60, 61-70, 71-80, 81 and above); B. The survival rate with pre- and post-menopausal patients; C. Compare the patient number with four blood types (A, B, O, AB) biannually for five years (left); The survival rate with four blood types (right). present with abnormal uterine bleeding, but so far there is non-specific symptoms [10]. Our patients generally present with variable symptoms and the foremost (45%) was abnormal vaginal bleeding like other studies [6]. If there are symptoms such as postmenopausal vaginal bleeding, abdominal pain, rapidly increased uterine mass, should be highly suspected uterine sarcoma. According to the uterine sarcoma ultrasonic performance combined with clinical symptoms and signs, the preoperative diagnosis rate of uterine sarcoma can be improved.
It is the first report that uterine sarcoma is more likely to happen at those females with type A or B blood. Furthermore, our results showed that the prognoses of patients with type A or B blood are poorer than that of other blood types. It seems reasonable that ABO blood type may have a close relationship with increased risk of many kinds of tumors, such as pancreatic cancer [11-13], gastrointestinal cancer [14], breast cancer [15,16] and oral cancer [17]. Studies performed suggested the association between blood types and other gynecological cancers, such as ovarian cancer and cervical cancer [18]. However, to the best of our knowledge, no previous studies have been conducted to investigate the possible association between ABO blood type and uterine sarcoma in the Chinese population. This is the first study showing a correlation between blood type and uterine sarcoma prevalence in the Chinese population, but it is necessary for us to embark on larger case-control study to evaluate their relationship.
For each histological types of uterine sarcomas, many clinical characteristics are different, so they were analyzed separately in our study. Our data showed that ESS is the predominant subtype in these 80 patients (47.5%) which is just compatible with the earlier literature [19]. LMS is known to be a common uterine sarcoma [20], and there’s 27.5% in our report, second to ESS. CS is not a common subtype and accounts for 22.5% in this study, corroborates the reports of other workers in this specialty [21]. Only two cases of undifferentiated sarcomas diagnosed in our hospital because of its rarity worldwide [22]. Usually patients with uterine sarcomas are diagnosed at older ages, our data co-relate well with the age distribution in other studies elsewhere [23]. There is age variation and distribution in occurrence of different histological subtypes. ESS occurs in younger patients than those of CS in our setting. Our result that ESS occurs in the peri-menopausal age group with an average age of 45 years, corroborated by the ages of our ESS patients in pre-menopausal group, which ranged from 36 to 54 years [24]. The figures recorded in this centre corroborates that CS is always presented at the age of 50 years and above [21]. Data regarding time of menopause as a risk factor for CS are inconclusive, but over half of cases occur after menopausal period in our data (mean age 66.3). In the series described by Kokawa et al. there was a similar proportion of stages III and IV (52%), as our data (50%) [5]. 63.6% of the patients in LMS group were diagnosed at stage I-II, while CS group was mainly diagnosed at advanced stages (stage III-IV, 66.6%). Moreover, stage of the disease has been found to be significant prognostic factor in most studies [8,25], including ours. Hysterectomy with or without salpingo-oophorectomy is the mainstay of treatment for all types of uterine sarcomas. The rate of lymph node metastasis of uterine sarcomas swung from 0% to 47% [26], so debate concerning the practical value of staging with lymphadenectomy is still ongoing [5]. However, additional pelvic lymphadenectomy is indicated for CS group, because of its high incidence of lymph node metastasis [27]. In our study, 88.9% CS cases underwent pelvic lymphadenectomy procedure and lymph node metastasis was found in 72.2% of them. For LMS and ESS, the incidence of involvement of lymph node is relatively less than that of CS.
In our study, overall survival rates are consistent with disease-specific rates. 5-year OS from other institution long ago was 24% [28], the figure in our study was 45%. The disparity may be attributed to different races, or that the survival rate has improved now. There is no consensus on prognostic factors of uterine sarcomas in the literature. Some studies reported that patients younger than age 50 years had longer survival [5]. In some studies, tumor size and depths of myometrial invasion had significant effect on the prognosis [29]. In our analysis, age, blood type, menopausal status, stage and pathologic type were proven to have independent influences on OS, but the operation range was not shown to be associated with OS. Many authors have come to the same conclusions as regards stage and age [5]. It is not clear from the literature whether histological type is an independent prognostic factor [19]. In our study, 5-years overall survival rates in CS, in LMS and in EES are calculated to be 11.1, 22.7 and 65.8% respectably, indicating that pathological type might be significant prognostic factor. Nowadays, it was reported that CS had poorer prognosis than other histological types of uterine sarcoma [30], is confirmed by our study. It’s worth mentioning that our data is the first study showing blood type as a prognostic factor of uterine sarcoma. Though surgery scopes were not significantly associated with OS in our study, to expand the scope of surgical methods can significantly reduce the rate of recurrence of uterine sarcoma.
Our study is the first of the kind to review clinical characteristics of uterine sarcoma in this setting with such size of sample and time span in China. Although limited by relative small sample size and retrospective nature, it gave us important findings from Chinese. In the future, large and multi-centered studies should be performed to help us get more data and better understand and treat uterine sarcoma.
Acknowledgements
We thank Lin Yao, Yujie Liu, Jinchan Chen, Wenyu Zhou for their constructive advices in the statistical analysis in this study. We also thank Yuehua Li, Dong Wu, Jun Ou, Dan Wang, Mingjuan Xu for their important input during the review process.
Disclosure of conflict of interest
None to declare.
Supporting Information
References
- 1.Parker WH. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2005;105:216–217. doi: 10.1097/01.AOG.0000150453.10906.b6. [DOI] [PubMed] [Google Scholar]
- 2.Akahira J, Tokunaga H, Toyoshima M, Takano T, Nagase S, Yoshinaga K, Tase T, Wada Y, Ito K, Niikura H, Yamada H, Sato A, Sasano H, Yaegashi N. Prognoses and prognostic factors of carcinosarcoma, endometrial stromal sarcoma and uterine leiomyosarcoma: a comparison with uterine endometrial adenocarcinoma. Oncology. 2006;71:333–340. doi: 10.1159/000107107. [DOI] [PubMed] [Google Scholar]
- 3.Bocker W. [WHO classification of breast tumors and tumors of the female genital organs: pathology and genetics] . Verh Dtsch Ges Pathol. 2002;86:116–119. [PubMed] [Google Scholar]
- 4.Chauveinc L, Deniaud E, Plancher C, Sastre X, Amsani F, de la Rochefordiere A, Rozemberg H, Clough KB. Uterine sarcomas: the Curie Institut experience. Prognosis factors and adjuvant treatments. Gynecol Oncol. 1999;72:232–237. doi: 10.1006/gyno.1998.5251. [DOI] [PubMed] [Google Scholar]
- 5.Kokawa K, Nishiyama K, Ikeuchi M, Ihara Y, Akamatsu N, Enomoto T, Ishiko O, Motoyama S, Fujii S, Umesaki N. Clinical outcomes of uterine sarcomas: results from 14 years worth of experience in the Kinki district in Japan (1990-2003) Int J Gynecol Cancer. 2006;16:1358–1363. doi: 10.1111/j.1525-1438.2006.00536.x. [DOI] [PubMed] [Google Scholar]
- 6.D’Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116:131–139. doi: 10.1016/j.ygyno.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Koivisto-Korander R, Butzow R, Koivisto AM, Leminen A. Clinical outcome and prognostic factors in 100 cases of uterine sarcoma: experience in Helsinki University Central Hospital 1990-2001. Gynecol Oncol. 2008;111:74–81. doi: 10.1016/j.ygyno.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Durnali A, Tokluoglu S, Ozdemir N, Inanc M, Alkis N, Zengin N, Sonmez OU, Kucukoner M. Prognostic factors and treatment outcomes in 93 patients with uterine sarcoma from 4 centers in Turkey. Asian Pac J Cancer Prev. 2012;13:1935–1941. doi: 10.7314/apjcp.2012.13.5.1935. [DOI] [PubMed] [Google Scholar]
- 9.Piura B, Rabinovich A, Yanai-Inbar I, Cohen Y, Glezerman M. Uterine sarcoma in the south of Israel: study of 36 cases. J Surg Oncol. 1997;64:55–62. doi: 10.1002/(sici)1096-9098(199701)64:1<55::aid-jso11>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Wickerham DL, Fisher B, Wolmark N, Bryant J, Costantino J, Bernstein L, Runowicz CD. Association of tamoxifen and uterine sarcoma. J. Clin. Oncol. 2002;20:2758–2760. doi: 10.1200/JCO.2002.20.11.2758. [DOI] [PubMed] [Google Scholar]
- 11.Wolpin BM, Chan AT, Hartge P, Chanock SJ, Kraft P, Hunter DJ, Giovannucci EL, Fuchs CS. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009;101:424–431. doi: 10.1093/jnci/djp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabir A. Comment on: Risk of pancreatic cancer in relation to ABO blood group and hepatitis C virus infection in Korea: a case-control study. J Korean Med Sci. 2013;28:1114. doi: 10.3346/jkms.2013.28.7.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo SM, Joo J, Lee WJ, Park SJ, Han SS, Kim TH, Koh YH, Kim HB, Hong EK. Risk of pancreatic cancer in relation to ABO blood group and hepatitis C virus infection in Korea: a case-control study. J Korean Med Sci. 2013;28:247–251. doi: 10.3346/jkms.2013.28.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong Y, Yang YS, Zhang XM, Su M, Wang J, Han JD, Guo MZ. ABO blood type, diabetes and risk of gastrointestinal cancer in northern China. World J Gastroenterol. 2012;18:563–569. doi: 10.3748/wjg.v18.i6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dede DS, Aksoy S, Dizdar O, Cerci P, Gullu I, Ozisik Y, Altundag K. Blood ABO groups and risk of breast cancer. Med Oncol. 2010;27:1433. doi: 10.1007/s12032-009-9346-1. [DOI] [PubMed] [Google Scholar]
- 16.Urun Y, Utkan G, Altundag K, Arslan O, Onur H, Arslan UY, Kocer M, Dogan I, Senler FC, Yalcin B, Demirkazik A, Akbulut H, Icli F. ABO and Rh blood groups frequency in women with HER2 positive breast cancer. J BUON. 2012;17:457–460. [PubMed] [Google Scholar]
- 17.Jaleel BF, Nagarajappa R. Relationship between ABO blood groups and oral cancer. Indian J Dent Res. 2012;23:7–10. doi: 10.4103/0970-9290.99029. [DOI] [PubMed] [Google Scholar]
- 18.Yuzhalin AE, Kutikhin AG. ABO and Rh blood groups in relation to ovarian, endometrial and cervical cancer risk among the population of South-East Siberia. Asian Pac J Cancer Prev. 2012;13:5091–5096. doi: 10.7314/apjcp.2012.13.10.5091. [DOI] [PubMed] [Google Scholar]
- 19.Wolfson AH, Wolfson DJ, Sittler SY, Breton L, Markoe AM, Schwade JG, Houdek PV, Averette HE, Sevin BU, Penalver M, et al. A multivariate analysis of clinicopathologic factors for predicting outcome in uterine sarcomas. Gynecol Oncol. 1994;52:56–62. doi: 10.1006/gyno.1994.1011. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Yang J, Huang H, Li Y, Miao Q, Lu X, Yang N, Huang Y, Chen J, Cao D, Wu M, Pan L, Lang J, Shen K. Management of intravenous leiomyomatosis with intracaval and intracardiac extension. Obstet Gynecol. 2012;120:1400–1406. doi: 10.1097/aog.0b013e31826ebb90. [DOI] [PubMed] [Google Scholar]
- 21.Gunthert AR. Sarcomas and mixed mesodermal tumors of the uterus. Ther Umsch. 2011;68:559–564. doi: 10.1024/0040-5930/a000214. [DOI] [PubMed] [Google Scholar]
- 22.Anyiam DC, Ukah CO, Onyiaorah IV, Okafor N. Sarcoma botyroides of the cervix in a HIV positive 45-year-old woman: a case report. Niger J Clin Pract. 2010;13:341–343. [PubMed] [Google Scholar]
- 23.Ghaemmaghami F, Karimi-Zarchi M, Gilani MM, Mousavi A, Behtash N, Ghasemi M. Uterine sarcoma: clinicopathological characteristics, treatment and outcome in Iran. Asian Pac J Cancer Prev. 2008;9:421–426. [PubMed] [Google Scholar]
- 24.Fekete PS, Vellios F. The clinical and histologic spectrum of endometrial stromal neoplasms: a report of 41 cases. Int J Gynecol Pathol. 1984;3:198–212. doi: 10.1097/00004347-198402000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Gadducci A. Prognostic factors in uterine sarcoma. Best Pract Res Clin Obstet Gynaecol. 2011;25:783–795. doi: 10.1016/j.bpobgyn.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Leitao MM, Sonoda Y, Brennan MF, Barakat RR, Chi DS. Incidence of lymph node and ovarian metastases in leiomyosarcoma of the uterus. Gynecol Oncol. 2003;91:209–212. doi: 10.1016/s0090-8258(03)00478-5. [DOI] [PubMed] [Google Scholar]
- 27.Temkin SM, Hellmann M, Lee YC, Abulafia O. Early-stage carcinosarcoma of the uterus: the significance of lymph node count. Int J Gynecol Cancer. 2007;17:215–219. doi: 10.1111/j.1525-1438.2006.00762.x. [DOI] [PubMed] [Google Scholar]
- 28.Kahanpaa KV, Wahlstrom T, Grohn P, Heinonen E, Nieminen U, Widholm O. Sarcomas of the uterus: a clinicopathologic study of 119 patients. Obstet Gynecol. 1986;67:417–424. [PubMed] [Google Scholar]
- 29.Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. The impact of tumor morcellation during surgery on the outcomes of patients with apparently early low-grade endometrial stromal sarcoma of the uterus. Ann Surg Oncol. 2011;18:3453–3461. doi: 10.1245/s10434-011-1751-y. [DOI] [PubMed] [Google Scholar]
- 30.Benito V, Lubrano A, Arencibia O, Andujar M, Alvarez E, Medina N, Falcon JM, Falcon O. Clinicopathologic analysis of uterine sarcomas from a single institution in the Canary Islands. Int J Gynaecol Obstet. 2009;107:44–49. doi: 10.1016/j.ijgo.2009.05.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


