Abstract
Collision tumor is an extremely rare tumor which defined as the concrescence of two distinct primaries neoplasms. We report here a case of collision tumor at lower third esophagus composed of small cell type neuroendocrine carcinoma (NEC), which is an very rare, highly aggressive and poorly prognostic carcinoma and squamous cell carcinoma (SqCC). In our case, pathologically, the small cell carcinoma display the characteristic of small, round, ovoid or spindle-shaped tumor cells with scant cytoplasm, which colliding with a moderately differentiated squamous cell carcinoma. Immunohistochemical staining demonstrated positive activities for CD56, synaptophysin, 34βE12, CK 5/6, ki-67 (70%-80%), but negative for CD99, chromogranin A, and TTF-1. Accurate diagnosis was made base on these findings.
Keywords: Collision tumor, neuroendocrine carcinoma, small cell type, squamous cell carcinoma, esophagus
Clinical summary
A 65-years-old male who was a former smoker and alcoholic drinker was presented at our hospital with a 10 day history of increasingly dysphagia. The upper gastrointestinal endoscopies have been performed revealed a circular neoplasm with superficial anabrosis caused esophageal stricture 30 cm from the incisors. Biopsy confirmed a squamous cell carcinoma (SqCC) of esophagus. Computerized Tomography (CT) scan of his chest demonstrated wall uneven thickening of middle segment of the esophagus. The patient expected further evaluation and surgical treatment. A size of 5 cm×3 cm×1.3 cm ulcerative mass and surrounding tissues was removed via a esophagectomy. Microscopically, the tumor invaded muscularis propria, and contained: small-cell carcinoma (consist of small, round, ovoid or spindle-shaped tumor cells with scant cytoplasm) as the major component (70%) and moderately differentiated SqCC (display an expansive growth pattern) were showed in Figure 1. Seven lymph nodes in total were involved. Immunohistochemical staining positive for neural cell adhesion molecule (NCAM/CD56), synaptophysin (Syn), 34βE12 (antibody for high-molecular weight cytokeratin 1, 5, 10, 14), cytokeratin 5/6 (CK5/6), ki-67 (70%-80%) were showed in Figure 2, but negative for CD99 (MIC2), chromogranin A (CgA), and thyroid transcription factor (TTF-1). Diagnosis of esophageal collision carcinoma (small cell type NEC and moderately differentiated SqCC) was confirmed by immunohistochemical findings. The clinical stage was finally classified as IIIA (T2N3M0) in the light of TNM/staging classification for esophageal carcinomas [1]. Base on World Health Organization (WHO) classification in 2010 for gastroenteropancreatic neuroendocrine neoplasms, the patient was classified as NEC grade 3 (Ki67 index >20%) [2]. After recovering from operation, the patient was sent to the oncology department to receive further therapy.
Figure 1.
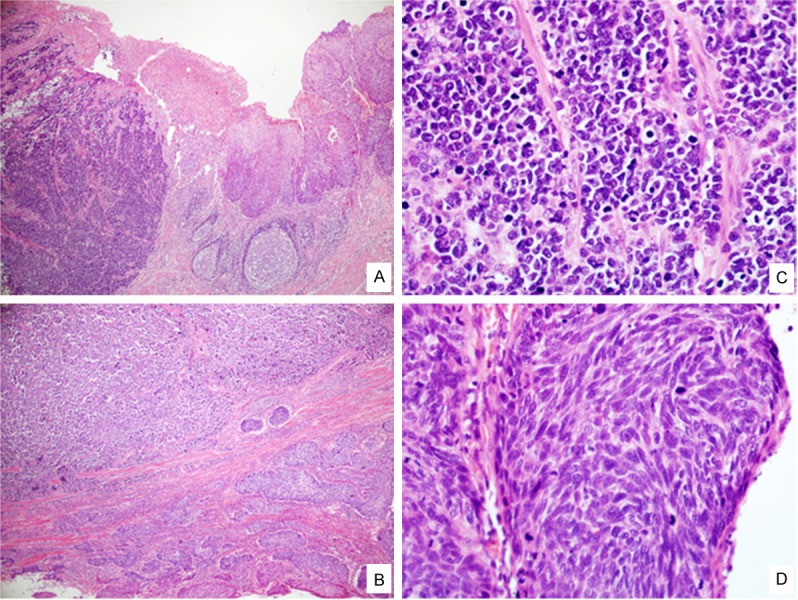
H&E Staining findings. The esophageal neoplasm was found to consist of two distinct lesions: small cell type NEC on left and SqCC on right in low-power view (A, ×40). Two components were separated by fibrocollagenous tissue (B, ×40). In the high-power view of (C) small cell type NEC and (D) SqCC (×400).
Figure 2.
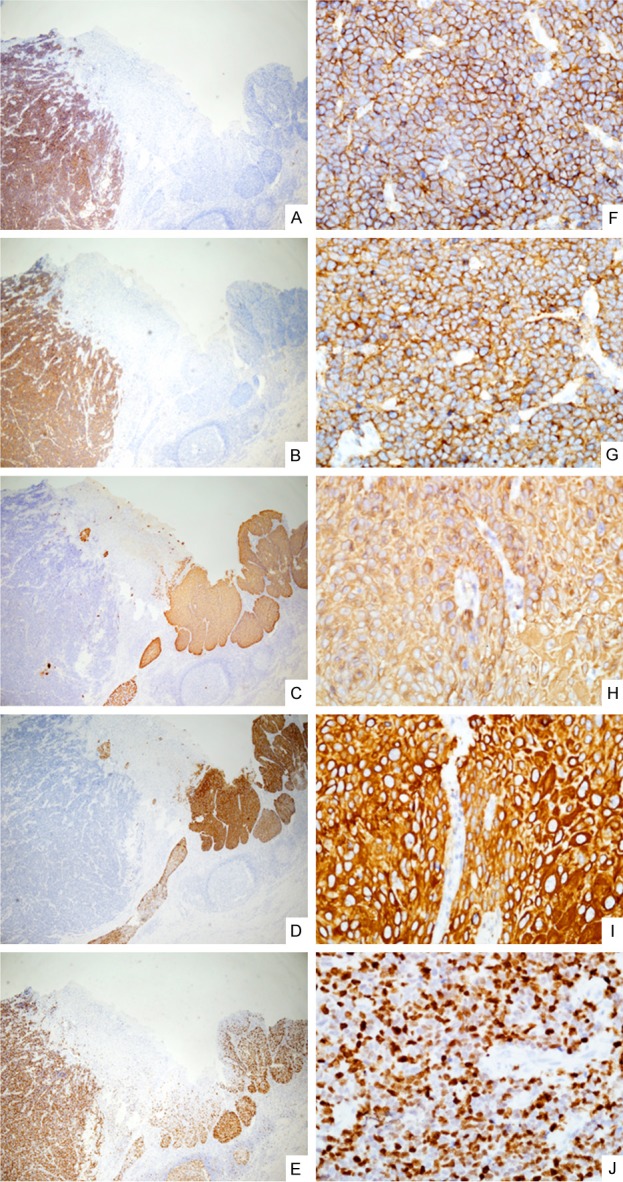
Immunohistochemical staining results. Positive for CD56 and Syn indicate small cell type NEC (A and B, ×40). Stains show a positive reaction in 34βE12, CK5/6, which identify SqCC (C and D, ×40). Strong positive for ki-67 (70%-80%, E, ×40). Magnifying views of A to E (F-J, ×400).
Discussion
Collision tumors are two different-origins tumors grow in proximity until they become juxtaposed, they need to be distinguished from composite tumors which characterized by two divergent lineages originating from the same neoplastic clonal proliferation [3]. Collision tumors have been described in various organs, such as lung [3], breast [4], urinary bladder [5], gastroesophageal junction [6], and esophagus, a fairly rare location [7,8]. The physiopathology of collision tumors is still unclear. The tumors cannot be easily detected, diagnosed and assigned to any of the established categories [6].
Neuroendocrine neoplasms are defined as neoplastic lesions, composed either by cells with a well-developed neuroendocrine phenotype or by cells with poorly developed but still recognizable and prominent neuroendocrine features [9]. The first case of esophageal NECs was reported by McKeown in 1952 [10]. NECs of esophagus are very rare, representing 0.04% to 4.6% of all gastrointestinal NETs [11,12]. Accounting for 0.5% to 5.9% of all esophageal cancers in Asian, and 1% to 2.8% in westerners [13,14]. Male-to-female ratio was 3.7-7 [13,14]. The reason for esophageal NECs is unclear, but it may related with tobacco and Barrett’s esophagitis [8,13-16].
NECs have been reported as a pure or mixed one [7,8,15]. Colliding with papillary adenocarcinoma, squamous cell carcinoma at esophagus have been reported, and NECs usually consist as the major part [14,15]. Classification of gastroenteropancreatic NENs was proposed by the WHO in 2010 are divided into low to intermediate-grade (grade 1-2) neuroendocrine tumors (NETs), and high-grade (grade 3) neuroendocrine carcinomas (NECs, large- or small-cell type), according to their histopathologic characterization (mitotic activity) and/or proliferation index (ki-67) [2,16]. The histological diagnosis of NENs is generally confirmed by immunohistochemistry neuroendocrine markers and thus to differentiate NETs from other tumor types [2,16,17]. CgA and Syn are the most common markers to confirm the endocrine nature of the neoplastic cells. CgA commonly demonstrate no or very mild in poorly differentiated NECs, but Syn can present in all NETs. Other general neuroendocrine markers such as protein cell product 9.5, CD56, Ecadherin, and p53 have been used in NETs but not as routine diagnostics [16,17].
Surgery is essential in many phases of NETs management, and in those with limited disease remains the primary method [8,9], but the results of surgery or radiotherapy alone generally were not satisfied compare with multi-modality therapies [9,13,15]. Personalized treatment should encouraged base on clinical characteristics, pathologic diagnosis, grade and staging classification.
NECs’ behavior can be highly aggressive and patients usually have a poor prognosis [9,11,13-15]. The median survival of patients after resection is 3.1 to 19 months in Asian and 5.3 to 20 months in westerners [14]. According to a study, patients with mixed NECs have more favorable prognosis than pure one [13].
The data on esophageal collision tumor are limited by the uncommon occurrence of this lesion. Careful assessment is essential before the final conclusion, and the treatments should warranted, depending on the type and degree of collision tumor encountered.
References
- 1.Wittekind C, Yamasaki S. eds. Digestive system tumours. In: Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 7th Edition. Hoboken, PA: Wiley-Blackwell; 2009. pp. 63–135. [Google Scholar]
- 2.Klöppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2011;18:S1–16. doi: 10.1530/ERC-11-0013. [DOI] [PubMed] [Google Scholar]
- 3.Nakata S, Nagata Y, Sugaya M, Yasuda M, Yamashita T, Takenoyama M, Hanagiri T, Morita M, Hamada T, Sugio K, Yasumoto K. Primary pulmonary collision cancer consisting of large cell carcinoma and adenocarcinoma. Ann Thorac Surg. 2005;80:340–342. doi: 10.1016/j.athoracsur.2003.12.053. [DOI] [PubMed] [Google Scholar]
- 4.Susnik B, Jordi Rowe J, Redlich PN, Chitambar C, Chang CC, Kampalath B. A unique collision tumor in breast: invasive ductal carcinoma and mucosa-associated lymphoid tissue lymphoma. Arch Pathol Lab Med. 2004;128:99–101. doi: 10.5858/2004-128-99-AUCTIB. [DOI] [PubMed] [Google Scholar]
- 5.Okumura K, Kato K, Furuhashi K, Suzuki K, Murase T. A collision cancer between urothelial carcinoma and malignant lymphoma of the urinary bladder: a case report. Hinyokika Kiyo. 2007;53:649–651. [PubMed] [Google Scholar]
- 6.Washizawa N, Kobayashi K, Kase H, Tokura N, Gotoh T, Iwasaki I, Tsujimoto S. Collision carcinoma at the esophagogastric junction. Gastric Cancer. 1999;2:240–243. doi: 10.1007/s101200050071. [DOI] [PubMed] [Google Scholar]
- 7.Wilson CI, Summerall J, Willis I, Lubin J, Inchausti BC. Esophageal collision tumor (large cell neuroendocrine carcinoma and papillary carcinoma) arising in a Barrett esophagus. Arch Pathol Lab Med. 2000;124:411–415. doi: 10.5858/2000-124-0411-ECTLCN. [DOI] [PubMed] [Google Scholar]
- 8.Dias AR, Sallum RA, Zalc N, Ctenas BB, Ribeiro U, Cecconello I. Squamous cell carcinoma and neuroendocrine carcinoma colliding in the esophagus. Clinics (Sao Paulo) 2010;65:114–7. doi: 10.1590/S1807-59322010000100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rindi G, Wiedenmann B. Neuroendocrine neoplasms of the gut and pancreas: new insights. Nat Rev Endocrinol. 2011;8:54–64. doi: 10.1038/nrendo.2011.120. [DOI] [PubMed] [Google Scholar]
- 10.McKeown F. Oat cell carcinoma of the oesophagus. J Pathol Bacteriol. 1952;64:889–891. doi: 10.1002/path.1700640420. [DOI] [PubMed] [Google Scholar]
- 11.Estrozi B, Bacchi CE. Neuroendocrine tumors involving the gastroenteropancreatic tract: a clinicopathological evaluation of 773 cases. Clinics (Sao Paulo) 2011;66:1671–5. doi: 10.1590/S1807-59322011001000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer. 1997;79:813–29. doi: 10.1002/(sici)1097-0142(19970215)79:4<813::aid-cncr19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Maru DM, Khurana H, Rashid A, Correa AM, Anandasabapathy S, Krishnan S, Komaki R, Ajani JA, Swisher SG, Hofstetter WL. Retrospective study of clinicopathologic features and prognosis of high-grade neuroendocrine carcinoma of the esophagus. Am J Surg Pathol. 1998;32:1404–1411. doi: 10.1097/PAS.0b013e31816bf41f. [DOI] [PubMed] [Google Scholar]
- 14.Huang Q, Wu H, Nie L, Shi J, Lebenthal A, Chen J, Sun Q, Yang J, Huang L, Ye Q. Primary high-grade neuroendocrine carcinoma of the esophagus: a clinicopathologic and immunohistochemical study of 42 resection cases. Am J Surg Pathol. 2013;37:467–83. doi: 10.1097/PAS.0b013e31826d2639. [DOI] [PubMed] [Google Scholar]
- 15.Gollard R, Ellis C, VanderHarten C. Small cell/neuroendocrine tumors of the esophagus: presentation of two cases and review of the literature. Tumori. 2010;96:780–3. doi: 10.1177/030089161009600524. [DOI] [PubMed] [Google Scholar]
- 16.Hirabayashi K, Zamboni G, Nishi T, Tanaka A, Kajiwara H, Nakamura N. Histopathology of gastrointestinal neuroendocrine neoplasms. Front Oncol. 2013;3:2. doi: 10.3389/fonc.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capelli P, Fassan M, Scarpa A. Pathology - grading and staging of GEP-NETs. Best Pract Res Clin Gastroenterol. 2012;26:705–717. doi: 10.1016/j.bpg.2013.01.003. [DOI] [PubMed] [Google Scholar]


