Abstract
Although the risk of malignant lymphoma in patients with atopic dermatitis (AD) remains controversial, an increased risk of malignant T-cell lymphoma in patients with AD has been reported. Primary cutaneous anaplastic large cell lymphoma (C-ALCL) is a relatively common distinct clinicopathological entity. However, occurrence of C-ALCL in patients with AD has been rarely reported. Herein, we describe the 5th reported case of C-ALCL occurring in a patient with AD and review the clinicopathological features. A 30-year-old Japanese male with a long-standing history of AD presented with a gradually enlarged nodular lesion in the right abdominal wall, which had spontaneously regressed without therapy. Two years later, multiple nodular lesions appeared in his trunk, and swelling of multiple lymph nodes was also detected. Histopathological studies demonstrated diffuse proliferation of large-sized lymphocytes with large convoluted nuclei containing conspicuous nucleoli and relatively rich cytoplasm in the skin and lymph node. Immunohistochemically, these lymphocytes were positive for CD30, CD8, and MUM1, and negative for CD3, CD4, and ALK1. Accordingly, a diagnosis of primary C-ALCL was made. The patient died of disease after various courses of chemotherapy. Our clinicopathological review revealed that the prognosis of C-ALCL occurring in patients with AD is poor because two of 5 patients died of disease. Therefore, albeit extremely rare, AD patients with C-ALCL should be monitored closely, and additional clinicopathological studies are needed to clarify the pathogenesis of C-ALCL occurring in patients with AD.
Keywords: Primary cutaneous anaplastic large cell lymphoma, atopic dermatitis, lymph node
Introduction
The risk of malignant lymphoma in patients with atopic dermatitis (AD) remains controversial. Recent study showed a two-fold increase of malignant lymphoma in patients with AD as compared to those without [1]. In contrast, one pooled analysis of data from 13 case-control studies demonstrated that overall risk of non-Hodgkin lymphoma was not associated with a history of AD (odds ratio 1.04), especially malignant B-cell lymphoma (odds ratio 0.96), however, AD was associated with a 2-fold increase in the risk of T-cell lymphoma due to an excess rate of occurrence of mycosis fungoides/Sezary syndrome [2].
Primary cutaneous CD30-positive lymphoproliferative disorders are a distinct clinicopathological disease entity and the second most common group of cutaneous T-cell lymphoma, accounting for approximately 30% of the cases [3]. This group includes primary cutaneous anaplastic large cell lymphoma (C-ALCL), lymphomatoid papulosis, and borderline cases according to the recent World Health Organization Classification [3]. C-ALCL is composed of large neoplastic cells with an anaplastic, pleomorphic morphology that express CD30 in the majority (>75%) of tumor cells [3]. This type of lymphoma mainly affects elderly males [3]. Only a few cases of primary C-ALCL occurring in patients with AD have been reported in the English language literature [4,5]. Herein, we report an additional case of C-ALCL in a patient with long-standing AD and review the clinicopathological features of this association.
Case report
A 30-year-old Japanese male with a long-standing history of AD presented with a gradually enlarged nodular lesion in the right abdominal wall. Physical examination revealed a well-circumscribed reddish nodule with erosion on the top of the nodule. Biopsy was performed, and subsequently, the nodule spontaneously regressed without any therapy.
Two years later, multiple nodular lesions appeared in his trunk, and computed tomography revealed swelling of multiple paraaortic and right inguinal lymph nodes. Laboratory tests demonstrated anemia and elevated soluble interleukin-2 receptor (red blood cells 3.16 × 1012/L (range 4.1-5.2), hemoglobin 8.7 g/L (range 13.4-17.0), white blood cells 10.6 × 109/L (3.0-8.0), platelets 429 × 109/L (150-400), and soluble interleukin-2 receptor 9,500 U/mL (135-483)). Biopsies of the skin from the right axilla and the inguinal lymph node were performed. After a diagnosis of malignant lymphoma, two courses of CHOP (cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisolone) therapy were performed. However, skin nodules and swelling of lymph nodes progressed, therefore, three courses of DeVIC (dexamethasone, carboplatin, etoposide, and ifosfamide) therapy were administered, which resulted in a mild decrease in size of the lymph nodes. Subsequently, three courses of GDP regimen (gemcitabine, dexamethasone, and cisplatin) were performed, however, lymph node swelling did not improve, and bone marrow suppression occurred. High-dose methotrexate/Ara-C therapy was performed, which led to a mild decrease in the size of lymph nodes. However, soluble interleukin-2 receptor was markedly increased (60,100 U/mL), thus, three courses of mLSG (VCAP/AMP/VECP) regimen were performed. In spite of this, swelling of the lymph nodes still did not improve, and he died of multiple organ failure and disseminated intravascular coagulation syndrome 13 months after the first course of chemotherapy.
Histopathological study of the first biopsy specimen from the abdominal wall revealed diffuse proliferation of large-sized lymphoid cells in the entire dermis, and no epidermotropic infiltration was noted (Figure 1A). These large-sized lymphocytes had large convoluted nuclei with conspicuous nucleoli and relatively rich slightly eosinophilic cytoplasm (Figure 1B). Infiltration of a few small-sized lymphocytes was noted, however, eosinophils were hardly seen.
Figure 1.
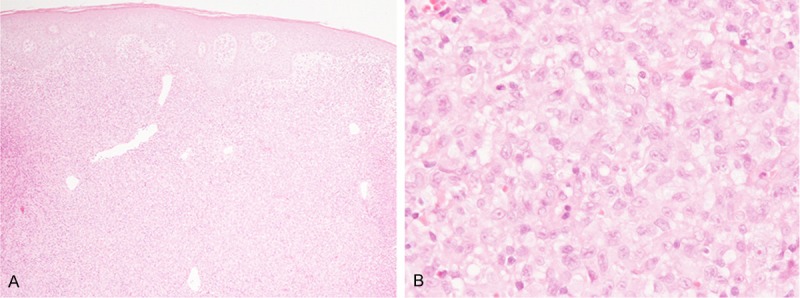
Histopathological features of the first biopsy specimen from the abdominal wall. A: Diffuse proliferation of atypical lymphocytes in the dermis without epidermotropic infiltration, HE, × 40. B: Proliferation of large-sized lymphocytes with large convoluted nuclei with conspicuous nucleoli and relatively rich eosinophilic cytoplasm, HE, × 400.
Immunohistochemical and in situ hybridization studies were performed using an autostainer (Ventana) by the same method as previously reported [6-10]. These large-sized lymphocytes were positive for CD30 and CD8, however, CD3, CD4, CD15, and ALK1 were negative. Moreover, these atypical cells were also positive for MUM-1, and granzyme B and TIA-1 were expressed in some of them. EBER was not expressed as assessed by in situ hybridization.
Accordingly, a diagnosis of CD30-positive lymphoproliferative disorder was made.
Histopathological examination of the second biopsy specimen from the axilla demonstrated diffuse proliferation of large-sized lymphocytes in the entire dermis and subcutis accompanied by epidermal erosion (Figure 2A). These lymphocytes had large convoluted nuclei with conspicuous nucleoli and relatively rich slightly eosinophilic cytoplasm (Figure 2B). Infiltration of small-sized lymphocytes and eosinophils was also observed among the large-sized lymphocytes (Figure 2B). Immunohistochemically, these large-sized lymphocytes were positive for CD30, CD8, and MUM-1, however, CD3, CD4,CD15, and ALK1 were negative. Moreover, granzyme B and TIA-1 were expressed in most of these lymphocytes (Figure 3). EBER was not expressed as assessed by in situ hybridization.
Figure 2.
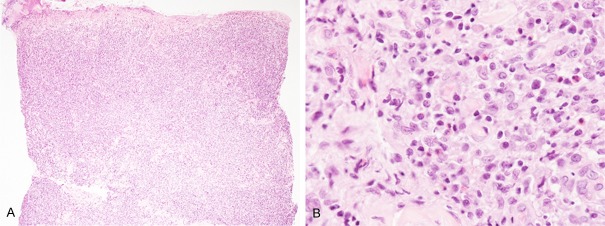
Histopathological features of the second biopsy specimen from the axilla. A: Diffuse proliferation of atypical lymphocytes in the dermis accompanying epidermal erosion, HE, × 40. B: Proliferation of large-sized lymphocytes with large convoluted nuclei with conspicuous nucleoli and relatively rich eosinophilic cytoplasm. Small-sized lymphocytes and eosinophils were scattered among the large-sized lymphocytes, HE, × 400.
Figure 3.
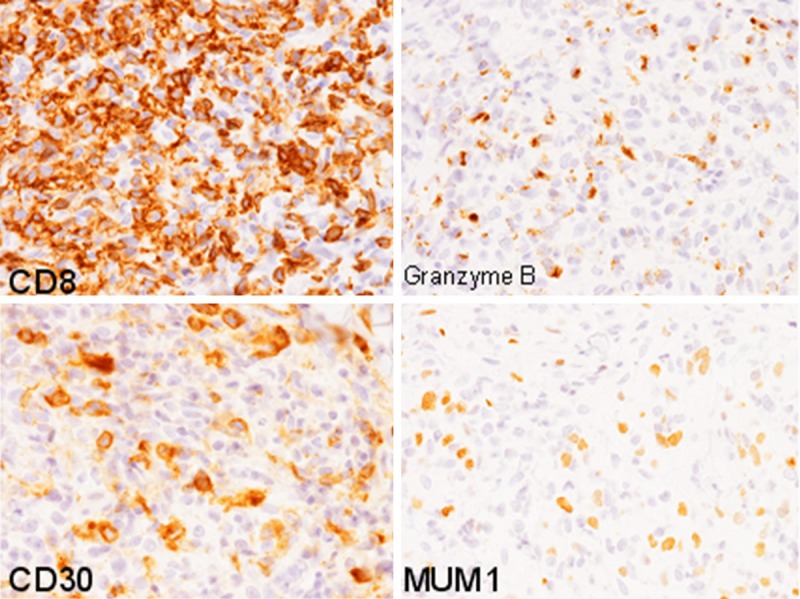
Immunohistochemical findings of the second biopsy specimen. CD8, CD30, and MUM-1 are expressed in the large-sized lymphocytes. Granzyme B is also expressed in most of these cells, × 400.
The biopsy specimen of the inguinal lymph node revealed diffuse proliferation of large-sized atypical lymphocytes and lack of preexisting lymph node architecture (Figure 4A). These atypical lymphocytes had large convoluted nuclei with conspicuous nucleoli and rich eosinophilic cytoplasm (Figure 4B). Eosinophils were scattered among these large-sized lymphocytes (Figure 4B). These large-sized lymphocytes were immunohistochemically identical to the ones found in the axilla biopsy specimen; they were diffusely positive for CD30, CD8, and MUM-1, negative for CD3, CD4, CD15, and ALK1, and granzyme B and TIA-1 were expressed in most of these lymphocytes (Figure 5). EBER was also negative as demonstrated by in situ hybridization.
Figure 4.
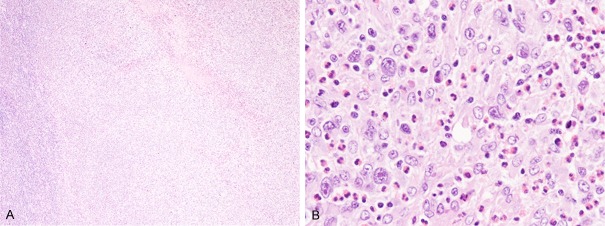
Histopathological features of the biopsy specimen from the inguinal lymph node. A: Diffuse proliferation of atypical lymphocytes without preexisting lymph node architecture, HE, × 40. B: Proliferation of large-sized lymphocytes with large convoluted nuclei with conspicuous nucleoli and relatively rich eosinophilic cytoplasm. Eosinophils are scattered among the large-sized neoplastic cells, HE, × 400.
Figure 5.
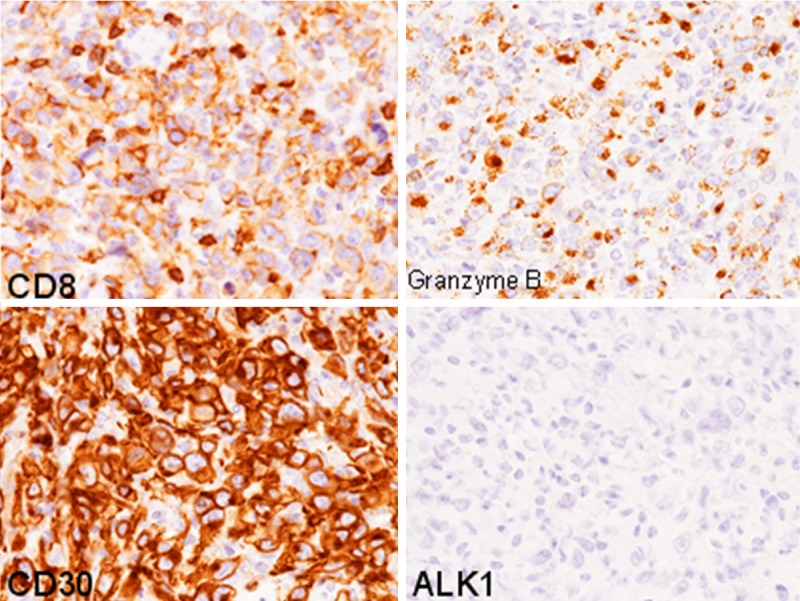
Immunohistochemical findings of the lymph node. CD8 and CD30 are expressed in the large-sized lymphocytes. Granzyme B is also expressed in most of these cells. ALK1 is negative, × 400.
Accordingly, an ultimate diagnosis of primary C-ALCL was made with consideration of the clinical information.
Discussion
In this report, we describe the fifth documented case of primary C-ALCL occurring in a patient with AD [4,5]. Table 1 summarizes the clinicopathological features of the 4 previously reported cases of C-ALCL occurring in patients with AD as well as the present one. This condition mainly affects young males (average age 32.6 years; range from 28 to 39 years; male/female 4/1) although primary C-ALCL typically affects older patients with a median age of approximately 60 years [3]. Moreover, all patients had a long-standing history of AD. The peculiar finding of these cases is a poor prognosis (Table 1). Two of five patients, including the present case, died of disease, and one case showed widespread lymphadenopathy after the initial treatment [5] although the prognosis of primary C-ALCL is usually favorable, with a 10-year disease-related survival of approximately 90% [3]. A recent study demonstrated that some clinical variants of C-ALCL, such as C-ALCL with leg involvement, show an aggressive clinical course [11,12]. Thus, AD patients with C-ALCL should also be monitored closely like those with C-ALCL with leg involvement. Moreover, a few cases of lymphomatoid papulosis occurring in patients with AD have also been documented, and these cases showed a good prognosis [13,14].
Table 1.
Clinicopathological features of primary cutaneous anaplastic large cell lymphoma occurring in patients with atopic dermatitis
| Case No. | Age/Gender | Clinical features | Outcome | Reference |
|---|---|---|---|---|
| 1 | 39/Male | Large ulcerated nodule in the back. | Free from disease. | [4] |
| 2 | 31/Male | Large grouped nodules in the elbow. | Died of disease. | [5] |
| 3 | 35/Female | Nodular lesions in the face and back. | Relapsed, however, free from disease after bone marrow transplant. | [5] |
| 4 | 28/Male | Multiple cutaneous tumors. | Free from disease. | [5] |
| Present Case | 30/Male | Multiple nodular lesions in the trunk. | Died of disease. |
The histopathological and immunohistochemical findings of the present case were fundamentally typical for C-ALCL. Histopathologically, C-ALCL shows diffuse or sheet growth of neoplastic large-sized lymphocytes [3]. These large-sized tumor cells have large nuclei with conspicuous nucleoli and rich eosinophilic cytoplasm [3]. Immunohistochemically, the majority of these neoplastic cells are CD30-positive and generally show a T-helper phenotype (CD3+, CD4+, CD8-) [3]. However, CD8+ cells with a cytotoxic phenotype are not uncommon (<5%) [3]. CD15 is usually negative. Moreover, the expression of MUM1, a member of the interferon regulatory factor family and normally expressed in plasma cells, late B-cells, and activated T-cells, is observed in C-ALCL and lymphomatoid papulosis [15]. Unlike systemic ALCL, C-ALCL does not carry translocations involving the ALK gene at chromosome 2, and ALK 1 is not expressed in C-ALCL [16]. Although the present case showed multiple lymph node swelling, this case was diagnosed as C-ALCL because the neoplastic cells lacked ALK1 expression and consideration of clinical history of spontaneous regression at the initial presentation.
Recently, it has been reported that CD30+ lymphocytes are up-regulated in AD lesion [17].CD30 is a TNF receptor superfamily member, and is consistently expressed in a subset of lymphocytes with a Th2 phenotype [18]. CD30 ligand (CD30L, also known as CD153) is a type II transmembrane glycoprotein and belongs to the TNF superfamily [19]. Mast cells express CD30L, and it has been reported that mast cell numbers are elevated in the lesional skin of AD [20]. Up-regulation of CD30+ T lymphocytes in the lesional skin of AD is thought to be induced by mast cells expressing CD30L [17]. Increased number and up-regulation of CD30+ T lymphocytes might be related to the occurrence of CD30-positive lymphoproliferative disorders in patients with AD, and further studies are needed to clarify the pathogenesis. Moreover, most of the previously reported cases of CD30-positive lymphoproliferative disorders in AD patients were treated with cyclosporine [4,5,13,14]. Therefore, occurrence of this type of lymphoproliferative disorder may be related to the use of this immunosuppression drug.
In summary, we describe the 5th reported case of primary C-ALCL occurring in a patient with AD. Our clinicopathological review revealed that the prognosis of C-ALCL occurring in patients with AD is poor because two of 5 patients died of disease. Therefore, albeit extremely rare, AD patients with C-ALCL should be monitored closely, and additional clinicopathological studies are needed to clarify the pathogenesis of C-ALCL occurring in patients with AD.
References
- 1.Arna A, Wentworth CE, Fernandez-Vidauree C, Schlienger RG, Conde E, Arellano FM. Incidence of cancer in the general population and in patients with or without atopic dermatitis in the U. K. Br J Dermatol. 2010;163:1036–1043. doi: 10.1111/j.1365-2133.2010.09887.x. [DOI] [PubMed] [Google Scholar]
- 2.Vajdic CM, Falster MO, de Sanjose S, Martinez-Maza O, Becker N, Bracci PM, Melbye M, Smedby KE, Engels EA, Turner J, Vineis P, Costantini AS, Holly EA, Kane E, Spinelli JJ, La Vecchia C, Zheng T, Chiu BC, Dal Maso L, Cocco P, Maynadie M, Foretova L, Staines A, Brennan P, Davis S, Severson R, Cerhan JR, Breen EC, Birmann B, Cozen W, Grulich AE. Atopic disease and risk of non-Hodgkin lymphoma: an InterLymph pooled analysis. Cancer Res. 2009;69:6482–6489. doi: 10.1158/0008-5472.CAN-08-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ralfkiaer E, Willemze R, Paulli M, Kadin ME. Primary cutaneous CD30-positive T-cell lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. pp. 300–301. [Google Scholar]
- 4.Kirby B, Owen CM, Blewitt RW, Yates VM. Cutaneous T-cell lymphoma developing in a patient on cyclosporine therapy. J Am Acad Dermatol. 2002;47:S165–S167. doi: 10.1067/mjd.2002.106357. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher CL, Orchard GE, Hubbard V, Whittaker SJ, Edelson RL, Russell-Jones R. CD30+ cutaneous lymphoma in association with atopic eczema. Arch Dermatol. 2004;140:449–454. doi: 10.1001/archderm.140.4.449. [DOI] [PubMed] [Google Scholar]
- 6.Ishida M, Iwai M, Yoshida K, Kagotani A, Okabe H. Sebaceous carcinoma associated with Bowen’s disease: a case report with emphasis on the pathogenesis of sebaceous carcinoma. Int J Clin Exp Pathol. 2013;6:3029–3032. [PMC free article] [PubMed] [Google Scholar]
- 7.Toriyama A, Ishida M, Amano T, Nakagawa T, Kaku S, Iwai M, Yoshida K, Kagotani A, Takahashi K, Murakami T, Okabe H. Leiomyomatosis peritonealis disseminata coexisting with endometriosis within the same lesions: a case report with review of the literature. Int J Clin Exp Pathol. 2013;6:2949–2954. [PMC free article] [PubMed] [Google Scholar]
- 8.Ishida M, Hodohara K, Yoshida K, Kagotani A, Iwai M, Yoshii M, Okuno K, Horinouchi A, Nakanishi R, Harada A, Yoshida T, Okabe H. Occurence of anaplastic large cell lymphoma following IgG4-related autoimmune pancreatitis and cholecystitis and diffuse large B-cell lymphoma. Int J Clin Exp Pathol. 2013;6:2560–2568. [PMC free article] [PubMed] [Google Scholar]
- 9.Ishida M, Yoshida K, Kagotani A, Iwai M, Yoshii M, Okuno K, Horinouchi A, Nakanishi R, Harada A, Yoshida T, Okuno T, Hodohara K, Okabe H. Anaplastic lymphoma kinase-positive large B-cell lymphoma: A case report with emphasis on the cytological features of the pleural effusion. Int J Clin Exp Pathol. 2013;6:2631–2635. [PMC free article] [PubMed] [Google Scholar]
- 10.Ishida M, Hodohara K, Yoshii M, Okuno H, Nakanishi R, Horinouchi A, Nakanishi R, Harada A, Iwai M, Yoshida K, Kagotani A, Yoshida T, Okabe H. Methotrexate-related Epstein-Barr virus-associated lymphoproliferative disorder occurring in the gingiva of a patient with rheumatoid arthritis. Int J Clin Exp Pathol. 2013;6:2237–2241. [PMC free article] [PubMed] [Google Scholar]
- 11.Benner MF, Willemze R. Applicability and prognostic value of the new TNM classification system in 135 patients with primary cutaneous anaplastic large cell lymphoma. Arch Dermatol. 2009;145:1399–1404. doi: 10.1001/archdermatol.2009.280. [DOI] [PubMed] [Google Scholar]
- 12.Ishida M, Fujii N, Okabe H. Primary cutaneous anaplastic large cell lymphoma with subsequent leg involvement. World J Dermatol. 2012;1:38–40. [Google Scholar]
- 13.Laube S, Stephens M, Smith AG, Whittaker SJ, Tan BB. Lymphomatoid papulosis in a patient with atopic eczema on long-term cyclosporine therapy. Br J Dermatol. 2005;152:1346–1348. doi: 10.1111/j.1365-2133.2005.06548.x. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura S, Takeda K, Hashimoto Y, Mizumoto T, Ishida-Yamamoto A, Iizuka H. Primary cutaneous CD30+ lymphoproliferative disorder in an atopic dermatitis patient on cyclosporine therapy. Indian J Dermatol Venereol Leprol. 2011;77:253. doi: 10.4103/0378-6323.77496. [DOI] [PubMed] [Google Scholar]
- 15.Wasco MJ, Fullen D, Su L, Ma L. The expression of MUM1 in cutaneous T-cell lymphoproliferative disorders. Hum Pathol. 2008;39:557–563. doi: 10.1016/j.humpath.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 16.DeCoteau JF, Butmarc JR, Kinney MC, Kadin ME. The t(2;5) chromosomal translocation is not a common feature of primary cutaneous CD30+ lymphoproliferative disorders: comparison with anaplastic large-cell lymphoma of nodal origin. Blood. 1996;87:3437–3441. [PubMed] [Google Scholar]
- 17.Fischer M, Harvima IT, Carvalho RF, Moller C, Naukkarinen A, Enblad G, Nilsson G. Mast cell CD30 ligand is upregulated in cutaneous inflammation and mediates degranulation-independent chemokine secretion. J Clin Invest. 2006;116:2748–2756. doi: 10.1172/JCI24274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Prete G, De Carli M, D’Elios MM, Daniel KC, Almerigogna F, Alderson M, Smith CA, Thomas E, Romagnani S. CD30-mediated signaling promotes the development of human T helper type 2-like T cells. J Exp Med. 1995;182:1655–1661. doi: 10.1084/jem.182.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith CA, Gruss HJ, Davis T, Anderson D, Farrah T, Baker E, Sutherland GR, Brannan CI, Coperland NG, Jenkins NA, Grabstein KH, Gliniak B, McAllster IB, Fanslow W, Alderson M, Falk B, Gimpel S, Gillis S, Din WS, Goodwin RG, Armitage RJ. CD30 antigen, a marker for Hodgkin’s lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell. 1993;73:1349–1360. doi: 10.1016/0092-8674(93)90361-s. [DOI] [PubMed] [Google Scholar]
- 20.Ackermann L, Harvima IT. Mast cells of psoriatic and atopic dermatitis skin are positive for TNF-alpha and their degranulation is associated with expression of ICAM-1 in the epidermis. Arch Dermatol Res. 1998;290:353–359. doi: 10.1007/s004030050317. [DOI] [PubMed] [Google Scholar]


