Abstract
The serotonin 5-HT2A receptor is a primary target of psychedelic hallucinogens such as lysergic acid diethylamine, mescaline, and psilocybin, which reproduce some of the core symptoms of schizophrenia. An incompletely resolved paradox is that only some 5-HT2A receptor agonists exhibit hallucinogenic activity, whereas structurally related agonists with comparable affinity and activity lack such a psychoactive activity. Using a strategy combining stable isotope labeling by amino acids in cell culture with enrichment in phosphorylated peptides by means of hydrophilic interaction liquid chromatography followed by immobilized metal affinity chromatography, we compared the phosphoproteome in HEK-293 cells transiently expressing the 5-HT2A receptor and exposed to either vehicle or the synthetic hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) or the nonhallucinogenic 5-HT2A agonist lisuride. Among the 5995 identified phosphorylated peptides, 16 sites were differentially phosphorylated upon exposure of cells to DOI versus lisuride. These include a serine (Ser280) located in the third intracellular loop of the 5-HT2A receptor, a region important for its desensitization. The specific phosphorylation of Ser280 by hallucinogens was further validated by quantitative mass spectrometry analysis of immunopurified receptor digests and by Western blotting using a phosphosite specific antibody. The administration of DOI, but not of lisuride, to mice, enhanced the phosphorylation of 5-HT2A receptors at Ser280 in the prefrontal cortex. Moreover, hallucinogens induced a less pronounced desensitization of receptor-operated signaling in HEK-293 cells and neurons than did nonhallucinogenic agonists. The mutation of Ser280 to aspartic acid (to mimic phosphorylation) reduced receptor desensitization by nonhallucinogenic agonists, whereas its mutation to alanine increased the ability of hallucinogens to desensitize the receptor. This study reveals a biased phosphorylation of the 5-HT2A receptor in response to hallucinogenic versus nonhallucinogenic agonists, which underlies their distinct capacity to desensitize the receptor.
Among the G Protein-Coupled Receptors (GPCRs)1 activated by serotonin (5-hydroxytryptamine, 5-HT), the 5-HT2A receptor continues to attract particular attention in view of its broad physiological role and implication in the actions of numerous psychotropic agents (1, 2). It is a primary target of widely used atypical antipsychotics such as clozapine, risperidone, and olanzapine, which act as antagonists or inverse agonists (1, 3). The activation of 5-HT2A receptors expressed in the prefrontal cortex has also been implicated in the psycho-mimetic effects of psychedelic hallucinogens, such as lysergic acid diethylamide (LSD), mescaline, and psilocybin, which are often used to model positive symptoms of schizophrenia (4–8). However, these psychoactive effects are not reproduced by structurally-related agonists, such as ergotamine and the anti-Parkinson agent lisuride, despite the fact that they exhibit comparable affinities and efficacies at 5-HT2A receptors (7, 9). This paradox was partially resolved by the demonstration that hallucinogens induce a specific transcriptomic signature because of the specific engagement of a Pertussis toxin-sensitive Gi/o/Src signaling pathway which is not activated by nonhallucinogenic agonists (7, 8). These findings suggest that hallucinogenic and nonhallucinogenic agonists induce different conformational states of the 5-HT2A receptor, and represent a striking example of functional selectivity that translates into contrasting pattern of mice behavior: induction of head-twitches by hallucinogenic but not by nonhallucinogenic agonists (9).
The differential influence of hallucinogenic versus nonhallucinogenic agonists on signaling pathways suggests that they trigger contrasting patterns of protein phosphorylation. To address this issue, we employed a quantitative phosphoproteomics strategy to directly compare the phosphoproteomes generated in HEK-293 cells by the synthetic hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) and the nonhallucinogenic 5-HT2A agonist lisuride. We found that DOI, but not lisuride, induced the phosphorylation of a serine residue (Ser280) located in the third intracellular loop of the receptor itself. The hallucinogen-specific phosphorylation of this residue was further validated in vitro and in vivo by using a phosphosite-specific antibody. These findings were followed by a series of experiments to determine the impact of Ser280 phosphorylation upon receptor desensitization and internalization.
EXPERIMENTAL PROCEDURES
Materials
Human Embryonic Kidney-293 (HEK-293) cells were from the European Collection of Cell Cultures, culture media from Invitrogen (Carlsbad, CA). Lisuride maleate was from Santa Cruz Biotechnologies (Santa Cruz, CA). All other chemicals were from Sigma Aldrich. Isotope-labeled amino acids for SILAC experiments were from Eurisotop (Saint Aubin, France).
The rabbit anti-phospho-Thr202/Tyr204-Erk1,2, and anti-total Erk1,2 antibodies were from Cell Signaling Technology (Danvers, MA), the rabbit anti-Hemagglutinin (HA) antibody from Zymed Laboratories Inc. (South San Francisco, CA), the rabbit anti-GFP antibody from Roche Diagnostics, the rabbit anti-5-HT2A receptor antibody from Immunostar (Hudson, MI) and the mouse anti-HA antibody conjugated to agarose beads from Sigma Aldrich. The anti-phosphoSer280-5-HT2A receptor antibody was generated by immunizing rabbits with the synthetic GTRAKLApSFSFL+C peptide coupled to Keyhole Limpet Hemocyanin (KLH, Eurogentec, Liege, Belgium).
The construct encoding the HA-tagged 5-HT2A receptor was described elsewhere (10). Following PCR amplification, the receptor cDNA was subcloned into the bicistronic plasmid pIRES2-EGFP (Clontech, Mountain View, CA) using the XhoI/BamhI restriction sites. This construct was transferred to pSinRep5 plasmid for Sindbis virus production (11). Plasmids encoding HA-tagged 5-HT2A receptor mutants (S280A and S280D) were generated using the Quick Change mutagenesis kit (Stratagene, La Jolla, CA). All constructs were confirmed by DNA sequencing.
Cell Cultures
HEK-293 cells, grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% dialyzed, heat-inactivated fetal calf serum and antibiotics, were transfected at 40–50% confluence using polyethyleneimine (PEI, Sigma-Aldrich), as previously described (12), and used 48 h after transfection. For stable isotope labeling by amino acids in cell culture (SILAC) experiments (13), cells were maintained for 2 weeks in DMEM deficient in lysine and arginine, supplemented with 10% dialyzed serum and either l-lysine/l-arginine for light label (K0R0, L) or l-Lysine-2HCl (2H4, 96–98%)/l-Arginine-HCl (13C6, 99%) for semi-heavy label (K4R6, M) or l-Lysine-2HCl (13C6, 99%; 15N2, 99%)/l-Arginine-HCl (13C6, 99%; 15N4, 99%) for heavy label (K8R10, H) (percentages represent the isotopic purity of the labeled amino acids). Under these conditions, analysis of semi-heavy amino acid incorporation at the protein level indicated a median ratio of 93% (first quartile at 88%, third quartile at 95%). A similar distribution was observed for the incorporation of the heavy amino acids.
Primary cultures of cortical neurons were prepared as described previously (14). Briefly, dissociated cells from the cerebral cortex of 17 day-old Swiss mouse embryos were plated on 6- or 96-well plates coated successively with poly-l-ornithine (mol. Wt. = 40,000; 15 μg/ml) and 10% fetal calf serum + 1 μg/ml laminin. The culture medium included a 1:1 mixture of DMEM and F-12 nutrient supplemented with 33 mm glucose, 2 mm glutamine, 13 mm NaHCO3, 5 mm HEPES buffer, pH 7.4, 5 IU/ml (5 mg/ml) penicillin-streptomycin, and a mixture of salt and hormones containing 100 μg/ml transferin, 25 μg/ml insulin, 20 nm progesterone, 60 nm putrescine, and 30 nm Na2SeO3. Cultures were infected 5 days after seeding with the Sindbis virus expressing HA-tagged 5-HT2A receptor and were used 7 days after seeding. At this stage, they were shown to contain at least 95% of neurons (14).
Global Quantitative Phosphoproteomics Analyses
HEK-293 cells grown in SILAC media and transiently expressing 5-HT2A receptors were serum-starved for 4 h and challenged for 15 min with either vehicle (L), or lisuride (1 μm, M), or DOI (1 μm, H). Cells were lysed in 0.5 ml of ice-cold lysis buffer (50 mm Tris-HCl, pH 7.5, 1 mm EGTA, 1% Triton X-100, 1 mm sodium orthovanadate, 50 mm sodium fluoride, 5 mm sodium pyrophosphate, 0.27 m sucrose, 1 mm DTT). Cell lysates were clarified by centrifugation at 15,000 × g (20 min at 4 °C) and protein concentration was determined using the Bradford reagent. Equal amounts of proteins (3 mg) from each condition were mixed, reduced with 10 mm DTT, alkylated with 50 mm iodoacetamide and precipitated on ice with trichloroacetic acid (25%, 20 min) before their digestion with trypsin (1/200, w/w) in 2 m urea, 25 mm triethylammonium bicarbonate pH 7.8. Digests were acidified in 1% TFA, desalted on a 1-g Sep-Pak cartridge (Waters, Milford, MA), and subjected to hydrophilic interaction liquid chromatography (HILIC) using a 4.6 × 250-mm TSKgel Amide-80 5-μm particle column (Tosoh Biosciences) and an Alliance e2695 HPLC system (Waters), as previously described (15). Nine mg of peptides were loaded in 80% solvent B (100% acetonitrile with 0.1% TFA). Solvent A consisted of 0.1% TFA in water. Peptides were eluted with a gradient consisting of 80% B held for 5 min followed by 80% B to 60% B in 40 min and finally 0% B for 5 min. Fourteen fractions were collected throughout the gradient and further enriched in phosphorylated peptides by immobilized metal affinity chromatography (IMAC) (15).
HILIC fractions were dried and resuspended in 25% acetonitrile/0.1% TFA and incubated for 3 h with 8 μl of Phos-Select beads (Sigma Aldrich) under agitation. Beads were rinsed twice with 100 μl of 25% acetonitrile/0.1% TFA and loaded on a microC18 ZipTip (Millipore). Phosphorylated peptides were then eluted in two steps with 30 μl of 0.4 m NH4OH and then with 30 μl of 50% acetonitrile. They were analyzed by nano-flow HPLC-nanoelectrospray ionization using a LTQ Orbitrap Velos mass spectrometer coupled to an Ultimate 3000 HPLC (Thermo Fisher Scientific). Desalting and preconcentration of samples were performed on-line on a Pepmap® precolumn (0.3 mm × 10 mm, Thermo Fisher Scientific, Courtaboeuf, France). A gradient consisting of 2–40% buffer B (3–33 min), 40–80% B (33–34 min), 80–0% B (49–50 min), and equilibrated for 20 min in 0% B (50–70 min) was used to elute peptides at 300 nL/min from a Pepmap® capillary (0.075 mm × 150 mm) reversed-phase column (LC Packings). Mass spectra were acquired using a top-10 collision-induced dissociation (CID) data-dependent acquisition (DDA) method. The LTQ-Orbitrap was programmed to perform a Fourier transform (FT) full scan (60,000 resolution) on 400–1400 Th mass range with the top ten ions from each scan selected for LTQ-MS/MS with multistage activation on the neutral loss of 24.49, 32.66, and 48.99 Th. FT spectra were internally calibrated using a single lock mass (445.1200 Th). Target ion numbers were 500,000 for FT full scan on the Orbitrap and 10,000 MSn on the LTQ.
The raw MS data were analyzed using the MaxQuant/Andromeda software (v. 1.2.2.5) (16) with a false discovery rate of less than 0.01 for peptides and phosphosites and a minimum peptide length of six amino acids. The mass accuracy of the precursor ions was improved by retention time-dependent mass recalibration. Andromeda was used to search the top eight per 100 Da peak lists against the human complete proteome set database (http://www.uniprot.org/uniprot/?query = organism:9606+keyword:1185) downloaded on February 22, 2012 (65,835 protein entries), combined with 248 frequently observed contaminants as well as reversed versions of all sequences. This version of the database contains both reviewed sequences from UniProtKB/Swiss-Prot and unreviewed sequences from UniProtKB/TrEMBL. Enzyme specificity was set to trypsin, additionally allowing cleavage N-terminal to proline and up to two missed cleavages. The search included cysteine carbamidomethylation as a fixed modification, protein N-terminal acetylation, oxidation of methionine and phosphorylation of Ser, Thr, and Tyr as variable modifications. Peptide identification was based on a search with a mass deviation of the precursor ion up to 7 ppm after recalibration, and the allowed fragment mass deviation was set to 0.5 Da. Identifications across different replicates and adjacent fractions was performed using the “match between runs” MaxQuant option with a 3 min time window. Quantification of SILAC triplex signals was performed by MaxQuant with standard settings. The phosphoSTY.txt file generated by MaxQuant was uploaded onto Perseus software (v. 1.2.0.17) to calculate B significance of phosphopeptide ratios in each of the three biological replicates (16). The new table was then uploaded onto the R environment in order to plot log H/M ratios against log M/L ratios and color-display the B significance count (p < 0.05) for each quantified phosphopeptide.
Targeted Analysis of 5-HT2A Receptor Phosphorylation in HEK-293 Cells
HEK-293 cells transiently expressing HA-tagged 5-HT2A receptors were lysed in 50 mm Tris-HCl, pH 7.5, 1 mm EGTA, 1% Triton X-100, 1 mm sodium orthovanadate, 50 mm sodium fluoride, 5 mm sodium pyrophosphate, 0.27 m sucrose, 1 mm DTT, and a protease inhibitor mixture (Roche). Samples were centrifuged at 15,000 × g for 30 min at 4 °C. Solubilized 5-HT2A receptors were immunoprecipitated with the agarose-conjugated anti-HA antibody (Sigma Aldrich). Immunoprecipitated HA-5-HT2A receptors were resolved by SDS-PAGE. Gel bands containing the receptor were excised and digested with trypsin (500 ng per condition). Peptides were analyzed by nano-LC-FT-MS/MS, top six per 30 Da windows peak lists were extracted using MSconvert 3.0 and searched with Mascot 2.4 against the same human Complete Proteome Set database, with phosphorylation of Ser, Thr, and Tyr as variable modifications, 7 ppm precursor mass tolerance, 0.5 Da fragment mass tolerance and trypsin/P digestion. MS2 spectra matching phosphorylated peptides with ion score over 15 were inspected using Prophossi software (17) for automatic annotation of unique transitions that pinpoint the position of phosphorylation sites. Ion signals corresponding to phosphorylated peptides were quantified from the maximal intensities measured in their ion chromatograms manually extracted using Qual browser v2.1 (Thermo Fisher Scientific) with a tolerance of 5 ppm for mass deviation, and normalized to signals of their nonphosphorylated counterparts. Ser280 phosphorylation of immunoprecipitated receptors was also analyzed by Western blotting using the phosphosite specific antibody.
Western Blotting
Proteins, resolved onto 10% polyacrylamide gels, were transferred to Hybond C nitrocellulose membranes (GE Healthcare). Membranes were immunoblotted with primary antibodies (anti phospho-Ser280 5-HT2A receptor, 1:300; anti phospho-Thr202/Tyr204-Erk1,2, 1:1000; anti Erk1,2, 1:1000; anti-HA, 1:1000; Anti-GFP, 1:1000; anti-RSK2, 1:1000) and then with either anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibodies (1:3000, GE Healthcare). Immunoreactivity was detected with an enhanced chemiluminescence method (ECLTM plus detection reagent, GE Healthcare) and immunoreactive bands were quantified by densitometry using the ImageJ software. In protein phosphorylation analyses, the amount of each phosphoprotein was normalized to the amount of the corresponding total protein detected in the sample.
Analysis of Ser280 Phosphorylation in Mice Prefrontal Cortex
Experiments were performed on wild type or 5-HT2A receptor-deficient mice (8) and conformed to European ethics standards (86/609-EEC) and to decrees of the French National Ethics Committee (N° 87/848) for the care and use of laboratory animals. Mice (∼30 g) were injected intraperitoneal with either vehicle (5% DMSO/5% Tween 80) or DOI or lisuride (10 mg/kg each). Thirty min after the onset of the treatment, mice were anesthetized with pentobarbital (100 mg/kg intraperitoneal, Ceva SA) and rapidly perfused transcardiacally with fixative solution containing 4% w/v paraformaldehyde in 0.1 m sodium phosphate buffer (pH 7.5) containing NaF (100 mm) and sodium orthovanadate (1 mm). Brains were post-fixed for 48 h in the same solution and stored at 4 °C. Fifty micrometers-thick sections were cut with a vibratome (Leica), permeabilized with 0.2% Triton X-100 in Tris buffer saline (TBS) for 20 min, saturated for 1 h with 10% goat serum in TBS containing 0.03% Triton X-100 and incubated for 48 h at 4 °C with primary antibodies (anti phospho-Ser280 5HT2A receptor, 1:100 or anti 5-HT2A receptor, 1:500) in TBS. After four washes, they were incubated for 1 h with an Alexa Fluor® 488-conjugated anti-rabbit antibody (1:1000, Invitrogen) in TBS. Immunofluorescent staining was observed with a Zeiss Axioimager Z1 microscope equipped with apotome. Images were acquired using the Axiovision 4.8 software driving an AxioCam MRm CCD camera (Carl Zeiss Microimaging).
Inositol Phosphate Production
Inositol phosphate production was analyzed as previously described (18).
Immunocytochemistry and Fluorescent Microscopy
HEK-293 cells transiently expressing HA-tagged 5-HT2A receptors and grown on glass cover-slips were incubated with the rabbit anti HA antibody (1/500, 30 min at 10 °C) and then with drugs for 1 h at 37 °C. Cells were fixed in 4% (w/v) paraformaldehyde, 4% sucrose in PBS for 20 min, quenched 4 times 10 min with PBS containing 4% sucrose and 0.1 m glycine, and incubated for 60 min at 4 °C with an Alexa Fluor® 594-coupled anti-rabbit antibody (1:1000 in PBS supplemented with 2% goat serum, Invitrogen) to label cell surface receptors. They were then rinsed three times PBS containing 2% goat serum, permeabilized with 0.2% (w/v) Triton X-100 in PBS containing 2% goat serum for 15 min and incubated for 30 min at 4 °C with the Alexa Fluor® 488-coupled anti-rabbit antibody (1:1000 in PBS supplemented with 2% goat serum, 0.05% Triton X-100) to label internalized receptors. After three washes, coverslips were mounted on glass slides in Mowiol® 4.88 (Calbiochem). Series of optical sections were collected with a Zeiss Axioimager Z1 microscope equipped with apotome. Images were acquired using the Axiovision 4.8 software driving an AxioCam MRm CCD camera (Carl Zeiss Microimaging).
ELISA
Quantification of receptor cell surface expression was performed by ELISA under nonpermeabilized conditions as previously described (18).
RESULTS
Phosphoproteome Changes Elicited by DOI and Lisuride in HEK-293 Cells
To directly compare the phosphorylation patterns generated upon 5-HT2A receptor stimulation by a hallucinogenic (DOI) and by a nonhallucinogenic (lisuride) agonist, we used the SILAC technology under three experimental conditions: light condition (vehicle-treated cells), semiheavy label (lisuride-treated cells), and heavy label (DOI-treated cells). As cultured neurons do not divide in vitro, a complete SILAC labeling prerequisite for unbiased quantification could not be achieved in these cultures. Therefore, we performed our phosphoproteomics screen in HEK-293 cells transiently expressing 5-HT2A receptors. We first examined whether this model recapitulates the biased signaling at 5-HT2A receptors initially described in neurons, i.e. specific activation of Gi/o signaling by hallucinogens (7). Exposure of cells to DOI or LSD induced comparable stimulation of inositol phosphate production and Extracellular-regulated kinase (Erk)1,2 phosphorylation to those elicited by two nonhallucinogenic agonists, lisuride and ergotamine, though activation of PLC by DOI and Erk1,2 phosphorylation elicited by LSD were slightly more pronounced (supplemental Figs. S1A and S2A). Pre-treating cells with Pertussis toxin (PTX) decreased PLC activation induced by DOI and LSD (supplemental Fig. S1B, S1C) and abolished Erk1,2 phosphorylation induced by both hallucinogens (supplemental Fig. S2A), whereas PTX treatment did not affect the lisuride and ergotamine responses (Figs. S1D, S1E, and S2A). PTX likewise prevented Erk1,2 phosphorylation induced by DOI and LSD without affecting lisuride and ergotamine responses in primary cultured cortical neurons (supplemental Fig. S2B). These observations indicate that, similarly to the observations in neurons, hallucinogens selectively engage Gi/o-operated signaling in HEK-293 cells, whereas nonhallucinogenic agonists do not.
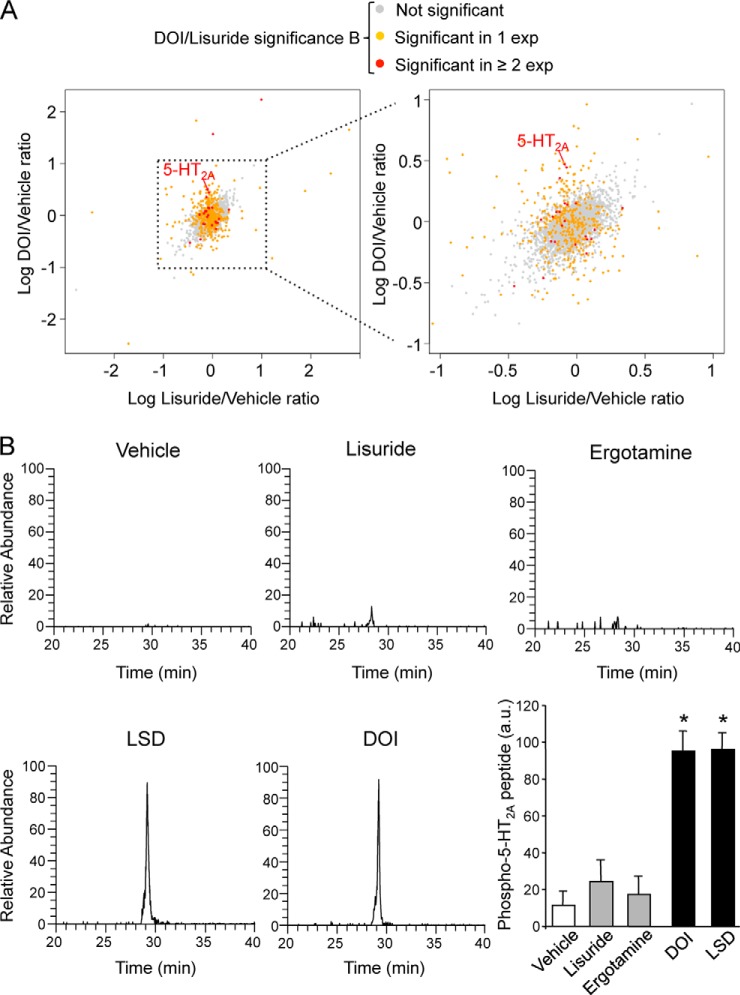
After a 15-min stimulation of stable isotope-labeled HEK-293 cells with either vehicle or DOI or lisuride, cells were harvested and 1:1:1 mixtures of differentially labeled samples were digested with trypsin and phosphopeptides were enriched by HILIC followed by IMAC. Analysis of phosphopeptide-enriched fractions by nano-LC-FT-MS/MS identified 5995 phosphorylated peptides with a false discovery rate of 1%. A total of 3349 phosphopeptides were robustly quantified in at least two out of the three biological replicates. As shown in Fig. 1A, the majority of them did not exhibit significant changes in abundance, assessed by statistical significance B (16), upon 5-HT2A receptor stimulation by DOI or lisuride (agonist/vehicle ratios ∼1). Only 30 phosphorylated peptides were significantly regulated by DOI versus vehicle and 24 following lisuride treatment (supplemental Table S1). Most importantly, 16 phosphopeptides were significantly different in abundance between DOI and lisuride-treated cells and 10 of them were significantly regulated by DOI, compared with vehicle (supplemental Table S1).
Fig. 1.
Hallucinogenic and nonhallucinogenic agonists differentially phosphorylate 5-HT2A receptor at Ser280. A, Results of the large-scale quantitative phosphoproteomic analysis comparing the phosphorylation events triggered by DOI and lisuride (1 μm each, 15 min) in HEK-293 cells transiently expressing 5-HT2A receptors. The x axis represents the relative abundance (expressed in log10 of the ratio) of each of the 3349 quantified phosphopeptides in lisuride versus vehicle-treated cells, the y axis their relative abundance in DOI versus vehicle-treated cells. B, HEK-293 cells transiently expressing HA-tagged 5-HT2A receptors were challenged for 15 min with vehicle or 1 μm of either LSD or DOI or lisuride, or ergotamine. Receptors were immunoprecipitated with the agarose bead-conjugated anti HA antibody, digested with trypsin and phosphorylated peptides were analyzed by MS/MS. The data illustrated show representative extracted ion chromatograms of the LApSFSFIPQSSISSEK peptide phosphorylated on a serine corresponding to Ser280 in the entire receptor sequence. Two other independent experiments performed on different sets of cultured cells yielded similar results. The histogram represents the means ± S.E. of ion signal intensities of the LApSFSFIPQSSISSEK peptide obtained in the three experiments. * p < 0.05 versus vehicle-treated cells.
Hallucinogens but Not Nonhallucinogenic Agonists Induce 5-HT2A Receptor Phosphorylation at Ser280 In Vitro and In Vivo
Among the phosphopeptides exhibiting the highest differences in abundance between DOI and lisuride-treated cells, we identified a peptide located in the third intracellular (i3) loop of the receptor itself and phosphorylated on three Ser residues (278LApSFSFIPQSpSISpSEK293, supplemental Table S1) corresponding to Ser280, Ser288, and Ser291 in the entire receptor sequence. To further analyze 5-HT2A receptor phosphorylation pattern and to confirm its differential phosphorylation by hallucinogenic and nonhallucinogenic agonists, receptors originating from vehicle- or agonist-treated cells were purified by immunoprecipitation and digested with trypsin. LC-MS/MS analysis of receptor digests identified several phosphorylated forms of the same peptide located in the receptor i3 loop (LASFSFIPQSSISSEK) (Table I). MS2 spectra matching phosphorylated peptides were inspected using Prophossi software (17) for automatic annotation of unique transitions that pinpoint the position of phosphorylation sites (supplemental Fig. S3). In addition to a unique monophosphorylated form (phosphorylated at Ser280), various doubly and triply phosphorylated forms corresponding to phosphorylation on Ser280 and either on Ser283 or Ser287 or Ser288 or Ser290 or Ser291 or on two of these residues) were also detected (Table I). Moreover, quantitative analysis of the corresponding ion signals from extracted ion chromatograms showed that the monophosphorylated peptide exhibited the highest relative abundance and that it was up-regulated by DOI or LSD exposure but not by lisuride or ergotamine (Table I, Fig. 1B, and supplemental Fig. S4). Though less abundant, the other multi-phosphorylated forms were likewise specifically up-regulated by hallucinogenic agonists (Table I and supplemental Fig. S4). In addition, these analyses identified another cluster of phosphorylated serines (Ser298 and Ser305) in a different receptor i3 loop peptide (298SIHREPGSYTGR309) (Table I and supplemental Fig. S4). Both mono-phosphorylated (at Ser298) and doubly phosphorylated forms of this peptide were detected. However, the basal level of phosphorylation of these residues was weakly increased by both hallucinogens and nonhallucinogenic agonists (Table I and supplemental Fig. S4).
Table I. List of phosphorylated peptides identified from purified 5-HT2A receptors by nano-LC-MS/MS. HEK-293 cells transiently expressing HA-tagged 5-HT2A receptors were exposed to either Vehicle or DOI or LSD or lisuride or ergotamine (1 μm each, 15 min). Solubilized receptors were immunoprecipitated with the anti HA antibody, resolved by SDS-PAGE and digested in-gel with trypsin. Peptides were analyzed by nano-LC-MS/MS using multistage activation on the neutral loss of phosphoric acid. MS/MS spectra were manually interpreted. For each peptide, the position of modified residue(s), the position in the protein sequence, experimental mass/charge, theoretical mass, mass deviation, Mascot score, and relative abundance compared with the non-phosphorylated peptide (site occupancy index: maximal intensity observed in the phosphorylated peptide extracted ion chromatogram/sum of the maximal intensities observed in the phosphorylated and the nonphosphorylated peptide extracted ion chromatograms) are indicated. The data are representative of three independent experiments. ND: not determined.
| Modified sequence | Start-end | Exp m/z (Th) | Mass (Da) | Δ mass (ppm) | Mascot score | Phosphorylation site occupancy index |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vehicle | LSD | DOI | Lisuride | Ergo | ||||||
| K.LAsFSFLPQSSLSSEK.L + Phospho (ST) | 278–293 | 904.4283 | 1806.8441 | −1.11 | 44 | 0.327 | 13.286 | 20.727 | 1.304 | 0.842 |
| K.LAsFSFLPQSSLSsEKLFQR.S + 2 Phospho (ST) | 278–297 | 811.3806 | 2431.1226 | −1.05 | 40 | 0.076 | 1.223 | 1.793 | 0.318 | 0.277 |
| K.LAsFSFLPQSSLsSEKLFQR.S + 2 Phospho (ST) | 278–297 | 811.3813 | 2431.1226 | −0.23 | 25 | 0.076 | 1.223 | 1.793 | 0.318 | 0.277 |
| K.LAsFSFLPQSSLSsEKLFQR.S + 3 Phospho (ST) | 278–297 | 838.035 | 2511.0889 | −2.22 | 21 | ND | 0.173 | 0.229 | 0.085 | 0.110 |
| R.sIHREPGSYTGR.R + Phospho (ST) | 298–309 | 480.5517 | 1438.6354 | −1.6 | 80 | 0.104 | 0.280 | 0.252 | 0.308 | 0.230 |
| R.SIHREPGsYTGR.R + Phospho (ST) | 298–309 | 480.5522 | 1438.6354 | −0.5 | 19 | 0.104 | 0.280 | 0.252 | 0.308 | 0.230 |
| R.sIHREPGsYTGRR.T + 2 Phospho (ST) | 298–310 | 559.2409 | 1674.7028 | −1.18 | 20 | 0.117 | 0.309 | 0.357 | 0.407 | 0.530 |
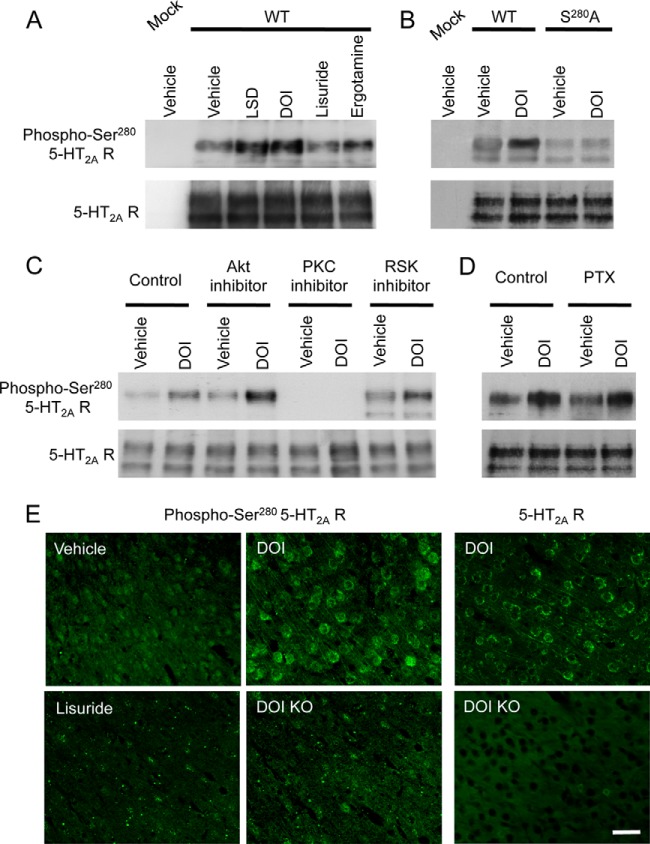
Given the apparent higher stoichiometry of Ser280 phosphorylation, compared with the phosphorylation of other residues, and the specific induction of its phosphorylation by hallucinogens, we produced a rabbit antibody against a phosphopeptide encompassing phosphorylated Ser280. We first validated the specificity of this antibody for the phosphorylated site by Western blotting using transfected HEK-293 cells. Although no immunoreactive signal was detected in blots from nontransfected cells, a clear signal was observed at the expected receptor size in blots obtained from cells expressing 5-HT2A receptor, and this immunoreactivity signal increased upon cell exposure to DOI or LSD, but not to ergotamine or lisuride (Fig. 2A). In contrast, DOI exposure did not increase the immunoreactive signal in blots obtained from cells expressing 5-HT2A receptors mutated on Ser280 (S280A, Fig. 2B and supplemental Fig. S5). Moreover, the signal observed in the absence of agonist treatment was lower in cells expressing mutant 5-HT2A receptors than in cells expressing wild type receptors (Fig. 2B). Collectively, these findings demonstrate a strong specificity of this antibody for phosphorylated Ser280 and further confirm the unique capacity of hallucinogens to promote phosphorylation of this residue, compared with nonhallucinogenic agonists.
Fig. 2.
Validation of the differential phosphorylation of Ser280 by hallucinogenic and nonhallucinogenic 5-HT2A agonists using a phosphosite antibody. A-B, An antibody raised against the GTRAKLApSFSFL+C peptide was validated in HEK-293 cells transiently expressing HA-5-HT2A receptors. Receptors were immunoprecipitated with the agarose bead-conjugated anti HA antibody. The generated antibody provided an immunoreactive signal in Western blots from cells expressing HA-5-HT2A receptors at a molecular weight corresponding to the signal obtained with the anti-HA antibody. This immunoreactive signal increased when cells were treated for 15 min with LSD or DOI but not with lisuride or ergotamine (1 μm each). Moreover, the signal was strongly attenuated in cells expressing Ser280A receptors (exposed or not to DOI), compared with cells expressing the wild type (WT) receptor. C-D, Impact of a 30-min cell pretreatment with NPC-15437 (20 μm, PKC inhibitor), or GSK690693 (1 μm, PKB inhibitor) or SL-0101–1 (10 μm, RSK inhibitor) and of a 18 h treatment with PTX (0.2 μg/ml) upon DOI-elicited Ser280 phosphorylation. Representative immunoblots of three independent experiments performed on different sets of cultured cells are illustrated. E, Immunofluorescence detection of cells positive for phospho-Ser280 5-HT2A receptor or 5-HT2A receptor in the prefrontal cortex of wild type and 5-HT2A receptor-deficient mice (four mice analyzed per condition) injected intraperitoneally with either vehicle or DOI or lisuride (10 mg/kg, intraperitoneal). Scale bar: 40 μm.
To explore whether biased 5-HT2A receptor phosphorylation occurs in vivo, we injected mice with either DOI or lisuride and examined 5-HT2A receptor phosphorylation in various brain regions known to express the receptor by immunohistochemisty. A robust immunostaining with the anti-phospho-Ser280 antibody was only detected in mice treated with DOI, but not in mice treated with vehicle or lisuride and the highest signal was found in middle layers of prefrontal cortex, which are known to express highest receptor densities (19) and which exhibited the strongest immunoreactive signal with a commercial antibody recognizing 5-HT2A receptor independently of its phosphorylation state (Fig. 2E). As expected and further supporting the specificity of our antibody for phospho-Ser280 5-HT2A receptor, no immunoreactive signal was detected in prefrontal cortex of DOI-treated 5-HT2A receptor knockout mice (Fig. 2E).
Phosphorylation of 5-HT2A Receptors by Hallucinogens is Protein Kinase C-dependent and Gi/o-independent
We next searched for consensus motifs of phosphorylation by kinases in the Ser280 flanking sequence by using two different algorithms: (1) Scansite, which defines scores for phosphorylation sites according to a matrix based on an oriented peptide library to determine the optimal substrates of protein kinases (20), and (2) Group-based Prediction System (GPS, v2.1), which classifies protein kinases into a hierarchical structure with four levels and trains its algorithm against the PhosphoELM database (21), in order to determine individual false discovery rate for each kinase (22). GPS found a strong consensus for Akt/protein kinase B (PKB) (5.7/3.8) and ribosomal S6 kinases (RSKs, 2.0/1.9), whereas Scansite indicated a strong consensus for phosphorylation by protein kinase C delta (PKCδ, score 0.3926, percentile 0.143%) and PKB (0.5238, 0.341%). Thus, pharmacological inhibitors of PKC (NPC-15437, 20 μm) (23), PKB (GSK690693, 1 μm) (24), and RSKs (SL-0101–1, 10 μm) (25) were tested for a potential effect on DOI-elicited Ser280 phosphorylation. Neither GSK690693 nor SL-0101–1 had any effect on Ser280 phosphorylation in response to DOI, indicating that RSKs and PKB are not involved in this phosphorylation (Fig. 2C). In contrast, pretreatment of cells with NPC-15437 strongly decreased basal Ser280 phosphorylation and abolished the DOI-elicited response, indicating that Ser280 phosphorylation induced by hallucinogens was dependent on PKC activity (Fig. 2C). Moreover, treatment of cells with PTX did not affect Ser280 phosphorylation elicited by DOI (Fig. 2D).
Hallucinogenic and Nonhallucinogenic Agonists Differentially Desensitize and Internalize the 5-HT2A Receptor
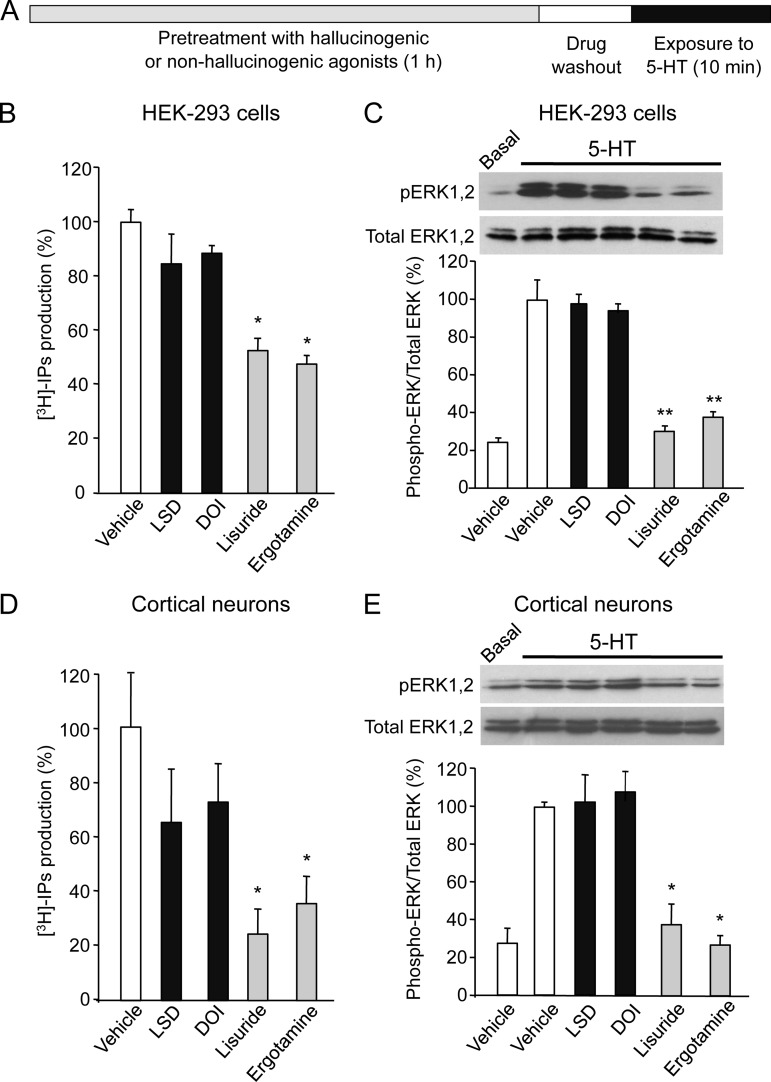
Given the role of PKC in 5-HT2A receptor desensitization and internalization and the importance of the 5-HT2A receptor i3 loop in the regulation of receptor responsiveness (26–29), we next examined whether hallucinogenic and nonhallucinogenic agonists differentially modulate receptor desensitization. Pretreatment of HEK-293 cells with either lisuride or ergotamine for 1 h, followed by extensive drug washout, inhibited inositol phosphate production induced by a further exposure of cells to 5-HT, whereas pretreating cells with DOI or LSD did not significantly affect the 5-HT response (Figs. 3A and 3B). The most pronounced difference in receptor desensitization (nonsignificant desensitization upon hallucinogen stimulation versus ∼50% desensitization upon receptor stimulation by nonhallucinogenic agonists) was observed after a 1 h treatment. After a 2 h treatment, both DOI and LSD desensitized the receptor, though to a differing extent (26 ± 4% desensitization) when compared with cells exposed for 2 h with lusuride or ergotamine (61 ± 5% desensitization). Therefore, 1 h preexposures to drugs were undertaken in further experiments. Treatment of HEK-293 cells with ergotamine and lisuride, but not with LSD and DOI, likewise induced strong desensitization of 5-HT2A receptor-operated Erk1,2 signaling (Fig. 3C). Hallucinogenic and nonhallucinogenic agonists produced a similar differential pattern of desensitization at 5-HT2A receptor-transduced signaling in primary cultures of cortical neurons (Figs. 3D and 3E).
Fig. 3.
Hallucinogenic and nonhallucinogenic agonists differentially desensitize 5-HT2A receptors. A, Schema of the experimental paradigm used to investigate the desensitization of 5-HT2A receptor induced by hallucinogenic and nonhallucinogenic agonists in HEK-293 cells and neurons. B and D, Effect of a 1 h pretreatment with either DOI or LSD or lisuride or ergotamine (1 μm each) upon inositol phosphate production elicited by 5-HT (10 μm) in HEK-293 cells (B) and neurons (D). Data, expressed in % of the 5-HT-elicited response in cells pretreated with vehicle are the means ± S.E. of values obtained in three independent experiments performed on different sets of cultured cells. * p < 0.05 versus vehicle-pretreated cells (ANOVA followed by Dunnett's test). C and E, Effects of the corresponding treatments upon 5-HT-elicited Erk1,2 phosphorylation, assessed by sequential immunoblotting with an antibody against phosphorylated Erk1,2 (Thr202-Tyr204) and an antibody recognizing Erk1,2 independently of their phosphorylation state. Immunoblots representative of three independent experiments are shown. Data, expressed as ratios of phosphorylated to total Erk1,2, represent the means ± S.E. of values obtained in the three independent experiments. * p < 0.05, ** p < 0.01 versus cells pretreated with vehicle.
As GPCR internalization is often important for desensitization, we also explored whether hallucinogenic and nonhallucinogenic agonists differentially affect receptor internalization by immunostaining cell-surface receptors in living cells before a 1 h exposure to a hallucinogenic or a nonhallucinogenic agonist. The proportion of internalized 5-HT2A receptors was much higher in cells treated with lisuride or ergotamine compared with cells exposed to DOI or LSD (Fig. 4A). The higher propensity of nonhallucinogenic agonists to internalize the receptor, compared with hallucinogens, was further confirmed by ELISA (Fig. 4B). Given the importance of the receptor i3 loop in its association with β-arrestins (30), we also compared the ability of the two agonist categories to promote β-arrestin2 recruitment by the receptor. 5-HT2A receptors recruited larger amounts of β-arrestin2 in cells treated ergotamine or lisuride than in cells exposed to LSD or DOI (Fig. 5C), corroborating their differential efficacy to promote receptor internalization.
Fig. 4.
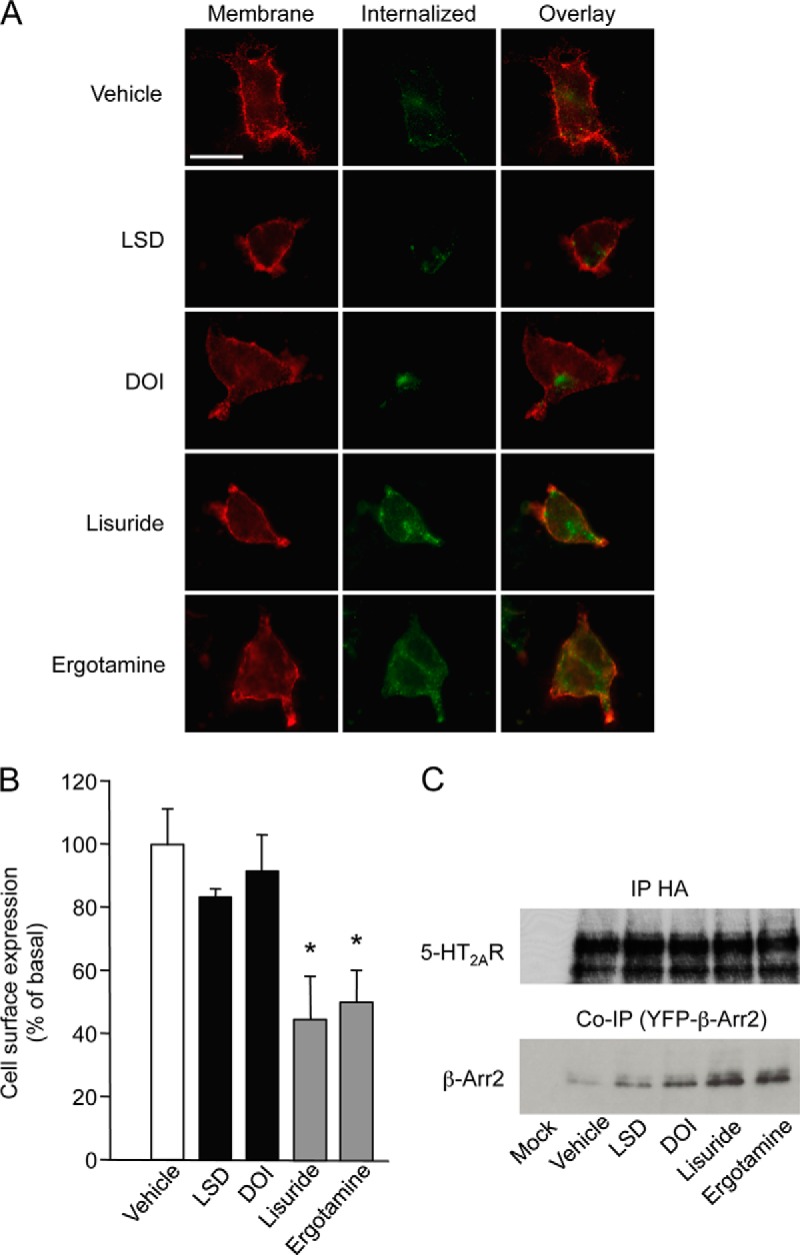
Hallucinogenic and nonhallucinogenic agonists differentially internalize 5-HT2A receptors. A, HEK-293 cells transiently expressing HA-tagged 5-HT2A receptors were treated for 30 min at 10 °C with a rabbit anti HA antibody and then with either vehicle or DOI or LSD or lisuride or ergotamine (1 μm each) for 1 h at 37 °C. Cell surface receptors were labeled with the Alexa Fluor® 594-coupled anti-HA antibody (red channel) and internalized receptor with the Alexa Fluor® 488-coupled anti-HA antibody (green channel). Double immunofluorescence staining of receptors in single cells is shown. Representative images of three independent experiments are illustrated. B, Quantification of cell surface expression of receptors in cells exposed to the same treatments was performed by ELISA in nonpermeabilizing conditions. Data are means ± S.E. of quadruplicate determinations performed in a representative experiment. Two other independent experiments yielded similar results. * p < 0.05 versus vehicle-treated cells (ANOVA followed by Dunnett's test). C, The data illustrated show the differential recruitment of β-arrestin2 by the 5-HT2A receptor (assessed by co-immunoprecipitation) in cells co-expressing HA-tagged 5-HT2A receptor and YFP-tagged β-arrestin2 and exposed to the same treatments. The Western blots illustrated are representative of three independent experiments.
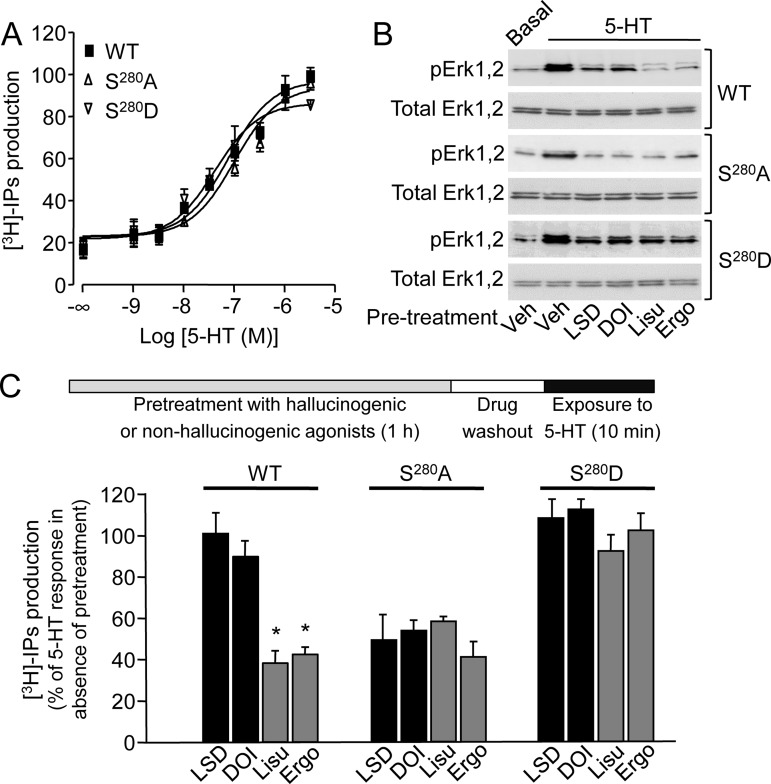
Fig. 5.
Ser280 phosphorylation underlies differential 5-HT2A receptor desensitization by hallucinogenic and nonhallucinogenic agonists. A, Inositol phosphate production induced by incremental concentrations of 5-HT in HEK-293 cells transiently expressing wild type or S280A or S280D 5-HT2A receptors. Data, expressed in % of the maximal 5-HT response in cells expressing wild type receptors are the means ± S.E. of quadruplicate determinations performed in a typical experiment. Two other experiments performed on different sets of cultured cells yielded similar results. B, Effect of a 1 h pretreatment with either vehicle (Veh) or DOI or LSD or lisuride or ergotamine (1 μm each) upon ERK1,2 phosphorylation elicited by 5-HT (10 μm) in HEK-293 cells expressing wild type or S280A or S280D 5-HT2A receptors. Immunoblots representative of three independent experiments are shown. C, Effect of the corresponding treatments upon inositol phosphate production elicited by 5-HT (10 μm) in HEK-293 cells expressing wild type or S280A or S280D 5-HT2A receptors. Data, expressed in % of the 5-HT-elicited response in cells pretreated with vehicle, are the means ± S.E. of values obtained in three independent experiments. * p < 0.05 versus vehicle-pretreated cells (ANOVA followed by Dunnett's test).
Ser280 Phosphorylation Underlies Differential Desensitization of the 5-HT2A Receptor by Hallucinogenic and Nonhallucinogenic Agonists
To directly determine the pertinence of Ser280 phosphorylation upon 5HT2A receptor desensitization, this residue was mutated into alanine or aspartate to inhibit or mimic its phosphorylation, respectively. Wild type, S280A and S280D 5-HT2A receptors displayed the same intrinsic efficacy in stimulating PLC and Erk1,2 upon activation by 5-HT (Figs. 5A and 5B). Nonetheless, the differential ability of hallucinogenic and nonhallucinogenic agonists to desensitize the receptor was not observed in cells expressing S280A or S280D 5-HT2A receptors: hallucinogenic as well as nonhallucinogenic agonists induced a strong desensitization of both PLC and Erk1,2 signaling in cells expressing the S280A receptor (to an extent comparable with that measured in cells expressing the wild type receptor after ergotamine or lisuride pretreatment), whereas all the four agonists tested induced a weak desensitization of receptor-operated signaling in cells expressing S280D 5-HT2A receptor, as observed in cells expressing wild type receptors following treatment with DOI or LSD (Figs. 5B and 5C).
DISCUSSION
The concept of functional selectivity or biased agonism was initially thought to reflect the ability of specific ligands of a given GPCR to induce or stabilize different active conformations capable of activating distinct signaling pathways (31–33). The difference in 5-HT2A receptor-operated signaling upon activation by hallucinogenic and nonhallucinogenic agonists represents one of the most remarkable examples of functional selectivity so far characterized (9). More recently, phosphorylation of GPCRs at specific sites has emerged as one mechanisms contributing to functional selectivity (34) and several studies have revealed the capacity of different ligands of a given receptor to promote preferential receptor phosphorylation at distinct sites (35–38). The present report likewise demonstrated the ability of a subset of 5-HT2A receptor agonists to induce phosphorylation of one well-defined site, despite a similar intrinsic efficacy to transduce signals. Biased 5-HT2A receptor phosphorylation elicited by the different agonists tested correlated with their behavioral outcomes and might represent one critical step underlying functional selectivity at these receptors.
Mass spectrometry analyses identified several phosphorylated forms of the same peptide located in the receptor i3 loop (LASFSFIPQSSISSEK). These included a unique monophosphorylated form (phosphorylated at Ser280), and several less abundant doubly or triply phosphorylated forms systematically phosphorylated at Ser280. All these phosphorylated peptides were up-regulated by hallucinogens but not by nonhallucinogenic agonists. Collectively, these observations clearly identify Ser280 phosphorylation as the primary event governing further phosphorylation of downstream serine residues. Differential phosphorylation of Ser280 upon receptor activation by hallucinogenic and nonhallucinogenic agonists was further established in vivo following systemic administration of these compounds to mice, by using a phosphosite specific antibody. Further supporting the relevance of Ser280 phosphorylation, the most prominent phospho-Ser280 immunoreactive signal in mice treated with a hallucinogenic agonist was detected in prefrontal cortex, the brain region involved in the psychomimetic effects of hallucinogens (8).
We identified another cluster of phosphorylated serines (Ser298 and Ser305) in a receptor i3 loop peptide (SIHREPGSYTGR). Both monophosphorylated (at Ser298) and doubly phosphorylated forms of this peptide were detected in the present study, in contrast with a previous large-scale analysis of synapse phosphoproteome in the mouse, which only identified phosphorylated Ser298 (39). The phosphorylation of Ser298 and Ser305 was weakly induced by both hallucinogens and nonhallucinogenic agonists. Moreover, our studies did not detect phosphorylation of Ser314, another serine located in the receptor i3 loop previously identified as a RSK2 substrate (26). Notably, phosphorylation of this residue was detected in vitro by incubating receptor i3 loop or the entire purified 5-HT2A receptor with recombinant RSK2, whereas the present study investigated the receptor phosphorylation state in HEK-293 cells. Further experiments suggested a role of Ser314 phosphorylation in attenuation of 5-HT2A receptor signaling induced by EGF and PDGF in a variety of cell types, including neurons (40). Our results suggest that in the absence of growth factors, Ser314 phosphorylation might occur at a lower stoichiometry than the other phosphorylated residues identified in the present study, even upon agonist stimulation of 5-HT2A receptors. Together with previous findings, they also identify the receptor i3 loop, which contains 18 potential phosphorylation sites for Ser/Thr kinases, as a hot spot of phosphorylation potentially important for regulating receptor functional activity.
In an effort to identify protein kinase(s) contributing to hallucinogen-elicited Ser280 phosphorylation, we found that it was dependent on PKC activity, though we cannot conclude at this stage whether PKC directly phosphorylates Ser280 or whether the phosphorylation of this residue is elicited by a closely related kinase different from RSK and PKB and activated by PKC. This observation was quite unexpected as both hallucinogenic and nonhallucinogenic agonists activate the PLC pathway and therefore PKC. Moreover, PTX treatment did not affect DOI-elicited Ser280 phosphorylation, indicating that biased 5-HT2A receptor phosphorylation is not triggered by a pathway (Gi/o-dependent) selectively engaged by hallucinogens but rather by a common pathway activated by both hallucinogenic and hallucinogenic agonists. We thus hypothesize that Ser280 might be accessible for PKC phosphorylation only in a 5-HT2A receptor conformation that is specifically stabilized by hallucinogens.
Another important finding of the present study is the different ability of hallucinogenic and nonhallucinogenic agonists to induce 5-HT2A receptor desensitization and internalization, observed in both HEK-293 cells and cortical neurons. To our knowledge, no study has so far compared the ability of hallucinogenic and nonhallucinogenic compounds to desensitize 5-HT2A receptors. Nonetheless, the present findings are consistent with a recent study, which demonstrated that DOI was less efficient than 5-HT to internalize eGFP-tagged 5-HT2A receptors stably expressed in HEK-293 cells (27). They also provide convergent evidence indicating that the different effects of hallucinogenic versus non hallucinogenic agonists upon receptor desensitization reflect their differential capacity to promote Ser280 phosphorylation: (1) mutating Ser280 into alanine or aspartate abolished the difference in the agonist effects upon receptor desensitization; (2) hallucinogens were able to desensitize S280A receptor to an extent comparable to that induced by nonhallucinogenic agonists in cells expressing wild type receptor and (3) nonhallucinogenic agonists did not promote desensitization of the Ser280D receptor mutant. Collectively, these observations establish a direct link between Ser280 phosphorylation and the low capacity of hallucinogens to desensitize the receptor and suggest that Ser280 is phosphorylated at a high stoichiometry following hallucinogen treatment. These findings contrast with a previous study which showed that mutating into alanine two serine residues, one (Ser421) located in the receptor C terminus and the other (Ser188) in the i2 loop, strongly reduced quipazine-mediated receptor desensitization, whereas the deletion of residues 280–296 or residues 280–310 in the i3 loop (i.e. Ser280 and downstream residues phosphorylated upon hallucinogen treatment) had no effect on the time course and extent of 5-HT2A receptor desensitization (41). However, it is likely that the agonist used to induce receptor desensitization (quipazine), which is devoid of hallucinogenic activity in humans (42), does not induce S280 phosphorylation, like lisuride and ergotamine. Phosphorylation of other residues (e.g. Ser188 and/or Ser421), though not detected in our MS/MS analyses, might thus underlie 5-HT2A receptor desensitization induced by any receptor agonist, whereas the specific phosphorylation of Ser280 (and/or of downstream serines in i3 loop) by hallucinogens might act as a brake limiting receptor desensitization. Alternatively, Ser280 phosphorylation might facilitate 5-HT2A receptor resensitization that occurs in the continuous presence of agonist, consistent with previous findings indicating that receptor resensitization, like Ser280 phosphorylation, is also dependent of PKC (28).
Initial studies on 5-HT2A receptor desensitization and internalization showed that they are both β-arrestin-independent (43), contrasting with what is more generally observed for numerous GPCRs. However, the situation is probably more complex than previously imagined, as a more recent study revealed a differential pattern of β-arrestin sensitivity for agonist-induced receptor internalization: though treatment with DOI or the 5-HT precursor l-5-hydroxy-tryptophan displayed similar efficacies to promote receptor internalization, DOI-induced receptor internalization was β-arrestin-independent, whereas 5-HT-induced internalization requires β-arrestins (44). β-arrestin-independent receptor internalization elicited by DOI treatment corroborates with the low ability of this compound to promote β-arrestin recruitment by the receptor (compared with nonhallucinogenic agonists), a property shared by LSD and likely reflecting the unique ability of hallucinogens to promote Ser280 phosphorylation. Whether β-arrestins contributes to receptor-internalization elicited by the nonhallucinogenic agonists remains to be elucidated.
In conclusion, our observations show that ligand identity not only determines the nature of 5-HT2A receptor-operated signaling but also the pattern of receptor phosphorylation at a site (Ser280) involved in desensitization and internalization. They highlight the power of quantitative phosphoproteomics to identify mechanisms underlying functional selectivity and of potential relevance to the behavioral responses induced by biased ligands. The clinical significance of the biased 5-HT2A receptor phosphorylation remains to be established. In this regard, it would be of considerable interest to explore in future studies how Ser280 phosphorylation is affected by the different classes of antipsychotics and by 5-HT2A receptor heterodimerization with mGlu2 metabotropic glutamate receptor, a process critical for hallucinogen psychomimetic activity (45, 46).
Supplementary Material
Footnotes
Author contributions: C.M., M.J.M., J.B., P.M., and F.V. designed research; S.K., S.M., P.M., and F.V. performed research; C.B. and L.P. contributed new reagents or analytic tools; S.K. and F.V. analyzed data; M.J.M., J.B., P.M., and F.V. wrote the paper.
* This work was supported by grants from la Fondation pour la Recherche Médicale (Contracts Equipe FRM 2005 and 2009), ANR (Contract n° ANR-08-MNPS-0011), CNRS, INSERM University Montpellier 1 and University Montpellier 2. Mass spectrometry experiments were carried out using facilities of the Functional Proteomic Platform of Montpellier Languedoc-Roussillon.
 This article contains supplemental Figs. S1 to S5 and Table S1.
This article contains supplemental Figs. S1 to S5 and Table S1.
Conflict of Interest: The authors declare that they have no conflict of interest.
1 The abbreviations used are:
- GPCR
- G protein-coupled receptor
- 5-HT
- 5-hydroxytryptamine, serotonin
- DOI
- 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane
- HILIC
- hydrophilic interaction liquid chromatography
- LSD
- lysergic acid diethylamine
- PKB
- Protein Kinase B
- PKC
- Protein Kinase C
- PLC
- phospholipase C
- PTX
- Pertussis toxin
- RSK
- Ribosomal S6 kinase
- SILAC
- stable isotope labeling by amino acids in cell culture.
REFERENCES
- 1. Meltzer H. Y., Massey B. W., Horiguchi M. (2012) Serotonin receptors as targets for drugs useful to treat psychosis and cognitive impairment in schizophrenia. Curr. Pharm. Biotechnol. 13, 1572–1586 [DOI] [PubMed] [Google Scholar]
- 2. Roth B. L., Berry S. A., Kroeze W. K., Willins D. L., Kristiansen K. (1998) Serotonin 5-HT2A receptors: molecular biology and mechanisms of regulation. Crit. Rev. Neurobiol. 12, 319–338 [DOI] [PubMed] [Google Scholar]
- 3. Gray J. A., Roth B. L. (2007) Molecular targets for treating cognitive dysfunction in schizophrenia. Schizophr. Bull. 33, 1100–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aghajanian G. K., Marek G. J. (1999) Serotonin and hallucinogens. Neuropsychopharmacology 21, 16S–23S [DOI] [PubMed] [Google Scholar]
- 5. Nichols D. E. (2004) Hallucinogens. Pharmacol. Ther. 101, 131–181 [DOI] [PubMed] [Google Scholar]
- 6. Gonzalez-Maeso J., Sealfon S. C. (2009) Psychedelics and schizophrenia. Trends Neurosci. 32, 225–232 [DOI] [PubMed] [Google Scholar]
- 7. Gonzalez-Maeso J., Weisstaub N. V., Zhou M., Chan P., Ivic L., Ang R., Lira A., Bradley-Moore M., Ge Y., Zhou Q., Sealfon S. C., Gingrich J. A. (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53, 439–452 [DOI] [PubMed] [Google Scholar]
- 8. Gonzalez-Maeso J., Yuen T., Ebersole B. J., Wurmbach E., Lira A., Zhou M., Weisstaub N., Hen R., Gingrich J. A., Sealfon S. C. (2003) Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J. Neurosci. 23, 8836–8843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gonzalez-Maeso J., Sealfon S. C. (2009) Agonist-trafficking and hallucinogens. Curr. Med. Chem. 16, 1017–1027 [DOI] [PubMed] [Google Scholar]
- 10. Becamel C., Gavarini S., Chanrion B., Alonso G., Galeotti N., Dumuis A., Bockaert J., Marin P. (2004) The serotonin 5-HT2A and 5-HT2C receptors interact with specific sets of PDZ proteins. J. Biol. Chem. 279, 20257–20266 [DOI] [PubMed] [Google Scholar]
- 11. Malinow R., Hayashi Y., Maletic-Savatic M., Zaman S. H., Poncer J. C., Shi S. H., Esteban J. A., Osten P., Seidenman K. (2010) Introduction of green fluorescent protein (GFP) into hippocampal neurons through viral infection. Cold Spring Harb. Protoc. 2010, pdb prot5406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dubois F., Vandermoere F., Gernez A. l., Murphy J., Toth R., Chen S., Geraghty K. M., Morrice N. A., MacKintosh C. (2009) Differential 14–3-3 affinity capture reveals new downstream targets of phosphatidylinositol 3-kinase signaling. Mol. Cell. Proteomics 8, 2487–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ong S. E., Blagoev B., Kratchmarova I., Kristensen D. B., Steen H., Pandey A., Mann M. (2002) Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics 1, 376–386 [DOI] [PubMed] [Google Scholar]
- 14. Weiss S., Pin J. P., Sebben M., Kemp D. E., Sladeczek F., Gabrion J., Bockaert J. (1986) Synaptogenesis of cultured striatal neurons in serum-free medium: a morphological and biochemical study. Proc. Natl. Acad. Sci. U.S.A. 83, 2238–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McNulty D. E., Annan R. S. (2008) Hydrophilic interaction chromatography reduces the complexity of the phosphoproteome and improves global phosphopeptide isolation and detection. Mol. Cell. Proteomics 7, 971–980 [DOI] [PubMed] [Google Scholar]
- 16. Cox J. R., Mann M. (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 26, 1367–1372 [DOI] [PubMed] [Google Scholar]
- 17. Martin D. M., Nett I. R., Vandermoere F., Barber J. D., Morrice N. A., Ferguson M. A. (2010) Prophossi: automating expert validation of phosphopeptide-spectrum matches from tandem mass spectrometry. Bioinformatics 26, 2153–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chanrion B., Mannoury la Cour C., Gavarini S., Seimandi M., Vincent L., Pujol J. F., Bockaert J., Marin P., Millan M. J. (2008) Inverse agonist and neutral antagonist actions of antidepressants at recombinant and native 5-hydroxytryptamine2C receptors: differential modulation of cell surface expression and signal transduction. Mol. Pharmacol. 73, 748–757 [DOI] [PubMed] [Google Scholar]
- 19. Miner L. A., Backstrom J. R., Sanders-Bush E., Sesack S. R. (2003) Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience 116, 107–117 [DOI] [PubMed] [Google Scholar]
- 20. Songyang Z., Blechner S., Hoagland N., Hoekstra M. F., Piwnica-Worms H., Cantley L. C. (1994) Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr. Biol. 4, 973–982 [DOI] [PubMed] [Google Scholar]
- 21. Dinkel H., Chica C., Via A., Gould C. M., Jensen L. J., Gibson T. J., Diella F. (2011) Phospho.ELM: a database of phosphorylation sites–update 2011. Nucleic Acids Res. 39, D261–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xue Y., Ren J., Gao X., Jin C., Wen L., Yao X. (2008) GPS 2.0, a Tool to Predict Kinase-specific Phosphorylation Sites in Hierarchy. Mol. Cell. Proteomics 7, 1598–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sullivan J. P., Connor J. R., Shearer B. G., Burch R. M. (1991) 2,6-Diamino-N-([1-oxotridecyl)-2-piperidinyl]methyl)hexanamide (NPC 15437): a selective inhibitor of protein kinase C. Agents Actions 34, 142–144 [DOI] [PubMed] [Google Scholar]
- 24. Heerding D. A., Rhodes N., Leber J. D., Clark T. J., Keenan R. M., Lafrance L. V., Li M., Safonov I. G., Takata D. T., Venslavsky J. W., Yamashita D. S., Choudhry A. E., Copeland R. A., Lai Z., Schaber M. D., Tummino P. J., Strum S. L., Wood E. R., Duckett D. R., Eberwein D., Knick V. B., Lansing T. J., McConnell R. T., Zhang S., Minthorn E. A., Concha N. O., Warren G. L., Kumar R. (2008) Identification of 4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-{[(3S)-3-piperidinylmethyl]oxy}-1H-imidazo[4,5-c]pyridin-4-yl)-2-methyl-3-butyn-2-ol (GSK690693), a novel inhibitor of AKT kinase. J. Med. Chem. 51, 5663–5679 [DOI] [PubMed] [Google Scholar]
- 25. Smith J. A., Poteet-Smith C. E., Xu Y., Errington T. M., Hecht S. M., Lannigan D. A. (2005) Identification of the first specific inhibitor of p90 ribosomal S6 kinase (RSK) reveals an unexpected role for RSK in cancer cell proliferation. Cancer Res. 65, 1027–1034 [PubMed] [Google Scholar]
- 26. Strachan R. T., Sheffler D. J., Willard B., Kinter M., Kiselar J. G., Roth B. L. (2009) Ribosomal S6 Kinase 2 Directly Phosphorylates the 5-Hydroxytryptamine 2A (5-HT2A) Serotonin Receptor, Thereby Modulating 5-HT2A Signaling. J. Biol. Chem. 284, 5557–5573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raote I., Bhattacharyya S., Panicker M. M. (2013) Functional selectivity in serotonin receptor 2A (5-HT2A) endocytosis, recycling, and phosphorylation. Mol. Pharmacol. 83, 42–50 [DOI] [PubMed] [Google Scholar]
- 28. Bhattacharyya S., Puri S., Miledi R., Panicker M. M. (2002) Internalization and recycling of 5-HT2A receptors activated by serotonin and protein kinase C-mediated mechanisms. Proc. Natl. Acad. Sci. U.S.A. 99, 14470–14475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berg K. A., Stout B. D., Maayani S., Clarke W. P. (2001) Differences in rapid desensitization of 5-hydroxytryptamine2A and 5-hydroxytryptamine2C receptor-mediated phospholipase C activation. J. Pharmacol. Exp. Ther. 299, 593–602 [PubMed] [Google Scholar]
- 30. Gelber E. I., Kroeze W. K., Willins D. L., Gray J. A., Sinar C. A., Hyde E. G., Gurevich V., Benovic J., Roth B. L. (1999) Structure and function of the third intracellular loop of the 5-hydroxytryptamine2A receptor: the third intracellular loop is alpha-helical and binds purified arrestins. J. Neurochem. 72, 2206–2214 [DOI] [PubMed] [Google Scholar]
- 31. Kenakin T. (2007) Collateral efficacy in drug discovery: taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol. Sci. 28, 407–415 [DOI] [PubMed] [Google Scholar]
- 32. Galandrin S., Oligny-Longpre G., Bouvier M. (2007) The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol. Sci. 28, 423–430 [DOI] [PubMed] [Google Scholar]
- 33. Urban J. D., Clarke W. P., von Zastrow M., Nichols D. E., Kobilka B., Weinstein H., Javitch J. A., Roth B. L., Christopoulos A., Sexton P. M., Miller K. J., Spedding M., Mailman R. B. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 320, 1–13 [DOI] [PubMed] [Google Scholar]
- 34. Reiter E., Ahn S., Shukla A. K., Lefkowitz R. J. (2012) Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu. Rev. Pharmacol. Toxicol. 52, 179–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nobles K. N., Xiao K., Ahn S., Shukla A. K., Lam C. M., Rajagopal S., Strachan R. T., Huang T. Y., Bressler E. A., Hara M. R., Shenoy S. K., Gygi S. P., Lefkowitz R. J. (2011) Distinct phosphorylation sites on the beta(2)-adrenergic receptor establish a barcode that encodes differential functions of beta-arrestin. Sci. Signal. 4, ra51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Butcher A. J., Prihandoko R., Kong K. C., McWilliams P., Edwards J. M., Bottrill A., Mistry S., Tobin A. B. (2011) Differential G-protein-coupled receptor phosphorylation provides evidence for a signaling bar code. J. Biol. Chem. 286, 11506–11518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Busillo J. M., Armando S., Sengupta R., Meucci O., Bouvier M., Benovic J. L. (2010) Site-specific phosphorylation of CXCR4 is dynamically regulated by multiple kinases and results in differential modulation of CXCR4 signaling. J. Biol. Chem. 285, 7805–7817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wisler J. W., DeWire S. M., Whalen E. J., Violin J. D., Drake M. T., Ahn S., Shenoy S. K., Lefkowitz R. J. (2007) A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc. Natl. Acad. Sci. U.S.A. 104, 16657–16662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trinidad J. C., Barkan D. T., Gulledge B. F., Thalhammer A., Sali A., Schoepfer R., Burlingame A. L. (2012) Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse. Mol. Cell. Proteomics. 11, 215–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Strachan R. T., Allen J. A., Sheffler D. J., Roth B. L. (2010) p90 Ribosomal S6 kinase 2, a novel GPCR kinase, is required for growth factor-mediated attenuation of GPCR signaling. Biochemistry 49, 2657–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gray J. A., Compton-Toth B. A., Roth B. L. (2003) Identification of two serine residues essential for agonist-induced 5-HT2A receptor desensitization. Biochemistry 42, 10853–10862 [DOI] [PubMed] [Google Scholar]
- 42. Fiorella D., Rabin R. A., Winter J. C. (1995) Role of 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. II: Reassessment of LSD false positives. Psychopharmacology (Berl) 121, 357–363 [DOI] [PubMed] [Google Scholar]
- 43. Bhatnagar A., Willins D. L., Gray J. A., Woods J., Benovic J. L., Roth B. L. (2001) The dynamin-dependent, arrestin-independent internalization of 5-hydroxytryptamine 2A (5-HT2A) serotonin receptors reveals differential sorting of arrestins and 5-HT2A receptors during endocytosis. J. Biol. Chem. 276, 8269–8277 [DOI] [PubMed] [Google Scholar]
- 44. Schmid C. L., Raehal K. M., Bohn L. M. (2008) Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc. Natl. Acad. Sci. U.S.A. 105, 1079–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gonzalez-Maeso J., Ang R. L., Yuen T., Chan P., Weisstaub N. V., Lopez-Gimenez J. F., Zhou M., Okawa Y., Callado L. F., Milligan G., Gingrich J. A., Filizola M., Meana J. J., Sealfon S. C. (2008) Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452, 93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moreno J. L., Muguruza C., Umali A., Mortillo S., Holloway T., Pilar-Cuellar F., Mocci G., Seto J., Callado L. F., Neve R. L., Milligan G., Sealfon S. C., Lopez-Gimenez J. F., Meana J. J., Benson D. L., Gonzalez-Maeso J. (2012) Identification of three residues essential for 5-hydroxytryptamine 2A-metabotropic glutamate 2 (5-HT2A.mGlu2) receptor heteromerization and its psychoactive behavioral function. J. Biol. Chem. 287, 44301–44319 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.