Abstract
We induced gallstones in C57L mice fed with a high cholesterol diet and examined the expression of bile salt export pump (BSEP) on the canalicular membrane of hepatocytes and its relation with PKCα and HAX-1.Twenty-four gallstone-prone C57L mice were randomly assigned to receive a high cholesterol diet or a regular diet. Gallstone formation was recorded. BSEP, PKCα and phospho-PKCα expression was examined by immunoblotting assays. Co-expression of BSEP and HAX-1 was studied by immunofluorescent microscopy and immunoprecipitations. Gallstones were formed in all 12 mice fed with the high cholesterol diet. In Gallstone group, BSEP levels on the canalicular membrane of hepatocytes were markedly lower while a significant increase was observed in phosphorylated PKCα. Immunofluorescent microscopy showed that BSEP and HAX-1 were co-localized on the canalicular membrane, which was apparently enhanced by feeding with the high cholesterol diet. The immunoprecipitation assays further demonstrated that BSEP and HAX-1 showed enhanced interaction in the hepatocytes of mice fed with the high cholesterol diet. Cholesterol gallstone formation is associated with downregulation of BSEP expression on the canalicular membrane of hepatocytes with increased phosphorylation of PKCα. BSEP and HAX-1 show enhanced interaction with one another on the canalicular membrane during gallstone formation.
Keywords: Gallstone, cholesterol, canaliculi, BSEP, HAX-1
Introduction
Gallstone formation is a complicated process and still remains incompletely elucidated. Substantial evidence indicates that cholesterol oversaturation due to reduced bile acid secretion by the liver is the primary cause of gallstones [1]. Transport of bile salt across bile canaliculi is an important driving force for bile secretion by the liver. The bile salt export pump (BSEP, ABCB11) on the canalicular membrane of hepatocytes is responsible for and a limiting step in bile acid secretion. BSEP belongs to the ATP binding cassette (ABC) superfamily and is implicated in cholesterol gallstone formation and BSEP is the most likely candidate of the gallstone Lithl gene [2]. However, the role of BSEP in cholelithogenesis remains controversial [3]. A study on BSEP transgenic mice showed that though BSEP overexpression increased biliary bile salt secretion, it did not affect cholelithogenesis in mice [4]. A study on gallstone-prone C57L mice revealed impaired transport function by BSEP and marked reduction in bile salt secretion in C57L mice fed with high cholesterol diet [5].
Bile secretion depends on the delivery and removal of transporter proteins to and from the canalicular membrane. Kubitz et al. showed that trafficking of BSEP to the canalicular membrane depended on the basal activity of such kinases as protein kinase C (PKC) in polarized hepatocytes in vitro [6]. Pérez et al. found that PKC was involved in internalizing BSEP during low levels of oxidative stress, resulting in reduced bile salt secretion [7]. HAX-1 is a 34-kDa polypeptide that interacts with a heterogeneous group of proteins. Ortiz et al. found that HAX-1 was also involved in BSEP internalization from the apical membrane in vitro [8]. However, there is no study on the relation between BSEP and PKC and HAX-1 in vivo.
In the current study, we induced gallstones in C57L mice fed with a high cholesterol diet and examined the expression of BSEP on the canalicular membrane of hepatocytes and its relation with PKCα and HAX-1.
Materials and methods
Animals
Twenty-four 8-week old male healthy C57L mice (Animal Experimental Center, Shengjing Hospital, China Medical University) and housed in environmentally controlled conditions (22°C, a 12 h light/dark cycle with the light cycle from 6:00 to 18:00 and the dark cycle from 18:00 to 6:00) with ad libitum access to standard laboratory chow and water. The mice were randomized into the gallstone group and control group with 12 mice per group. Mice in the gallstone group were fed with a high cholesterol die containing 2% cholesterol, 0.5% cholic acid and 15% fat for 8 weeks. The control mice were fed with a regular diet. Then, mice anesthetized intraperitoneally with 10% chloral hydrate and after a mid-abdomen incision was made, gallstones were recorded and biles were collected. Blood was collected via the inferior vena cava and after centrifugation at 5000 rpm for 10 min the supernatant was stored for subsequent biochemical assays. Liver specimens were snap-frozen at -80°C for further assays. The study protocol was approved by the Institution Review Board of Shengjing Hospital, China Medical University and all the animal experiments were performed according to the guidelines of the Animal Care and Use Committee of the US National Institute of Health (NIH) for experimental use of laboratory animals.
Biochemical assays
Serum total cholesterol, total bile acids, triglycerides, HDL and LDL contents, and bile total cholesterol and total bile acids were examined by automatic biochemical analyzer UnieelDxC800 (Beckman Coulter, US).
Western blotting assays
Membrane proteins were extracted from 200 g liver tissues. Immunoblotting assays were performed as previously depicted [9] and the following antibodies were used for the procedure: anti-BSEP (sc-17294, Santa Cruz Biotechnology,Santa Cruz, CA) antibody, anti-PKCα (sc-8393, Santa Cruz Biotechnology) antibody, anti-phospho-PKCα (Thr638/641, 9375P, Cell Signaling Technology, Danvers, MA) antibody. Protein expression was normalized against β-actin. Protein bands were visualized by chemiluminescence (ChemiScope2850, CLiNX Sci-ence Instruments) and densitometry was done using the Gel-Pro-Analyzer 3.1 software.
Immunofluorescent microscopy
Immunostaining of liver tissue sections was conventionally performed and antibodies against the following molecules were used: anti-BSEP antibody (sc-17294, Santa Cruz Biotechnology) and anti-HAX-1 antibody (610824, BD Biosciences) at 4°C overnight. DAPI was used to stain nuclei. The following secondary antibodies were used: FITC-conjugated donkey anti-goat secondary antibody, and Cy3-conjugated goat anti-mouse secondary antibody. The slides were photographed using a Zeiss 510 laser confocal microscope (Zeiss Fluorescent Microsystems, Göttingen, Germany) at 600×.
Immunoprecipitations
To test interaction between BSEP and HAX-1, we incubated proteins of indicated cells with anti-BSEP (sc-17294, Santa Cruz Biotechnology) or anti HAX-1 antibodies (610824, BD Biosciences) and protein A/G-agarose beads (Pierce, Rockfrod, IL) at 4°C with constant rotation for overnight and then were analyzed by blotting with anti-BSEP or anti-HAX-1 antibodies. Whole cell lysates were used as controls.
Statistical analysis
Data were expressed as x̅ ± s.d. and analyzed using SPSS version 19.0 (SPSS Inc, Chicago, IL). Student’s t test was used for independent samples and P≤0.05 was considered statistically significant.
Results
Serum and bile cholesterol contents are elevated in mice fed with high cholesterol diet with formation of gallstones
The serum cholesterol content was 4.19±1.02 mmol/L in the gallstone group, which was markedly higher than that of the control group (2.01±0.15 mmol/L; t=7.36, P<0.01) (Table 1). The total serum bile acid content was 53.24±21.08 μmol/L for the gallstone group (vs. the control group, 6.61±2.86 μmol/L; t=7.26, P<0.01). The serum triglyceride content was 0.21±0.23 mmol/L for the gallstone group, which was not markedly higher than that of the control group (0.38±0.20 mmol/L; t=1.808, P>0.05). The serum HDL content in the gallstone group (2.12±0.27 mmol/L) was significantly higher than that of the control group (1.75±0.16 mmol/L; t=3.806, P<0.05) and the serum LDL content (2.67±1.28 mmol/L) was also noticeably higher than that of the control group (0.30±0.07 mmol/L; t=5.852, P<0.01). The bile cholesterol content in the gallstone group (6.87 mmol/L) was markedly higher than that of the control group (2.43 mmol/L) while the bile total bile acid content (48288.0 μmol/L) was significantly lower than that of the control group (61411.2 μmol/L). Gallstones were formed in all 12 mice fed with the high cholesterol diet (Figure 1A, 1B). Infrared spectroscopy of the gallstones revealed cholesterol peaks at 1054 cm-1, 1383 cm-1 and 2933 cm-1 and no pigment peaks (Figure 1C), indicating that the gallstones were predominantly cholesterol.
Table 1.
Serum and bile contents of cholesterol, triglycerides, HDL, LDL and bile acids in C57/L mice fed with high cholesterol or regular diet, bile acids (μmol/L)
| Group | Serum | Bile | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Cholesterol | Triglyceride | HDL | LDL | Total bile acids | Cholesterol | Total bile acids | |
| Gallstone | 4.19±1.02 | 0.21±0.23 | 2.12±0.27 | 2.67±1.28 | 53.24 ± 21.08 | 6.87 | 48288.0 |
| Control | 2.01±0.15 | 0.38±0.20 | 1.75±0.16 | 0.30±0.07 | 6.61±2.86 | 2.43 | 61411.2 |
Figure 1.
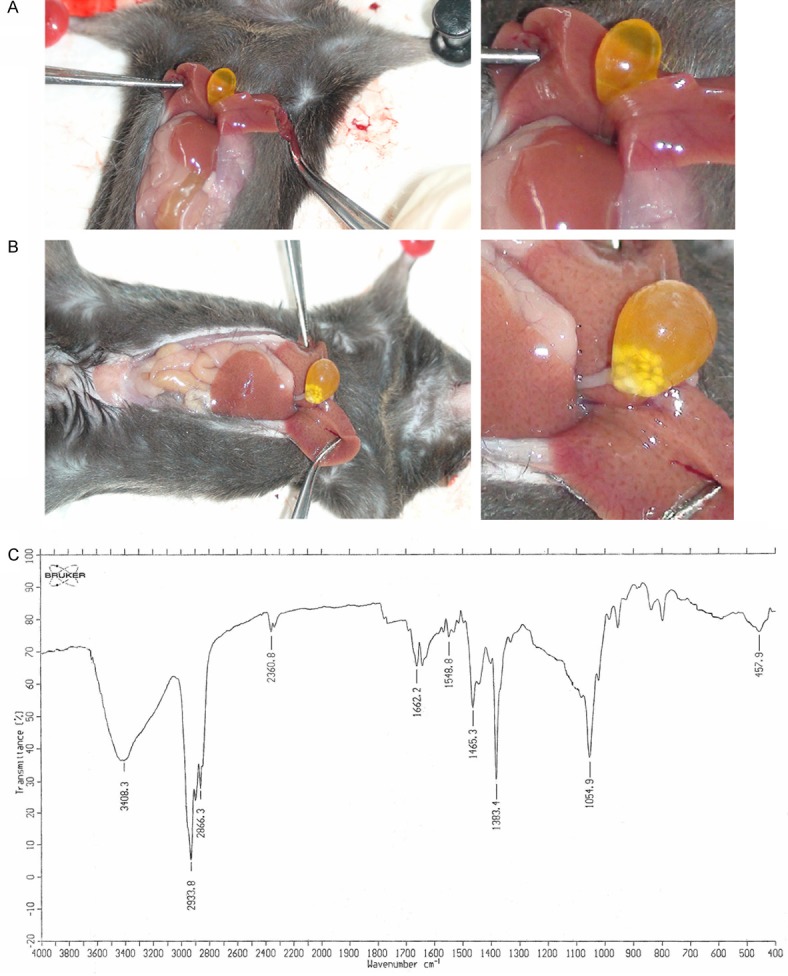
A. Gallbladder with no stone formation. B. Gallbladder with stone formation. C. Infrared spectroscopy of cholesterol gallstones.
BSEP downregulation on the canalicular membrane of hepatocytes is associated with increased phosphorylation of PKCα
We examined the expression of BSEP on the canalicular membrane of hepatocytes by Western blotting assays. We found that BSEP expression levels on the canalicular membrane of hepatocytes of mice fed with the high cholesterol diet (0.67±0.04) were markedly lower than those of the control group (0.99±0.12; t=14.302, P<0.01) (Figure 2A, 2C). We further investigated whether BSEP downregulation was associated with changes in PKCα expression. Western blotting assays revealed no apparent difference in the levels of PKCα on the canalicular membrane of hepatocytes of mice fed with the high cholesterol diet (1.04±0.04) from that of the control group mice (1.03±0.03; t=0.525, P>0.05) (Figure 2B, 2C). However, we observed a significant increase in the phosphorylation level of PKCα on the canalicular membrane of hepatocytes of mice fed with the high cholesterol diet (1.49±0.14) compared with that of the control mice (0.95±0.05; t=6.136, P<0.01) (Figure 2B, 2C).
Figure 2.
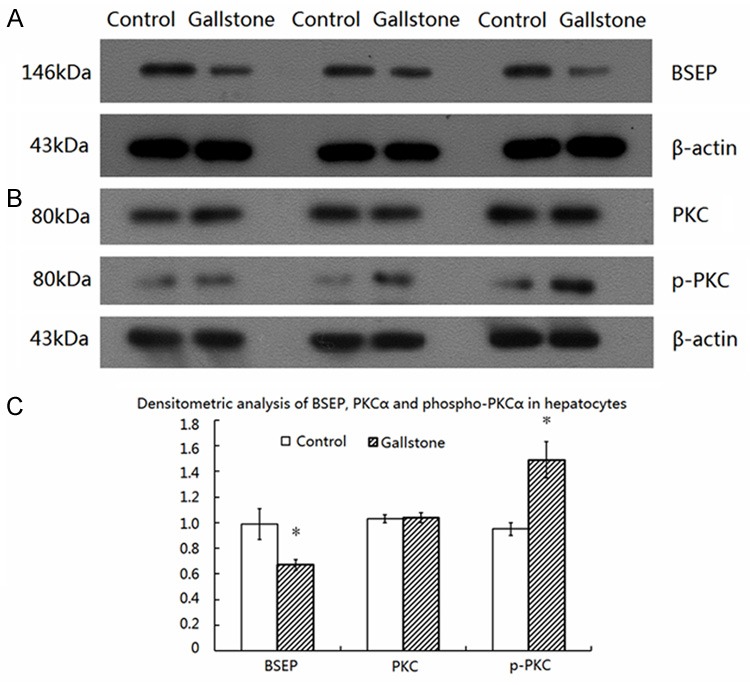
BSEP downregulation on the canalicular membrane of hepatocytes is associated with increased phosphorylation of PKCα. A. Immunoblotting of BSEP in hepatocytes. B. Immunoblotting of PKCα and phospho-PKCα in hepatocytes. C. Densitometric analysis of BSEP, PKCα and phospho-PKCα in hepatocytes. *P<0.01 vs. Control.
BSEP interacts with HAX-1 on the canalicular membrane of hepatocytes
Our immunofluorescent microscopy showed that BSEP and HAX-1 were co-localized on the canalicular membrane of hepatocytes of mice fed with a regular diet (Figure 3A). The co-expression of BSEP and HAX-1 was apparently increased on the canalicular membrane of hepatocytes of mice fed with the high cholesterol diet (Figure 3B). The finding prompted us to examine whether BSEP and HAX-1 interacted in the hepatocytes. Our immunoprecipitation assays demonstrated that BSEP and HAX-1 interacted at low levels in the hepatocytes of mice fed with a regular diet (Figure 3C). This interaction was significantly increased in the hepatocytes of mice fed with the high cholesterol diet.
Figure 3.
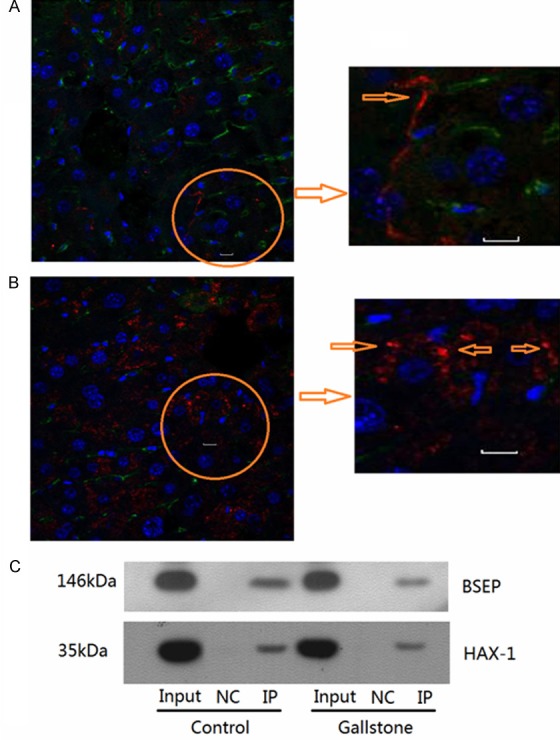
BSEP interacts with HAX-1 on the canalicular membrane of hepatocytes. Immunofluorescent microscopy of BSEP and HAX-1 on the canalicular membrane of hepatocytes. Inset of the circle area is shown on the right. Blue arrow indicates co-expressed BSEP and HAX-1. A. The control mice. B. The mice fed with the high cholesterol diet. C. Immunoprecipitation of BSEP and HAX-1 in hepatocytes.
Discussion
Cholesterol cholelithiasis is a prevalent digestive disease, exerting a considerable financial and social toll worldwide. However, the mechanisms of gallstone formation including cholesterol cholelithiasis still remain poorly defined. Bile formation depends mainly on the sequential action of several membrane lipid transport proteins that are located on the canalicular membrane of hepatocytes and BSEP is a limiting step in bile acid secretion and is explicitly implicated in cholesterol gallstone formation. Our study demonstrated markedly increased serum and bile cholesterol content in mice fed with the high cholesterol diet with formation of cholesterol gall stones. Furthermore, BSEP expression was downregulated on the canalicular membrane of hepatocytes of mice fed with the high cholesterol diet. This finding is consistent with the finding by a previous study which showed that a high cholesterol diet for gallstone-prone C57L mice impaired transport function by BSEP reduced bile salt secretion [5].
The regulatory mechanisms of BSEP during cholesterol gallstone formation are incompletely understood. Schwartz et al. found that mice with hypercholesterolemia fed with a high cholesterol diet exhibited increased expression of PKCα in epithelial cells [10] and believed that PKCα overexpression in hypercholesterolemia was a major cause of epithelial dysfunction [11]. Kubitz et al. showed that trafficking of BSEP to the canalicular membrane depended on PKC in polarized hepatocytes in vitro [6]. It remains unclear whether BSEP downregulation on the canalicular membrane of hepatocytes of mice fed with the high cholesterol diet was associated with PKCα overexpression. After modification, BSEP is stored in the BSEP circulating pool [12], and expressed on the canalicular membrane of hepatocytes under the regulation of multiple factors [12].
We found no apparent difference in PKCα expression on the canalicular membrane of hepatocytes of mice fed with the high cholesterol diet or regular diet. By contrast, we found markedly increased phosphorylation of PKCα expression on the canalicular membrane of hepatocytes of mice fed with the high cholesterol diet, suggesting that the high cholesterol diet induced activation of PKCα. The PKCα activator, thymeleatoxin, induces bile stasis in rats [13]. In a mouse model with estradiol 17β-D-glucuronide-induced bile stasis, BSEP expression was downregulated on the canalicular membrane of hepatocytes and taurocholic acid secretion was markedly reduced with concurrent translocation of PKCα to the canalicular membrane [14]. It has also been shown that bile stasis induced by multiple drugs is associated with impaired secretory function of hepatocytes, which is related to activation PKCα [15]. A study found that BSEP was localized in domains enriched in caveolin-1 on the canalicular membrane of hepatocytes [16] and PKCα was shown to be localized in the same region [17], suggesting that and BSEP might interact with one another within the region. Our immunofluorescent microscopy also showed that BSEP and PKCα within the same region, which was further enhanced by high cholesterol diet. These findings together suggest that BSEP and PKCα are closely associated during cholesterol cholelithiasis.
In MDCK II cells, BSEP was found to partner with Hax-1 upon serine phosphorylation [8], initiating clathrin-dependent endocytosis. Here, we present the first direct evidence by immunofluorescent microscopy and co-immunoprecipitations that BSEP and HAX-1 were co-expressed on the canalicular membrane of hepatocytes and interacted with one another in the hepatocytes, which were further enhanced by feeding with the high cholesterol diet. These findings together lead us to speculate that activated PKCα might act on BSEP and with serine phosphorylation of BSEP lead to the interaction of BSEP and HAX-1, thus initiating clathrin-dependent endocytosis and translocation of BSEP from the canalicular membrane to the BSEP circulating pool.
In conclusion, we demonstrate that cholesterol gallstone formation is associated with downregulation of BSEP expression on the canalicular membrane of hepatocytes with increased phosphorylation of PKCα. BSEP and HAX-1 show enhanced interaction with one another on the canalicular membrane during gallstone formation. Our findings reveal that an interacting network of membrane lipid transport proteins and kinases located on the canalicular membrane of hepatocytes is involved in gallstone formation. Additional studies are required to further delineate their interactions and the underlying mechanisms.
Acknowledgements
Supported by NSFC (Natural Science Foundation of China) (81100313), Dr Research Startup Fund in Liaoning Province (20111105).
Disclosure of conflict of interest
None.
References
- 1.Van Berge-Henegouwen GP, Venneman NG, Portincasa P, Kosters A, Van Erpecum KJ, Groen AK. Relevance of hereditary defects in lipid transport proteins for the pathogenesis of cholesterol gallstone disease. Scand J Gastroenterol Suppl. 2004:60–69. doi: 10.1080/00855920410011022. [DOI] [PubMed] [Google Scholar]
- 2.Stieger B, Meier Y, Meier PJ. The bile salt export pump. Pflugers Arch. 2007;453:611–620. doi: 10.1007/s00424-006-0152-8. [DOI] [PubMed] [Google Scholar]
- 3.Dikkers A, Tietge UJ. Biliary cholesterol secretion: more than a simple ABC. World J Gastroenterol. 2010;16:5936–5945. doi: 10.3748/wjg.v16.i47.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang HH, Lammert F, Schmitz A, Wang DQ. Transgenic overexpression of Abcb11 enhances biliary bile salt outputs, but does not affect cholesterol cholelithogenesis in mice. Eur J Clin Invest. 2010;40:541–551. doi: 10.1111/j.1365-2362.2010.02300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller O, Schalla C, Scheibner J, Stange EF, Fuchs M. Expression of liver plasma membrane transporters in gallstone-susceptible and gallstone-resistant mice. Biochem J. 2002;361:673–679. doi: 10.1042/0264-6021:3610673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubitz R, Sutfels G, Kuhlkamp T, Kolling R, Haussinger D. Trafficking of the bile salt export pump from the Golgi to the canalicular membrane is regulated by the p38 MAP kinase. Gastroenterology. 2004;126:541–553. doi: 10.1053/j.gastro.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Perez LM, Milkiewicz P, Elias E, Coleman R, Sanchez Pozzi EJ, Roma MG. Oxidative stress induces internalization of the bile salt export pump, Bsep, and bile salt secretory failure in isolated rat hepatocyte couplets: a role for protein kinase C and prevention by protein kinase A. Toxicol Sci. 2006;91:150–158. doi: 10.1093/toxsci/kfj113. [DOI] [PubMed] [Google Scholar]
- 8.Ortiz DF, Moseley J, Calderon G, Swift AL, Li S, Arias IM. Identification of HAX-1 as a protein that binds bile salt export protein and regulates its abundance in the apical membrane of Madin-Darby canine kidney cells. J Biol Chem. 2004;279:32761–32770. doi: 10.1074/jbc.M404337200. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Jiang Y, Wan Y, Zhang L, Tang W, Ma J, Wu S, Cheng W. Medroxyprogestogen enhances apoptosis of SKOV-3 cells via inhibition of the PI3K/Akt signaling pathway. J Biomed Res. 2013;27:43–50. doi: 10.7555/JBR.27.20120051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz IF, Ingbir M, Chernichovski T, Reshef R, Chernin G, Litvak A, Weinstein T, Levo Y, Schwartz D. Arginine uptake is attenuated, through post-translational regulation of cationic amino acid transporter-1, in hyperlipidemic rats. Atherosclerosis. 2007;194:357–363. doi: 10.1016/j.atherosclerosis.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 11.Monti M, Donnini S, Giachetti A, Mochly-Rosen D, Ziche M. Delta PKC inhibition or varepsilonPKC activation repairs endothelial vascular dysfunction by regulating eNOS post-translational modification. J Mol Cell Cardiol. 2010;48:746–756. doi: 10.1016/j.yjmcc.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kipp H, Pichetshote N, Arias IM. Transporters on demand: intrahepatic pools of canalicular ATP binding cassette transporters in rat liver. J Biol Chem. 2001;276:7218–7224. doi: 10.1074/jbc.M007794200. [DOI] [PubMed] [Google Scholar]
- 13.Kubitz R, Saha N, Kuhlkamp T, Dutta S, Vom Dahl S, Wettstein M, Haussinger D. Ca2+-dependent protein kinase C isoforms induce cholestasis in rat liver. J Biol Chem. 2004;279:10323–10330. doi: 10.1074/jbc.M306242200. [DOI] [PubMed] [Google Scholar]
- 14.Crocenzi FA, Sanchez Pozzi EJ, Ruiz ML, Zucchetti AE, Roma MG, Mottino AD, Vore M. Ca(2+)-dependent protein kinase C isoforms are critical to estradiol 17beta-D-glucuronide-induced cholestasis in the rat. Hepatology. 2008;48:1885–1895. doi: 10.1002/hep.22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wimmer R, Hohenester S, Pusl T, Denk GU, Rust C, Beuers U. Tauroursodeoxycholic acid exerts anticholestatic effects by a cooperative cPKC alpha-/PKA-dependent mechanism in rat liver. Gut. 2008;57:1448–1454. doi: 10.1136/gut.2007.140871. [DOI] [PubMed] [Google Scholar]
- 16.Ismair MG, Hausler S, Stuermer CA, Guyot C, Meier PJ, Roth J, Stieger B. ABC-transporters are localized in caveolin-1-positive and reggie-1-negative and reggie-2-negative microdomains of the canalicular membrane in rat hepatocytes. Hepatology. 2009;49:1673–1682. doi: 10.1002/hep.22807. [DOI] [PubMed] [Google Scholar]
- 17.Yang Z, Sun W, Hu K. Adenosine A(1) receptors selectively target protein kinase C isoforms to the caveolin-rich plasma membrane in cardiac myocytes. Biochim Biophys Acta. 2009;1793:1868–1875. doi: 10.1016/j.bbamcr.2009.10.007. [DOI] [PubMed] [Google Scholar]


