Abstract
In Lewy body disease, Lewy pathology (LP: the accumulation of α-synuclein in neuronal perikarya and processes as Lewy bodies and Lewy neurites and dots, respectively) is observed in the central and peripheral nervous systems. Previous autopsy or biopsy studies of individuals with Lewy body diseases (LBDs) indicated that LP could be observed in the peripheral nerves of the gastrointestinal (GI) systems. The aim of this study is to clarify whether examination of GI and biliary surgical specimens would be useful for diagnosing LBD. We analyzed eight patients diagnosed clinically with LBD and with medical histories of GI or biliary surgery at our hospital. LP was identified by using α-synuclein immunohistochemistry in GI and biliary surgical specimens obtained before, at or after the clinical onset of LBD. LP was frequently observed in Auerbach’s plexus, Meissner’s plexus and the subserosal nerve fascicles within the GI and biliary surgical specimens. LP was observed in the specimens obtained 7 years before the onset of LBD. Our approach does not require any invasive procedures for patients. The immunohistochemical analysis of anti- α-synuclein antibody to archival GI or biliary surgical specimens from patients with clinically suspected LBD may contribute to clinical diagnosis of LBD.
Keywords: α-synuclein, gastrointestinal and biliary tract, Lewy body disease, Lewy pathology, surgical specimen
Introduction
The presence of Lewy pathology (LP: the accumulation of α-synuclein in neuronal perikarya and processes as Lewy bodies (LBs) and Lewy neurites (LNs), respectively) is important for the diagnosis of Lewy body diseases (LBDs) such as Parkinson’s disease (PD), Parkinson’s disease with dementia (PDD), dementia with LBs (DLB), and pure autonomic failure. LBD is clinically diagnosed on the basis of the patient’s neurological presentation [1], biochemical examination [2], and imaging findings [3]. However, the definitive diagnosis of LBD is made only by postmortem study.
LP is usually observed in the brainstem, basal ganglia, limbic system and cerebral neocortex of LBD individuals [4,5]. LP is also present in the sympathetic and parasympathetic peripheral nervous systems. It is generally accepted that the presence of LP in the peripheral autonomic nervous system is associated with signs of autonomic failure in LBD patients, such as orthostatic hypotension and dysmotility of the gastrointestinal (GI) tract [6-11]. Therefore, biopsy analyses of the peripheral autonomic nervous system may help to diagnose LBD. In a recent biopsy study of subjects with PD, a specific microdissection technique showed that LP was present in the colonic mucosa and submucosa [12]. However, this technique is difficult to apply in routine surgical histopathology and it is still difficult to confirm the diagnosis of LBD pathologically by using biopsy materials [12-16]. Because these biopsy studies were performed on the colon and skin, it might be difficult to obtain enough tissue materials to identify LP in the nerve fibers.
In contrast to use biopsy analyses, Minguez-Castellanos et al. suggested that abdominopelvic surgical specimens might be useful to identify LP for the diagnosis of LBDs [17]. Our study therefore focused on the usefulness of the GI and biliary surgical specimens for diagnosis of LBDs. We investigated the presence of LP in surgical specimens obtained from patients with GI or biliary disorders using conventional and immunohistochemical staining.
Materials and methods
Tissue source
We selected eight patients who had been clinically diagnosed with LBD (six DLB patients and two PDD patients) and who had undergone surgery for GI or biliary problems at Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology between 2007 and 2011 (Table 1). Two patients had medical histories of GI or biliary surgery before they were clinically diagnosed with LBD. The other six were diagnosed with LBD before their GI or biliary surgery.
Table 1.
Lewy pathology in surgical specimens from eight LBD patients
| Patient No. | Diagnosis | Age at diagnosis (years) mean, 83±4.0 [SD] | Gender | MMSE | Hoehn & Yahr stage | Parkinsonism | Autonomic symptoms | Surgical specimens | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Age at surgery (years)/Age at diagnosis of LBD | Surgical site | Pathological diagnosis | Lewy pathology | ||||||||
| 1 | DLB | 85 | M | 10 | IV | Postural instability | Syncope, dysuria, oligohidrosis | 71/78 | Stomach | Adenocarcinoma | + (LBs, LNs) |
| 2 | PDD | 76 | M | 17 | V | Bradykinesia tremor postural instability | Constipation, orthostatic hypotension | 72/74 | Gallbladder | Chronic cholecystitis | + (LNs) |
| 3 | DLB | 86 | M | 17 | V | Bradykinesia postural instability | Constipation | 86/86 | Small intestine | Strangulated ileus, intussusception with submucosal tumor | + (LBs, LNs) |
| 4 | DLB | 84 | F | 0 | V | Bradykinesia postural instability | Constipation, orthostatic hypotension | 84/84 | Terminal ileum to sigmoid colon | Ischemic colitis | + (LNs) |
| 5 | DLB | 86 | F | 16 | III | Postural instability | Oligohidrosis, orthostatic hypotension | 86/86 | Stomach | Adenocarcinoma | + (LNs) |
| 6 | PDD | 77 | F | 22 | IV | Bradykinesia postural instability | Constipation | 77/77 | Stomach | Adenocarcinoma | + (LBs, LNs) |
| 7 | DLB | 85 | M | 14 | III | Bradykinesia postural instability tremor | Constipation | 85/85 | Sigmoid colon | Adenocarcinoma | – |
| 8 | DLB | 82 | M | 25 | IV | Bradykinesia postural instability | Syncope, oligohidrosis | 88/85 | Duodenum Gallbladder | Duodenal ulcer, amyloidosis, chronic cholecystitis | – |
LBD, Lewy body disease; DLB, dementia with Lewy bodies; PDD, Parkinson’s disease with dementia; M, male; F, female; MMSE, Mini-Mental State Examination; LBs, Lewy bodies; LNs, Lewy neurites.
We also analyzed surgical specimens of GI and biliary systems from 10 autopsy subjects who had no LP in the central and peripheral nervous systems (Table 2). LP from these autopsy subjects had also been analyzed in our published paper [18].
Table 2.
Clinical and pathological data on 10 autopsy subjects
| Patient No. | Age (years) mean, 82±6.8 [SD] | Gender | MMSE | Parkinsonism | Autopsy diagnosis (No Lewy pathology was found in the central nervous system or peripheral autonomic nervous system.) | Surgical specimens | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Age at death (years)/Age at surgery | Surgical site | Pathological diagnosis | Lewy pathology | ||||||
| 1 | 76 | M | 30 | – | Lung cancer | 76/75 | Stomach | Adenocarcinoma | – |
| 2 | 88 | F | 24 | – | Rupture of abdominal aortic aneurysm | 88/88 | Stomach | Adenocarcinoma | – |
| 3 | 75 | M | 25 | + | Progressive supranuclear palsy, pneumonia | 75/75 | Small intestine | Perforation, ulcer | – |
| 4 | 83 | M | 30 | – | Systemic amyloidosis | 83/83 | Stomach | Adenocarcinoma | – |
| 5 | 69 | M | 28 | – | Recurrence of rectal cancer, multiple metastasis | 69/65 | Rectum | Adenocarcinoma | – |
| 6 | 86 | F | 24 | – | Acute exacerbation of chronic subdural hematoma, cerebral herniation | 86/84 | Sigmoid colon | Adenocarcinoma | – |
| 7 | 91 | F | 8 | – | Lung cancer, pneumonia, dementia with grains | 91/83 | Stomach | Adenocarcinoma | – |
| 8 | 79 | M | N/A | – | Malignant lymphoma, invasive pulmonary aspergillosis, lung cancer | 79/79 | Descending colon | Adenocarcinoma | – |
| 9 | 86 | F | 9 | – | Alzheimer’s disease, dementia with grains, pneumonia, primary biliary cirrhosis | 86/77 | Ascending colon | Adenocarcinoma | – |
| 10 | 83 | F | N/A | N/A | Diffuse alveolar damage | 83/83 | Sigmoid colon | Diverticulitis | – |
MMSE, Mini-Mental State Examination; M, male; F, female; N/A, not available; +, present; –, absent.
Informed consent was obtained from the patient or the patient’s relatives at the time of surgery or autopsy. The study protocol was approved by the ethical committee of the Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology.
Clinical information
Clinical information, including the presence or absence of parkinsonism and dementia, as well as other clinical symptoms, Hoehn and Yahr stage and Mini-Mental State Examination was obtained from medical charts. Diagnoses of DLB and PDD were confirmed in accordance with the third report of the DLB consortium [19]. PDD was differentiated from DLB by applying the “12-month” rule mentioned in the Consensus Guidelines of the consortium on DLB international workshop [1].
Histology
LP was analyzed in archival paraffin blocks stored at our department of pathology. All materials had therefore been prepared by using the same methodology, namely fixation in 20% buffered formalin for at least 24 h and then embedding in paraffin. The total numbers and anatomic sites of the archival paraffin blocks that were available for study were listed in Table 3 (since LP was not detected in case 7 and 8, we avoided listing both patients in Table 3). Six-micron-thick sections were cut and stained with H&E and immunohistochemical methods.
Table 3.
Frequency of Lewy pathology in each nerve fiber area of the gastrointestinal and biliary surgical specimens
| Patient No. | Total number of blocks | Number of blocks having Lewy pathology/Total number of blocks | |||
|---|---|---|---|---|---|
|
| |||||
| Meissner’s plexuses | Auerbach’s plexuses | Subserosal nerve fascicles | Total | ||
| 1 | 6 | 6/6 (100%) | 6/6 (100%) | 6/6 (100%) | 6/6 (100%) |
| 2 | 2 | * | * | 1/2 (50%) | 1/2 (50%) |
| 3 | 13 | 11/13 (85%) | 13/13 (100%) | 7/13 (54%) | 13/13 (100%) |
| 4 | 21 | † | 8/21 (38%) | 10/21 (48%) | 13/21 (62%) |
| 5 | 8 | 3/8 (38%) | 5/8 (63%) | 3/8 (38%) | 5/8 (63%) |
| 6 | 10 | 4/10 (40%) | 10/10 (100%) | 7/10 (70%) | 10/10 (100%) |
| Total | 60 | 24/37*,† (65%) | 42/58* (72%) | 34/60 (57%) | 48/60 (80%) |
Meissner’s and Auerbach’s plexuses were absent in the gallbladder in patient 2.
Meissner’s plexuses were not identified in the terminal ileum to sigmoid colon in patient 4 because of severe submucosal ischemia with inflammatory cells infiltration.
Under microscopic examination, we identified and analyzed nerve fibers in Meissner’s submucosal nerve plexus, Auerbach’s myenteric nerve plexus and the subserosal nerve fascicles.
Immunohistochemistry
The following antibodies were used: phosphorylated α-synuclein (pSyn#64, monoclonal [20], and PSer129, polyclonal; both kind gifts from Dr. T Iwatsubo [21], pSyn#64 was available from Wako, Osaka, Japan), phosphorylated neurofilament (SMI31, monoclonal; Sternberger Immunochemicals, Bethesda, MA, USA), and tyrosine hydroxylase (anti-tyrosine hydroxylase, monoclonal; Calbiochem-Novabiochem Corporation, Darmstadt, Germany). Immunohistochemistry was performed with a Ventana BenchMark GX autostainer (Ventana Medical Systems, Tucson, AZ, USA) and an I-View Universal DAB Detection Kit (Roche, Basel, Switzerland) in accordance with the manufacturer’s instructions. Sections were counterstained with hematoxylin.
We considered immunoreactivity for pSyn#64 in rounded and intracellular clear dots, intracytoplasmic inclusions, and threads in the nerve fibers to be a positive indicator of LP. However, one drawback with pSyn#64 is that intracytoplasmic granules of mast cells and perivascular small particles may be immunoreactive. If we suspect nonspecific immunoreactive deposits in pSyn#64 immunohistochemistry, we evaluated the results by additionally using polyclonal PSer129 antibody, with which no nonspecific immunoreactivity is detected. Therefore, the immunohistochemistry results were routinely based on those of pSyn#64 antibody unless otherwise specified (e.g. Figure 1H, 1I).
Figure 1.
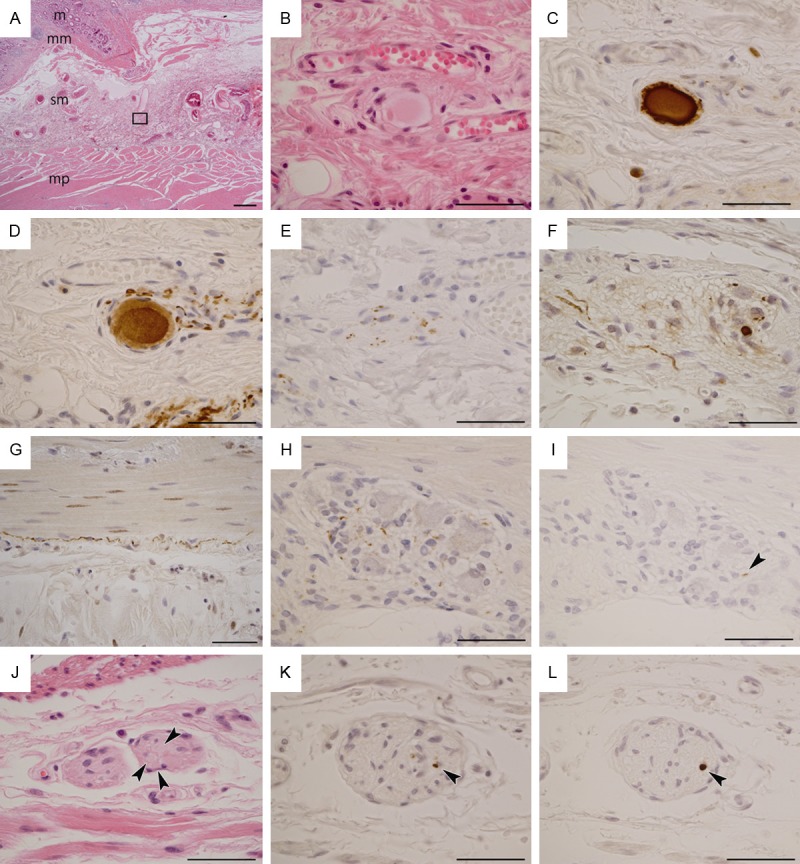
Photomicrographs of Lewy pathology in surgical specimens of Lewy body disease. See Table 1 for the clinical details of the patients. A-E: Photomicrographs from patient 1. F-I: Photomicrographs from patient 3. J-L: Photomicrographs from patient 6. A: Section of the stomach; mucosa (m), muscularis mucosae (mm), submucosa (sm) and muscularis propria (mp). Rectangle corresponds to panel B, including Meissner’s submucosal nerve plexus. B: An oval-shaped hyaline structure formed in the plexus. C: The hyaline structure shows reactivity against monoclonal phosphorylated α-synuclein antibody (pSyn#64). D: The hyaline structure also shows reactivity against phosphorylated neurofilament (SMI31) antibody. In addition, the structure is apparently located in an SMI31-immunoreactive ganglion cell. E: The plexus shows punctate anti-tyrosine hydroxylase immunoreactivity. F: pSyn#64-immunoreactive neurites and small deposits in Auerbach’s myenteric nerve plexus in the small intestine. G: pSyn#64-immunoreactive long neurites in a subserosal nerve fascicle of the small intestine. H: pSyn#64-immunoreactive small deposits in Auerbach’s myenteric nerve plexus of the small intestine. I: A short and small polyclonal phosphorylated α-synuclein (PSer129)-immunoreactive neurite (arrowhead) in the same plexus as shown in H. J: Arrowheads indicate round hyaline bodies (Lewy bodies) in Auerbach’s myenteric nerve plexus of the surgically removed stomach. K: A small pSyn#64 immunoreactive deposit in Auerbach’s myenteric nerve plexus of the stomach (arrowhead). L: A PSer129 immunoreactive round region in the same plexus as shown in K (arrowhead). A: Scale bar = 500 μm; B-L: Scale bar = 50 μm. A, B, J: Hematoxylin and eosin staining. D: SMI31; E: Tyrosine hydroxylase; C, F, G, H, K: pSyn#64; I, L: PSer129.
Frequencies of LP-positive blocks
We obtained two slides from each archival surgical block for H&E staining and immunohistochemistry. In addition, some sections were inappropriate for identifying nerve fibers because of the subjects’ disease conditions. In fact, when the sections were heavily infiltrated or where normal cells were replaced by tumor cells, inflammatory cells or necrotic lesions, it was difficult to detect nerve fibers in the GI mucosa and submucosa. Therefore, we expected that analyzing all blocks prepared from each subject would increase the possibility of identifying LP. We counted the number of blocks in which LP was found in the nerve fibers and calculated the proportion of LP-positive blocks.
Statistical analysis
Statistical analysis was performed with Fisher’s exact test for comparison of categorical data. A P-value lower than 0.05 was considered statistically as significant.
Results
Clinical information
Clinical information on each individual with LBD is summarized in Table 1. Besides parkinsonism, the individuals showed dysfunctions of the autonomic nervous system. Cognitive impairment was evident in all patients.
Six out of eight LBD patients were clinically diagnosed with DLB and the other two were diagnosed with PDD on the basis of the Consensus Guidelines [1]. There were no neurological signs or symptoms at the time of surgery in patients 1 and 2. Patients 3 to 7 were known to have DLB or PDD at the time of surgery. Patient 8 was diagnosed with LBD 3 years after undergoing abdominal surgery.
Histology of surgical specimens
Six out of eight LBD patients (75%; patients 1 to 6) had α-synuclein-immunoreactive LP in their surgical specimens (Table 1). LBs were identified in patients 1, 3 and 6 with H&E staining (Figure 1A, 1B, 1J). There was no LP in patients 7 and 8.
We observed α-synuclein-immunoreactive LNs and small oval-shaped dots along the axons of the nerve fibers in patients 1 to 6 (Figure 1C, 1F-I, 1K and 1L). In patient 1, a Lewy body-like hyaline body was observed in a ganglion cell of Meissner’s submucosal plexus (Figure 1A and 1B). The body was immunoreactive for α-synuclein (Figure 1C) and SMI-31 (Figure 1D). In addition, the nerve fibers observed in patient 1 showed dot-like positivity for tyrosine hydroxylase (Figure 1E). LP was observed in Meissner’s submucosal plexus (Figure 1C), in Auerbach’s myenteric plexus (Figure 1F) and in the subserosal nerve fascicles (Figure 1G) in each of the six patients. Patient 1 had LP in Meissner’s and Auerbach’s plexuses and in the subserosal nerve fibers of stomach tissue that had been resected because of his stomach cancer 7 years before the onset of DLB, who also suffered from severe autonomic dysfunction. Patient 2 had LP in the gallbladder, which had been resected because of chronic cholecystitis 2 years before the onset of PDD. In patients 3 to 7, the surgery and the diagnosis of LBD had occurred simultaneously. Although the surgical specimens were obtained from patient 8 three years after the diagnosis of DLB, no LP was found in the duodenum or gallbladder.
No LP was present in the surgical specimens of GI or biliary systems from 10 autopsy subjects who had no LP in the central and peripheral nervous systems (Table 2).
Frequencies of LP-positive blocks
The numbers and proportions of blocks in which α-synuclein-immunoreactive deposits were present in each patient are shown in Table 3. In three patients (1, 3 and 6), LP was identified in all available blocks. The percentage of LP-positive blocks was 50% in patient 2, 62% in patient 4 and 63% in patient 5. The distributions of LP in each layer of the GI and biliary tracts are summarized in Table 3. We focus here on the six LBD patients in whose surgical specimens we found LP. From these six patients, a total of 60 blocks were available. LP was identified in 48 blocks (80%). In patient 4, there was no Meissner’s plexus in the terminal ileum to sigmoid colon because of severe ischemic colitis, and in patient 2, this plexus was absent in the gallbladder, so across all six patients, the available number of blocks that included Meissner’s plexus was reduced to 37. Auerbach’s plexus and subserosal nerve fascicles were identified in 58 and 60 blocks, respectively. LP was seen in 24/37 (65%) blocks with Meissner’s plexus, 42/58 (72%) blocks with Auerbach’s plexus, and 34/60 (57%) blocks with subserosal nerve fascicles.
There were no statistical differences in the percentage occurrences of LP among the three nerve regions.
Discussion
Our study yielded three important results:
1. LP was identified by using α-synuclein immunohistochemistry in GI and biliary surgical specimens obtained before or at the same time as the clinical onset of LBD.
2. LP was frequently observed in Auerbach’s plexus, Meissner’s plexus and the subserosal nerve fascicles within the GI and biliary surgical specimens.
3. LP could be observed even if the specimens had been obtained 7 years before the onset of LBD.
Many researchers have reported that LP is detectable at various anatomic sites in LBD patients [7,8,22-30]. In addition, ideal biopsy sites have been intensely investigated in order to reach a diagnosis of LBD [12-16,27,31-33]. Minguez-Castellanos et al., studying surgical specimens, found α-synuclein aggregates in 26% of vesicoprostatic organs and 4% of digestive tracts [17]. A recent autopsy study revealed the presence of LP in multiple organs in individuals with LBD [22]. The same authors suggested that there was a rostrocaudal gradient of LP in the GI tract, i.e., the lower esophagus had the greatest LP involvement (33%) and the colon and rectum the lowest (6%). Moreover, LP is less likely to be detected in the GI tract than in organs such as the submandibular glands [22,25] and heart [29]. Kupsky et al. found LBs in the surgically resected megacolon of a patient with PD [34]. Sunwoo et al. reported that patients with postoperative delirium after total gastrectomy had a higher frequency of phosphorylated α-synuclein pathology in their gastric surgical specimens than those without [35]. However, information about LP in surgical specimens of GI and biliary tracts obtained for reasons not related to parkinsonism is not enough. Our results suggest that whatever the surgical specimen it must be analyzed by using α-synuclein immunohistochemistry in patients with suspected parkinsonism.
The tissue condition of collected GI and biliary specimens may affect the detection of LP. We found LP in Meissner’s plexus, Auerbach’s plexus and the subserosal nerve fascicles (Table 3). Because the mucosa and submucosa are vulnerable to the effects of tumor invasion, ischemia and inflammation, we recommend analyzing the subserosal nerve fascicles for LP observation besides Meissner’s and Auerbach’s plexuses. One previous autopsy analysis revealed LP more frequently in Auerbach’s plexus than in the other nerve plexuses of the GI tract [36]. Another investigator found LP less frequently in the nerve fibers of the serosa than in those of Meissner’s and Auerbach’s plexuses [37]. However, we found no significant differences in the frequency of LP among Meissner’s plexus, Auerbach’s plexus and the subserosal nerve fascicles of the GI tract. In general, there is more abundant mesenteric adipose tissue in the lower GI tract than in the upper GI tract, and it is easy to find nerve fascicles in subserosal adipose tissue.
We found no LP in the mucosal layer of any patients. Pouclet and Lebouvier et al. found that three out of nine PD patients had LNs in the colonic mucosa with their microdissection technique [12]. This discrepancy may be associated with differences in the methodology used to observe the nerve fibers. In fact, their methodology is difficult to apply to surgical specimens obtained for other medical reasons. The Gastro 2009 International Working Group for GI neuromuscular pathology reported that tangential sections are rarely employed in diagnostic histopathologic practice and have no well-established benefit except when examination of larger areas of a plexus is needed [38]. Because surgical pathologists usually cut surgical specimens of the GI tract vertically against the mucosal surface, they can evaluate only small numbers of mucosal nerve fibers. However, this method makes it easy to observe Auerbach’s plexus because of the abundance of its autonomic nerve fibers and ganglion cells in the muscularis propria. Because most GI and biliary surgical specimens contain muscularis propria, we were more easily able to observe any LP present in GI and biliary surgical specimens than could other authors in biopsy specimens.
It is important to know when LP in surgical specimens has developed in relation to the time of clinical onset of LBD. Shannon et al. reported that biopsied colonic materials obtained from three patients 2 to 5 years before the first motor PD signs revealed α-synuclein-immunoreactive deposits in nerve fibers [16]. Recently, Hilton et al. reported that a gastric biopsy taken 8 years before diagnosis showed occasional linear deposits of phosphorylated α-synuclein [31]. In six of our patients (75%) with LP, the surgical specimens were obtained 7, 2 or 0 years before the onset of LBD. Thus, it is possible to detect LP in surgical specimens obtained several years before the onset of LBD.
Many investigators have reported a correlation between severity of autonomic dysfunctions and LP in the GI tract [9,10,17,24,30,39]. Our results confirmed these previous studies. In fact, our six patients whose surgical specimens had LP showed autonomic dysfunction. Constipation was a particularly common clinical presentation in these patients.
Our study had some limitations. We had no chance to analyze the appropriate surgical specimens from individuals who were neuropathologically diagnosed as LBD. In addition, we could not obtain autopsies of two deceased individuals of the present study. Further studies are needed to verify and broaden our results.
On the basis of our results, we would like to emphasize the following approaches to support the diagnosis of LBD using surgical specimens:
1) Obtain a surgical history.
2) If surgical specimens are available, stain them by using α-synuclein immunohistochemistry.
3) All available blocks must be considered for immunohistochemical analysis.
4) In particular, Auerbach’s and Meissner’s plexuses and the subserosal nerve fascicles must be intensely investigated to detect small α-synuclein-immunoreactive deposits.
In conclusion, we demonstrated the clinical usefulness of surgical specimens for finding LP by using α-synuclein immunohistochemistry. Detection of LP in GI and biliary surgical specimens may help us to support a clinical diagnosis of LBD. Our methodology does not require any invasive procedures for patients. Further analyses may enable early medical intervention in individuals with LBD.
Acknowledgements
This work was partly supported by Grants-in-Aid from the Research Committee of CNS Degenerative Diseases (H23-nanchi-ippan-015), Abnormal protein propagation (H25-Shinkei- Kin, Ippan-002), the Ministry of Health, Labor and Welfare of Japan, Kiban B (24300133), the Comprehensive Brain Science Network (221S003), the Ministry of Education, Culture, Sports, Science and Technology of Japan, and National Center for Geriatrics and Gerontology Fund (23-42), Obu, Japan. The authors thank Dr. Kinuko Suzuki (Department of Neuropathology, Tokyo Metropolitan Institute of Gerontology) for insightful comments and useful discussions, and Dr. Takeshi Iwatsubo (Department of Neuropathology, the University of Tokyo, Tokyo, Japan) for the kind gifts of antibodies. We also thank Mr. Naoo Aikyo, Ms. Mieko Harada, Ms. Yuuki Kimura, and Ms. Nobuko Naoi for technical help.
Disclosure of conflict of interest
We declare no conflict of interest.
References
- 1.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 2.Dickson DW, Fujishiro H, DelleDonne A, Menke J, Ahmed Z, Klos KJ, Josephs KA, Frigerio R, Burnett M, Parisi JE, Ahlskog JE. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol. 2008;115:437–444. doi: 10.1007/s00401-008-0345-7. [DOI] [PubMed] [Google Scholar]
- 3.Orimo S, Ozawa E, Nakade S, Sugimoto T, Mizusawa H. (123)I-metaiodobenzylguanidine myocardial scintigraphy in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;67:189–194. doi: 10.1136/jnnp.67.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosaka K, Yoshimura M, Ikeda K, Budka H. Diffuse type of Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree--a new disease? Clin Neuropathol. 1984;3:185–192. [PubMed] [Google Scholar]
- 5.Okazaki H, Lipkin LE, Aronson SM. Diffuse intracytoplasmic ganglionic inclusions (Lewy type) associated with progressive dementia and quadriparesis in flexion. J Neuropathol Exp Neurol. 1961;20:237–244. doi: 10.1097/00005072-196104000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Amino T, Orimo S, Itoh Y, Takahashi A, Uchihara T, Mizusawa H. Profound cardiac sympathetic denervation occurs in Parkinson disease. Brain Pathol. 2005;15:29–34. doi: 10.1111/j.1750-3639.2005.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloch A, Probst A, Bissig H, Adams H, Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol. 2006;32:284–295. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 8.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goetz CG, Lutge W, Tanner CM. Autonomic dysfunction in Parkinson’s disease. Neurology. 1986;36:73–75. doi: 10.1212/wnl.36.1.73. [DOI] [PubMed] [Google Scholar]
- 10.Horimoto Y, Matsumoto M, Akatsu H, Ikari H, Kojima K, Yamamoto T, Otsuka Y, Ojika K, Ueda R, Kosaka K. Autonomic dysfunctions in dementia with Lewy bodies. J Neurol. 2003;250:530–533. doi: 10.1007/s00415-003-1029-9. [DOI] [PubMed] [Google Scholar]
- 11.Wakabayashi K, Takahashi H, Ohama E, Ikuta F. Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol (Berl) 1990;79:581–583. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- 12.Pouclet H, Lebouvier T, Coron E, Des Varannes SB, Neunlist M, Derkinderen P. A comparison between colonic submucosa and mucosa to detect Lewy pathology in Parkinson’s disease. Neurogastroenterol Motil. 2012;24:e202–5. doi: 10.1111/j.1365-2982.2012.01887.x. [DOI] [PubMed] [Google Scholar]
- 13.Dabby R, Djaldetti R, Shahmurov M, Treves TA, Gabai B, Melamed E, Sadeh M, Avinoach I. Skin biopsy for assessment of autonomic denervation in Parkinson’s disease. J Neural Transm. 2006;113:1169–1176. doi: 10.1007/s00702-005-0431-0. [DOI] [PubMed] [Google Scholar]
- 14.Lebouvier T, Neunlist M, Bruley des Varannes S, Coron E, Drouard A, N’Guyen JM, Chaumette T, Tasselli M, Paillusson S, Flamand M, Galmiche JP, Damier P, Derkinderen P. Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS One. 2010;5:e12728. doi: 10.1371/journal.pone.0012728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pouclet H, Lebouvier T, Coron E, des Varannes SB, Rouaud T, Roy M, Neunlist M, Derkinderen P. A comparison between rectal and colonic biopsies to detect Lewy pathology in Parkinson’s disease. Neurobiol Dis. 2012;45:305–309. doi: 10.1016/j.nbd.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord. 2012;27:716–719. doi: 10.1002/mds.25020. [DOI] [PubMed] [Google Scholar]
- 17.Minguez-Castellanos A, Chamorro CE, Escamilla-Sevilla F, Ortega-Moreno A, Rebollo AC, Gomez-Rio M, Concha A, Munoz DG. Do alpha-synuclein aggregates in autonomic plexuses predate Lewy body disorders?: a cohort study. Neurology. 2007;68:2012–2018. doi: 10.1212/01.wnl.0000264429.59379.d9. [DOI] [PubMed] [Google Scholar]
- 18.Funabe S, Takao M, Saito Y, Hatsuta H, Sugiyama M, Ito S, Kanemaru K, Sawabe M, Arai T, Mochizuki H, Hattori N, Murayama S. Neuropathologic analysis of Lewy-related alpha-synucleinopathy in olfactory mucosa. Neuropathology. 2013;33:47–58. doi: 10.1111/j.1440-1789.2012.01329.x. [DOI] [PubMed] [Google Scholar]
- 19.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 20.Saito Y, Ruberu NN, Sawabe M, Arai T, Kazama H, Hosoi T, Yamanouchi H, Murayama S. Lewy body-related alpha-synucleinopathy in aging. J Neuropathol Exp Neurol. 2004;63:742–749. doi: 10.1093/jnen/63.7.742. [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. Alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 22.Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray MT, Munoz DG, Gray DA, Schlossmacher MG, Woulfe JM. alpha-synuclein in the appendiceal mucosa of neurologically intact subjects. Mov Disord. 2013 doi: 10.1002/mds.25779. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Cersosimo MG, Benarroch EE. Pathological correlates of gastrointestinal dysfunction in Parkinson’s disease. Neurobiol Dis. 2012;46:559–564. doi: 10.1016/j.nbd.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Del Tredici K, Hawkes CH, Ghebremedhin E, Braak H. Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson’s disease. Acta Neuropathol. 2010;119:703–713. doi: 10.1007/s00401-010-0665-2. [DOI] [PubMed] [Google Scholar]
- 26.Fumimura Y, Ikemura M, Saito Y, Sengoku R, Kanemaru K, Sawabe M, Arai T, Ito G, Iwatsubo T, Fukayama M, Mizusawa H, Murayama S. Analysis of the adrenal gland is useful for evaluating pathology of the peripheral autonomic nervous system in lewy body disease. J Neuropathol Exp Neurol. 2007;66:354–362. doi: 10.1097/nen.0b013e3180517454. [DOI] [PubMed] [Google Scholar]
- 27.Ikemura M, Saito Y, Sengoku R, Sakiyama Y, Hatsuta H, Kanemaru K, Sawabe M, Arai T, Ito G, Iwatsubo T, Fukayama M, Murayama S. Lewy body pathology involves cutaneous nerves. J Neuropathol Exp Neurol. 2008;67:945–953. doi: 10.1097/NEN.0b013e318186de48. [DOI] [PubMed] [Google Scholar]
- 28.Iwanaga K, Wakabayashi K, Yoshimoto M, Tomita I, Satoh H, Takashima H, Satoh A, Seto M, Tsujihata M, Takahashi H. Lewy body-type degeneration in cardiac plexus in Parkinson’s and incidental Lewy body diseases. Neurology. 1999;52:1269–1271. doi: 10.1212/wnl.52.6.1269. [DOI] [PubMed] [Google Scholar]
- 29.Orimo S, Amino T, Itoh Y, Takahashi A, Kojo T, Uchihara T, Tsuchiya K, Mori F, Wakabayashi K, Takahashi H. Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol. 2005;109:583–588. doi: 10.1007/s00401-005-0995-7. [DOI] [PubMed] [Google Scholar]
- 30.Singaram C, Ashraf W, Gaumnitz EA, Torbey C, Sengupta A, Pfeiffer R, Quigley EM. Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. Lancet. 1995;346:861–864. doi: 10.1016/s0140-6736(95)92707-7. [DOI] [PubMed] [Google Scholar]
- 31.Hilton D, Stephens M, Kirk L, Edwards P, Potter R, Zajicek J, Broughton E, Hagan H, Carroll C. Accumulation of alpha-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 2014;127:235–41. doi: 10.1007/s00401-013-1214-6. [DOI] [PubMed] [Google Scholar]
- 32.Lebouvier T, Coron E, Chaumette T, Paillusson S, Bruley des Varannes S, Neunlist M, Derkinderen P. Routine colonic biopsies as a new tool to study the enteric nervous system in living patients. Neurogastroenterol Motil. 2010;22:e11–14. doi: 10.1111/j.1365-2982.2009.01368.x. [DOI] [PubMed] [Google Scholar]
- 33.Shannon KM, Keshavarzian A, Mutlu E, Dodiya HB, Daian D, Jaglin JA, Kordower JH. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov Disord. 2012;27:709–715. doi: 10.1002/mds.23838. [DOI] [PubMed] [Google Scholar]
- 34.Kupsky WJ, Grimes MM, Sweeting J, Bertsch R, Cote LJ. Parkinson’s disease and megacolon: concentric hyaline inclusions (Lewy bodies) in enteric ganglion cells. Neurology. 1987;37:1253–1255. doi: 10.1212/wnl.37.7.1253. [DOI] [PubMed] [Google Scholar]
- 35.Sunwoo MK, Hong JY, Choi J, Park HJ, Kim SH, Lee PH. Alpha-Synuclein pathology is related to postoperative delirium in patients undergoing gastrectomy. Neurology. 2013;80:810–813. doi: 10.1212/WNL.0b013e3182840782. [DOI] [PubMed] [Google Scholar]
- 36.Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol (Berl) 1988;76:217–221. doi: 10.1007/BF00687767. [DOI] [PubMed] [Google Scholar]
- 37.Annerino DM, Arshad S, Taylor GM, Adler CH, Beach TG, Greene JG. Parkinson’s disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol. 2012;124:665–80. doi: 10.1007/s00401-012-1040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knowles CH, De Giorgio R, Kapur RP, Bruder E, Farrugia G, Geboes K, Gershon MD, Hutson J, Lindberg G, Martin JE, Meier-Ruge WA, Milla PJ, Smith VV, Vandervinden JM, Veress B, Wedel T. Gastrointestinal neuromuscular pathology: guidelines for histological techniques and reporting on behalf of the Gastro 2009 International Working Group. Acta Neuropathol. 2009;118:271–301. doi: 10.1007/s00401-009-0527-y. [DOI] [PubMed] [Google Scholar]
- 39.Cersosimo MG, Raina GB, Pecci C, Pellene A, Calandra CR, Gutierrez C, Micheli FE, Benarroch EE. Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. J Neurol. 2013;260:1332–1338. doi: 10.1007/s00415-012-6801-2. [DOI] [PubMed] [Google Scholar]


