Abstract
It is well established that patients with immunosuppression have a higher risk of development of lymphoproliferative disorders (LPDs), and Epstein-Barr virus (EBV) is associated with development of LPDs. Aplastic anemia (AA) is an immune-mediated hematological disorder, and immunosuppression therapy (IST), such as antithymocyte globulin (ATG), is widely used for treatment of AA. However, occurrence of LPD without bone marrow transplantation has been extremely rarely documented in patients with IST for AA. Herein, we report the 6th documented case of EBV-associated LPD after IST for AA and review the clinicopathological features of this extremely rare complication. A 46-year-old Japanese female was admitted for evaluation of progressive pancytopenia. Bone marrow biopsy revealed fatty marrow with marked decrease of trilineage cells, and bone marrow aspiration demonstrated no dysplastic changes. IST with rabbit ATG was administered, after which, she developed high fever. Bone marrow aspiration showed increase of atypical plasma cells with mildly enlarged nuclei and irregular nuclear contour. These atypical plasma cells were EBER-positive. Accordingly, a diagnosis of EBV-positive plasmacytic LPD was made. Most cases of LPDs are B-cell origin, and plasmacytic LPD is a rare subtype. The current report is the second case of plasmacytic LPD in patients with IST for AA. Therefore, detailed histopathological and immunohistochemical analyses are needed for correct diagnosis and treatment, and additional studies are needed to clarify the clinicopathological features of EBV-LPD after IST for AA.
Keywords: Epstein-Barr virus-associated lymphoproliferative disorder, antithymocyte globulin therapy, aplastic anemia
Introduction
It is well established that patients with immunosuppression have a higher risk of development of lymphoproliferative disorders (LPDs), and immunodeficiency-associated LPDs are classified into four categories according to the recent World Health Organization Classification: lymphoproliferative diseases associated with primary immune disorders, lymphomas associated with HIV infection, post-transplant LPDs, and other iatrogenic immunodeficiency-associated LPDs [1]. Epstein-Barr virus (EBV) is known to be associated with development of LPDs, which is referred to as EBV-associated LPDs [1].
Aplastic anemia is an immune-mediated hematological disorder characterized by pancytopenia in the peripheral blood and hypocellularity of trilineage cells in the bone marrow [2]. Antithymocyte globulin (ATG) is widely used for treatment of AA, and the majority of patients show hematological improvement after T-cell depletion by ATG [2,3]. Cyclosporine A (CsA) is also an immunosuppressive drug used for AA [3]. Immunosuppressive therapy (IST) shows high efficacy for AA, however, it is associated with several complications, such as the occurrence of myelodysplastic syndrome and acute myelogenous leukemia [4,5]. Further, albeit extremely rare, occurrence of LPDs after IST for AA without bone marrow transplantation has been documented [6-10]. Herein, we describe the 6th documented case of EBV-associated LPD after IST for AA and review the clinicopathological features of this extremely rare complication.
Case report
A 46-year-old Japanese female was admitted to our hospital for evaluation of progressive pancytopenia. She had been pointed out thrombocytopenia 6 years earlier, and progressive pancytopenia had developed 1 year earlier. Laboratory tests showed pancytopenia (red blood cells 3.35 × 1012/L (range 3.8-4.8), white blood cells 1.9 × 109/L (3.0-8.0), and platelets 26 × 109/L (150-400)). Bone marrow biopsy revealed fatty marrow with marked decrease of trilineage cells (Figure 1A). Accordingly, a diagnosis of AA was made. Thus, steroid therapy was administered, however, pancytopenia did not improve. Laboratory investigations at the time of admission showed continuation of pancytopenia (hemoglobin 11.6 g/dL (range 11.3-15.0), red blood cells 3.57 × 1012/L, white blood cells 2.0 × 109/L (neutrophils 9.5% and lymphocytes 84.5%), platelets 15 × 109/L, and reticulocytes 15‰). Liver and renal function tests were within normal ranges. Bone marrow aspiration revealed hypocellular bone marrow with decrease of megakaryocytes, however, no dysplastic signs were detected in the trilineage hematopoietic cells (Figure 1B). Bone marrow biopsy showed normocellular bone marrow with erythroid hyperplasia, and no neoplastic signs were detected (Figure 1C). Chromosomal analysis of the bone marrow demonstrated an abnormal karyotype with trisomy 8.
Figure 1.
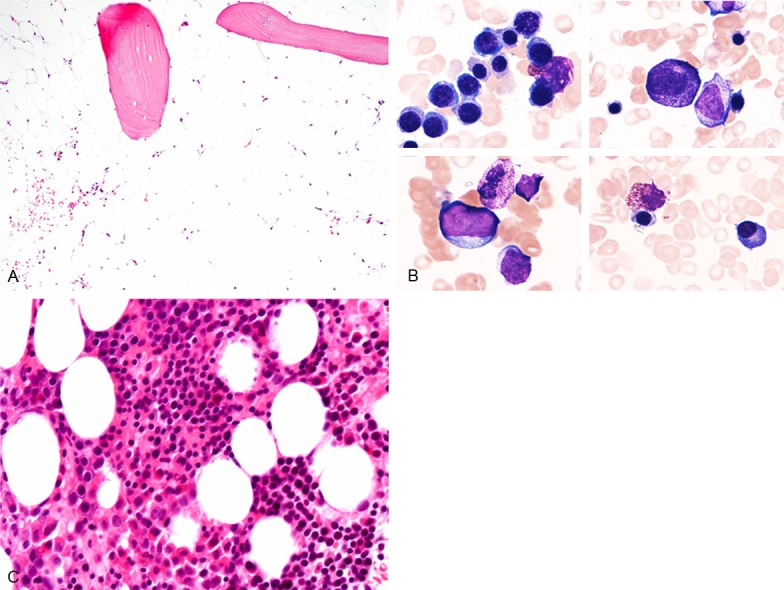
Histopathological and cytomorphological features of the bone marrow. A. First biopsy specimen reveals fatty marrow with marked decrease of trilineage cells, HE, × 100. B. First bone marrow aspiration specimen demonstrates hypocellular marrow without dysplastic change in the trilineage hematopoietic cells, Giemsa, × 1000. C. Second biopsy specimen shows normocellular marrow with increase of erythroid cells, and no neoplastic signs are detected, HE, × 200.
According to these findings, she was diagnosed with AA (very severe form according to the classification by Camitta et al. [11]), and was administered immunosuppressive therapy (IST) with rabbit ATG (3.75 mg/kg/day), CsA (drug blood level of 150-250 mg/kg), and prednisolone (1.2-2.5 mg/kg).
On the 26th day of IST, she developed high fever and exanthema. Serum levels of lactate dehydrogenase, aspartate aminotransferase, and alanine aminotransferase had gradually increased. Bone marrow aspiration was performed again because atypical lymphocytes were detected in the peripheral blood (5% of leukocytes) on the 33rd day of IST.
The third bone marrow aspirate specimen demonstrated hypocellular marrow with decrease of megakaryocytes. Atypical large-sized plasma cells with irregular cytoplasmic and nuclear contours were detected (11.8% of all bone marrow cells) (Figure 2A). Bone marrow clot section showed hypercellular marrow with increase of atypical plasma cells with mildly enlarged nuclei and irregular nuclear contour (Figure 2B).
Figure 2.
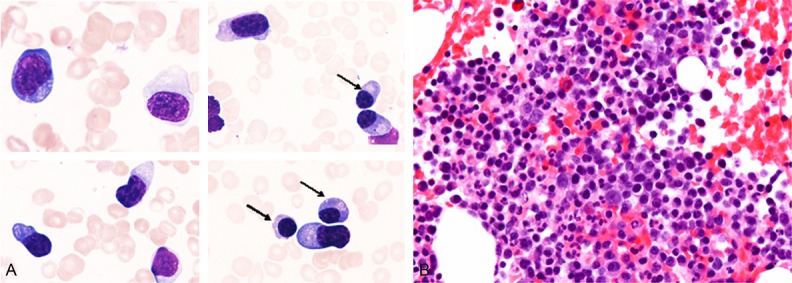
Cytomorphological and histopathological features of the bone marrow after immunosuppression therapy for aplastic anemia. A. The presence of atypical large-sized plasma cells with irregular cytoplasmic and nuclear contour (as compared to normal-appearing plasma cells (arrows)), Giemsa, × 1000. B. Hypercellular bone marrow with increase of atypical plasma cells with mildly enlarged nuclei and irregular nuclear contour, HE, × 400.
Immunohistochemical and in situ hybridization studies were performed using an autostainer (Ventana) by the same method as previously reported [12-16]. Many CD138-positive plasma cells were present in the bone marrow, however, only a few CD3- and CD20-positive lymphocytes were observed (Figure 3). In situ hybridization analysis revealed many cells positive for EBV encoded small RNAs (EBER) in the bone marrow (Figure 3), which corresponded to the distribution of CD138-positive cells.
Figure 3.
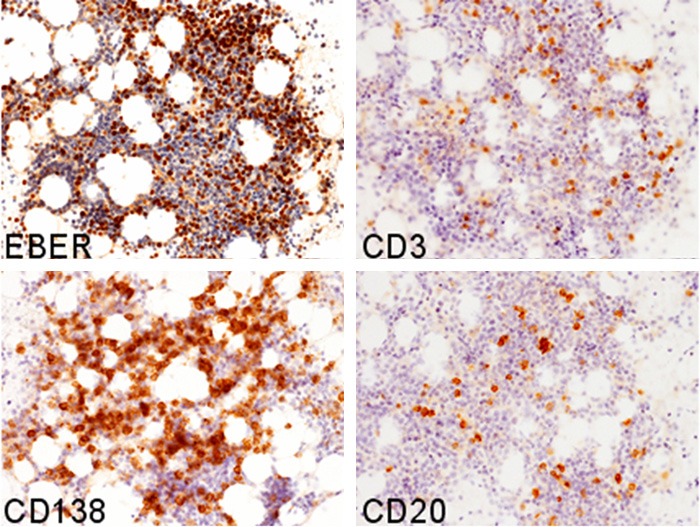
Immunohistochemical and in situ hybridization features of the bone marrow after immunosuppression therapy for aplastic anemia. Abundant EBER- and CD138-positive cells are observed, however, only a few CD3- and CD20-positive cells are present, × 200.
Polymerase chain reaction for EBV-DNA revealed 7.9 × 106 copies/mL (range <100) in the peripheral blood. Moreover, reactivation of varicella-zoster virus (VZV) was detected in the biopsy of the skin eruption and cytomegalovirus (CMV) reactivation was indicated by the biopsy of the gastric ulcer. Serum C7-HRP was positive (12/50,000 cells).
According to these results, an ultimate diagnosis of EBV-associated plasmacytic LPD with reactivation of VZV and CMV after rabbit ATG therapy for aplastic anemia was made.
After the diagnosis, IST was discontinued, and antivirus therapy for CMV was administrated. Subsequently, the EBV-DNA viral load decreased, and serum C7-HRP reduced to below the cutoff level. She underwent frequent blood transfusions without chemotherapy, and has been free from LPD recurrence for 30 months after the diagnosis.
Discussion
LPDs are defined as lymphoid or plasmacytic proliferative lesions that develop as a consequence of immunosuppression, such as under conditions following organ or bone marrow transplantation [1]. Iatrogenic immunodeficiency-associated LPDs occur in a setting of IST with drugs including methotrexate and infliximab [1]. EBV is associated with development of LPDs, and approximately 30-50% of LPDs in RA patients treated with methotrexate are EBV-positive [17,18]. Although the occurrence of myelodysplastic syndrome and acute myelogenous leukemia after IST for AA has been recognized [4,5], development of LPD is extremely rare without bone marrow transplantation. Scheinberg et al. reported that none of the 78 patients in their study with severe AA who had received different IST developed EBV-LPD [19]. Table 1 summarizes the clinicopathological features of the five previously reported cases of EBV-LPD after IST for AA as well as the present one [6-10]. This disorder predominantly affects middle-aged people (male/female 4/2), CsA is frequently used as an immunosuppressive drug, and the most common histopathology of the disorder is EBV-LPD (4 cases). The prognosis of EBV-LPD after IST for AA may be favorable because most cases, including the present one, had no recurrence of EBV-LPD after cessation of IST. Calistri et al. reported a case of infectious mononucleosis after ATG treatment for AA [7]. The patient complained of enlarged and painful cervical lymph nodes after the 20th day of ATG therapy. Although histopathological analysis of the lymph nodes was not performed, the presence of atypical lymphocytes and high EBV DNA in the peripheral blood led to a diagnosis of infectious mononucleosis [7]. The most commonly affected organ of this disorder is the lymph node [7,8,10]. Sugimoto-Sekiguchi et al. reported a very interesting case who developed general malaise and appetite loss after IST (ATG and CsA) for AA. Because of a positive occult blood test of the stool, he underwent colonoscopy, which revealed multiple tumorous lesions in the colon. Biopsy from a colon tumor demonstrated EBV-LPD [10]. Although it has been well recognized that post-transplant EBV-LPDs frequently occur in extranodal sites [16], their case was the first one of EBV-LPD after IST for AA without bone marrow transplantation occurring in the extranodal site [10]. The current case is the first report of EBV-LPD affecting the bone marrow after IST for AA. Moreover, a few cases of non-EBV-associated LPD after IST for AA have also been documented [20,21].
Table 1.
Clinicopathological features of Epstein-Barr virus-associated lymphoproliferative disorder after antithymocyte globulin therapy for aplastic anemia
| Case No. | Age/Gender | Source of ATG | Immunosuppression drugs | Histopathology | EBV infected cells | Reactivated virus | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 36/Male | Horse | Cyclosporine A | EBV-LPD | Not available | Not available | [6] |
| 2 | 38/Male | Horse | Cyclosporine A, predonisolone | Infectious mononucleosis | B-cell | None | [7] |
| 3 | 42/Female | Rabbit | Cyclosporine A | DLBCL | B-cell | Not available | [8] |
| 4 | 55/Male | Horse | None | EBV-LPD | Plasma cell | CMV | [9] |
| 5 | 55/Male | Rabbit | Cyclosporine A | EBV-LPD | B-cell | None | [10] |
| Present Case | 46/Female | Rabbit | Cyclosporine A, predonisolone | EBV-LPD | Plasma cell | CMV, VZV |
ATG, antithymocyte globulin; CMV, cytomegalovirus; DLBCL, diffuse large B-cell lymphoma; EBV, Epstein-Barr virus; LPD, lymphoproliferative disorder; VZV, varicella-zoster virus.
Rabbit ATG was used in three cases including the present one, and horse ATG was administered in the remaining cases. It has been estimated that rabbit ATG has a greater degree and duration of T-cell depletion, and shows more immunosuppressive efficacy than horse ATG [22]. Therefore, the risk of complication including the development of EBV-LPD may be increased in the cases who receive rabbit ATG than in those with horse ATG therapy.
Most cases of iatrogenic EBV-LPDs are of B-cell origin [1]. According to the analysis of 135 reported cases of MTX-related LPDs with detailed histopathological features, the most common subtype is diffuse large B-cell lymphoma (64/135 cases), followed by Hodgkin lymphoma and lymphoid proliferation of Hodgkin-like features (33/135 cases) [1]. Albeit rare, plasmacytic LPD has been reported in patients following organ transplantation [23,24]. This type of disorder is an unusual form of monomorphic post-transplant LPDs [1,23,24] that is histopathologically characterized by the proliferation of monomorphic plasma cells expressing CD138 and lacking CD20 expression, and resembles multiple myeloma [23]. Karuturi et al. analyzed the clinicopathological features of plasmacytic post-transplant LPD cases [23], and they found that among 7,428 cases who had received solid organ transplantation from 1988 to 2012, 210 patients (3%) developed LPDs, and 9 of them had plasmacytic LPD (4% of LPD cases) in their series [23]. All patients presented with extranodal lesion and often subcutaneous tumor as well, and seven of these 9 cases were EBV-positive [23]. Moreover, Trappe et al. reported 8 cases of plasmacytic LPD after solid organ transplantation [24], and while three of these 8 cases were EBV-positive, none of them had bone marrow involvement [24]. Plasmacytic LPD occurring in patients with iatrogenic immunodeficiency is extremely rare. A case of plasmacytic LPD after ATG therapy for AA has been reported [9], and the current report is the second documented case of plasmacytic LPD after IST for AA. The frequency of plasmacytic LPD in patients who receive ATG therapy for AA can be high (2/6 cases of EBV-LPD), therefore, additional clinicopathological study is needed.
In the present case, chromosomal analysis of the bone marrow specimen diagnosed as AA demonstrated trisomy 8. In the series of 600 adult AA cases reported by Kim et al., 95.3% of cases showed a normal karyotype, and the most frequent abnormality was trisomy 8, followed by monosomy 7/deletion of 7q and deletion of 1q [25]. Although trisomy 8 is also observed in cases of hypoplastic myelodysplastic syndrome [26], no dysplastic change was observed in the trilineage hematopoietic cells of the bone marrow of this case, therefore, a diagnosis of AA was made.
In the present case, concurrent reactivation of CMV and VZV was observed in a setting of development of EBV-LPD. Albeit extremely rare, concurrent reactivation of multiple viruses with development of EBV-LPD without transplantation has been reported [27], and CMV reactivation in a patient with EBV-LPD after IST for AA has also been documented (Table 1) [9]. Therefore, monitoring for virus reactivation other than EBV is also important in patients who receive IST, including ATG therapy.
In conclusion, we describe an extremely rare case of plasmacytic EBV-LPD after IST for AA. Albeit rare, plasmacytic LPD can occur in patients who receive IST without transplantation, and multiple virus reactivations can develop. Therefore, detailed histopathological and immunohistochemical analyses are needed for correct and rapid diagnosis and treatment, and additional studies are needed to clarify the clinicopathological features of EBV-LPD after IST for AA.
Disclosure of conflict of interest
None.
References
- 1.Gaulard P, Swerdlow SH, Harris NL, Jaffe ES, Sundström C. Other iatrogenic immunodeficiency-associated lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. pp. 350–351. [Google Scholar]
- 2.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teramura M, Kimura A, Iwase S, Yonemura Y, Nakao S, Urabe A, Omine M, Mizoguchi H. Treatment of severe aplastic anemia with antithymocyte globulin and cyclosporine A with or without G-CSF in adults: a multicenter randomized study in Japan. Blood. 2007;110:1756–1761. doi: 10.1182/blood-2006-11-050526. [DOI] [PubMed] [Google Scholar]
- 4.Socie G, Henry-Amar M, Bacigalupo A, Hows J, Tichelli A, Ljungman P, McCann SR, Frickhofen N, Vant’s Veer-Korthof E, Gluckman E. Malignant tumors occurring after treatment of aplastic anemia. European Bone Marrow Transplantation-Severe Aplastic Anemia Working Party. N Eng J Med. 1993;329:1152–1157. doi: 10.1056/NEJM199310143291603. [DOI] [PubMed] [Google Scholar]
- 5.Socie G, Rosenfeld S, Frickhofen N, Gluckman E, Tichelli A. Late clonal diseases of treated aplastic anemia. Semin Hematol. 2000;37:91–101. [PubMed] [Google Scholar]
- 6.Raghavachar A, Ganser A, Freund M, Heimpel H, Hermann F, Schrezenmeier H. Long-term interleukin-3 and intensive immunosuppression in the treatment of aplastic anemia. Cytokines Mol Ther. 1996;2:215–223. [PubMed] [Google Scholar]
- 7.Wondergem MJ, Stevens SJ, Janssen JJ, Oudejans JJ, Ossenkoppele GJ, Middeldorp JM, Zweegman S. Monitoring of EBV reactivation is justified in patients with aplastic anemia treated with rabbit ATG as a second course of immunosuppression. Blood. 2008;111:1739. doi: 10.1182/blood-2007-09-111534. [DOI] [PubMed] [Google Scholar]
- 8.Calistri E, Tiribelli M, Battista M, Michelutti A, Corbellino M, Viale P, Fanin R, Damiani D. Epstein-Barr virus reactivation in a patient treated with anti-thymocyte globulin for severe aplastic anemia. Am J Hematol. 2006;81:355–357. doi: 10.1002/ajh.20560. [DOI] [PubMed] [Google Scholar]
- 9.Viola GM, Zu Y, Baker KR, Aslam S. Epstein-Barr virus-related lymphoproliferative disorder induced by equine anti-thymocyte globulin therapy. Med Oncol. 2011;28:1604–1608. doi: 10.1007/s12032-010-9635-8. [DOI] [PubMed] [Google Scholar]
- 10.Sugimoto-Sekiguchi H, Tashiro H, Shirasaki R, Arai T, Yamamoto T, Oka Y, Akiyama N, Kawasugi K, Shirafuji N. Colonic EBV-associated lymphoproliferative disorder in a patient treated with rabbit antithymocyte globulin for aplastic anemia. Case Rep Gastrointest Med. 2012;2012:395801. doi: 10.1155/2012/395801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camitta BM, Thomas ED, Nathan DG, Santos G, Gordon-Smith EC, Gale RP, Rappeport JM, Storb R. Severe aplastic anemia: a prospective study of the effect of early marrow transplantation on acute mortality. Blood. 1976;48:63–70. [PubMed] [Google Scholar]
- 12.Ishida M, Iwai M, Yoshida K, Kagotani A, Okabe H. Sebaceous carcinoma associated with Bowen’s disease: a case report with emphasis on the pathogenesis of sebaceous carcinoma. Int J Clin Exp Pathol. 2013;6:3029–3032. [PMC free article] [PubMed] [Google Scholar]
- 13.Toriyama A, Ishida M, Amano T, Nakagawa T, Kaku S, Iwai M, Yoshida K, Kagotani A, Takahashi K, Murakami T, Okabe H. Leiomyomatosis peritonealis disseminata coexisting with endometriosis within the same lesions: a case report with review of the literature. Int J Clin Exp Pathol. 2013;6:2949–2954. [PMC free article] [PubMed] [Google Scholar]
- 14.Ishida M, Hodohara K, Yoshida K, Kagotani A, Iwai M, Yoshii M, Okuno K, Horinouchi A, Nakanishi R, Harada A, Yoshida T, Okabe H. Occurence of anaplastic large cell lymphoma following IgG4-related autoimmune pancreatitis and cholecystitis and diffuse large B-cell lymphoma. Int J Clin Exp Pathol. 2013;6:2560–2568. [PMC free article] [PubMed] [Google Scholar]
- 15.Ishida M, Yoshida K, Kagotani A, Iwai M, Yoshii M, Okuno K, Horinouchi A, Nakanishi R, Harada A, Yoshida T, Okuno T, Hodohara K, Okabe H. Anaplastic lymphoma kinase-positive large B-cell lymphoma: A case report with emphasis on the cytological features of the pleural effusion. Int J Clin Exp Pathol. 2013;6:2631–2635. [PMC free article] [PubMed] [Google Scholar]
- 16.Ishida M, Hodohara K, Yoshii M, Okuno H, Nakanishi R, Horinouchi A, Nakanishi R, Harada A, Iwai M, Yoshida K, Kagotani A, Yoshida T, Okabe H. Methotrexate-related Epstein-Barr virus-associated lymphoproliferative disorder occurring in the gingiva of a patient with rheumatoid arthritis. Int J Clin Exp Pathol. 2013;6:2237–2241. [PMC free article] [PubMed] [Google Scholar]
- 17.Hoshida Y, Xu JX, Fujita S, Nakamichi I, Ikeda J, Tomita Y, Nakatsuka S, Tamaru J, Iizuka A, Takeuchi T, Aozasa K. Lmyphoproliferative disorders in rhematoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medicaton. J Rheumatol. 2007;34:322–331. [PubMed] [Google Scholar]
- 18.Salloum E, Cooper DL, Howe G, Lacy J, Tallini G, Crouch J, Schultz M, Murren J. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rhematoid arthritis and other rheumatic diseases. J. Clin. Oncol. 1996;14:1943–1949. doi: 10.1200/JCO.1996.14.6.1943. [DOI] [PubMed] [Google Scholar]
- 19.Scheinberg P, Fisher SH, Li L, Nunez O, Wu CO, Sloand EM, Cohen JI, Young NS, John Barrett A. Distinct EBV and CMV reactivation patterns following antibody-based immunosuppressive regimens in patients with severe aplastic anemia. Blood. 2007;109:3219–3224. doi: 10.1182/blood-2006-09-045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorr V, Doolittle G, Woodroof J. First report of a B cell lymphoproliferative disorder arising in a patient treated with immune suppressants for severe aplastic anemia. Am J Hematol. 1996;52:108–113. doi: 10.1002/(SICI)1096-8652(199606)52:2<108::AID-AJH7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki Y, Niitsu N, Hayama M, Katayama T, Ishii R, Osaka M, Miyazaki K, Danbara M, Horie R, Yoshida T, Nakamura N, Higashihara M. Lymphoproliferative disorders after immunosuppressive therapy for aplastic anemia: a case report and literature review. Acta Haematol. 2009;121:21–26. doi: 10.1159/000209225. [DOI] [PubMed] [Google Scholar]
- 22.Atta EH, Dias DS, Marra VL, de Azevedo AM. Comparison between horse and rabbit antithymocyte globulin as first-line treatment for patients with severe aplastic anemia: a single-center retrospective study. Ann Hematol. 2010;89:851–859. doi: 10.1007/s00277-010-0944-y. [DOI] [PubMed] [Google Scholar]
- 23.Karuturi M, Shah N, Frank D, Fasan O, Reshef R, Ahya VN, Bromberg M, Faust T, Goral S, Schuster SJ, Stadtmauer EA, Tsai DE. Plasmacytic post-transplant lymphoproliferative disorder: a case series of nine patients. Transpl Int. 2013;26:616–622. doi: 10.1111/tri.12091. [DOI] [PubMed] [Google Scholar]
- 24.Trappe R, Zimmermann H, Fink S, Reinke P, Dreyling M, Pascher A, Lehmkuhl H, Gärtner B, Anagnostopoulos I, Riess H. Plasmacytoma-like post-transplant lymphoproliferative disorder, a rare subtype of monomorphic B-cell post-transplant lymphoproliferation, is associated with a favorable outcome in localized as well as in advanced disease: a prospective analysis of 8 cases. Haematologica. 2011;96:1067–1071. doi: 10.3324/haematol.2010.039214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SY, Lee JW, Lee SE, Cho BS, Kim M, Eom KS, Kim YJ, Kim HJ, Lee S, Min CK, Cho SG, Kim DW, Han K, Min WS. The characteristics and clinical outcome of adult patients with aplastic anemia and abnormal cytogenetics at diagnosis. Genes Chromosomes Cancer. 2010;49:844–850. doi: 10.1002/gcc.20793. [DOI] [PubMed] [Google Scholar]
- 26.Koh Y, Lee HR, Song EY, Kim HK, Kim I, Park S, Park MH, Kim BK, Yoon SS, Lee DS. Hypoplastic myelodysplastic syndrome (h-MDS) is a distinctive clinical entity with poorer prognosis and frequent karyotypic and FISH abnormalities compared to aplastic anemia (AA) Leuk Res. 2010;34:1344–1350. doi: 10.1016/j.leukres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Ohta M, Taga T, Nomura A, Kato H, Takano T, Maruo Y, Takeuchi Y, Ishida M, Ohta S. Epstein-Barr virus-related lymphoproliferative disorder, cytomegalovirus reactivation and varicella zoster virus encephalitis during treatment of meduuloblastoma. J Med Virol. 2011;83:1582–1584. doi: 10.1002/jmv.22136. [DOI] [PubMed] [Google Scholar]


