Abstract
Background and Aim: Dicer is one of the most important components in microRNA biogenesis. Although studies have revealed the aberrantly expression of Dicer in types of cancer, the results were greatly controversial. Here we aimed to study the expression of Dicer in gastric cancer (GC) and further explored the possible roles of Dicer in cancer progression. Methods: The alteration of Dicer-expression in GC and its clinical significance was retrospectively studied with immunohistochemical analyses on 377 cases of cancer tissues using tissue microarray (TMA). Dicer mRNA and protein levels were also examined in 8 paired of GC tissues and non-neoplastic surrounding gastric epithelium with real time RT-PCR and western blot. Results: We found that Dicer was reduced in GC tissues in both mRNA and protein levels. Moreover, down-regulation of Dicer was correlated highly with tumor differentiation (P<0.05) and lymph node invasion (P<0.05) in GC tissues, which suggested an essential role of Dicer in cancer invasion. Conclusions: Considering that Dicer might be closely related to progression of GC, we proposed that Dicer might offer a promising target for prevention of metastatic progression in GC.
Keywords: Dicer, gastric cancer, immunohistochemical analyses
Introduction
Gastric cancer (GC) is one of the leading causes of cancer-related death worldwide. A better knowledge of the pathogenesis and metastatic process of GC will provide clues that may lead to the development of new therapeutic strategies to control cancer malignant progression.
In recent years, many studies have proposed the important roles of components involved in the RNA interference (RNAi) pathway and microRNAs (miRNAs) biogenesis in tumorigenesis and cancer progression. It was reported that these miRNA biogenesis-relating proteins have been linked to human diseases, in which they can function as a group to mark differentiation states or individually as bona fide oncogenes or tumor suppressors [1,2]. Thus, the study of those miRNA biogenesis components is likely to be important.
Dicer, the key enzyme in the RNAi and miRNA pathways, is a highly conserved RNase III type enzyme which could be found in almost all eukaryotes [3]. Although studies revealed that Dicer was aberrantly expressed in types of cancer, the results were greatly controversial. For example, Dicer has been found to be down-regulated in non-small cell lung carcinoma (NSCLC) [4], breast cancer [5], and ovarian cancer tissues [6]. Furthermore, reduced mRNA expression of Dicer was significantly associated with advanced tumor stages and decreased survival [7,8]. In contrast, other studies showed that Dicer was significantly up-regulated in precursor lesions of lung adenocarcinomas and 81% of prostate cancers, and increased Dicer was related to advanced stages [9] or reduced disease-free survival [10,11]. Although the present results were inconsistent with each other, nevertheless, these findings supported the hypothesis that altered levels of Dicer may have an essential role in carcinogenesis and cancer progression, which were worth studying.
To study the influence of Dicer in GC, here we examined the alteration of Dicer-expression in GC and its correlation with clinicopathological characteristics of GC patients.
Materials and methods
Patients and tissues
For immunohistochemistry (IHC) study, we retrospectively reviewed 377 consecutive GC patients who underwent potentially curative surgery without preoperative chemotherapy or radiotherapy during the period of 2002-2011 at Suzhou Municipal Hospital. The medical records such as the demographic and clinicopathological data were collected from the electronic clinical records. Tumor location and size were derived from the surgical report. The clinical stage was determined according to the 7th edition of AJCC cancer staging manual [12]. And histologic grading of the specimens were graded into well, moderately, poorly differentiated and undifferentiated carcinoma according to the World Health Organization classification. Owing to tissue availability and the possible confounding factors from stage IV such as performance status, we only included stage I, II and III patients in the analysis. In addition, 10 “non-neoplastic” surrounding gastric epithelium specimens were also applied for Dicer immunostaining. For examination of mRNA levels, 8 paired of GC and adjacent non-neoplastic tissues were obtained in Jinling Hospital between October 2011 and January 2012. Tissue samples were immediately frozen in liquid nitrogen and stored at -80°C until use.
This prospective pilot study was conducted within the framework of the Declaration of Helsinki and approved by the ethics and scientific committees of Suzhou Municipal Hospital and Jinling Hospital. Informed consent was obtained from all study subjects.
Tissue microarray (TMA) construction and IHC analysis
A paraffin TMA was constructed containing tissue from the tumor center in triplicates using a manual tissue arrayer (Beecher Instruments Inc., Sun Prairie, WI, USA), as described previously [13]. For IHC staining, 4 μm sections from each paraffin block were stained with antibodies reacting with Dicer (1:250, Abcam, Hamburg, Germany). Staining was performed on an automated system (Bench-Mark XT, Ventana Medical System, Tucson, AZ, USA) with a final detection of immunoreactivity by the diaminobenzidine (DAB) substrate. All slides were counterstained with Hematoxylin. System controls were performed and did not yield a positive result.
Evaluation of the IHC results
Evaluation of Dicer staining was carried out by two independent observers, and assessed by applying a semiquantitative immunoreactivity scoring system (IRS) with the sum of stained area and intensity scores. Specifically, a score of 0 was assigned to a stained area with 0% reactivity, 1 for an area with >1% to ≤10% of tumor cells, 2 for >11% to ≤ 50% tumor cells, 3 for >51% to ≤80% tumor cells and 4 for >81% tumor cells. For the staining intensity, a score of 0 was assigned for absent staining (negative control), 1 for a weak staining obviously stronger than the negative control level, 2 for moderately intense staining, and 3 for intense staining. Finally, cases with IRS <6 were identified as Dicer negative, whereas cases with IRS ≥6 were grouped together as Dicer-positive.
Real-time RT-PCR
Dicer mRNA in 8 paired of frozen tumor samples and matched nonneoplastic gastric mucosa was examined with real time RT-PCR according to standard protocols. The primers used were as follows: Dicer-F: 5’-GAGCTGTCCTATCA-GATCAGGG-3’, Dicer-R: 5’-ACTTGTTGAGCAACCT-GGTTT-3’ (GenBank accession number NM_177438); β-actin-F: 5’-CAACTGGGACGACATGG-AGAAAAT-3’, β-actin-R: 5’-CCAGAGGCGTACAGG-GATAGCAC-3’ (GenBank accession number NM_001101). All the samples were analyzed in triplicates and the mean value was calculated and expressed as cycle threshold (CT). The relative amount of target gene expression was analyzed by the 2-ΔCt-method and the final data was multiplied by a factor of 10 to better illustrate mRNA quantities.
Western blot analysis
Total proteins were prepared by standard procedures as described previously [14]. Protein concentrations were determined by using BCA protein quantification assay. Equal amounts of proteins (20 μg) were applied to a gradient SDS-PAGE gel. After electrophoresis, the protein was transferred onto nitrocellulose paper, incubated with BSA in TBS overnight at 4°C and then probed with specific antibodies recognizing target proteins and visualized with DAB. The intensity of each protein was normalized to β-actin by densitometry with Image J analysis software and multiplied by 10 for better illustration. All reactions were performed in triplicates.
Statistical analysis
The independent-sample t-test or ANOVA followed by LSD method and post-hoc Dunnett’s t-tests was used to evaluate the significance of the differences in the mRNA and protein expression levels. The chi-squared test and if appropriate Fisher’s exact test were applied to test for associations of clinicopathological parameters and Dicer expression in GC specimens. All tests were performed two-tailed at a 5% level of significance using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA).
Results
Expression of Dicer in GC and nonneoplastic epithelia tissues
IHC analysis demonstrated that Dicer protein expression decreased significantly in GC compared to its abundance in the corresponding nonneoplastic epithelia tissues (P=0.010). Among the 377 tested cancer samples, only 39.5% (149/377) stained positive, while 80% (8/10) of nonneoplastic epithelia tissues showed positive staining. Cytoplasmic localization of Dicer expression was detected primarily in the non-neoplastic foveolar epithelium cells, while could also be found in some stromal or inflammatory cells (Figure 1A, 1B).
Figure 1.
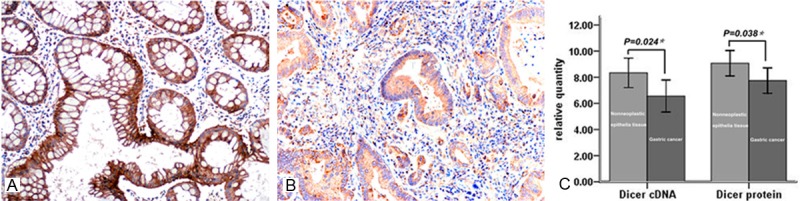
Downregulation of Dicer in gastric cancer (GC). Representative slides of immunohistochemical staining for Dicer in nonneoplastic epithelial-gastric specimens (A, ×200) and GC (B, ×200). (C) Decreased Dicer mRNA and protein in GC tissue specimens was identified by real time RT-PCR and western blot analysis (Dicer mRNA 6.56±1.466 in GC vs. 8.34±1.348 in corresponding nonneoplastic epithelia tissues, P=0.024; Dicer protein 7.74±1.165 in GC vs. 9.07±1.158 in corresponding nonneoplastic epithelia tissues, P=0.038). Results were indicated as the mean±SD of three separate experiments.
Comparative analysis of Dicer expression was further conducted in 8 tumor specimens and paired nonneoplastic mucosa using western blot and real time RT-PCR. The analysis revealed the lower expression levels of Dicer mRNA (6.56±1.466 vs. 8.34±1.348, P=0.024) and protein (7.74±1.165 vs. 9.07±1.158, P=0.038) in GC tissues compared to those in corresponding nonneoplastic epithelia tissues, confirming our IHC results (Figure 1C). It is of note that the levels of Dicer protein were correlated with the mRNA expression levels, indicating that the down-regulation of Dicer in GC may be caused by transcriptional downregulation.
The correlation between the decreased expression of Dicer and clinicopathological features in GC
Down-regulation of Dicer had no correlation with patients’ gender (P=0.391), age (P=0.233), morphology (P=0.104), tumor location (P=0.061), tumor size (P=0.764), infiltration depth (P=0.827) or clinical stage (P=0.624) (Table 1). However, negative Dicer expression was more common in GC patients with poorly differentiation (P=0.037) (Figure 2A-C) or lymph node metastasis (P=0.044). These results may indicate the potential role of Dicer in the malignant progression of GC.
Table 1.
Correlation of Dicer expression with clinicopathological characteristics of gastric cancer patients
| Clinicopathological variables | N | Dicer expression (%) | χ2 | P value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Negative | Positive | |||||
| Overall frequency | Nonneoplastic | 10 | 2 (20) | 8 (80) | 6.620 | 0.010* |
| Gastric cancer | 377 | 228 (60.5) | 149 (39.5) | |||
| Age (years)† | ≥64 | 199 | 126 (63.3) | 73 (36.7) | 1.421 | 0.233 |
| >64 | 178 | 102 (57.3) | 76 (42.7) | |||
| Gender | Male | 269 | 159 (59.1) | 110 (40.9) | 0.737 | 0.391 |
| Female | 108 | 69 (63.9) | 39 (36.1) | |||
| Tumor site | Distal 1/3 | 126 | 85 (67.5) | 41 (32.5) | 5.580 | 0.061 |
| Middle 1/3 | 100 | 52 (52) | 48 (48) | |||
| Proximal 1/3 | 151 | 91 (60.3) | 60 (39.7) | |||
| Gross morphology | Ulcerocancer | 287 | 182 (63.4) | 105 (36.6) | 4.517 | 0.104 |
| Polypoid type | 47 | 25 (53.2) | 22 (46.8) | |||
| Diffuse type | 43 | 21 (48.8) | 22 (51.2) | |||
| Tumor size (centimeter)† | <4.86 | 206 | 126 (61.2) | 80 (38.8) | 0.090 | 0.764 |
| ≥4.86 | 171 | 102 (59.6) | 69 (40.4) | |||
| Histologic grading§ (tumor differentiation) | well | 32 | 15 (46.9) | 17 (53.1) | 6.557 | 0.037* |
| moderate | 129 | 71 (55.0) | 58 (45.0) | |||
| poorly/undifferentiated | 216 | 142 (65.7) | 74 (34.3) | |||
| Tumor infiltration‡ | T1 | 12 | 8 (66.7) | 4 (33.3) | 0.381 | 0.827 |
| T2 | 74 | 43 (58.1) | 31 (41.9) | |||
| T3 | 291 | 177 (60.8) | 114 (39.2) | |||
| Lymph node invasion‡ | N0 | 118 | 62 (51.7) | 58 (48.3) | 9.823 | 0.044* |
| N1 | 119 | 70 (58.8) | 49 (41.2) | |||
| N2 | 67 | 45 (67.2) | 22 (32.8) | |||
| N3a | 61 | 44 (72.1) | 17 (27.9) | |||
| N3b | 12 | 9 (75.0) | 3 (25.0) | |||
| Clinical stage‡ | ІA+ІB | 28 | 15 (53.6) | 13 (46.4) | 0.944 | 0.624 |
| IIA+IIB | 227 | 136 (59.9) | 91 (40.1) | |||
| IIIA+IIIB+IIIC | 122 | 77 (63.1) | 45 (36.9) | |||
P<0.05.
Patients’ age and tumor size was determined by the mean value of the cases in our present research.
Cancer staging was determined according to the 7th edition of AJCC cancer staging manual.
Histologic grading of the specimens was graded according to the World Health Organization classification.
Figure 2.
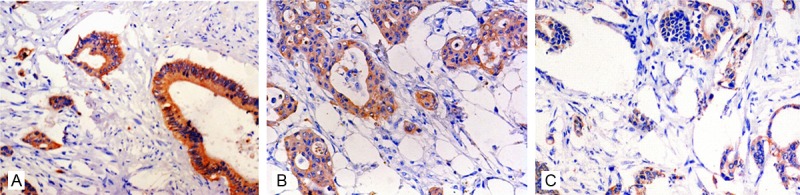
The immunoreactivity of Dicer was much higher in GC tissues with well differentiation (A, ×200) than that in moderate (B, ×200) and poorly (C, ×200) differentiated tumor tissues.
Discussion
Previous studies have identified that apart from genetic and epigenetic changes in classical oncogenes and tumor suppressor genes, miRNAs, mediated by miRNA biogenesis-relating proteins such as Dicer, Drosha, Ago2 and DGCR8, may also play essential roles in tumor biology and may even contribute to malignant transformation [15-17]. Lately, two different studies about Dicer were performed in GC, however, with contradictory results. In the first study, Dicer was found up-regulated in cases of GC [18], while according to the second study, Dicer mRNA and levels of the corresponding protein were found down-regulated in GC tissues [19]. In our preliminary studies, we have identified the significant alterations of Ago2 and DGCR8 in the aggressive clinical course of GC [17], but the special role of Dicer in tumor growth, invasion, or metastasis of GC still need better elucidation.
Here we first examined the expression levels of Dicer in GC and non-neoplastic gastric epithelium tissues with IHC. We found that Dicer was mainly existed in non-neoplastic epithelial cells, while could also be detected in some stromal and inflammatory cells. The cytoplasmic expression of Dicer in tumor cells was downregulated compared to that of non-neoplastic gastric epithelium specimens. With these results, we proposed that the wide distribution of Dicer may involve in kinds of physiological responses as well as in early cancer formation,and the dysregulation of Dicer may be important in tumor transformation. Our results were consistent with some of the previous reports as reduction of Dicer was found in NSCLC, breast cancers as well as ovarian cancer tissues. In contrast, other studies indicated the up-regulation of Dicer in prostate adenocarcinoma and ovarian serous carcinoma. Whether these discrepancies were attributed to tissue-specific differences and/or the degree of aggressiveness of the cancer tissues should be further studied.
To explore the possible role of Dicer in GC progression, we further observed the correlations of Dicer with clinicopathological features of GC patients. We found that reduction of Dicer was more common in GC cases with poor tumor differentiation and lymph node invasion, which might be hypothesized as a putative modulator of tumor progression. Moreover, the association of Dicer with tumor differentiation and lymph node invasion, both of which had been taken as important factors correlated with unfavorable outcomes of GC patients, may also show a possibility of Dicer as an indicator for GC prognosis. Inconsistent with our results, Tchernitsa et al. identified that upregulation of Dicer in GC tumors was associated with tumor type and local tumor growth [18]. This discrepancy may be caused by the different editions of TNM stage classification partly. On the other hand, the different detection methods, along with the variability in sample-sizes and the heterogeneity in the source of sample populations, may also be the reasons of the variations in the aberrant expression of Dicer that have been detected within the same tumor type and across different cancers. Further investigations on larger sample size and functional analyses of Dicer in GC are warranted.
In summary, we demonstrated that the reduction of Dicer in GC was correlated to tumor differentiation and lymph node invasion, which may suggest an essential role of Dicer in GC development and progression. The exact mechanisms still need further illustration.
Acknowledgements
This work was supported by research grants from the National Natural Science Foundation of China (NO. 81171391, NO. 81101815), China Postdoctoral Science Foundation (NO. 201150M1575), Special Program of the Postdoctoral Science Foundation of China (NO. 2012T50895) and Maixin Pathology Foundation (NO. m1204).
Disclosure of conflict of interest
None.
References
- 1.De Santa F, Iosue I, Del Rio A, Fazi F. microRNA biogenesis pathway as a therapeutic target for human disease and cancer. Curr Pharm Des. 2013;19:745–764. [PubMed] [Google Scholar]
- 2.Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and function. Thromb Haemost. 2012;107:605–610. doi: 10.1160/TH11-12-0836. [DOI] [PubMed] [Google Scholar]
- 3.Park JE, Heo I, Tian Y, Simanshu DK, Chang H, Jee D, Patel DJ, Kim VN. Dicer recognizes the 5’ end of RNA for efficient and accurate processing. Nature. 2011;475:201–205. doi: 10.1038/nature10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiosea S, Jelezcova E, Chandran U, Luo J, Mantha G, Sobol RW, Dacic S. Overexpression of Dicer in Precursor Lesions of Lung Adenocarcinoma. Cancer Res. 2007;67:2345–2350. doi: 10.1158/0008-5472.CAN-06-3533. [DOI] [PubMed] [Google Scholar]
- 5.Grelier G, Voirin N, Ay AS, Cox DG, Chabaud S, Treilleux I, Léon-Goddard S, Rimokh R, Mikaelian I, Venoux C, Puisieux A, Lasset C, Moyret-Lalle C. Prognostic value of Dicer expression in human breast cancers and association with the mesenchymal phenotype. Br J Cancer. 2009;101:673–683. doi: 10.1038/sj.bjc.6605193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pampalakis G, Diamandis EP, Katsaros D, Sotiropoulou G. Down-regulation of dicer expression in ovarian cancer tissues. Clin Biochem. 2010;43:324–327. doi: 10.1016/j.clinbiochem.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Merritt WM, Lin YG, Han LY, Kamat AA, Spannuth WA, Schmandt R, Urbauer D, Pennacchio LA, Cheng JF, Nick AM, Deavers MT, Mourad-Zeidan A, Wang H, Mueller P, Lenburg ME, Gray JW, Mok S, Birrer MJ, Lopez-Berestein G, Coleman RL, Bar-Eli M, Sood AK. Dicer, Drosha, and Outcomes in Patients with Ovarian Cancer. N Engl J Med. 2008;359:2641–2650. doi: 10.1056/NEJMoa0803785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karube Y, Tanaka H, Osada H, Tomida S, Tatematsu Y, Yanagisawa K, Yatabe Y, Takamizawa J, Miyoshi S, Mitsudomi T, Takahashi T. Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci. 2005;96:111–115. doi: 10.1111/j.1349-7006.2005.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiosea S, Jelezcova E, Chandran U, Acquafondata M, McHale T, Sobol RW, Dhir R. Up-Regulation of Dicer, a Component of the MicroRNA Machinery, in Prostate Adenocarcinoma. Am J Pathol. 2006;169:1812–1820. doi: 10.2353/ajpath.2006.060480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flavin RJ, Smyth PC, Finn SP, Laios A, O’Toole SA, Barrett C, Ring M, Denning KM, Li J, Aherne ST, Aziz NA, Alhadi A, Sheppard BL, Loda M, Martin C, Sheils OM, O’Leary JJ. Altered eIF6 and Dicer expression is associated with clinicopathological features in ovarian serous carcinoma patients. Mod Pathol. 2008;21:676–684. doi: 10.1038/modpathol.2008.33. [DOI] [PubMed] [Google Scholar]
- 11.Faber C, Horst D, Hlubek F, Kirchner T. Overexpression of Dicer predicts poor survival in colorectal cancer. Eur J Cancer. 2011;47:1414–1419. doi: 10.1016/j.ejca.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th edition. New York, NY: Springer; 2010. [Google Scholar]
- 13.Zhang J, Zhan N, Dong WG. Altered expression of selenium-binding protein 1 in gastric carcinoma and precursor lesions. Med Oncol. 2011;28:951–957. doi: 10.1007/s12032-010-9564-6. [DOI] [PubMed] [Google Scholar]
- 14.Liu QS, Zhang J, Liu M, Dong WG. Lentiviral-mediated miRNA against liver-intestine cadherin suppresses tumor growth and invasiveness of human gastric cancer. Cancer Sci. 2010;101:1807–1812. doi: 10.1111/j.1349-7006.2010.01600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mott JL. MicroRNAs involved in tumor suppressor and oncogene pathways: implications for hepatobiliary neoplasia. Hepatology. 2009;50:630–637. doi: 10.1002/hep.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeshita D, Zenno S, Lee WC, Nagata K, Saigo K, Tanokura M. Homodimeric structure and double-stranded RNA cleavage activity of the C-terminal RNase III domain of human dicer. J Mol Biol. 2007;374:106–120. doi: 10.1016/j.jmb.2007.08.069. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Fan XS, Wang CX, Liu B, Li Q, Zhou XJ. Up-regulation of Ago2 expression in gastric carcinoma. Med Oncol. 2013;30:628. doi: 10.1007/s12032-013-0628-2. [DOI] [PubMed] [Google Scholar]
- 18.Tchernitsa O, Kasajima A, Schäfer R, Kuban RJ, Ungethüm U, Györffy B, Neumann U, Simon E, Weichert W, Ebert MP, Röcken C. Systematic evaluation of the miRNA-ome and its downstream effects on mRNA expression identifies gastric cancer progression. J Pathol. 2010;222:310–319. doi: 10.1002/path.2759. [DOI] [PubMed] [Google Scholar]
- 19.Zheng ZH, Sun XJ, Fu WN, Guan Y, Gao F, Wang Y, Sun KL. Decreased expression of DICER1 in gastric cancer. Chin Med J (Engl) 2007;120:2099–2104. [PubMed] [Google Scholar]


