Abstract
Breast carcinoma with osteoclastic giant cells (OGCs) are uncommon. Here, we report a 46-year-old woman with a painless lump in her left breast that has been proved clinically and radiographically. Microscopical examination showed OGCs accompanying invasive ductal carcinoma. Immunohistochemical assay revealed that OGCs derived from macrophages. Despite positive lymph node metastasis, the patient has been well without evidence of recurrence or metastasis one year after the operation. To date, the influence of OGCs on the prognosis of patients is still controversial. Our case may provide insights into further understanding beast carcinoma with OGCs.
Keywords: Breast carcinoma, osteoclastic giant cells, tumor metastasis, prognosis, immunohistochemistry
Introduction
Breast carcinoma with osteoclastic giant cells (OGCs) is rare and decribed in less than 2% of breast cancer patients [1,2]. Up to date, a few cases reported in the medical literature. Breast carcinoma with osteoclast-like giant cells was first described by Rosen in 1979 [3]. This tumor is characterized by the presence of OGCs, the nature of which remains controversial. We herein reported a case of breast carcinoma with OGCs accompanied by lymph node metastasis; we discussed its clinical, imaging, light microscopic and immunohistochemical features.
Case presentation
A 46-year-old woman was admitted to our hospital with one-week history of a painless lump in the upper of her left breast. The patient denied any systemic symptoms, weight loss or bone pain. She had no known family history of breast, uterine, or ovarian malignancy. Physical examination revealed that a hard mass was about 4 cm in diameter at 12 o’clock in the left breast and 5 cm away from the nipple. Further, we further touched a well-circumscribed firm mass with good mobility in the left axillary.
Laboratory studies showed 14.52 G/L leukocyte count, 10.40 G/L neutrophils count, 5.57 U/mL CA-199 (gastrointestinal cancer marker) and 13.4 U/mL CA153 (breast cancer marker). All other routine lab parameters were within normal limits. Incisional breast biopsy was performed and frozen pathologic diagnosis was beast carcinoma. Then the patient underwent modified radical mastectomy with axillary clearance, following sentinel lymph node biopsy. Post-operation course were smooth. Adjuvant chemotherapy was administered postoperatively. At the time of writing she has been well without evidence of recurrence or metastasis one year after the operation.
Imaging findings
The woman underwent a radiological examination including breast mammography and ultrasound. Mammography showed an irregular high-density mass shadow in the upper outer quadrant of her left breast (Figure 1A). The tumor was 3.0 × 2.6 cm in size, spicule sign edging infiltration and along the duct infiltration in the direction of the nipple. The region around the tumor showed disordered structure, increased local blood supply and wide transparent halo sign. No clear calcification was found within and around the lesion (Breast Imaging Reporting and Data System (BI-RADS) category 4). Ultrasound revealed an irregularly shaped hypoechoic mass without posterior echo enhancement (Figure 1B). Invasive breast carcinoma accompany axillary lymph node metastasis was suspected on ultrasonography.
Figure 1.
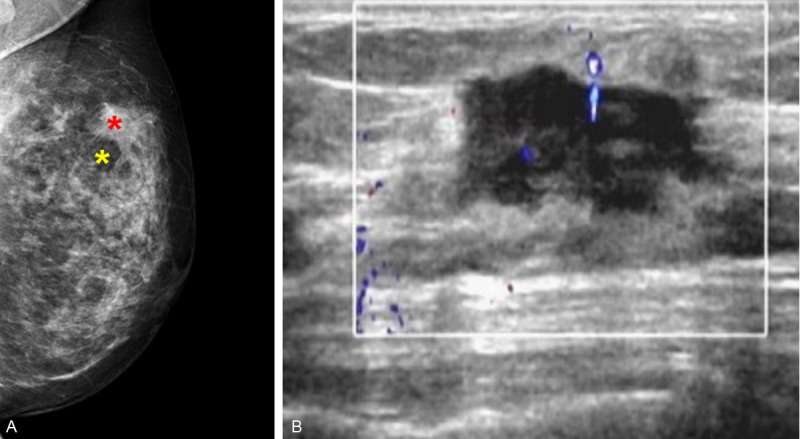
Imaging features. A. Mammography showed an irregular high-density mass shadow in the upper outer quadrant of her left breast. The tumor was 3.0 × 2.6 cm in size (Red star). The region around the tumor showed wide transparent halo sign (Yellow star). B. Ultrasound revealed an irregularly shaped hypoechoic mass without posterior echo enhancement.
Pathological findings
Grossly, the tumor was a well-defined, red-brown mass with bleeding. Microscopically, sections showed neoplastic cells arranged in cords, sheets, and glandular pattern with low degree of pleomorphism and infrequent mitosis (Figure 2). The stroma was collagenized and fresh hemorrhages as well as abundant hemosiderin-laden macrophages were also noted. Besides these, many OGCs and a few mononuclear histiocytes were seen in the vascular stroma, more so at the periphery. The giant cells had abundant eosinophilic cytoplasm and contained numerous small uniform nuclei. The nuclei were uniform and round to oval with some convolution in shape. The size and nuclear number of OGCs often vary from 80 per 10 high power fields with a number of nuclei up to 12 in one cell. No granulomas, necrosis or inflammatory infiltrate were observed. Three sentinel lymph nodes were inspected for intraoperative rapid histology, and only one positive node was observed. Similar histological signs could be found between primary and metastatic lesions, such as the organization of carcinoma cells, osteoclastic-like giant cells, scattered hemorrhage and focal flake hemosiderin deposition. The 12/12 axillary lymph nodes resected from the axillary tail were free of tumor.
Figure 2.
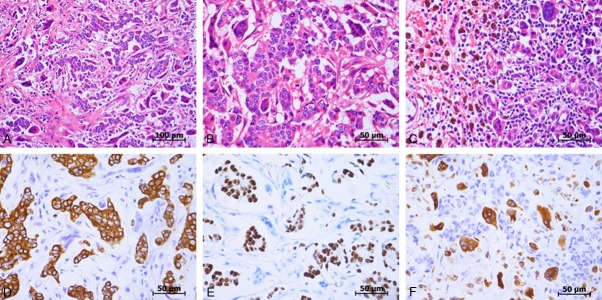
Histological features. A. Low power view of the primary tumor. B. High power view of the primary tumor. C. High power view of lymph node metastases. D. Immunohistochemical positive reaction of carcinoma cells for CK. E. Immunohistochemical positive reaction of carcinoma for PR. F. Immunohistochemical positive reaction of OGCs for CD68.
Immunohistochemically, the OGCs were positive for CD68, a histiocytic marker and negative for E-cadherin and cytokeratin (CK), epithelial markers. In contrast, cancer cells were negative for CD68, but positive for E-cadherin and CK, suggesting that those cells resulted from dedifferentiation of ductal carcinoma and retained the nature of carcinoma. The cancer cells were also positive for estrogen receptor (ER; weak, 95%), progesterone receptor (PR; strong, 95%) and negative for p53 and human epidermal growth factor receptor type 2 (Her-2; 0+), while the OGCs were negative for ER, PR and Her-2. Following the WHO classification, the diagnosis was breast carcinoma with OGCs.
Discussion
Carcinoma with osteoclastic giant cells (OGCs) has been described in several organs such as gallbladder [4], liver [5] and thyroid [6], other than pancreas [7] and urinary tract [8]. To date approximately 200 cases of carcinoma with osteoclastic giant cells have been reported. Carcinoma with osteoclastic (or osteoclast-like) giant cells of the breast constitutes only 0.5-1.2% of breast carcinoma [1,2]. OGCs can occur in invasive ductal, lobular, papillary, or squamous types of breast carcinoma [2,9,10]. In approximately one third of cancers with osteoclast-like giant cells there is axillary node metastasis, leading to a worse prognosis for patients with this form of carcinoma [11]. Here, we reported a case of breast carcinoma with OGCs accompany lymph node metastasis.
Initial diagnosis of mammary carcinoma is often based on fine needle aspiration or core needle biopsy. Cytologic finding of osteoclast-like giant cells include large cells with abundant cytoplasm and centrally located nuclei ranging in size and number [12]. There are also prominent, associated nucleoli. Diagnosis of osteoclast-like giant cells can be extremely difficult on cytologic examination as these cells can be bland in appearance and have a similar appearance to foreign-body giant cells associated with fat necrosis. As a result, malignant cells may be missed, leading to a false negative diagnosis [2]. Sometimes, the OGCs may be missed, causing a cytological mimicker of benign tumors such as fibroadenoma [13].
Breast carcinoma with OGCs is characterized by the presence of OGCs admixed with malignant epithelial cells. They often showed hyperchromatic nuclei that are atypical with occasional small nucleoli and fine chromatin structure. Mitotic figures are typically rare [14]. OGCs also presented in many other lesions and diseases, such as tuberculosis, sarcoidosis and granulomatous mastitis. However, in contrast, breast carcinoma has no histological features consistent with granulomatous disease [2].
The mechanism for formation of osteoclast-like giant cells is still unknown. A recent study showed that secretion of specific cytokines, such as VEGF and MMP12, leaded to a characteristic inflammatory and hypervascular stroma, which is commonly observed in breast carcinoma with OGCs, regardless of histology of tumoral cells. Therefore, appearance of OGCs may not be antitumoral immunological reactions, but rather pro-tumoral differentiation of macrophage responding to hypervascular microenvironments induced by breast cancer [15].
To our best knowledge, no formal studies have been performed to investigate the role of OGCs in overall survival. The OGCs are of a different origin than the carcinoma and are possibly a reactive infiltrate [16,17]. Patients with benign giant cells adjacent to bone that expressed a CD68 pattern were found to have good prognosis. The presence of OGCs suggests a less aggressive tumor with a good outcome [13]. Several related analysis in other types of breast cancer, however, suggested a less favorable prognosis for patients with carcinoma with OGCs [11,17]. In the present case, the patient was alive with no evidence of recurrence 1 year after the operation despite positive lymph node metastasis.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 81272762, 81201635), Guangdong Natural Science Funds for Distinguished Young Scholar (S20120011334), Guangdong Natural Science Foundation (S2013010014254, S2012040006418).
Disclosure of conflict of interest
None.
References
- 1.Holland R, van Haelst UJ. Mammary carcinoma with osteoclast-like giant cells. Additional observations on six cases. Cancer. 1984;53:1963–1973. doi: 10.1002/1097-0142(19840501)53:9<1963::aid-cncr2820530927>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 2.Cai N, Koizumi J, Vazquez M. Mammary carcinoma with osteoclast-like giant cells: a study of four cases and a review of literature. Diagn Cytopathol. 2005;33:246–251. doi: 10.1002/dc.20341. [DOI] [PubMed] [Google Scholar]
- 3.Rosen PP. Multinucleated mammary stromal giant cells: a benign lesion that simulates invasive carcinoma. Cancer. 1979;44:1305–1308. doi: 10.1002/1097-0142(197910)44:4<1305::aid-cncr2820440421>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Akatsu T, Kameyama K, Kawachi S, Tanabe M, Aiura K, Wakabayashi G, Ueda M, Shimazu M, Kitajima M. Gallbladder carcinoma with osteoclast-like giant cells. J Gastroenterol. 2006;41:83–87. doi: 10.1007/s00535-005-1726-5. [DOI] [PubMed] [Google Scholar]
- 5.Wada Y, Takami Y, Tateishi M, Ryu T, Momosaki S, Saitsu H. Adenocarcinoma of the liver with osteoclast-like giant cells. Pathol Int. 2013;63:476–478. doi: 10.1111/pin.12090. [DOI] [PubMed] [Google Scholar]
- 6.Lee JS, Lee MC, Park CS, Juhng SW. Fine needle aspiration cytology of anaplastic carcinoma with osteoclastlike giant cells of the thyroid. A case report. Acta Cytol. 1996;40:1309–1312. doi: 10.1159/000334027. [DOI] [PubMed] [Google Scholar]
- 7.Hirano H, Morita K, Tachibana S, Okimura A, Fujisawa T, Ouchi S, Nakasho K, Ueyama S, Nishigami T, Terada N. Undifferentiated carcinoma with osteoclast-like giant cells arising in a mucinous cystic neoplasm of the pancreas. Pathol Int. 2008;58:383–389. doi: 10.1111/j.1440-1827.2008.02240.x. [DOI] [PubMed] [Google Scholar]
- 8.McCash SI, Unger P, Dillon R, Xiao GQ. Undifferentiated carcinoma of the renal pelvis with osteoclast-like giant cells: a report of two cases. APMIS. 2010;118:407–412. doi: 10.1111/j.1600-0463.2010.02608.x. [DOI] [PubMed] [Google Scholar]
- 9.Mukkamala A, Blight C, Azher Q, Danish R. Breast Carcinoma with Osteoclastic Giant Cells. Breast J. 1999;5:149–150. doi: 10.1046/j.1524-4741.1999.00141.x. [DOI] [PubMed] [Google Scholar]
- 10.Kurokawa K, Mouri Y, Asano A, Kamei K, Iwata Y, Isogai M, Saga S, Ichihara S. Pleomorphic carcinoma with osteoclastic giant cells of the breast: immunohistochemical differentiation between coexisting neoplastic and reactive giant cells. Pathol Int. 2009;59:91–97. doi: 10.1111/j.1440-1827.2008.02334.x. [DOI] [PubMed] [Google Scholar]
- 11.Cai G, Simsir A, Cangiarella J. Invasive mammary carcinoma with osteoclast-like giant cells diagnosed by fine-needle aspiration biopsy: review of the cytologic literature and distinction from other mammary lesions containing giant cells. Diagn Cytopathol. 2004;30:396–400. doi: 10.1002/dc.20069. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Alonso P. Breast carcinoma with osteoclast-like giant cells diagnosed by fine-needle aspiration cytology. Diagn Cytopathol. 2012;40:148–149. doi: 10.1002/dc.21615. [DOI] [PubMed] [Google Scholar]
- 13.Jogai S, Al-Jassar A, Amir T, Temmim L. Metaplastic mammary carcinoma with osteoclastic giant cells: a cytological mimicker of fibroadenoma. Cytopathology. 2004;15:334–336. doi: 10.1111/j.1365-2303.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 14.Iacocca MV, Maia DM. Bilateral infiltrating lobular carcinoma of the breast with osteoclast-like giant cells. Breast J. 2001;7:60–65. doi: 10.1046/j.1524-4741.2001.007001060.x. [DOI] [PubMed] [Google Scholar]
- 15.Shishido-Hara Y, Kurata A, Fujiwara M, Itoh H, Imoto S, Kamma H. Two cases of breast carcinoma with osteoclastic giant cells: are the osteoclastic giant cells pro-tumoural differentiation of macrophages? Diagn Pathol. 2010;5:55. doi: 10.1186/1746-1596-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryska A, Reynolds C, Keeney GL. Benign tumors of the breast with multinucleated stromal giant cells. Immunohistochemical analysis of six cases and review of the literature. Virchows Arch. 2001;439:768–775. doi: 10.1007/s004280100470. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Moriki T, Hiroi M, Nakayama H. Invasive lobular carcinoma of the breast with osteoclastlike giant cells. A case report. Acta Cytol. 1998;42:734–741. doi: 10.1159/000331836. [DOI] [PubMed] [Google Scholar]


