Abstract
In this study, we analyzed the immunohistochemical and molecular profiles of an unusual RCC showed coexistent absence of INI1 and BRG1 expression, rhabdoid morphology, and poor prognosis. Histologically, the tumor had rhabdoid features, which were demonstrated by large round to polygonal cells with eccentric nuclei, prominent nucleoli, and eosinophilic cytoplasm varying from abundant to scanty. Immunohistochemically, the tumor were positive for BRM, PBRM1, ARID1A, CD10, CKpan, Vimentin, carbonic anhydrase IX (CA-IX), and P504S (AMACR) but negative for INI1, BRG1, HMB45, melan A, CK7, CD117, Ksp-cadherin, TFEB, TFE3, and Cathepsin K. We detected all three exons status of the VHL gene of the tumor and observed 1 somatic mutations in 1st exon. Chromosome 3p deletion, coupled with polysomy of chromosome 3 was also found. Based on these findings, it is further indicated that in some cases, rhabdoid RCC may arise from clear cell RCC. SWI/SNF chromatin remodeling complex may be an attractive candidate for being the “second hit” in RCCs and may play an important role during tumor progression. The role of SWI/SNF complex in rhabdoid RCC should be further studied on a larger number of cases.
Keywords: INI1, SMARCB1, BRG1, SMARCA4, rhabdoid, renal cell carcinoma, SWI/SNF complex, immunohistochemistry
Introduction
Rhabdoid renal cell carcinomas (RCC) are rare and have been recently identified as a morphologic variant of RCC associated with aggressive behavior [1-11]. In a previous study, Gokden and colleagues found that 5% of RCC exhibited rhabdoid features and most of them were found to have a nonrhabdoid carcinoma component, in most cases described as clear cell RCC [1]. Although there are approximately 60 reported cases of RCC with rhabdoid morphology in the English literature, the potential molecular connection associated with the rhabdoid cytologic phenotype and the aggressive biologic behavior of these tumors have not yet been elucidated [1-11].
The yeast switch in mating type (SWI)/sucrose nonfermentation (SNF) complex is one of several chromatin-remodeling complexes, including the INI1 (also known as SMARCB1, SNF5 and BAF47), ARID1A (also known as BAF250A and SMARCF1), PBRM1 (also known as BAF180) and BRM (also known as SMARCA2)/BRG1 (also known as SMARCA4) subunits [12,13]. It has been shown that the SWI/SNF complex plays critical roles for growth control and cancer development and complete loss of a SWI/SNF subunit can promote cancer formation [12,13]. In previous studies, loss of INI1 or BRG1 expression has been described in malignant tumors with rhabdoid morphology including pediatric renal and extrarenal malignant rhabdoid tumors, atypical teratoid/rhabdoid tumors of the central nervous system, epithelioid sarcoma, renal medullary carcinoma and a subset (15%) of renal collecting duct carcinoma, suggesting that these protein act an important role in human cancer [12-16].
In this study, we reported a case of rhabdoid RCC showing coexistent loss of INI1 and BRG1 expression that implicate a possible role of SWI/SNF complex in the biological mechanisms driving these tumors.
Case report
Clinical history
A 65-year-old man with no significant past medical history presented with 3 months history of intermittent lumbar pain. Abdominal computed tomography (CT) scan demonstrated a 5.5×4 cm sized mass in the upper pole of the left kidney. A malignant tumor was suspected and a total nephrectomy was performed without chemotherapy or radiation therapy after surgery. The patient died of the disease 1 year after diagnosis.
Histopathological and immunohistochemical findings
Morphologically, the tumor displayed all areas with a rhabdoid histologic appearance, which were demonstrated by large round to polygonal cells with eccentric nuclei, prominent nucleoli, and eosinophilic cytoplasm varying from abundant to scanty. Densely eosinophilic nuclear pseudoinclusions were noted. These cells were arranged partly in an alveolar arrangement with delicate fibrovascular septa, compared with classic clear cell RCC with a delicate sinusoidal vascular network (Figure 1A and 1B).
Figure 1.
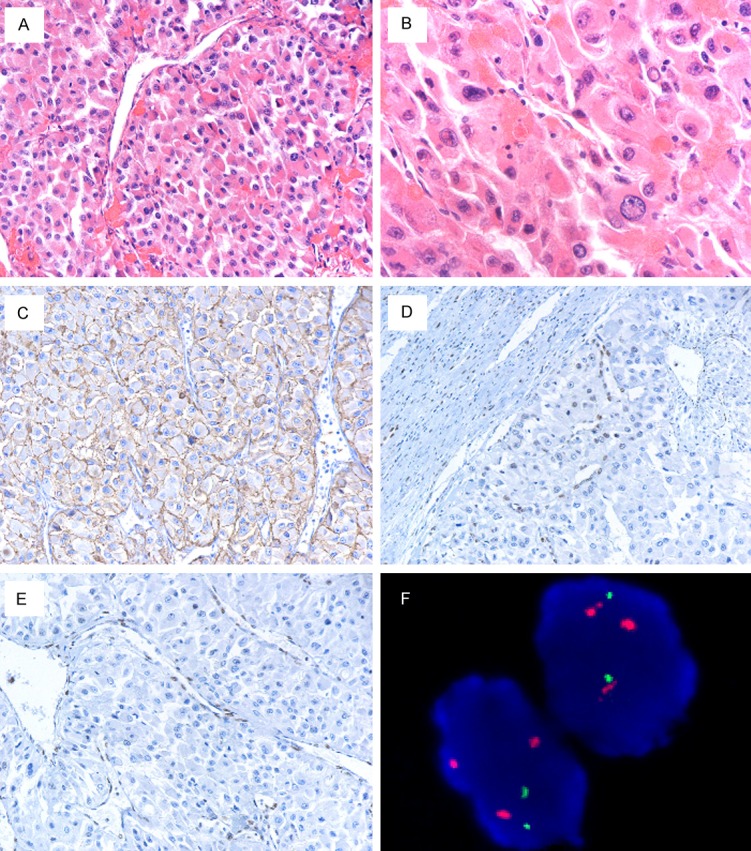
(A) The tumor was arranged partly in a large alveolar arrangement with delicate fibrovascular septa, compared with classic clear cell RCC with a delicate sinusoidal vascular network. (B) Neoplastic cells were large round to polygonal with eccentric nuclei, prominent nucleoli, and abundant eosinophilic cytoplasm. Densely eosinophilic nuclear pseudoinclusions were observed. (C) The tumor showed strong labeling for CA-IX. Immunostaining for INI1 (D) and BRG1 (E) was entirely negative. (F) The tumor cells showed 3p loss with polysomy of chromosome 3. FISH showed nuclei with multiple hybridization signals of centromeric probe for chromosome 3 (Spectrum Orange) and two signals of subtelomeric probe for 3p25 (Spectrum Green).
Immunoreaction was performed using the labelled streptavidin–biotin method and overnight incubation as previously described [17,18]. Immunohistochemically, the tumor cells demonstrated moderately (2+) or strongly (3+) positive staining for BRM, PBRM1, ARID1A, CD10, CKpan, Vimentin, carbonic anhydrase IX (CA-IX), and P504S (AMACR) but negative for INI1, BRG1, HMB45, melan A, CK7, CD117, Ksp-cadherin, TFEB, TFE3, and Cathepsin K. The presence of Ki-67 protein demonstrated a high proliferation rate (Figure 1C-E).
Molecular analysis
VHL sequence analysis of VHL gene and fluorescence in situ hybridization (FISH) detection for chromosome 3p deletion were performed as recently described [19,20]. We detected all three exons status of the VHL gene and observed 1 somatic mutation in 1st exon (Figure 2). The tumor demonstrated chromosome 3p deletion, coupled with polysomy of chromosome 3 (Figure 1F).
Figure 2.
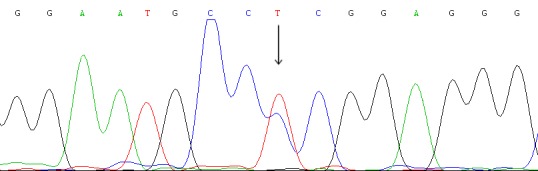
Analysis of the VHL gene mutation of the case. One synonymous mutation was identified: c.219C>T (Pro2Pro).
Discussion
We analyzed the immunohistochemical and molecular profiles of an unusual RCC showed coexistent absence of INI1 and BRG1 expression, rhabdoid morphology, and poor prognosis.
Histologically, the tumor had rhabdoid features, which were demonstrated by large round to polygonal cells with eccentric nuclei, prominent nucleoli, and eosinophilic cytoplasm varying from abundant to scanty. Densely eosinophilic nuclear pseudoinclusions were noted. These cells were arranged partly in a large alveolar arrangement with delicate fibrovascular septa, compared with classic clear cell RCC with a delicate sinusoidal vascular network. When reviewing published data with histopathologic description, RCC with rhabdoid features has been recently reported as a morphologic variant of RCC in few series and universally recognized as a highly aggressive neoplasm [1-11]. Although there are approximately 60 reported cases of RCC with rhabdoid morphology in the English literature, it is uncertain whether this subset of RCC has distinct immunophenotype, molecular genetic features and origin [1-11]. The possibility of a molecular connection between the rhabdoid cytologic phenotype and the aggressive biologic behavior has not yet been elucidated.
Immunohistochemically, the tumor were positive for BRM, PBRM1, ARID1A, CD10, CKpan, Vimentin, carbonic anhydrase IX (CA-IX), and P504S (AMACR) but negative for INI1, BRG1, HMB45, melan A, CK7, CD117, Ksp-cadherin, TFEB, TFE3, and Cathepsin K. The immunophenotype of this subset is generally similar to that of clear cell RCC. At the molecular level, it was estimated that approximately 33% to 75% of all sporadic clear cell RCC harbor VHL defects [21-23]. Chromosome 3p deletion and the inactivation of the von Hippel-Lindau (VHL) tumor suppressor gene are the most common genetic alterations observed in this subtype [20,24,25]. We detected all three exons status of the VHL gene of the tumor and observed 1 somatic mutations in 1st exon. Chromosome 3p deletion, coupled with polysomy of chromosome 3 was also found. These molecular findings further indicated that in some cases, rhabdoid RCC may arise from clear cell RCC. Existing evidence indicates that VHL inactivation is considered as a necessary but not sufficient step for clear cell RCC development and progression [26]. Indeed, exome sequencing has recently unveiled additional genes mutated in clear cell RCC. Several of those encode histone and chromatin regulators and include SETD2, KDM6A, KDM5C, BAP1, and PBRM1 [26].
INI1 and BRG1 protein is key member of the SWI/SNF complex, which also includes other important subunits such as BRM, ARID1A, and PBRM1 and mediates gene expression by shifting the position of histones, thereby making the DNA more accessible to transcription factors and key cellular proteins [12,13]. Several distinct tumors are associated with the loss expression of this protein family. For example, BRM has been found to be inactivated in 10-20% of many solid tumor types including lung, breast, colon, esophageal, ovarian, bladder, prostate, gastric and head/neck tumors [27]. More recently, the ARID1A subunit of SWI/SNF complexes was also recently found to be specifically mutated in 50% of ovarian clear cell carcinomas and 30% of endometrioid carcinomas [28,29]. BRG1 mutations and loss expression have been identified in primary lung cancers [13]. Mutations in PBRM1 were identified in 41% of renal cell carcinomas, making PBRM1 the second major clear cell RCC cancer gene [26,30]. Moreover, loss of INI1 or BRG1 expression has been described in malignant tumors with rhabdoid morphology including pediatric renal and extrarenal malignant rhabdoid tumors, atypical teratoid/rhabdoid tumors of the central nervous system, epithelioid sarcoma, and renal medullary carcinoma [12-14,16,31]. In the present study, there was a complete absence of INI1 and BRG1 in the case of rhabdoid RCCs. These findings suggest a potential role of INI1 and BRG1 in the acquisition of this distinct histopathological appearance and the extremely aggressive behavior. Based on these findings, SWI/SNF chromatin remodeling complex may be an attractive candidate for being the “second hit” in RCCs and may play an important role during tumor progression. The question whether genetic alterations of other members of the SWI/SNF chromatin remodeling complex might play a role in those rare cases of rhabdoid RCC remains to be determined.
In conclusion, we have reported a case of RCC with rhabdoid features showed absence of INI1 and BRG1 expression, and poor prognosis. The role of SWI/SNF complex in rhabdoid RCC should be further studied on a larger number of cases.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81101933; Q Rao); and (81372743; Xiao-Jun Zhou); Natural Science Foundation of Jiangsu Province, China (BK2010463; Q Rao); and Maixin fund (m1203; Shan-Shan Shi).
Disclosure of conflict of interest
None.
References
- 1.Gokden N, Nappi O, Swanson PE, Pfeifer JD, Vollmer RT, Wick MR, Humphrey PA. Renal cell carcinoma with rhabdoid features. Am J Surg Pathol. 2000;24:1329–1338. doi: 10.1097/00000478-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kuroiwa K, Kinoshita Y, Shiratsuchi H, Oshiro Y, Tamiya S, Oda Y, Naito S, Tsuneyoshi M. Renal cell carcinoma with rhabdoid features: an aggressive neoplasm. Histopathology. 2002;41:538–548. doi: 10.1046/j.1365-2559.2002.01427.x. [DOI] [PubMed] [Google Scholar]
- 3.Shannon B, Stan Wisniewski Z, Bentel J, Cohen RJ. Adult rhabdoid renal cell carcinoma. Arch Pathol Lab Med. 2002;126:1506–1510. doi: 10.5858/2002-126-1506-ARRCC. [DOI] [PubMed] [Google Scholar]
- 4.Shannon BA, Cohen RJ. Rhabdoid differentiation of chromophobe renal cell carcinoma. Pathology. 2003;35:228–230. doi: 10.1080/0031302031000123209. [DOI] [PubMed] [Google Scholar]
- 5.Lee L, Marsh WL Jr, Wen P. Pathologic quiz case: an 82-year-old woman with a renal mass. Renal cell carcinoma with rhabdoid features. Arch Pathol Lab Med. 2004;128:109–110. doi: 10.5858/2004-128-109-PQC. [DOI] [PubMed] [Google Scholar]
- 6.Leroy X, Zini L, Buob D, Ballereau C, Villers A, Aubert S. Renal cell carcinoma with rhabdoid features: an aggressive neoplasm with overexpression of p53. Arch Pathol Lab Med. 2007;131:102–106. doi: 10.5858/2007-131-102-RCCWRF. [DOI] [PubMed] [Google Scholar]
- 7.Kapoor A, Tutino R, Kanaroglou A, Hotte SJ. Treatment of adult rhabdoid renal cell carcinoma with sorafenib. Can Urol Assoc J. 2008;2:631–634. doi: 10.5489/cuaj.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman-Fredricks JR, Herrera L, Bracho J, Gomez-Fernandez C, Leveillee R, Rey L, Jorda M. Adult renal cell carcinoma with rhabdoid morphology represents a neoplastic dedifferentiation analogous to sarcomatoid carcinoma. Ann Diagn Pathol. 2011;15:333–337. doi: 10.1016/j.anndiagpath.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Humphrey PA. Renal cell carcinoma with rhabdoid features. J Urol. 2011;186:675–676. doi: 10.1016/j.juro.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 10.De Vincenzo F, Zucali PA, Ceresoli GL, Colombo P, Simonelli M, Lorenzi E, Perrino M, Gianoncelli L, De Sanctis R, Graziotti P, Santoro A. Response to sunitinib in an adult patient with rhabdoid renal cell carcinoma. J. Clin. Oncol. 2011;29:e529–531. doi: 10.1200/JCO.2011.34.8284. [DOI] [PubMed] [Google Scholar]
- 11.Kats-Ugurlu G, Maass C, Van Herpen C, De Waal R, Oosterwijk E, Mulders P, Hulsbergen-van de Kaa C, Leenders W. Better effect of sorafenib on the rhabdoid component of a clear cell renal cell carcinoma owing to its higher level of vascular endothelial growth factor-A production. Histopathology. 2011;59:562–564. doi: 10.1111/j.1365-2559.2011.03923.x. [DOI] [PubMed] [Google Scholar]
- 12.Reisman D, Glaros S, Thompson EA. The SWI/SNF complex and cancer. Oncogene. 2009;28:1653–1668. doi: 10.1038/onc.2009.4. [DOI] [PubMed] [Google Scholar]
- 13.Wilson BG, Roberts CW. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer. 2011;11:481–492. doi: 10.1038/nrc3068. [DOI] [PubMed] [Google Scholar]
- 14.Cheng JX, Tretiakova M, Gong C, Mandal S, Krausz T, Taxy JB. Renal medullary carcinoma: rhabdoid features and the absence of INI1 expression as markers of aggressive behavior. Mod Pathol. 2008;21:647–652. doi: 10.1038/modpathol.2008.44. [DOI] [PubMed] [Google Scholar]
- 15.Elwood H, Chaux A, Schultz L, Illei PB, Baydar DE, Billis A, Sharma R, Argani P, Epstein JI, Netto GJ. Immunohistochemical analysis of SMARCB1/INI-1 expression in collecting duct carcinoma. Urology. 2011;78:474, e471–475. doi: 10.1016/j.urology.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 16.Calderaro J, Moroch J, Pierron G, Pedeutour F, Grison C, Maille P, Soyeux P, De la Taille A, Couturier J, Vieillefond A, Rousselet MC, Delattre O, Allory Y. SMARCB1/INI1 inactivation in renal medullary carcinoma. Histopathology. 2012;61:428–435. doi: 10.1111/j.1365-2559.2012.04228.x. [DOI] [PubMed] [Google Scholar]
- 17.Rao Q, Liu B, Cheng L, Zhu Y, Shi QL, Wu B, Jiang SJ, Wang Y, Wang X, Yu B, Zhang RS, Ma HH, Lu ZF, Tu P, Wang JD, Zhou XJ. Renal cell carcinomas with t(6;11)(p21;q12): A clinicopathologic study emphasizing unusual morphology, novel alpha-TFEB gene fusion point, immunobiomarkers, and ultrastructural features, as well as detection of the gene fusion by fluorescence in situ hybridization. Am J Surg Pathol. 2012;36:1327–1338. doi: 10.1097/PAS.0b013e31825aafb5. [DOI] [PubMed] [Google Scholar]
- 18.Rao Q, Cheng L, Xia QY, Liu B, Li L, Shi QL, Shi SS, Yu B, Zhang RS, Ma HH, Lu ZF, Tu P, Zhou XJ. Cathepsin K expression in a wide spectrum of perivascular epithelioid cell neoplasms (PEComas): a clinicopathological study emphasizing extrarenal PEComas. Histopathology. 2013;62:642–650. doi: 10.1111/his.12059. [DOI] [PubMed] [Google Scholar]
- 19.Gobbo S, Eble JN, Grignon DJ, Martignoni G, MacLennan GT, Shah RB, Zhang S, Brunelli M, Cheng L. Clear cell papillary renal cell carcinoma: a distinct histopathologic and molecular genetic entity. Am J Surg Pathol. 2008;32:1239–1245. doi: 10.1097/PAS.0b013e318164bcbb. [DOI] [PubMed] [Google Scholar]
- 20.Gobbo S, Eble JN, Maclennan GT, Grignon DJ, Shah RB, Zhang S, Martignoni G, Brunelli M, Cheng L. Renal cell carcinomas with papillary architecture and clear cell components: the utility of immunohistochemical and cytogenetical analyses in differential diagnosis. Am J Surg Pathol. 2008;32:1780–1786. doi: 10.1097/PAS.0b013e31818649ed. [DOI] [PubMed] [Google Scholar]
- 21.Ma X, Yang K, Lindblad P, Egevad L, Hemminki K. VHL gene alterations in renal cell carcinoma patients: novel hotspot or founder mutations and linkage disequilibrium. Oncogene. 2001;20:5393–5400. doi: 10.1038/sj.onc.1204692. [DOI] [PubMed] [Google Scholar]
- 22.Van Houwelingen KP, Van Dijk BA, Hulsbergen-van de Kaa CA, Schouten LJ, Gorissen HJ, Schalken JA, van den Brandt PA, Oosterwijk E. Prevalence of von Hippel-Lindau gene mutations in sporadic renal cell carcinoma: results from The Netherlands cohort study. BMC Cancer. 2005;5:57. doi: 10.1186/1471-2407-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao Q, Chen JY, Wang JD, Ma HH, Zhou HB, Lu ZF, Zhou XJ. Renal cell carcinoma in children and young adults: clinicopathological, immunohistochemical, and VHL gene analysis of 46 cases with follow-up. Int J Surg Pathol. 2011;19:170–179. doi: 10.1177/1066896909354337. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006;49:798–805. doi: 10.1016/j.eururo.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 25.Cairns P. Renal cell carcinoma. Cancer Biomark. 2010;9:461–473. doi: 10.3233/CBM-2011-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pawlowski R, Muhl SM, Sulser T, Krek W, Moch H, Schraml P. Loss of PBRM1 expression is associated with renal cell carcinoma progression. Int J Cancer. 2013;132:11–17. doi: 10.1002/ijc.27822. [DOI] [PubMed] [Google Scholar]
- 27.Glaros S, Cirrincione GM, Muchardt C, Kleer CG, Michael CW, Reisman D. The reversible epigenetic silencing of BRM: implications for clinical targeted therapy. Oncogene. 2007;26:7058–7066. doi: 10.1038/sj.onc.1210514. [DOI] [PubMed] [Google Scholar]
- 28.Jones S, Wang TL, Shih Ie M, Mao TL, Nakayama K, Roden R, Glas R, Slamon D, Diaz LA Jr, Vogelstein B, Kinzler KW, Velculescu VE, Papadopoulos N. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, Yang W, Heravi-Moussavi A, Giuliany R, Chow C, Fee J, Zayed A, Prentice L, Melnyk N, Turashvili G, Delaney AD, Madore J, Yip S, McPherson AW, Ha G, Bell L, Fereday S, Tam A, Galletta L, Tonin PN, Provencher D, Miller D, Jones SJ, Moore RA, Morin GB, Oloumi A, Boyd N, Aparicio SA, Shih Ie M, Mes-Masson AM, Bowtell DD, Hirst M, Gilks B, Marra MA, Huntsman DG. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varela I, Tarpey P, Raine K, Huang D, Ong CK, Stephens P, Davies H, Jones D, Lin ML, Teague J, Bignell G, Butler A, Cho J, Dalgliesh GL, Galappaththige D, Greenman C, Hardy C, Jia M, Latimer C, Lau KW, Marshall J, McLaren S, Menzies A, Mudie L, Stebbings L, Largaespada DA, Wessels LF, Richard S, Kahnoski RJ, Anema J, Tuveson DA, Perez-Mancera PA, Mustonen V, Fischer A, Adams DJ, Rust A, Chan-on W, Subimerb C, Dykema K, Furge K, Campbell PJ, Teh BT, Stratton MR, Futreal PA. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011;469:539–542. doi: 10.1038/nature09639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Q, Galli S, Srinivasan R, Linehan WM, Tsokos M, Merino MJ. Renal medullary carcinoma: molecular, immunohistochemistry, and morphologic correlation. Am J Surg Pathol. 2013;37:368–374. doi: 10.1097/PAS.0b013e3182770406. [DOI] [PMC free article] [PubMed] [Google Scholar]


