Abstract
Objective: Intermittent hypoxia was introduced to mimic obstructive sleep apnea-hypopnea syndrome (OSAHS) in rats. Then, bone mass, bone strength and bone turnover were evaluated, and the influence of genistein on bone mass reduction was investigated in these rats. Methods: OSAHS animal model was established via chronic intermittent hypoxia, and genistein (2.5 mg/kg/day) was used to treat OSAHS rats. The bone mineral density (BMD), bone Histomorphometric indicators, bone biomechanics and expressions of genes related to bone formation and resorption (Runx2, Col I, ALP, Osteocalcin, OPG, RANKL and TRAP-5b) were measured after treatment. Results: The BMD in OSAHS+OVX group was significantly lower than that in OVX group (P<0.05). The BMD in OSAHS+OVX+Genistein group was markedly increased when compared with OSAHS+OVX group (P<0.05), accompanied by partial improvement of the OSAHS induced damage to the lumbar biomechanics. In OSAHS+OVX group, the expressions of Runx2, Col I, ALP and Osteocalcin were significantly reduced when compared with OVX group, and rats in OSAHS+OVX+Genistein group had significantly higher expressions of Runx2, Col I, ALP and Osteocalcin and reduced TRAP-5b expression as compared to OSAHS+OVX group (P<0.05). Conclusions: Genistein can improve the reduction in bone mass and bone strength due to OSAHS in OVX rats, which may be attributed to the increase in bone formation and inhibition of bone resorption. Our findings suggest that genistein may be used to treat and prevent osteoporosis in postmenopausal women with OSAHS.
Keywords: Obstructive sleep apnea-hypopnea syndrome, bone loss, genistein, hypoxia
Introduction
Sleep apnea syndrome is also known as obstructive sleep apnea hypopnea syndrome (OSAHS). OSAHS refers to pauses in breathing occurring 30 times or more within 7-h sleep or apnea hypopnea index (AHI) of ≥5 times/h, accompanied by clinical symptoms such as sleepiness. During the OSAHS, repeated hypoxemia and hypercapnia may cause neurological dysfunction, disturbance of catecholamines, endothelin and renin - angiotensin system, endocrine dysfunction and changes in hemodynamics, resulting in damage to multiple systems including cardiovascular system, nervous system, urinary system, mental system, hematological system and endocrine system. Statistics reveal that the prevalence of OSAHS is about 5% in general population [1], and that in males is about 2 times of it in females [2]. It was reported that the incidence of OSHAS in postmenopausal women is significantly higher than that in premenopausal women [3-5]. An epidemiological study shows the incidence of OSAHS in postmenopausal women is at least 2 times of that in premenopausal women [3]. These findings suggest that clinicians should concern the occurrence of OSAHS and the detrimental effect of OSAHS in post-menopausal women.
OSAHS induced hypoxia and other pathological conditions (such as oxidative stress and metabolic abnormality) may influence the normal bone metabolism. In males with OSAHS, the C-terminal peptide of type I collagen (a marker of bone resorption) increases significantly in the urine and may return to normal level after continuous positive airway pressure for 3 months, but the osteocalcin (OC) and alkaline phosphatase (ALP) (two markers of bone formation) remain unchanged in the serum. This suggests that OSAHS patients present with increased bone resorption, but the bone formation is not influenced [6]. In postmenopausal women, the estrogen reduces and the osteolytic ability increases, leading to increased prevalence of osteoporosis. Whether OSAHS further deteriorates abnormal bone remodeling in postmenopausal women is still poorly understood.
Hormone replacement therapy (HRT) can improve the bone loss and prevent osteoporosis in postmenopausal women [7]. However, HRT still has risks for side effects [8]. Thus, more and more investigators pay attention to the Traditional Chinese Medicine with few side effects, such as phytoestrogens (genistein, epimedium, etc.). Genistein is derived from beans and has been confirmed to possess anti-oxidative, anti-proliferation, estrogen-like and immunoregulatory activities [9,10]. A lot of (Several) studies have confirmed that genistein can improve the trabecular bone loss in ovariectomized mammalians to prevent and treat osteoporosis [11]. However, no studies have been conducted to investigate whether genistein can improve hypoxia induced bone metabolic abnormality.
In the present study, intermittent hypoxia was employed to INDUCE OSAHS, aiming to investigate the influence of intermittent hypoxia on bone mass and bone quality in ovariectomized rats. In addition, whether genistein was helpful to improve the reduction in bone mass secondary to intermittent hypoxia in ovariectomized rats was also investigated. Our results showed intermittent hypoxia could deteriorate the reduction in bone mass, the damage to bone microarchitecture and bone biomechanics in ovariectomized rats. In addition, genistein could effectively reverse the detrimental effects of intermittent hypoxia on bone mass and bone microarchitecture. Detection of expressions of genes related to bone formation and bone resorption showed the genistein induced improvement of bone mass and bone microarchitecture was partially attributed to the inhibition of bone resorption and promotion of bone formation.
Materials and methods
Grouping
A total of 35 female SD rats aged 8 weeks were randomly assigned into 4 groups: control group, OVX group, OVX+hypoxia group, and OVX+hypoxia+genistein group. In control group, rats did not receive treatment. Rats in the remaining 3 groups received oophorectomy. Two weeks after surgery, rats in OVX+hypoxia group and OVX+hypoxia+genistein group were exposed to intermittent hypoxia for 35 days to induce OSAHS. At the same time, rats in OVX+hypoxia+genistein groups received intraperitoneal injection of genistein at 2.5 mg/kg/day. Rats in other groups were intraperitoneally injected with DMSO of equal volume.
OSAHS modeling
Rats were placed in a chamber which was alternatively filled with nitrogen and compressed air for 30 s (compressed air for 15 s and nitrogen for 15 s). The alteration between nitrogen and air was achieved via a timing solenoid valve. This alteration was done twice every minute for 8 h per day for 5 per week for a total of 5 weeks. Digital oxygen analyzer (CYS-1, XLYBC) was used to monitor the oxygen concentration. Hypoxia was defined as oxygen concentration of 1%.
Detection of femoral and vertebral bone density
Rats in each group were anesthetized and then sacrificed by cervical dislocation. The L1-4 vertebrae and bilateral femora were collected, followed by detection of bone mass density (BMD, g/cm2) with a bone density meter. The mean of BMD of bilateral femora was used as the BMD of the specific animal for further analysis.
Bone trabecular morphometry
The vertebrae were fixed in 4% formalin followed by decalcification in EDTA-G solution (14.5 g EDTA, 1.25 g NaOH, and 15 ml glycerol were dissolved in distilled water and the pH was adjusted to 7.1-7.3. The solution was then made up to 100 ml and stored at 5°C). Then, sagittal segments were obtained, dehydrated in a series of ethanol, transparentized in xylene, embedded in paraffin and sectioned followed by HE staining. Sections were observed under a light microscope (Nikon E600, Japan) at 400. The middle segments were photographed and analyzed with Image-Pro Plus IPP Mediaplayer (USA). The bone trabecular area, width and interval were determined. In addition, the ratio of trabecular area to total area was calculated as percentages.
Bone biomechanics
Femoral three-point bending test and lumbar compression test were employed to detect the strength of cortical bone and cancellous bone. The muscles on fresh femoral and lumbar vertebrae were removed, and both tests were done with three-point bending instrument (INSTRON-5543 USA; accuracy: 0.04%; detection range: 10-1100) and compression instrument (span: 18 mm; rate: 10.0 mm/min; temperature: 23°C; humidity: 60-70%).
Real-time PCR
The calvaria were collected, and surrounding tissues were removed, placed in liquid nitrogen and grounded. The total RNA was extracted in the presence of Trizol according to manufacturer’s instructions. The RNA quality was determined after agarose gel electrophoresis. The first strand cDNA was synthesized with 500 ng of RNA, 50 μM oligo dT primer, 100 μM random 6 mers, 0.5 μl of prime script RT enzyme mix I and 2 μl of 5× primescript buffer at 65°C for 5 min and then on ice for 2 min. Products were stored at -20°C. Then, PCR was performed for amplification with 1.2 μLof cDNA, 2.5 μL of 10× pcr buffer, 2 μL of 25 mmol/L magnesium, 0.2 μL of 25 mmol/L dNTPs, 0.5 μL of 10 μmol/L forward primer, 0.5 μL of 10 μmol/L reverse primer, 0.5 μL of 50× SYBR fluorescent dye, 0.3 μL of 5 U/μL Taq polymerase and ultra-pure water (final volume: 25 μL). The PCR conditions were as follows: predenaturation at 95°C for 2 min, and 40 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 20 s and extension at 72°C for 45 s. Then, melting curve was delineated and analyzed. After PCR, the fluorescence signals were analyzed with a software, and the amplification and melting curves of target genes and internal reference gene were delineated. The primers were as follows: Cbfa1:TCTTCTGTCCCGTCACCTCC (forward), GCTCAC-GTCGCTCATCTTGC (reverse); ALP: TATGGCTCA-CCTGCTTCACGG (forward), GCTGTCCATTGTG-GGCTCTTG (reverse); type I collagen: CTACA-GCACGCTTGTGGATGG (forward), CAGATTGGGA-TGGAGGGAGTT (reverse); Osteocalcin: GAGA-GAGATGGCACACAGTAGG (forward), GGAGGGT-AGGACACAATCAGAG (reverse); OPG: ATCAGT-TGGTGGGAATGAAGAT (forward), TTATCAAAT-AGCCTGCCTCACT (reverse); RANKL: GAGCGAAGACACAGAAGCACTA (forward), ATTGATGGTG-AGGTGAGCAAAC (reverse); TRAP5b: ATGCTA-AAGAAATCGCCAGAAC (forward), AGAACACATC-CTCAAAGGTCTC (reverse).
Results
Genistein improves bone mass reduction secondary to intermittent hypoxia
The lumbar BMD reduced markedly in OVX rats, but the femoral BMD remained unchanged. After intermittent hypoxia, the lumbar and femoral BMD of reduced further in OVX rats. Intraperitoneal injection of Genistein could improve the reduction in BMD in OVX rats after hypoxia exposure, but the BMD was still lower than that in control rats (Table 1).
Table 1.
Lumbar and femoral BMD in rats of different groups
| Number | Lumbar BMD | Femoral BMD | |
|---|---|---|---|
| CON | 8 | 0.2684±0.008 | 0.2674±0.007 |
| OVX | 8 | 0.2546±0.010* | 0.2630±0.010 |
| OVX+hypoxia | 8 | 0.2454±0.007*,# | 0.2514±0.009*,# |
| OVX+hypoxia+genistein | 8 | 0.2588±0.010& | 0.2646±0.010& |
Footnote: CON: control group; OVX: Ovariectomized rats; OVX+hypoxia: Ovariectomized rats with intermittent hypoxia; OVX+hypoxia+genistein: Ovariectomized rats with intermittent hypoxia and genistein treatment.
P<0.05 vs. CON;
P<0.05 vs. OVX group;
P<0.05 vs. OVX+Hypoxia group.
Genistein improves histomorphometric parameters of rats with intermittent hypoxia
The trabecular bone area ratio and trabecular width in ovariectomized rats reduced, but the mean trabecular interval increased, which were deteriorated after intermittent hypoxia. Genistein could improve the above histomorphometric parameters in rats with intermittent hypoxia (Table 2 and Figure 1).
Table 2.
Histomorphometric parameters in rats of different groups
| Number | Trabecular bone area ratio | Mean trabecular width | Mean trabecular interval | |
|---|---|---|---|---|
| CON | 8 | 31.14±2.74 | 54.06±3.21 | 121.46±8.46 |
| OVX | 8 | 29.32±3.68* | 52.60±3.30* | 131.61±4.71* |
| OVX+Hypoxia | 8 | 28.41±2.79*,# | 48.85±2.72*,# | 133.37±5.90*,# |
| OVX+Hypoxia+genistein | 8 | 29.15±2.32& | 51.26±2.65& | 132.24±3.52& |
Footnote: CON: control group; OVX: Ovariectomized rats; OVX+hypoxia: Ovariectomized rats with intermittent hypoxia; OVX+hypoxia+genistein: Ovariectomized rats with intermittent hypoxia and genistein treatment.
P<0.05 vs. CON;
P<0.05 vs. OVX group;
P<0.05 vs. OVX+Hypoxia group.
Figure 1.
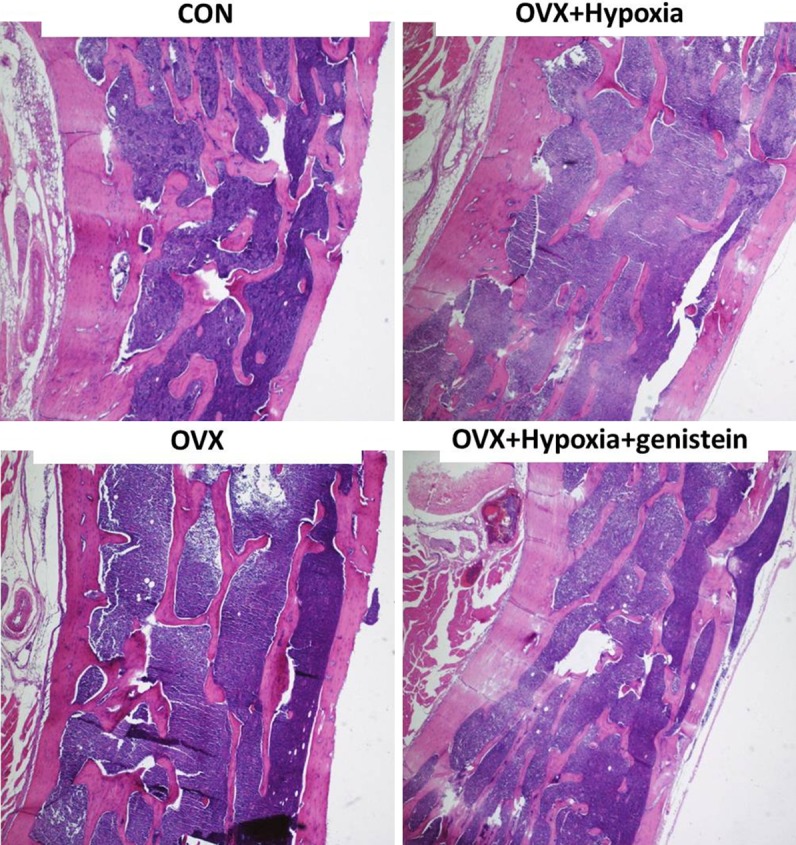
Lumbar histomorphometry in rats of different groups (HE staining).
Genistein improves bone biomechanics of rats after intermittent hypoxia
The lumbar maximum compressive load and femoral maximum three-point bending load were employed to evaluate the bone biomechanics. Results showed the lumbar maximum compressive load reduced, but the femoral maximum three-point bending load remained unchanged in ovariectomized rats. Both parameters deteriorated after intermittent hypoxia, and genistein could partially improve the changes in these biomechanical parameters in ovariectomized rats with intermittent hypoxia (Table 3).
Table 3.
Bone biomechanics of rats in different groups
| Number | Lumbar maximum compressive load | Femoral maximum three-point bending load | |
|---|---|---|---|
| CON | 16 | 311.27±9.20 | 128.37±7.27 |
| OVX | 16 | 289.26±7.58* | 126.57±3.26 |
| OVX+Hypoxia | 16 | 254.83±6.09*,# | 116.47±5.81*,# |
| OVX+Hypoxia+genistein | 16 | 271.25±5.47& | 124.81±4.61& |
Footnote: CON: control group; OVX: Ovariectomized rats; OVX+hypoxia: Ovariectomized rats with intermittent hypoxia; OVX+hypoxia+genistein: Ovariectomized rats with intermittent hypoxia and genistein treatment.
P<0.05 vs. CON;
P<0.05 vs. OVX group;
P<0.05 vs. OVX+Hypoxia group.
Genistein increases expression of osteogenesis related genes
To investigate the mechanisms related to the increase in BMD and improvement of bone microarchitecture after genistein treatment, the skull was collected, and total RNA was extracted. Real time PCR was performed to detect the expressions of osteolysis and osteogenesis related genes. As shown in Figure 2, the Runx2 expression was increased by 2.8 folds, Col I by 12.4 folds, ALP by 4.2 folds, OPG by 2.7 folds, and TRAP-5b (osteolysis related gene) by 6.4 folds, but the expressions of OC and RANKL remained unchanged in OVX rats when compared with control rats. After intermittent hypoxia, when compared with OVX rats, the expressions of Runx2, Col I and ALP (genes related to osteogenesis) were inhibited, and the extent of increase in TRAP-5b expression also decreased (from 6.4 folds to 4.3 folds). In addition, genistein increased the expressions of osteogenesis related genes to different extents (Runx2: from 1.1 folds to 2.5 folds; Col I: from 4.5 folds to 10.5 folds; ALP: from 2.4 folds to 5.3 folds), accompanied by inhibition of TRAP-5b expression (from 4.3 folds to 2.1 folds).
Figure 2.
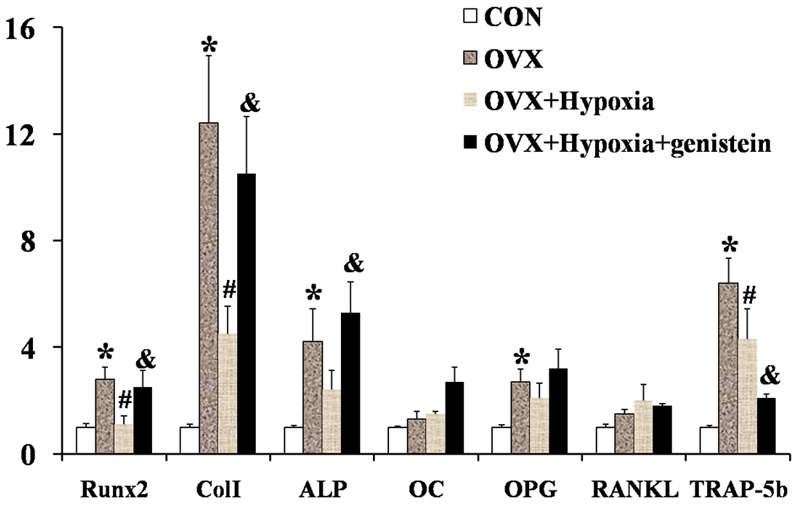
Expressions of genes related to bone turnover.
Discussion
Our results showed intermittent hypoxia (1% oxygen) could further reduced the bone mass and bone strength in OVX rats, and genistein partially reversed the detrimental effect of intermittent hypoxia on bone mass and bone strength. The present study for the first time investigated the influence of intermittent hypoxia on bone mass, bone microarchitecture and bone strength, and reported that genistein could improve the abnormal bone metabolism of OVX rats undergoing intermittent hypoxia.
Under the normal condition, the arterial oxygen concentration is about 12% and venous/capillary oxygen concentration is about 5%. The blood oxygen concentration fluctuates within a narrow range via precise regulatory mechanisms. When the blood oxygen concentration reduces, cells fail to produce enough ATP to main the normal functions under the hypoxic condition, and thus pathological processes occur. The reduced oxygen concentration of blood may influence the functions of osteoclasts and osteoblasts. In mouse bone marrow and human peripheral mononuclear cells (hPBMCs), hypoxia can significantly increase the amount and volume of osteoclasts [12,13], but has no influence on the bone resorption ability of mature osteoclasts [12]. In addition, hypoxia can also increase the expressions of vascular endothelial growth factor (VEGF) and interleukin 6 (IL-6), two factors promoting the functions of osteoclasts [13]. In previous studies, continuous hypoxia was introduced in the establishment of animal model, which has risk for causing cell death and influences the observation of osteoclast functions [14]. In the present study, intermittent hypoxia was employed to mimic the hypopnea during OSAHS, and the markers for bone resorption were detected in the bone. Results showed the influence of intermittent hypoxia on osteoclasts was consistent with that of continuous hypoxia: hypoxia of both patterns could promote the functions of osteoclasts. A lot of studies have been conducted to investigate the influence of hypoxia on osteoblasts. In vitro studies reveal that hypoxia can reduce the Cbfa1 expression [15-17] and increase ATP release (ATP may block the bone mineralization and stimulate osteolysis) [18], which then reduce the differentiation and maturation of osteoblasts. In studies on bone marrow stromal stem cells (BMSCs), results showed hypoxia could stimulate lipogenesis [19]. Adipocytes and osteoblasts have the same precursor cells. Thus, the increased lipogenesis may be accompanied by reduced differentiation into osteoblasts. Chronic intermittent hypoxia may reduce the proliferation of osteoblasts, synthesis of collagen and ALP expression to decrease osteogenesis [14]. There is evidence showing that hypoxia may inhibit collagen synthesis, which is attributed to the reduction of oxygen dependent enzymes such as proline hydroxylase and lysine oxidase [20]. However, the influence of hypoxia on bone formation is still controversial. Some studies show hypoxia may promote the growth of osteoblasts [21], reduce the Sclerostin expression in osteoblasts and promote Wnt signaling pathway to elevate bone formation [22]. Our results showed intermittent hypoxia could reduce the expressions of Cbfa1, bone ALP and type I collagen.
The hypopnea state during sleep apnea usually causes acidosis, and the acidosis induced damage to bone system has been reported [23]. Reduced pH may increase the bone resorption significantly. There is evidence showing that the increment of pH value of 0.1 may increase the bone resorption by 1 fold [24]. Acidosis is also necessary for the initiation of bone resorption [14]. In the presence H+, osteoclasts may be further activated by osteolytic factors (such as PTH and 1,25(OH)D) to induce bone resorption. In addition, acidosis may also influence the functions of osteoblasts. Mild reduction in pH value may induce the reduction in the expression of extracellular matrix including collagens [25,26]. Moreover, acidosis also affects the mineralization of matrix and cause lysis of mineralized hydroxyapatites. Experiments showed the concentrations of Ca2+ and PO3- released from hydroxyapatites increased by 2 folds and 4 folds, respectively, when compared with those at baseline [27].
In recent years, studies on phytoestrogens show genistein can be used to effectively prevent against osteoporosis. There is evidence showing that genistein can reduce the generation and activity of osteoclasts [11,28] and promote the apoptosis of mature osteoclasts [29] to inhibit bone resorption, which prevent against trabecular bone lost in OVX rats [30]. Besides the inhibition of osteoclasts, genistein may also increase the differentiation and mineralization of osteoblasts and protein synthesis in these cells [31,32]. During the differentiation of BMSCs, genistein can reduce the differentiation of these cells into adipocytes and increase their differentiation into osteoblasts to elevate the amount of osteoblasts [33]. In addition, genistein may also bind to estrogen receptor (ER) on osteoblasts to exert osteogenic effect [34,35]. Although the affinity of genistein to ERβ is higher than that to ERα [36], the effects of genistein are realized via ERα [37]. Our results indicated that genistein not only improved the BMD in OVX rats, but also alleviated the reduction in BMD of OVX rats undergoing intermittent hypoxia. Besides the direct effect on osteoclasts and osteoblasts, genistein may also exert anti-inflammatory effect [38] and improve stress [39] to attenuate the hypoxia induced damage to bone metabolism. Thus, genistein may exert comprehensively protective effects on hypoxia induced damage to bone metabolism.
Epidemiological studies have confirmed that estrogen is related to OSAHS and occurrence and development of cardiovascular complications and may exert protective effects on these conditions. However, whether hormone replacement therapy can be used to prevent and treat OSAHS is required to be further studied. Hormone replacement therapy has risk for side effects. Thus, we should weight the oros and cons of hormone replacement therapy and individualized therapy is then applied. In addition, the side effects during the treatment should be closely monitored and promptly treated. Thus, to develop more safe and effective drugs is currently a key point in studies. Phytoestrogens and estrogen-like drugs (such as: estrogen receptor modulators) may provide a new modality for the treatment of OSAHS in postmenopausal women.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (NO: 30872726) and Project of Science and Technology Commission of Shanghai Municipality (NO: 13ZR1438000).
Disclosure of conflict of interest
None.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Kapsimalis F, Kryger MH. Gender and obstructive sleep apnea syndrome, part 1: Clinical features. Sleep. 2002;25:412–419. [PubMed] [Google Scholar]
- 3.Anttalainen U, Saaresranta T, Aittokallio J, Kalleinen N, Vahlberg T, Virtanen I, Polo O. Impact of menopause on the manifestation and severity of sleep-disordered breathing. Acta Obstet Gynecol Scand. 2006;85:1381–1388. doi: 10.1080/00016340600935649. [DOI] [PubMed] [Google Scholar]
- 4.Banhiran W, Junlapan A, Assanasen P, Chongkolwatana C. Physical predictors for moderate to severe obstructive sleep apnea in snoring patients. Sleep Breath. 2014;18:151–8. doi: 10.1007/s11325-013-0863-y. [DOI] [PubMed] [Google Scholar]
- 5.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 6.Tomiyama H, Okazaki R, Inoue D, Ochiai H, Shiina K, Takata Y, Hashimoto H, Yamashina A. Link between obstructive sleep apnea and increased bone resorption in men. Osteoporos Int. 2008;19:1185–1192. doi: 10.1007/s00198-007-0556-0. [DOI] [PubMed] [Google Scholar]
- 7.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 8.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 9.Le Marchand L. Cancer preventive effects of flavonoids--a review. Biomed Pharmacother. 2002;56:296–301. doi: 10.1016/s0753-3322(02)00186-5. [DOI] [PubMed] [Google Scholar]
- 10.Valsecchi AE, Franchi S, Panerai AE, Rossi A, Sacerdote P, Colleoni M. The soy isoflavone genistein reverses oxidative and inflammatory state, neuropathic pain, neurotrophic and vasculature deficits in diabetes mouse model. Eur J Pharmacol. 2011;650:694–702. doi: 10.1016/j.ejphar.2010.10.060. [DOI] [PubMed] [Google Scholar]
- 11.Reinwald S, Weaver CM. Soy isoflavones and bone health: a double-edged sword? J Nat Prod. 2006;69:450–459. doi: 10.1021/np058104g. [DOI] [PubMed] [Google Scholar]
- 12.Arnett TR, Gibbons DC, Utting JC, Orriss IR, Hoebertz A, Rosendaal M, Meghji S. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol. 2003;196:2–8. doi: 10.1002/jcp.10321. [DOI] [PubMed] [Google Scholar]
- 13.Utting JC, Flanagan AM, Brandao-Burch A, Orriss IR, Arnett TR. Hypoxia stimulates osteoclast formation from human peripheral blood. Cell Biochem Funct. 2010;28:374–380. doi: 10.1002/cbf.1660. [DOI] [PubMed] [Google Scholar]
- 14.Arnett TR. Acidosis, hypoxia and bone. Arch Biochem Biophys. 2010;503:103–109. doi: 10.1016/j.abb.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Ontiveros C, Irwin R, Wiseman RW, McCabe LR. Hypoxia suppresses runx2 independent of modeled microgravity. J Cell Physiol. 2004;200:169–176. doi: 10.1002/jcp.20054. [DOI] [PubMed] [Google Scholar]
- 16.Salim A, Nacamuli RP, Morgan EF, Giaccia AJ, Longaker MT. Transient changes in oxygen tension inhibit osteogenic differentiation and Runx2 expression in osteoblasts. J Biol Chem. 2004;279:40007–40016. doi: 10.1074/jbc.M403715200. [DOI] [PubMed] [Google Scholar]
- 17.Yang DC, Yang MH, Tsai CC, Huang TF, Chen YH, Hung SC. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PLoS One. 2011;6:e23965. doi: 10.1371/journal.pone.0023965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orriss IR, Knight GE, Utting JC, Taylor SE, Burnstock G, Arnett TR. Hypoxia stimulates vesicular ATP release from rat osteoblasts. J Cell Physiol. 2009;220:155–162. doi: 10.1002/jcp.21745. [DOI] [PubMed] [Google Scholar]
- 19.Fink T, Abildtrup L, Fogd K, Abdallah BM, Kassem M, Ebbesen P, Zachar V. Induction of adipocyte-like phenotype in human mesenchymal stem cells by hypoxia. Stem Cells. 2004;22:1346–1355. doi: 10.1634/stemcells.2004-0038. [DOI] [PubMed] [Google Scholar]
- 20.Utting JC, Robins SP, Brandao-Burch A, Orriss IR, Behar J, Arnett TR. Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp Cell Res. 2006;312:1693–1702. doi: 10.1016/j.yexcr.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Lennon DP, Edmison JM, Caplan AI. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. J Cell Physiol. 2001;187:345–355. doi: 10.1002/jcp.1081. [DOI] [PubMed] [Google Scholar]
- 22.Genetos DC, Toupadakis CA, Raheja LF, Wong A, Papanicolaou SE, Fyhrie DP, Loots GG, Yellowley CE. Hypoxia decreases sclerostin expression and increases Wnt signaling in osteoblasts. J Cell Biochem. 2010;110:457–467. doi: 10.1002/jcb.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemann J Jr, Litzow JR, Lennon EJ. The effects of chronic acid loads in normal man: further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest. 1966;45:1608–1614. doi: 10.1172/JCI105467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnett TR, Spowage M. Modulation of the resorptive activity of rat osteoclasts by small changes in extracellular pH near the physiological range. Bone. 1996;18:277–279. doi: 10.1016/8756-3282(95)00486-6. [DOI] [PubMed] [Google Scholar]
- 25.Frick KK, Bushinsky DA. Chronic metabolic acidosis reversibly inhibits extracellular matrix gene expression in mouse osteoblasts. Am J Physiol. 1998;275:F840–847. doi: 10.1152/ajprenal.1998.275.5.F840. [DOI] [PubMed] [Google Scholar]
- 26.Frick KK, Bushinsky DA. In vitro metabolic and respiratory acidosis selectively inhibit osteoblastic matrix gene expression. Am J Physiol. 1999;277:F750–755. doi: 10.1152/ajprenal.1999.277.5.F750. [DOI] [PubMed] [Google Scholar]
- 27.Brandao-Burch A, Utting JC, Orriss IR, Arnett TR. Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcif Tissue Int. 2005;77:167–174. doi: 10.1007/s00223-004-0285-8. [DOI] [PubMed] [Google Scholar]
- 28.Liao QC, Xiao ZS, Qin YF, Zhou HH. Genistein stimulates osteoblastic differentiation via p38 MAPK-Cbfa1 pathway in bone marrow culture. Acta Pharmacol Sin. 2007;28:1597–1602. doi: 10.1111/j.1745-7254.2007.00632.x. [DOI] [PubMed] [Google Scholar]
- 29.Uchiyama S, Yamaguchi M. Genistein and zinc synergistically stimulate apoptotic cell death and suppress RANKL signaling-related gene expression in osteoclastic cells. J Cell Biochem. 2007;101:529–542. doi: 10.1002/jcb.21208. [DOI] [PubMed] [Google Scholar]
- 30.Nian H, Ma MH, Nian SS, Xu LL. Antiosteoporotic activity of icariin in ovariectomized rats. Phytomedicine. 2009;16:320–326. doi: 10.1016/j.phymed.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Chen X, Garner SC, Quarles LD, Anderson JJ. Effects of genistein on expression of bone markers during MC3T3-E1 osteoblastic cell differentiation. J Nutr Biochem. 2003;14:342–349. doi: 10.1016/s0955-2863(03)00056-1. [DOI] [PubMed] [Google Scholar]
- 32.Morris C, Thorpe J, Ambrosio L, Santin M. The soybean isoflavone genistein induces differentiation of MG63 human osteosarcoma osteoblasts. J Nutr. 2006;136:1166–1170. doi: 10.1093/jn/136.5.1166. [DOI] [PubMed] [Google Scholar]
- 33.Ming LG, Chen KM, Xian CJ. Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling. J Cell Physiol. 2013;228:513–521. doi: 10.1002/jcp.24158. [DOI] [PubMed] [Google Scholar]
- 34.Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M, Masamune Y. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull. 2001;24:351–356. doi: 10.1248/bpb.24.351. [DOI] [PubMed] [Google Scholar]
- 35.Wang TT, Sathyamoorthy N, Phang JM. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis. 1996;17:271–275. doi: 10.1093/carcin/17.2.271. [DOI] [PubMed] [Google Scholar]
- 36.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 37.Hertrampf T, Gruca MJ, Seibel J, Laudenbach U, Fritzemeier KH, Diel P. The bone-protective effect of the phytoestrogen genistein is mediated via ER alpha-dependent mechanisms and strongly enhanced by physical activity. Bone. 2007;40:1529–1535. doi: 10.1016/j.bone.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Danciu C, Soica C, Csanyi E, Ambrus R, Feflea S, Peev C, Dehelean C. Changes in the anti-inflammatory activity of soy isoflavonoid genistein versus genistein incorporated in two types of cyclodextrin derivatives. Chem Cent J. 2012;6:58. doi: 10.1186/1752-153X-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ondricek AJ, Kashyap AK, Thamake SI, Vishwanatha JK. A comparative study of phytoestrogen action in mitigating apoptosis induced by oxidative stress. In Vivo. 2012;26:765–775. [PubMed] [Google Scholar]


