Abstract
Background: The characteristics of pathologically measured (PMS) and endoscopically measured sizes (EMS) of the colorectal polyps (CRPs) is poorly understood, particularly in polypoid unremarkable mucosa (PUM), sessile serrated adenoma (SSA), and high-grade dysplasia (HGD). Methods: To characterize the discordance and correlation between the PMS and EMS of CRPs including PUM, SSA, HGD, hyperplastic polyp (HP) and adenomas, we conducted this prospective observational study on the polyps collected between August 2012 and December 2013. Results: PMS was significantly smaller than EMS in the 497 qualified CRPs regardless of the sites (left, transverse and right colorectum) or EMS (≥1 cm and <1 cm) subgroups. The PMS and EMS discordance was associated with a diagnosis of HP and adenoma (versus PUM, SSA or HGD), single fragment (versus multiple), 3 of the 8 endoscopists and PMS<1 cm (versus ≥1 cm). Despite a good correlation between EMS and PMS in the adenomas (ĸ=0.626, 95% confidence intervals [CI], 0.505-0.746) and a moderate correlation in the serrated polyps (SPs) including HP and SSA, (ĸ=0.424, 95% CI, 0.244-0.604), 40.4% (23/57) of the adenomas and 63.6% (21/33) of the SPs with EMS≥1 cm might warrant longer follow-up intervals since their PMS were <1 cm. The PMS and EMS had linear correlations except in CRPs with HGD or EMS≥1 cm. Conclusions: The discordance between PMS and EMS is associated with the pathologic diagnosis, fragment number, endoscopists and PMS, and may lead to different follow-ups in a considerable portion of adenomas and SPs.
Keywords: Polyp, surveillance, measurement, colonoscopy, pathology, cancer
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer related deaths in the US [1]. Colonoscopy could effectively reduce CRC risks by detecting and removing the CRC precursor lesions, mainly adenomas [2,3]. After endoscopic resection of a colorectal polyp (CRP), an endoscopic surveillance is recommended by both the US and UK guidelines [4-6]. Specifically, the CRP size, polyp number, and polyp histology (villous versus tubular adenoma, and presence of high-grade dysplasia, in the US guidelines only) are used to determine CRC risks and follow-up intervals. However, the preferred method for CRP size assessment was not specified in the US guidelines [4,7], while the UK ones mentioned using the endoscopically measured size (EMS) [5]. EMS in fact was used in the earlier studies demonstrating that adenomas ≥1 cm had high risks for CRC [8,9].
The preferred CRP size assessment method is controversial. EMS has been reported both larger [10,11] and smaller [12,13] than the corresponding pathologically measured size (PMS). And most of the comparison studies only included adenomas and hyperplastic polyps (HP), and had a modest to acceptable sample size (31-230 CRPs) [10,11,14-18]. The PMS and EMS of adenoma with high-grade dysplasia (HGD), sessile serrated adenoma (SSA), and polypoid unremarkable mucosa (PUM) to our best knowledge have not been explored yet despite their clinical significance [4-6]. In addition, only 2 studies investigated the factors associated with the discordance and correlation between PMS and EMS of CRP [11,19]. Large-scale, prospective studies ideally also including PUM, SSA and HGD are needed to better characterize the PMS and EMS of CRPs.
EMS was originally used in the hallmark trials on optimal CRP surveillance intervals [3,9]. In the meantime, PMS is preferred by some for its higher reliability, accuracy and simplicity, and has been recommended by the European colorectal cancer screening pathology quality assurance guidance [11,14,15,17]. Before the trials stratifying CRC risk with PMS become available, EMS seems more directly associated with CRP classification, risk prognostication and follow-up interval determination. Therefore, a potential linear correlation between PMS and EMS would help estimate EMS and determine proper follow-up interval, when EMS is not available. No studies have examined the linear correlation between PMS and EMS.
We therefore conducted this prospective study to investigate the discordance and potential linear correlation between the PMS and EMS in 497 qualified CRPs. HGD, adenoma, SSA, HP and PUM were all included to fill the aforementioned knowledge gap on SSA and HGD.
Materials and methods
Patients and study design
An IRB approval was obtained from University Medical Center of Princeton at Plainsboro. We prospectively included all qualified colonoscopy cases, for symptoms or screening, incurred at the same institution from August 2012 to December 2013. All of the patients had provided written informed consents for the colonoscopic procedures. The inclusion criteria were: 1. No history of or current diagnosis of inflammatory bowel diseases; 2. EMS data were available in the endoscopic report; 3. The endoscopic report indicated that the CRP was completely removed and retrieved; 4. All CRPs were collected between August 2012 to December 2013, except the cases with PUM between March to December 2013. No patients underwent additional or unnecessary colonoscopies or had alterations in their management as part of the study. The following information of the qualified cases was collected: patient’s age, gender, lesion site, pathologic diagnosis, EMS, PMS and the endoscopist initials.
Endoscopic procedure and pathological evaluation
Video endoscopes (Olympus Optical Co., Tokyo, Japan) were used for all procedures. The patients were prescribed polyethylene glycol lavage bowel preparation or equivalent and the examiners cleaned the colon during instrument insertion and withdrawal as much as possible. The CRPs were identified mainly during withdrawal. The EMS was rendered by using the largest diameter of each CRP in situ (to the nearest mm) with wide-open biopsy forceps as the reference. Eight endoscopists (four with a more-than-ten-year colonoscopy experience) with qualified adenoma detection rates participated in the study. The method of retrieval was at the endoscopist’s discretion: retrieval net or tripod.
Pathologic evaluations were performed by a gastrointestinal and liver pathology fellowship trained pathologist (LZ). All collected specimens were fixed in 10% formalin within 1 hour of the removal and then fixed for a minimum of 4 hours. Then the fixed specimens were cut into 2-mm slices according to standard pathology laboratory protocols. By using a micrometer, the PMS for the CRPs with single tissue fragment was the greatest dimension of the lesions, and the PMS for the CRPs with multiple fragments were the aggregation of the lesions’ greatest dimensions. The histological diagnoses, including PUM, HP, SSA, adenoma and HGD, were determined according to the Vienna classification system [20]. The endoscopists and the pathologists were blinded to each other’s measurements.
Statistical analysis
The data were entered manually and analyzed by using STATA version 12.0 (Stata Corp, College Station, Texas, USA). The paired continuous data were compared by using the paired Student’s t test. Linear regression analysis (LRA) was used to assess the correlation between PMS and EMS. The association between categorical data and interrater agreement were analyzed by using χ2 test and ĸ statistics respectively. A two-tailed P<0.05 was considered statistically significant.
Results
As shown in Figure 1, 1073 CRPs were identified through the colonoscopic procedures. Of them, 12 were not assessed for PMS and 564 cases had no available endoscopic reports. Finally, 497 CRP with both EMS and PMS were included for the analysis. The average patient age was 60.64 year old (±10.73 SD) and 273 (54.9%) of CRPs were taken from a male. We found 149 (30.0%) CRPs located in the left colon, 108 (21.7%) in the transverse colon and 240 (48.3%) in the right colon and rectum.
Figure 1.
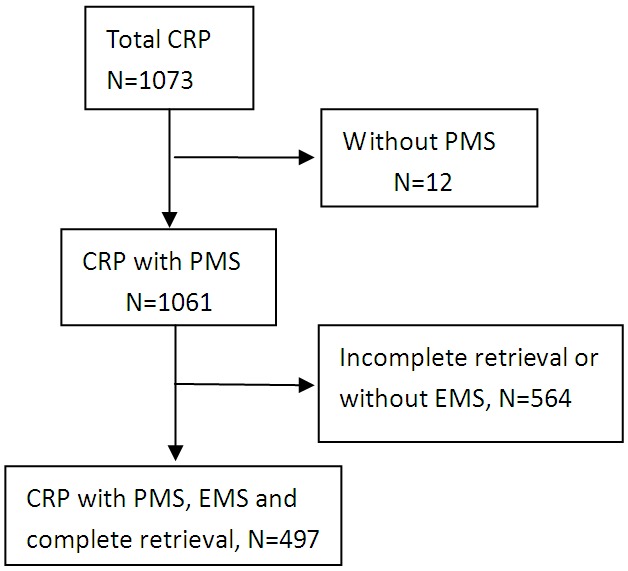
Flow chart of the study. PMS: pathologically measured size; EMS: endoscopically measured size.
In the qualified 497 CRPs, the EMS (0.71±0.995 cm, Mean±SD) was significantly larger than the PMS (0.549±0.455 cm) (P<0.001). Compared with HP, the PMSs of HGD, adenoma and SSA were larger (P<0.001 for all). Compared with adenoma, the PMS of HGD (P=0.1343) and SSA (P=0.1602) were not statistically different. Our LRA showed that EMS and PMS significantly correlated with each other (Figure 2, coefficient 0.180 and P<0.001).
Figure 2.
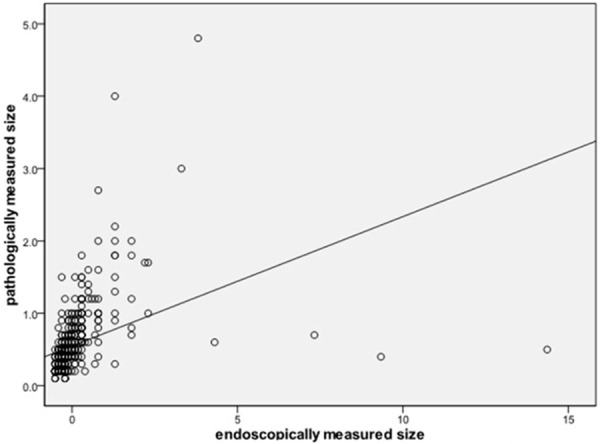
Linear regression analysis showed that EMS and PMS significantly correlated with each other. Equation: y=0.180x+0.421; R2=0.154.
We classified 37 CRPs as PUM, 105 as HP, 84 as SSA, 262 as adenoma and 9 as HGD. All of the PUMs were described as a polyp or prominent fold in the endoscopic report. The mean EMS of HP and adenoma were 0.458 cm (±0.262 SD) and 0.784 cm (±1.183 SD) respectively, significantly larger than the paired PMS (0.372±0.218 cm, P<0.001 and 0.597±0.486 cm, P=0.007; respectively). No statistical differences between EMS and PMS were detected in the PUMs (P=0.097), SSA (P=0.197) or HGD (P=0.115). By using LRA, linear correlations between EMS and PMS were found in the PUM (coefficient 0.056, P=0.003), HP (coefficient 0.466, P<0.001), SSA (coefficient 0.882, P<0.001) and adenoma groups (coefficient 0.137, P<0.001), but not in the HGD group (coefficient 0.049, P=0.686).
The 397 CRPs with an EMS<1 cm had a mean EMS of 0.46 cm (±0.191SD), significantly larger than the paired PMS (0.41±0.223 cm, P<0.001, Table 1). For the 100 CRPs with an EMS≥1 cm, there was also a statistical difference between the EMS and PMS (1.73±1.871 cm vs. 1.09±0.690 cm, P=0.001). Our LRA showed a linear correlation between the EMS and PMS in the CRPs with an EMS<1 cm (coefficient 0.703,P<0.001), but not in the CRPs with an EMS≥1 cm (coefficient 0.028, P=0.457). When taken 1 cm as a cutoff in PMS per the US and British guidelines and recommendation [4-6], 337 CRPs were classified into the small-size group, with a mean PMS of 0.43 cm (±0.223 SD) and a mean EMS of 0.61 cm (±0.975 SD) (P<0.001). Among the rest 60 CRPs with their PMSs≥1 cm, the EMS was similar to the PMS (1.45±0.816 cm versus 1.45±0.704 cm, P=0.968). However, the EMS had a linear association with the PMS in the CRPs with a PMS<1 cm or ≥1 cm, with different coefficients (coefficient 0.050, P<0.001 and coefficient 0.564, P<0.001, respectively).
Table 1.
Factors associated with the discordance and correlation between the pathologic and endoscopic measurements of colorectal polyp sizes
| Category (n, %) | PMS | EMS | P value | LRA Coefficient | P value |
|---|---|---|---|---|---|
| All (497, 100%), mean (SD) | 0.549 (0.455) | 0.71 (0.995) | 0.000 | 0.180 | 0.000 |
| Sites (n, %) | |||||
| Left colon (149, 30.0%) | 0.645 (0.568) | 0.746 (0.736) | 0.013 | 0.579 | 0.000 |
| Transverse (108, 21.7%) | 0.506 (0.348) | 0.613 (0.434) | 0.000 | 0.581 | 0.000 |
| Right colon and rectum (240, 48.3%) | 0.510 (0.409) | 0.740 (1.273) | 0.004 | 0.073 | 0.000 |
| Pathologic Diagnosis (n, %) | |||||
| PUM (37, 7.4%) | 0.338 (0.150) | 0.678 (1.279) | 0.097 | 0.056 | 0.003 |
| HP (105, 21.1%) | 0.372 (0.218) | 0.458 (0.262) | 0.000 | 0.466 | 0.000 |
| SSA (84, 16.9%) | 0.686 (0.558) | 0.726 (0.549) | 0.197 | 0.882 | 0.000 |
| Adenoma (262, 52.7%) | 0.597 (0.486) | 0.784 (1.183) | 0.007 | 0.137 | 0.000 |
| HGD (9, 1.8%) | 0.844 (0.456) | 1.711 (1.473) | 0.115 | 0.049 | 0.686 |
| Fragment number (n, %) | |||||
| Single (278, 55.9%) | 0.387 (0.326) | 0.56 (0.626) | 0.000 | 0.236 | 0.000 |
| Multiple (219, 44.1%) | 0.755 (0.510) | 0.91 (1.297) | 0.060 | 0.127 | 0.000 |
| Endoscopists (n, %) | |||||
| A (51, 10.3%) | 0.48 (0.306) | 0.48 (0.322) | 1.000 | 0.465 | 0.000 |
| B (72, 14.5%) | 0.57 (0.422) | 0.78 (0.571) | 0.000 | 0.667 | 0.000 |
| C (35, 7.0%) | 0.53 (0.356) | 0.68 (0.433) | 0.001 | 0.67 | 0.000 |
| D (147, 29.6%) | 0.46 (0.380) | 0.68 (1.525) | 0.071 | 0.050 | 0.015 |
| E (23, 4.6%) | 0.49 (0.198) | 0.60 (0.158) | 0.000 | 0.949 | 0.000 |
| F (12, 2.4%) | 0.63 (0.407) | 0.80 (0.724) | 0.152 | 0.51 | 0.000 |
| G (62, 12.5%) | 0.62 (0.363) | 0.89 (1.035) | 0.031 | 0.123 | 0.005 |
| H (12, 2.4%) | 0.49 (0.315) | 0.66 (0.231) | 0.005 | 1.164 | 0.000 |
| EMS (n, %) | |||||
| EMS≥1 cm (100, 20.1%) | 1.09 (0.690) | 1.73 (1.871) | 0.001 | 0.028 | 0.457 |
| EMS<1 cm (397, 79.9%) | 0.41 (0.223) | 0.46 (0.191) | 0.000 | 0.703 | 0.000 |
| PMS (n, %) | |||||
| PMS≥1 cm (60, 12.1%) | 1.45 (0.704) | 1.45 (0.816) | 0.968 | 0.564 | 0.000 |
| PMS<1 cm (437, 87.9%) | 0.43 (0.206) | 0.61 (0.975) | 0.000 | 0.050 | 0.000 |
PUM: Polypoid unremarkable mucosa; PMS: pathologically measured size; EMS: endoscopically measured size; LRA: linear regression analysis; SD: standard deviation; HP: hyperplastic polyp; SSA: sessile serrated adenoma; HGD: high-grade dysplasia.
Our results showed that 278 CRPs had singe fragment and 201 had multiple fragments. In the single-fragment group, the EMS was larger than the PMS (P<0.001, Table 1) with a linear correlation between the two (coefficient 0.236 and P<0.001). Among the 219 multiple-fragment CRPs, the EMS was also possibly larger than the PMS (0.91±1.297 cm vs. 0.755±0.510 cm), but with no statistical significance (P=0.060), while LRA showed that the EMS and PMS correlation was statistically significant (coefficient 0.127, P<0.001).
The correlation and discordance between EMS and PMS were operator-dependent. Five of the 8 (62.5%) participated endoscopists estimated an EMS significantly larger than the PMS, while the other 3 estimated an EMS similar to the corresponding PMS (Table 1). The EMS were correlated with the PMS with regards to each individual endoscopist (coefficient 0.050~1.164 and P<0.001~0.015). The endoscopists’ practicing years (≥10 years versus <10 years) were not associated with the linear correlation between EMS and PMS (data not shown).
We last sought the association between the follow-up recommendations based on the PMS and EMS with 1 cm as the size cutoff, according to the US and British guidelines and recommendation [4-6,21]. The EMS and PMS were found associated with each other in the adenoma and serrated polyps (SPs) by using χ2 test (P<0.001, Table 2). There was a good correlation between the EMS and PMS in the adenomas (ĸ=0.626, 95% confidence intervals [CI], 0.505 to 0.746) and a moderate correlation in the SPs (ĸ=0.424, 95% CI, 0.244 to 0.604). However, 40.4% (23/57) of the adenomas and 63.6% (21/33) of the SPs with EMS≥1 cm would be reclassified as low-risk lesions based on a PMS<1 cm, and hence warrant a longer follow-up interval, suggesting that a considerable portion of adenomas and SPs may have been mis-sized and hence misclassified.
Table 2.
The impact of the pathologically and endoscopically measured lesion sizes on the surveillance intervals (based on the size cutoff of 1 cm)
| Adenoma*,# | Serrated polyps*,¶ | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| PMS<1 cm (n=221) | PMS≥1 cm (n=41) | Total (n=262) | PMS<1 cm (n=173) | PMS≥1 cm (n=16) | Total (n=189) | |
| EMS<1 cm, n (%) | 198 (96.6%) | 7 (3.4%) | 205 (100%) | 152 (97.4%) | 4 (2.6%) | 156 (100%) |
| EMS≥1 cm, n (%) | 23 (40.4%) | 34 (59.6%) | 57 (100%) | 21 (63.6%) | 12 (36.4%) | 33 (100%) |
PMS: pathologically measured size; EMS: endoscopically measured size; Serrated polyps include hyperplastic polyp, traditional serrated adenoma and sessile serrated adenoma;
a P value less than 0.001 in the χ2 tests;
ĸ=0.626 (95% confidence intervals [CI], 0.505 to 0.746);
ĸ=0.424 (95% CI, 0.244 to 0.604).
Discussion
Comparison and linear correlation between the EMS and PMS of CRPs
PMS of adenomas is an important factor for predicting cancer risks [8,9] and determining the appropriate follow-up strategy [4,5,21]. Recent studies consider PMS as the “golden standard” and preferred it over EMS [10,15,18,22]. However, most of the prior studies are limited by a modest to acceptable study size and/or retrospective study in nature. Not surprisingly, some studies showed EMS was larger than PMS [10,11] and the others showed otherwise [12,16]. Our large sample size (497 CRP) may offer more statistical power and shed light on the best measurement method for CRP CRC risk stratification.
We found that EMS was larger than PMS regardless of the CRP site (left, transverse and right colon) or PMS subgrouping (≥ and <1 cm). The definition/nature of the EMS and PMS may have contributed to their difference. PMS is based on the histologic evidence of CRP lesions, and may be different from the EMS which is endoscopic findings. In fact, we have noted that adenomas often were only a portion of the resected CRP (Figure 3). We propose that PMS is the bona fide lesion size of the completely resected CRPs, would better predict their biological behaviors than EMS, and therefore should be preferred over EMS for CRC risk stratification. Our proposal is supported by majority of the studies comparing PMS and EMS of adenomas and HPs [14-16,22] and the European guidelines for CRP quality assurance [23], and may also be applied to PUM, SSA and HGD. In the cases of incomplete polypectomy, PMS may be smaller than bona fide CRP size and EMS may be more reliable.
Figure 3.
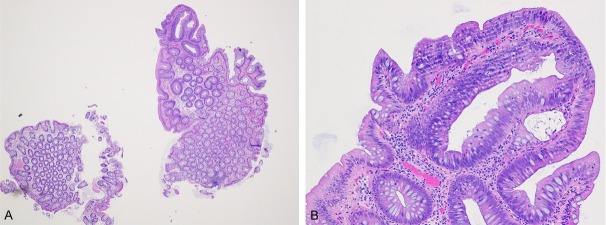
A small tubular adenoma measuring 2 mm is only a portion of the colonic polyp measuring 7 mm under endoscopy. A (x 20) B (x 200).
There are several other possible reasons for the PMS and EMS differences. First, the optical distortion caused by distance and visual angle would affect the endoscopic and microscopic measurements. Second, the wide-opened forceps, commonly used reference for EMS, may not always be well with the largest dimension of the lesion, causing measurement bias. Its guiding effect may also be limited when comparing a CRP much larger than the forceps’ wide-opened mouth. A ruler may help but will still face the problem of alignment. Therefore, it is not surprising that PMS has less variation than the EMS assisted by a ruler [14]. Third, the grasp and removal of an excised CRP may sometimes cause deformation, probably leading to a different PMS. Fourth, it has been reported that there would be a shrinkage after fixation [14]. Of note, recent studies also found formalin fixation of CRP specimens did not affect CRP sizes [10,11,16]. Last, in this single-center study, the institutional endoscopists’ and/or the pathologist’s performance and environmental factors may have potentially biased the results. A multi-center may address these biases.
The different PMS and EMS may cause mis-measurement and reclassification of CRPs despite the good and moderate agreements between PMS and EMS in adenomas and SPs,respectively. Consistent with an early report of 61 CRPs [11], we showed 40% of the adenomas with EMS≥1 cm had a PMS<1 cm and would be reclassified from a high-risk adenoma to a low-risk one based on the size, while only 3.4% of the adenomas would be upgraded from a low-risk adenoma to a high-risk one. The recommended follow-up interval for those down-graded adenomas would therefore be changed from 3 years to 5-10 years [4,21]. Similarly, 63.6% of SP with EMS≥1 cm would warrant a longer follow-up interval (from 3 years to 10 years) for their PMSs<1 cm, according to the US guidelines [6,21]. Considerable economic and clinical consequences may be resulted in. Moreover, EMS of 1 cm was used in the earlier trials stratifying adenoma and HP risks for CRC [3,9]. Our data hence suggest that the bona fide adenoma size cutoff (PMS) for higher CRC risk may be smaller than 1 cm. Despite the enthusiasm in and supporting data for using PMS as the preferred CRP size measurement method, more high quality evidence (i.e. randomized, prospective, multi-center studies) in our opinion are needed to demonstrate the usefulness of PMS for CRC risk assessment. Before those lines of evidence become available, one feasible approach is to use the here described linear association between PMS and EMS and related factors to predict the EMS with a known PMS. In fact, our linear regression models allow prediction of EMS based on the CRP types (pathologic diagnosis), PMS, and endoscopists, and hence a direct use of PMS for CRC risk prediction.
Factors associated with the PMS and EMS discordance and correlation
The EMSs of PUM and SSA were found similar to the PMSs in this study and had a linear correlation with the PMSs, which to our best knowledge has not been reported before. Compared with PUM, SSAs were more precisely measured under the endoscopy, probably due to a sessile endoscopic morphology and the larger sizes. Given the important role of SSA in CRC development [24-26], large sessile lesions may have been examined more carefully under endoscopy, and given additional examinations, such as staining and narrow band image. In addition, the border of SSA may be easier to identify for its sessile endoscopic morphology than that of pedunculated CRPs.
HGD is considered a criterion for high-risk adenoma by the US, but not the UK, guidelines and warrants a follow-up in 3 years [4,21]. However, the adenomas with HGD have not been subject to the PMS and EMS comparison. Despite only 9 (1.8%) cases of HGD included in this study, we for the first time demonstrated no significant differences between the PMS and EMS in the adenomas with HGD, suggesting the PMS and EMS may be interchangeable in those cases. Interestingly, no linear correlation between those two was established in our LRA. Because HGD histology trumps the size of adenoma, at least in the US guidelines, the sizes of adenomas with HGD seem less important. However, there are no data on the potential synergistic effects of adenoma size and presence of HGD on CRC risk. It would be very interesting to study whether the CRPs with adenomas ≥1 cm and HGD will have higher CRC risks than that with HGD or adenomas ≥1 cm alone.
Besides the pathologic diagnosis of CRP, we also identified 3 factors associated with the PMS and EMS discordance including tissue fragment number, endoscopist and PMS subgrouping (≥1 cm versus <1 cm). Those factors may help predict the PMS based on EMS or vice versa, and identify the causes of PMS and EMS discordance. In contrast to our findings, a study of 61 CRPs including only HP and adenoma showed that PMS and EMS discordance is not dependent on endoscopist and pathologic diagnosis [11]. We speculate the different findings may be attributed to addition of PUM, SSA and HGD in our study, different sample sizes, and different measurement performances of the pathologists and the endoscopists in the studies. Our data and the earlier work, however, agreed on that the PMS and EMS discordance is independent of CRP location [11].
There was a significant difference between the EMS and the PMS in the single-fragment CRP, but not in the multiple-fragment ones. The multiple fragments in a CRP polypectomy specimen imply that the lesion could not be excised as a whole, probably due to the difficulties in endoscopic manipulation and/or a size larger than the single-fragmented CRP ones (P<0.0001). Both of the manipulation difficulties and larger sizes may result in incomplete resection and retrieval of the CRP. Meanwhile, the pathologists had to deal with the problems of aggregating the fragments together for PMS,leading to an additional bias. So our results on multiple-fragment CRPs should be interpreted and applied to clinical practice with caution.
Study limitations and conclusions
There are several limitations of this study. First, this study was conducted at a single medical center, causing potential environmental and operator biases. Second, we did not measure the size of excised CRPs before fixation, so we could not render any information on the effect of the fixation. However, previous work has shown that fixation had little impact on the PMS [14,16]. Third, we did not record the methods of CRP resection/polypectomy, which might also influence the PMS. Last, the sample size of HGD is relatively small although this is the first report in this regard.
In summary, this prospective study of PUM, SSA, HP, adenoma and HGD show that PMS is significantly smaller than EMS despite the linear correlation between PMS and EMS. Therefore, the bona fide CRPs size (PMS) cutoff for CRC risk stratification may be smaller than the currently recommended 1 cm. The discordance between PMS and EMS is associated with several factors including pathologic diagnosis, fragment number, endoscopists and PMS, and may contribute to different follow-ups in a considerable portion of adenomas and SPs.
Acknowledgements
We thank Kerry A. O’Rourke at Rutgers Robert Wood Johnson Library of Health Sciences for her assistance in literature retrieval.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Imperiale TF, Glowinski EA, Lin-Cooper C, Larkin GN, Rogge JD, Ransohoff DF. Five-year risk of colorectal neoplasia after negative screening colonoscopy. N Engl J Med. 2008;359:1218–1224. doi: 10.1056/NEJMoa0803597. [DOI] [PubMed] [Google Scholar]
- 3.Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR United States Multi-Society Task Force on Colorectal C. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP, Lucassen A, Jenkins P, Fairclough PD, Woodhouse CR. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–689. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 6.Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF, O’Brien MJ, Odze RD, Ogino S, Parry S, Snover DC, Torlakovic EE, Wise PE, Young J, Church J. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315–1329. doi: 10.1038/ajg.2012.161. quiz 1314, 1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR, Pickhardt P, Rex DK, Thorson A, Winawer SJ. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 8.Winawer SJ, Zauber AG, O’Brien MJ, Ho MN, Gottlieb L, Sternberg SS, Waye JD, Bond J, Schapiro M, Stewart ET, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med. 1993;328:901–906. doi: 10.1056/NEJM199304013281301. [DOI] [PubMed] [Google Scholar]
- 9.Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med. 1992;326:658–662. doi: 10.1056/NEJM199203053261002. [DOI] [PubMed] [Google Scholar]
- 10.Morales TG, Sampliner RE, Garewal HS, Fennerty MB, Aickin M. The difference in colon polyp size before and after removal. Gastrointest Endosc. 1996;43:25–28. doi: 10.1016/s0016-5107(96)70255-9. [DOI] [PubMed] [Google Scholar]
- 11.Schoen RE, Gerber LD, Margulies C. The pathologic measurement of polyp size is preferable to the endoscopic estimate. Gastrointest Endosc. 1997;46:492–496. doi: 10.1016/s0016-5107(97)70002-6. [DOI] [PubMed] [Google Scholar]
- 12.Fennerty MB, Davidson J, Emerson SS, Sampliner RE, Hixson LJ, Garewal HS. Are endoscopic measurements of colonic polyps reliable? Am J Gastroenterol. 1993;88:496–500. [PubMed] [Google Scholar]
- 13.Schwartz E, Catalano MF, Krevsky B. Endoscopic estimation of size: improved accuracy by directed teaching. Gastrointest Endosc. 1995;42:292–295. doi: 10.1016/s0016-5107(95)70124-9. [DOI] [PubMed] [Google Scholar]
- 14.Turner JK, Wright M, Morgan M, Williams GT, Dolwani S. A prospective study of the accuracy and concordance between in-situ and postfixation measurements of colorectal polyp size and their potential impact upon surveillance. Eur J Gastroenterol Hepatol. 2013;25:562–567. doi: 10.1097/MEG.0b013e32835d1f2d. [DOI] [PubMed] [Google Scholar]
- 15.Eichenseer PJ, Dhanekula R, Jakate S, Mobarhan S, Melson JE. Endoscopic mis-sizing of polyps changes colorectal cancer surveillance recommendations. Dis Colon Rectum. 2013;56:315–321. doi: 10.1097/DCR.0b013e31826dd138. [DOI] [PubMed] [Google Scholar]
- 16.Moug SJ, Vernall N, Saldanha J, McGregor JR, Balsitis M, Diament RH. Endoscopists’ estimation of size should not determine surveillance of colonic polyps. Colorectal Dis. 2010;12:646–650. doi: 10.1111/j.1463-1318.2009.01870.x. [DOI] [PubMed] [Google Scholar]
- 17.Hayes SJ. Assessment of colorectal adenomatous polyp size measured during pathological examination highlights the importance of accuracy. Gastrointest Endosc. 2009;70:540–541. doi: 10.1016/j.gie.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Gopalswamy N, Shenoy VN, Choudhry U, Markert RJ, Peace N, Bhutani MS, Barde CJ. Is in vivo measurement of size of polyps during colonoscopy accurate? Gastrointest Endosc. 1997;46:497–502. doi: 10.1016/s0016-5107(97)70003-8. [DOI] [PubMed] [Google Scholar]
- 19.Rubio CA, Grimelius L, Lindholm J, Hamberg H, Porwit A, Elmberger G, Hoog A, Kanter L, Eriksson E, Stemme S, Orrego A, Saft L, Petersson F, De La Torre M, Ekstrom C, Astrom K, Rundgren A, Djokic M, Chandanos E, Lenander C, Machado M, Nilsson P, Mattsson L. Reliability of the reported size of removed colorectal polyps. Anticancer Res. 2006;26:4895–4899. [PubMed] [Google Scholar]
- 20.Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Flejou JF, Geboes K, Hattori T, Hirota T, Itabashi M, Iwafuchi M, Iwashita A, Kim YI, Kirchner T, Klimpfinger M, Koike M, Lauwers GY, Lewin KJ, Oberhuber G, Offner F, Price AB, Rubio CA, Shimizu M, Shimoda T, Sipponen P, Solcia E, Stolte M, Watanabe H, Yamabe H. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lieberman DA. Colon polyp surveillance: clinical decision tool. Gastroenterology. 2014;146:305–306. doi: 10.1053/j.gastro.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 22.Schachschal G, Mayr M, Treszl A, Balzer K, Wegscheider K, Aschenbeck J, Aminalai A, Drossel R, Schroder A, Scheel M, Bothe CH, Bruhn JP, Burmeister W, Stange G, Bahr C, Kiesslich R, Rosch T. Endoscopic versus histological characterisation of polyps during screening colonoscopy. Gut. 2014;63:458–65. doi: 10.1136/gutjnl-2013-304562. [DOI] [PubMed] [Google Scholar]
- 23.Quirke P, Risio M, Lambert R, von Karsa L, Vieth M. Quality assurance in pathology in colorectal cancer screening and diagnosis-European recommendations. Virchows Arch. 2011;458:1–19. doi: 10.1007/s00428-010-0977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crockett SD, Snover DC, Ahnen DJ, Baron JA. Sessile Serrated Adenomas: An evidence-based guide to management. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.10.035. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Beggs AD, Jones A, Shepherd N, Arnaout A, Finlayson C, Abulafi AM, Morton DG, Matthews GM, Hodgson SV, Tomlinson IP. Loss of expression and promoter methylation of SLIT2 are associated with sessile serrated adenoma formation. PLoS Genet. 2013;9:e1003488. doi: 10.1371/journal.pgen.1003488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bettington M, Walker N, Clouston A, Brown I, Leggett B, Whitehall V. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367–386. doi: 10.1111/his.12055. [DOI] [PubMed] [Google Scholar]


