Abstract
Neisseria gonorrhoeae (GC) is a human-specific pathogen, and the agent of a sexually transmitted disease, gonorrhea. There is a critical need for new approaches to study and treat GC infections because of the growing threat of multidrug-resistant isolates and the lack of a vaccine. Despite the implied role of the GC cell envelope and membrane vesicles in colonization and infection of human tissues and cell lines, comprehensive studies have not been undertaken to elucidate their constituents. Accordingly, in pursuit of novel molecular therapeutic targets, we have applied isobaric tagging for absolute quantification coupled with liquid chromatography and mass spectrometry for proteome quantitative analyses. Mining the proteome of cell envelopes and native membrane vesicles revealed 533 and 168 common proteins, respectively, in analyzed GC strains FA1090, F62, MS11, and 1291. A total of 22 differentially abundant proteins were discovered including previously unknown proteins. Among those proteins that displayed similar abundance in four GC strains, 34 were found in both cell envelopes and membrane vesicles fractions. Focusing on one of them, a homolog of an outer membrane protein LptD, we demonstrated that its depletion caused loss of GC viability. In addition, we selected for initial characterization six predicted outer membrane proteins with unknown function, which were identified as ubiquitous in the cell envelopes derived from examined GC isolates. These studies entitled a construction of deletion mutants and analyses of their resistance to different chemical probes. Loss of NGO1985, in particular, resulted in dramatically decreased GC viability upon treatment with detergents, polymyxin B, and chloramphenicol, suggesting that this protein functions in the maintenance of the cell envelope permeability barrier. Together, these findings underscore the concept that the cell envelope and membrane vesicles contain crucial, yet under-explored determinants of GC physiology, which may represent promising targets for designing new therapeutic interventions.
Neisseria gonorrhoeae (GC)1 is an obligate human pathogen and the etiological agent of gonorrhea, a sexually transmitted disease. GC infection remains a significant health and economic burden worldwide (1). It is also the second most commonly reported infectious disease in the United States (2). Gonorrhea ranges from clinically asymptomatic to local genital infections to disseminated bloodstream infections. Asymptomatic infections often have devastating consequences on women's health including pelvic inflammatory disease, ectopic pregnancy, and infertility (3). Additionally, GC infections facilitate transmission and acquisition of HIV (4). For all of these reasons it is critical to provide effective treatments against gonorrhea. Currently, a dual therapy with cephalosporin and either azithromycin or doxycycline is recommended (5). However, over the past several years treatment failures associated with GC strains displaying decreased susceptibility to extended spectrum cephalosporins have been reported from various parts of the world (6–9). This is especially concerning because no other antibiotics are clinically useful in these cases, and because no appropriate vaccine exists (10). The escalating problem of the spread of antimicrobial resistance in GC, and the importance of the development of new approaches to study, treat, and prevent GC infection, have been recognized by the World Health Organization and by the Centers for Disease Control and Prevention (11, 12).
We propose that largely unexplored proteins localized to bacterial cell envelope and naturally released membrane vesicles are particularly promising as potential novel molecular targets for therapeutic interventions against gonorrhea. The small fraction of known components of the GC cell envelope (outer membrane, periplasm, cytoplasmic membrane) plays a fundamental role in establishing infection by enabling the microbes to adhere to and invade host cells, facilitating nutrient acquisition, host tissue destruction, and suppression of immune responses (3, 13–15). Further, GC, like many other Gram-negative bacteria, produces membrane vesicles (MVs), which are nano-sized bilayered proteolipids (16). Naturally produced MVs are potentially an effective way to deliver toxins, enzymes, and other effectors to host tissues. Additionally, evidence from various studies support that MVs participate in intercellular communication and horizontal gene transfer (16–21). In GC, MVs are necessary for biofilm formation, which is thought to play an important role in asymptomatic infection in women, resistance to antimicrobial agents, and suppression of host immune defenses (22–24). MVs may also contribute to the serum resistance by providing an enhanced ability to bind and eliminate bactericidal factors (17).
Although the potential importance of proteins localized to the GC cell envelope and MVs has been reported previously (25, 26), only two proteomic studies have been published addressing GC membrane composition (27, 28). Most studies have focused on extensive characterization of factors involved in direct host cell interaction: protruding surface proteins (pili), outer membrane adhesins Opa, porins P.IA and P.IB, lipooligosaccharide, and several iron utilization proteins (3, 4, 15, 29–32). Many of these vital virulence factors undergo phase and/or antigenic variation, making them poor drug or vaccine targets. Therefore, the pursuit for novel and constitutively expressed proteins—therapeutic targets in GC—is of utmost importance.
Accordingly, in this study we applied global and unbiased proteomics to compare the composition of both the cell envelopes and MVs isolated from four GC strains: FA1090, F62, MS11, and 1291. Specifically, we used isotope tagging for relative and absolute quantification (iTRAQ) coupled with multidimensional liquid chromatography and tandem mass spectrometry (2D-LC/MS/MS). This approach allowed us to determine a uniformly and differentially expressed repertoire of proteins. Focusing on a homolog of LPS transport protein, LptD (OstA, Imp), which was identified in both the cell envelopes and MVs fractions, and ubiquitously expressed among analyzed strains, we showed that its depletion led to loss of GC viability. Finally, we selected for initial characterization six predicted outer membrane proteins, which were present at similar levels in the GC cell envelopes. We generated Δngo1344, Δngo1955, Δngo1985, Δngo2111, Δngo2121, and Δngo2139 mutant strains and examined their sensitivity toward different cell envelope-perturbing agents as well as chloramphenicol. These studies showed that the lack of NGO1985 resulted in dramatically decreased GC viability, suggesting that this protein functions in the maintenance of the cell envelope permeability barrier. Overall, these findings further support our hypothesis that the conserved proteins may represent promising targets for designing new therapeutic interventions.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions
GC strains FA1090, F62, MS11, and 1291 used in this study are described in detail in Table I. Additionally we used strain FA19 (33), and a few isolates (LGB1, LG14, LG20, and LG26) collected from two public health clinics in Baltimore between 1991–1994. The strains were grown on either solid media, GCB agar in 5% CO2 atmosphere at 37 °C, or in liquid media, GCBL, at 37 °C as previously described (34–36). GCB agar is GC Medium Base agar (Difco, Detroit, MI) with Kellogg's supplements, whereas GCBL is a GC Medium Base broth containing Kellogg's supplements and sodium bicarbonate at a final concentration of 0.042%. For anaerobic growth, GC was grown as described previously in an anaerobic jar using GasPak Plus anaerobic system with palladium catalyst (BD) and indicator strip (BBL) at 37 °C for 24 h in the presence of 5 μl of 20% sodium nitrite spotted on a paper disc placed in the middle of the GCB plates (37, 38).
Table I. Summary of recognized differences between GC isolates utilized in this study.
| Characteristic | FA1090 | F62 | MS11 | 1291 | Reference |
|---|---|---|---|---|---|
| Source | DGIa | Urethra | Endocervix | Urethra | (30) |
| Serum susceptibility | Resistant | Sensitive | Sensitive | Intermediate | (30, 57, 107, 108) |
| Genetic island | Absent | Absent | Present | Present | (109) |
| Iron utilizationb | Tbp+, Lf− | Tbp+, Lf− | Tbp+, Lf+ | n/a | (110) |
a DGI–Disseminated GC Infection.
b Tbp–Transferrin binding protein; Lf–Lactoferrin binding protein; (+) – present; (−) - absent.
To examine the proteome of the cell envelope and MVs, GC strains FA1090, F62, MS11, and 1291 were streaked at the same time on GCB plates and incubated for 20 h in 5% CO2 at 37 °C. Subsequently, bacteria were swabbed from plates and suspended in prewarmed GCBL medium to an optical density at 600 nm (OD600) of 0.1 and cultured in Fernbach flasks with shaking at 220 rpm. Growth was monitored every hour by determining the turbidity of the cultures (OD600). All cultures were harvested at the late-logarithmic phase of growth (at OD600 about 0.8). Cells were separated from culture supernatants by low speed centrifugation (6000 × g), and both membrane fractions and MVs were prepared for proteomic analysis as described below.
Preparation of the GC Cell Envelope Fraction
Preparation of the cell envelope fraction was accomplished according to (39, 40) with the following modifications. Pellet of bacterial cells obtained from 1.0 L of each GC strain cultured as described above was suspended in phosphate buffered saline pH 7.5, (PBS, Li-Cor, Lincoln, NE) supplemented with Complete Protease inhibitor mixture tablets EDTA-free (buffer A, Roche). Then, GC cells were lysed by three passages through French pressure cell at 12,000 psi, and the unbroken bacterial cells and debris were removed by centrifugation at 7000 × g for 30 min at 4 °C. Cell lysates obtained from respective GC cultures were subsequently diluted four times with freshly prepared ice-cold 0.1 m sodium carbonate (pH 11 without adjustment). The suspensions were sonicated (2 × 20 s, 5 Watts) and incubated for 1 h at 4 °C with a gentle stirring. Following another two cycles of sonication (20 s/cycle, 5 Watts), the crude membrane fractions were collected by ultracentrifugation in a Beckman Type 70 Ti rotor at 170,000 × g, 1 h, 4 °C. To remove the residual membrane associated proteins, the pellets were washed with ice-cold buffer A, sonicated (3 × 15 s, 5 Watts), and separated at 100,000 × g, 2 h, 4 °C in a Beckman SW41Ti rotor. These procedures were repeated twice and finally the pellets containing membrane proteins were suspended in 1 ml of PBS containing 0.2% SDS. The total concentration of proteins in each sample was assessed with 2-D Quant Kit (GE Health Care).
Preparation of Naturally Released MVs
The naturally released MVs were prepared using optimized protocol for GC (18). Briefly, supernatants (about 1.0 L per each culture) obtained from the same GC cultures used for cell envelope extractions, were first passed through the 0.22 μm filter units (ThermoFisher Scientific) and to prevent proteolysis, Complete Protease inhibitor mixture tablet EDTA-free was added. Subsequently, the supernatants were subjected to ultracentrifugation at 210,000 × g, 3 h, 4 °C in a Beckman Type 45 Ti rotor. The pellets containing MVs fraction were washed with PBS and finally reconstituted in PBS containing 0.2% SDS. The total protein concentration in MVs was measured using 2D Quant Kit.
Transmission Electron Microscopy
Native MVs isolated from culture supernatants of GC strains FA1090, F62, MS11, and 1291 as described above, were reconstituted in PBS, adsorbed on a formvar/carbon coated copper grid for 5 min, and stained with 2% ammonium molybdate for 1 min. Grids were air-dried and MVs were observed using FEI Titan 80–200 transmission electron microscope at the Oregon State University Electron Microscopy Facility.
iTRAQ Labeling, Two-Dimensional Liquid Chromatography, and Mass Spectrometry
Proteins obtained from the cell envelopes and MVs extractions (100 μg and 40 μg, respectively) were precipitated overnight at −20 °C in 90% acetone and washed twice with ice-cold acetone. iTRAQ (AB Sciex) labeling was performed according to the manufacturer's recommendations. The following iTRAQ tags were used to label peptides in appropriate GC strains: 114 for FA1090, 115 for F62, 116 for MS11, and 117 for 1291. The reactions were carried out for 1 h at room temperature and were stopped by the addition of 250 μl of 0.1% Trifluoroacetic acid (TFA). Labeled peptides were pooled and purified on C18 column (Honeywell Burdick & Jackson). Subsequent procedures were performed at the Proteomic Core at Fred Hutchinson Cancer Research Center, Seattle, WA. iTRAQ labeled peptides were separated by strong cation exchange (SCX) using a Paradigm MG4 HPLC (Michrom Bioresources/Bruker). Peptide separations were performed using a Zorbax300 SCX column (2.1 mm × 150 mm; Agilent Technologies, Santa Clara, CA) with an in-line guard cartridge. Peptides were eluted using SCX A and SCX B buffers with a flow rate of 200 μl/min using the following gradient: 2% B from 0 to 5 min, 8% B to 5.1 min, 18% B to 20 min, 34% B to 32 min, 60% B to 40 min, 98% B to 40.1 min and held to 50 min, 2% B to 50.1 min and held to the end of the run at 60 min. SCX A buffer contained 5 mm KH2PO4 pH 2.7, 30% (v/v) acetonitrile (ACN) and SCX B buffer contained SCX A buffer with 500 mm KCl. Fractions were collected at 2 min intervals throughout the run and pooled to a reduced number of fractions for reversed-phase (RP-HPLC-MS) analysis based on ultraviolet absorption at 220 nm. The pooled fractions were dried in a vacuum concentrator. Each pooled fraction was reconstituted with 0.1% TFA (100 μl) and desalted using SepPak C18 cartridges (Waters, Milford, MA). Peptides were eluted with 50% ACN/0.1% TFA and concentrated in a vacuum concentrator. Subsequently, peptides were separated using Eksigent nano-two-dimensional high-pressure liquid chromatography (2D HPLC, AB Sciex). In-line de-salting was accomplished using a reversed-phase trap column (100 μm × 20 mm) packed with Magic C18AQ (5-μm 200 Å resin; Michrom BioResources) followed by peptide separations on a reversed-phase column (75 μm × 250 mm) packed with Magic C18AQ (5-μm 100 Å resin; Michrom BioResources) directly mounted to the Dionex Probot MALDI Spotter (ThermoFisher Scientific). A 90 min gradient from 7% to 35% ACN in 0.1% TFA at a flow rate of 500 nL/min was used for chromatographic separations. The gradient of solvent B was as follows: 0 min, 5% B; 2 min, 7% B; 92 min, 35% B; 93 min, 50% B; 102 min 50% B; 103 min, 95% B; 108 min, 95% B; 109 min, 5% B; 129 min, 5% B; and 130 min, 5% B. The column effluent was mixed in a micro Tee with matrix (5 mg/ml alpha-cyano 4-hydroxy cinnamic acid (Sigma-Aldrich) with 2% Ammonium Citrate (Sigma-Aldrich) and 40 fmol/μl Glu-Fibrinopeptide B (in-house synthesis) delivered at 1 μl/min. Fractions were spotted at 10 s intervals onto a stainless steel MALDI target plate (AB Sciex). MS and MS/MS spectra were acquired on an Applied Biosystems 4800 Proteomics Analyzer (TOF/TOF) (AB Sciex) in positive ion reflection mode with a 200 Hz Nd:YAG laser operating at 355 nm, and accelerating voltage with 400 ns delay. MS spectra were obtained with minimal laser energy in order to maintain the best resolution. MS spectra for the entire sample set were collected first, and on each sample spot MS/MS spectra were collected for the 20 most intense peaks above the signal-to-noise ratio threshold of 20 using a collision energy of 2 keV and air as the collision gas. Both MS and MS/MS data were acquired on the sample spots using an internal calibration with Glu-Fibrinopeptide B.
Proteomic Data Analysis
Data analysis was performed using the Paradigm search algorithm, which is a part of the ProteinPilot software (version 4.0) (AB Sciex) against the SwissProt target-decoy database for N. gonorrhoeae FA1090 with 1963 protein entries (downloaded on January 17, 2012). The user-defined search parameters were selected as follows: Sample Type: iTRAQ 4plex, Cys. Alkylation: MMTS, Digestion: Trypsin, Instrument: 4800, ID Focus: Biological Modifications, Search Effort: Thorough, FDR Analysis: Yes, User Modified Parameter Files: No. All other search parameters, such as number of missed and/or nonspecific cleavages allowed and the mass tolerance of the precursor and fragment ions, are hard-coded into the ProteinPilot software for analyzing MALDI 4800 data. The ProGroup Algorithm built within ProteinPilot software was used to perform the statistical analysis on the identified peptides to determine the minimal set of identifications. Data were normalized by bias correction built in ProteinPilot. The mass spectrometry proteomics data have been deposited in the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository ProteomeXchange with the dataset identifier PXD000549. The annotated spectra can be examined using MS-Viewer, which is available on the public Protein Prospector website at the following uniform resource locator: http://prospector2.ucsf.edu/prospector/cgi-bin/msform.cgi?form=msviewer. The search keys for the individual data sets are as follows: ikgoxmdesw (cell envelope Exp.1; x5616y5bsg (cell envelope Exp.2); nyiveb6jjz (MVs Exp1); and zv4dvvldqm (MVs Exp. 2). Unprocessed and unfiltered results from Protein Pilot can be found in Microsoft Excel files in the supplemental Material S1–S4. All statistical analyses were performed as we described previously (41). Briefly, only proteins identified with 1% FDR with at least one peptide of 95% confidence or more were recorded. For protein quantification, only proteins with associated p values corresponding to the calculated iTRAQ ratios were included. Proteins were considered ubiquitously expressed if the iTRAQ ratios were between 0.5 and 2, and were designated as differentially expressed when the values were below 0.5 or above 2 with the corresponding p values < 0.05 in both biological experiments. The relative protein abundance heat maps were constructed using MultiExperiment Viewer (MeV version 4.8) (42).
Bioinformatics Analyses
Subcellular localization of identified proteins was assessed using PSORTb 3.0.2 (43), SOSUIGramN (44), and CELLO 2.5 (45, 46), and majority-votes strategy was used for protein assignment. In cases where PSORTb, SOSUIGramN, and CELLO 2.5 predicted different subcellular localizations for a particular protein, the protein was allocated to a group of “unknown subcellular localization.” Furthermore, the predicted amino acid sequences of identified proteins were analyzed for the presence and location of signal peptides cleavage sites using both SignalP v.4.1 (47) and TatP v1.0 (48) servers. To phylogenetically classify identified proteins the Clusters of Orthologous Groups (COGs) functional categories were assigned using COGnitor, WebMGA (49, 50), and J. Craig Venter Institute-Comprehensive Microbial Resource (http://cmr.jcvi.org/tigr-scripts/CMR/). The predicted amino acid sequences of selected, newly identified proteins were also analyzed using protein BLAST similarity searches (www.ncbi.nlm.gov.blast).
Genetic Manipulations
The genome sequence of GC strain FA1090 was used as a template to design primers. Chromosomal DNA purified from FA1090 using Promega Wizard Genomic DNA Purification Kit was used in PCR reactions. Reactions were performed using Q5 High-Fidelity DNA Polymerase (New England BioLabs, Ipswich, MA) with oligonucleotides synthesized by Integrated DNA Technologies. PCR products were verified by sequencing at the Center for Genome Research and Biocomputing at OSU. The sequences of all oligonucleotides designed and used in this study are listed in the supplemental Table S1.
Construction of GC Strains Expressing RpsM-His, NGO1985-His, and NGO2054-His From Their Native Chromosomal Loci
Splicing by overlap extension PCR (SOE PCR) (51) was used to engineer an epitope-encoding tail, 6×His-tag, at the 3′ end of individual genes ngo1821, ngo1985, and ngo2054 in their respective loci of the FA1090 chromosome. The nucleotide sequence encoding 6×His-tag followed by a stop codon was included within the corresponding oligonucleotides. PCR reactions were used to amplify 500 bp upstream and downstream from the stop codon of each gene. Both fragments were spliced using a set of the external primers. The individual SOE PCR products were used to transform strain FA1090 in liquid culture as described (52). The presence of the His-tag-encoding sequence at the 3′ end of the particular gene was confirmed by PCR reaction using a forward primer specific to the His-tag, NGO-His, and a reverse primer designed to the individual gene; RpsM-His-down-R, NGO1985-His-down-R, or NGO2054-His-down-R.
Construction of Conditional lptD Mutant Strain
The conditional lptD mutant strain, FA1090 PlaclptD, was constructed as follows. First, a gene encoding LptD (NGO1715) including an upstream region encoding native ribosome binding site was amplified with primers NGO1715-C-F and NGO1715-C-R. The 2422 bp PCR product was subjected to digestion with endonuclease FseI and subcloned into ScaI/FseI-pGGC4, to yield pGCC4-lptD, resulting in the placement of the lptD gene behind the Plac promoter. This vector uses the Neisserial Complementation System and contains an isopropyl-β-d-thiogalactoside (IPTG)-inducible promoter, thus allowing controlled expression of a cloned gene (53). Subsequently, the fragment containing lacI repressor gene, Plac promoter and 630 bp of lptD gene was amplified with primers NGO1715-Down-F and NGO1715-Down-R, digested with BamHI and SalI and ligated into pUC18K, creating pUC18K-PlaclptD (54). Then the upstream region of lptD was amplified using primers NGO1715-Up-F and NGO1715-Up-R. The resulting 516 bp product was digested with EcoRI and KpnI, and cloned into pUC18K-PlaclptD. This final construct containing nonpolar kanamycin resistance cassette apha-3 (54) flanked by homologous regions for recombination and allelic exchange (upstream region of lptD, and PlaclptD) was used to introduce the mutation onto the FA1090 chromosome. The plasmid was linearized by digestion with NdeI and transformation was conducted as described previously with the exception that the growth media were supplemented with IPTG (100 μm final concentration) (55). Transformants were plated on GCB agar with 40 μg/ml kanamycin and 100 μm IPTG and selected FA1090 PlaclptD clones were verified by PCR.
Construction of In-frame Deletion Mutants Δngo1985, Δngo1344, Δngo1955, Δngo2111, Δngo2121, and Δngo2139
All strains containing in-frame deletions of individual genes were constructed using a scheme of genetic manipulations briefly described below. The length of individual PCR products as well as restriction enzymes used in cloning procedures are listed in the supplemental Table S1. The upstream regions of particular genes (about 500 bp) were amplified with primers designated as NGO-up-F and NGO-up-R (where F-forward and R-reverse). Subsequently, PCR products were digested and cloned into pUC18K vector, yielding pUC18K-ngo-up. Similarly, the corresponding downstream regions of individual genes (about 500 bp) were first amplified using primers NGO-down-F and NGO-down-R. The resulting PCR products were subjected to digestion with appropriate restriction enzymes and cloned into pUC18K-ngo-up. Thus, these final constructs, pUC18K-Δngo, contained kanamycin resistance cassette (54) flanked by homologous regions for recombination and allelic exchange. The specific pUC18K-Δngo was linearized with NdeI and transformation of GC was conducted as described (55). The presence of desired mutation on the FA1090 chromosome was examined with respective primers listed in the supplemental Table S1. In all PCR reactions, chromosomal DNA isolated from wild-type bacteria was used as a control.
To Construct the Complemented Strain, Δngo1985/Placngo1985, first, the 643 bp fragment of ngo1985 with a native ribosome-binding site was amplified with primers NGO1985-C-F and NGO1985-C-R. The PCR product was subjected to digestion with endonuclease FseI and subcloned into ScaI/FseI-pGGC4, to yield pGCC4-Plac ngo1985, which was subsequently used to complement the Δngo1985 strain as described above.
Effect of LptD Depletion on GC Physiology
To examine the effect of LptD depletion on GC physiology, FA1090 PlaclptD were plated from freezer stocks onto GCB plates supplemented with 100 μm IPTG. The following day, single nonpiliated colonies were passaged onto fresh GCB plates containing 100 μm IPTG and incubated ∼18 h in 5% CO2 atmosphere at 37 °C. A sterile Dacron swab was used to collect the bacteria from plates and suspend them to OD600 = 0.1 in a prewarmed to 37 °C GCBL. After three washes in GCBL, bacteria were divided and cultured in the presence or absence of IPTG (as indicated) for 5 h with shaking (220 rpm) at 37 °C in GCBL. Bacterial growth was monitored every hour by both measuring absorbance at OD600 and assessing cell viability by spotting 10 μl of 10-fold serial dilutions of GC cultures onto GCB agar plates with or without IPTG. The number of colonies was examined after 22 h, and colony-forming units, CFU, were calculated per ml of bacterial culture. These experiments were performed on four separate occasions and mean values and standard deviations of the mean (S.E.) are presented. Bacterial colonies were visualized with a Zeiss AxioObserver.D1 microscope at A-Plan 10× magnification 0.25 P hase Contrast 1.
Phenotypic Analysis of Knockout Strains for Sensitivity to Different Chemical Probes
The individual knockout strains Δngo1985, Δngo1344, Δngo1955, Δngo2111, Δngo2121, and Δngo2139, the complemented strain Δngo1985/Plac1985, as well as isogenic parent strain, FA1090, were plated from freezer stocks and nonpiliated colonies were passaged as described above. All GC strains were collected from plates, and suspended to 5 × 105 CFU/ml in a prewarmed to 37 °C GCBL supplemented with 100 μm IPTG. Subsequently, 10 μl of undiluted and 10-fold serial dilutions of cell suspensions were spotted on solid media containing 100 μm IPTG and with or without the addition of various compounds (as indicated in the text). Bacterial growth was examined after 22 h. The relative viability of each GC strain was calculated by comparing CFUs/ml on GCB plates supplemented with different chemicals to the CFUs/ml on control plates. These experiments were performed in biological triplicates and mean values with calculated S.E. are presented.
Purification of Recombinant Soluble Domain of AniA and Generation of Rabbit Polyclonal Antibody
The gene encoding AniA (NGO1276) lacking the N-terminal palmitoylation signal was amplified, cloned, and purified as described previously in a study by Boulanger et al., where the three-dimensional crystal structure of AniA was resolved (56). Subsequently, 3 mg (1 mg/ml) of purified, recombinant soluble form of AniA was submitted to Pacific Immunology Corporation, where it was used to immunize two rabbits to generate polyclonal antibodies.
SDS-PAGE, Coomassie Staining and Immunoblotting Analyses
Cell envelopes, MVs, and cytoplasmic proteins were extracted from GC strains as described in the sections above. Whole cell lysates were derived from GC grown on GCB plates either anaerobically or aerobically, as specified in the text. When bacterial colonies reached approximately the same size, all strains were harvested, suspended in prewarmed GCBL, and the cell density was assessed turbidometrically at OD600. Cytoplasmic proteins, cell envelopes, and MVs (15 μg of proteins loaded per lane), or whole cell lysates matched by equivalent OD600 units, were boiled in SDS sample buffer in the presence of 50 mm dithiotreitol and resolved in 4–20% Tris-glycine gradient gels (Bio-Rad, Hercules, CA). When indicated, purified recombinant AniA (250 ng) was loaded as a positive control. The gels were either stained with Colloidal Coomassie Blue G-250, and imaged using GelDoc MP (Bio-Rad) or subjected to electrotransfer of proteins onto 0.2 μm nitrocellulose membrane (Bio-Rad) using Trans-blot Turbo (Bio-Rad). The membranes were blocked in 5% milk in PBS supplemented with 0.1% Tween 20, followed by incubation with polyclonal rabbit antisera against either AniA (1:5000) or Ng-MIP (1:10,000), monoclonal mouse antisera against MtrE (1:1000), or monoclonal mouse antibody against 6×His epitope tag (1:1000; ThermoFisher Scientific). The horseradish peroxidase conjugate of either goat anti-rabbit IgG antibody (BioRad) or goat anti-mouse IgG antibody (ThermoFisher Scientific) was used as secondary antisera at 1:10,000 dilution. Immunoblots were developed using Clarity Western ECL-Substrate (BioRad) on Chemi-DocTM MP System (BioRad).
RESULTS AND DISCUSSION
Rationale and Experimental Design
To identify the potential target proteins for development of new pharmacological interventions against GC infections, we compared the proteomes of cell envelopes and MVs derived from four GC isolates: FA1090, F62, MS11, and 1291. We chose these strains for the following reasons: (1) they display several notable differences that might contribute to their fitness in a human host including resistance to human serum, ability to use iron from human transferrin and lactoferrin for growth, and presence of the GC genetic island (Table I) (30, 34, 57–61); (2) their genomes have been sequenced (62); and (3) they have been used in many laboratories and human volunteer studies over the past 20 years (61).
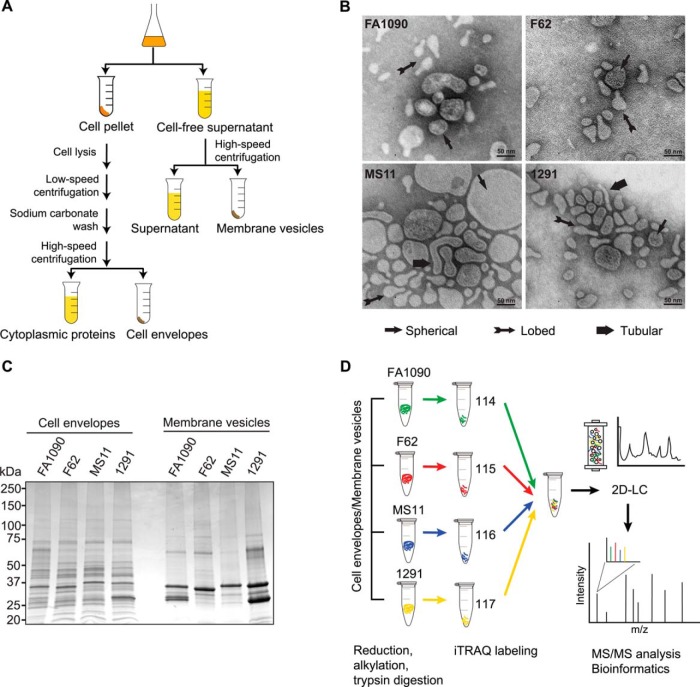
Our experimental design for profiling of the GC cell envelope and MVs is outlined in Fig. 1. Bacterial cultures (1.0 L volume) of FA1090, F62, MS11, and 1291 were propagated simultaneously under standard laboratory growth conditions as described under “Experimental Procedures.” The culture supernatants containing naturally elaborated MVs were separated from bacterial cells by low speed centrifugation at the late-logarithmic phase of growth (Fig. 1A). This stage of growth has been proven for GC to accumulate native MVs (17). Subsequently, cell pellets were used for isolation of membrane proteins by a sodium carbonate extraction procedure (40). This method facilitates efficient enrichment of membrane proteins and ensures the suitability of the samples for mass spectrometry analysis (39, 40, 63). To provide enough material for proteomic analyses of MVs, the entire amount of each cell-free culture supernatant (about 1.0 L) was filtered to remove any remaining bacterial cells and subjected to ultracentrifugation using optimized protocol for GC (18). Transmission electron microscopy of the harvested MVs fractions demonstrated the presence of spherical, tubular, and lobed MVs with wide variation in dimensions (Fig. 1B). Similar morphological types of MVs released by GC were reported previously (64). The examination of the purified cell envelopes and MVs by 1 D SDS-PAGE revealed, as expected, the presence of common and distinct proteins, and the differences were particularly apparent in the MVs fraction (Fig. 1C). The most prominent proteins migrated at about 35 and 25 kDa, likely corresponding to the GC major porins PorIB (P.IB) and PorIII, respectively (65, 66).
Fig. 1.
Flowchart describing the proteomic strategy for a discovery of potential therapeutic targets in GC. A, Workflow of preparation cell envelopes and MVs from GC strains: FA1090, F62, MS11, and 1291. Strains were cultured aerobically in GCBL medium at 37 °C to reach OD600 = 0.8. Bacterial cells and culture supernatants were separated by low-speed centrifugation. Subsequently, cells were lysed and the crude membrane fractions were obtained by cold sodium carbonate extraction at high alkaline pH and ultracentrifugation steps. Cell-free culture supernatants were subjected to high-speed centrifugation at 210,000 × g for 3 h to pellet MVs. B, Native MVs purified from strains FA1090, F62, MS11, and 1291 were negatively stained, and visualized by transmission electron microscopy. The different morphological classes of MVs, indicated by arrows, included spherical, lobed, and tubular. C, Profiles of isolated GC cell envelopes and MVs proteins. Cell envelopes and MVs fractions were prepared from aerobically grown cultures of strains FA1090, F62, MS11, and 1291. Samples were normalized by the total protein concentration and 15 μg were loaded per lane of 1D SDS-PAGE 4–20% Tris-glycine gel. Proteins were visualized by staining with Colloidal Coomassie Blue G250. The migration of molecular weight markers and their masses in kilodaltons (kDa) are indicated on the left. D, Outline of four-plex iTRAQ labeling coupled with 2D-LC/MS/MS. The total amounts of 100- and 40-μg of the cell envelope and MVs proteins, respectively, were reduced, alkylated, and trypsinized. The following iTRAQ tags were used to subsequently label peptides derived from respective GC strains: 114 for FA1090, 115 for F62, 116 for MS11, and 117 for 1291. After labeling the samples were pooled and subjected to SCX fractionation followed by a reversed-phase separation (2D-LC) and protein identification, and quantification using MALDI-TOF MS/MS. Integrated bioinformatics and statistical analyses were applied including ProteinPilot software.
Comprehensive identification of cell envelope and MVs proteins represents a challenge because they are commonly hydrophobic, have a basic charge, and are often of high molecular weight. Thus, it is widely accepted that two-dimensional gel electrophoresis has very limited capabilities for the separation of integral membrane proteins (21, 67). Instead, a multidimensional protein identification technology that separates proteins using a combination of two different kinds of liquid chromatography (2D-LC) prior to protein identification significantly reduces the complexity of the sample at the peptide level, resulting in the identification of a greater number of proteins. Accordingly, we used a comprehensive proteomic platform including iTRAQ coupled with 2D-LC/MS/MS to identify and quantitatively compare proteins in both the GC cell envelope and naturally elaborated MVs. This proteomic approach allows for simultaneous comparison of up to eight samples of complex protein mixtures (68). In a four-plex experiment, proteins in each sample are subjected to digestion by trypsin or other proteolytic enzymes, and the obtained peptides are labeled at their free amine group with one of the four available mass tag labels: 114, 115, 116, and 117 (Fig. 1D). While, in an eight-plex experimental approach, in addition to the latter tags, 113, 118, 119, and 121 are also used. Comparison of the intensity of these reporter labels permits the relative quantification of identical peptides in each digest and thus the proteins from which they originate (68). In our studies, the four-plex iTRAQ was used on two separate occasions to label either 100 or 40 μg of cell envelope or MVs proteins, respectively, obtained from each GC strain (Fig. 1D). Thus, four independent iTRAQ experiments were performed in total. The following iTRAQ tags were used for respective GC strains: 114 for FA1090, 115 for F62, 116 for MS11, and 117 for 1291. After labeling, the samples were pooled and subjected to SCX fractionation followed by a reversed-phase separation and protein identification, and quantification using MALDI-TOF MS/MS (Fig. 1D).
Strategy for Rigorous Proteomic Profiling of the GC Cell Envelopes and MVs
The proteome mining of the GC cell envelopes revealed 765 and 568 proteins in two independent iTRAQ experiments (Fig. 2A and supplemental Table S2). Further, 308 and 213 proteins were detected in the MVs fractions (Fig. 2B and supplemental Table S3). All of these proteins were identified with <1% false discovery rate. There were 533 and 168 common proteins in the cell envelopes and MVs, respectively, identified in all four GC strains in both proteomic studies (Fig. 2A and 2B).
Fig. 2.
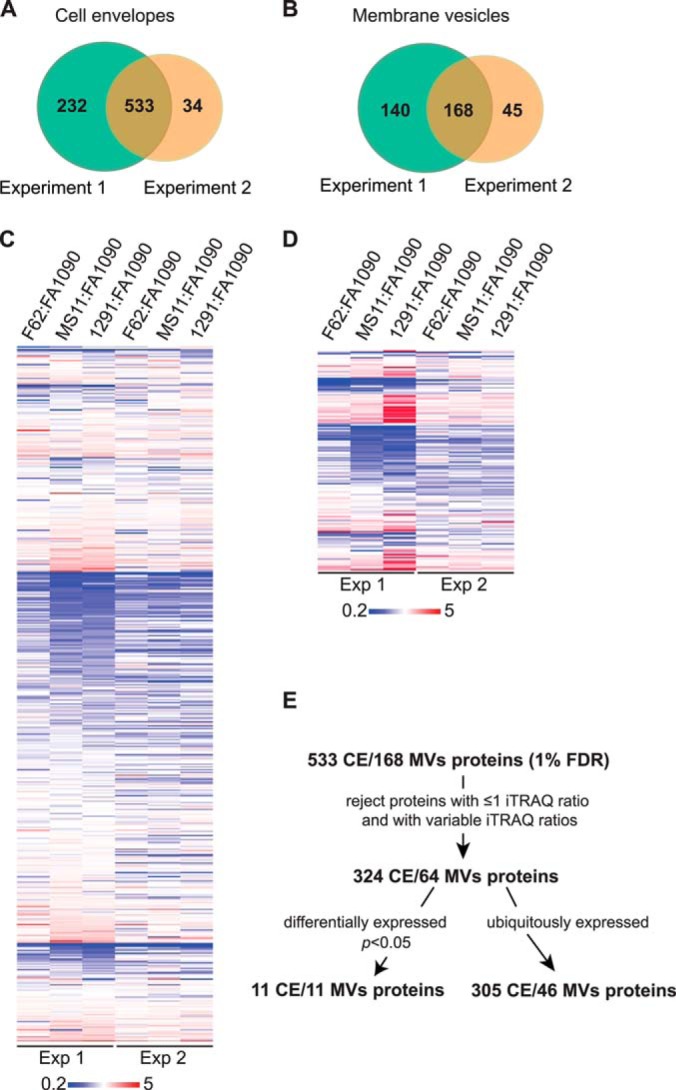
Quantitative proteomics profiling of the cell envelopes and MVs isolated from GC strains FA1090, MS11, F62, and 1291. A, Venn diagram of proteins identified in the cell envelopes in biological replicate experiments. A total of 765 and 567 individual protein species was identified in Experiment 1 and 2, respectively. B, Distribution of proteins identified in MVs in two independent experiments (Experiments 1 and 2). A total number of 308 and 213 proteins was identified, respectively. C, and D, Heat maps illustrating the relative abundance of common proteins identified in both biological experiments in the cell envelopes and MVs, respectively. To quantify the abundance of the proteins, strain FA1090 was arbitrarily chosen as the reference strain. The color scale covers fivefold down-regulation (blue), via no change (white), to fivefold up-regulation (red). E, Flowchart outlining the strategy for rigorous analysis of data obtained during proteomic profiling of the GC cell envelopes (CE) and MVs.
To quantify the relative abundance of identified proteins, strain FA1090 was arbitrarily selected as the reference strain and the iTRAQ ratios were calculated as follows: F62/FA1090 (115/114), MS11/FA1090 (116/114), and 1291/FA1090 (117/114). Similarly to our previous study (41) a >2.0-fold change in the iTRAQ ratios was chosen as a criterion for differential protein abundance (Fig. 2C and 2D). Subsequently, proteins without associated iTRAQ ratios, quantified in only one experiment, and displaying variable reporter ratios were eliminated (Fig. 2E). Hence, only these proteins were considered, which in two independent experiments were consistently identified as either ubiquitously (iTRAQ ratios of 0.5<proteins<2) or differentially expressed (down- and up-regulated with calculated p < 0.05 and the reporters ratios of either <0.5 or >2, respectively). This rigorous strategy eliminated nearly 40 and 68% of proteins identified in the cell envelope and MVs fractions, and the total number of quantified protein species decreased to 316 and 57, respectively (Fig. 2E, Tables II–V and supplemental Tables S4–S5). These proteins were subjected to further computational analysis.
Table II. Ubiquitous proteins identified by iTRAQ in the GC cell envelope.
| Accession | Name | Gene | Average ratio ± S.D.a |
Sb | Tc | ||
|---|---|---|---|---|---|---|---|
| F62:FA1090 | MS11:FA1090 | 1291:FA1090 | |||||
| Extracellular | |||||||
| Q5F7V4 | Lipoprotein | NGO1063 | 1.31 ± 0.26 | 1.45 ± 0.17 | 1.18 ± 0.1 | Y | N |
| Outer membrane | |||||||
| Q5F5F6 | Adhesin mafA 1/4 | mafA1/NGO1067 mafA4/NGO1972 | 0.63 ± 0.03 | 1.1 ± 0.01 | 1.47 ± 0.32 | N | N |
| Q5F9W0 | Competence lipoprotein | bamD, comL/NGO0277 | 1.07 ± 0.09 | 1.25 ± 0.39 | 1.06 ± 0.02 | Y | N |
| Q5F679 | Efflux pump protein, fatty acid resistance | emrA/NGO1683 | 1.33 ± 0.22 | 0.98 ± 0.3 | 1.42 ± 0.11 | N | Y |
| Q5F651 | LPS-assembly protein LptD | lptD, imp, ostA/NGO1715 | 1.14 ± 0.19 | 1.34 ± 0.29 | 1.23 ± 0.1 | Y | N |
| Q5F6J5 | OmpA/MotB domain-containing protein | NGO1559 | 1.33 ± 0.2 | 1.64 ± 0.31 | 1.1 ± 0.16 | Y | N |
| Q5F5W8 | Outer membrane protein assembly factor BamA | bamA/NGO1801 | 1.27 ± 0.21 | 1.15 ± 0.39 | 1.16 ± 0.03 | Y | N |
| Q5F6I1 | Outer membrane protein PIII | rmp/NGO1577 | 1.22 ± 0.27 | 1.22 ± 0.17 | 0.73 ± 0.02 | Y | N |
| Q5F7A4 | Nitrite reductased | aniA/NGO1276 | 1.46 ± 0.26 | 1.15 ± 0.24 | 1.13 ± 0.31 | Y | Y |
| Q5F8E4 | Periplasmic protein | NGO0834 | 1.49 ± 0.14 | 1.16 ± 0.09 | 0.8 ± 0.07 | Y | N |
| Q5F7F3 | Peptidyl-prolyl cis-trans isomerase Ng-MIPe | mip/NGO1225 | 1.15 ± 0 | 0.94 ± 0.09 | 1.17 ± 0.07 | Y | Y |
| Q5F6Q7 | Phospholipase | pldA/NGO1492 | 1.42 ± 0.14 | 1.37 ± 0.01 | 1.15 ± 0 | N | N |
| Q5FAG7 | Pilus-associated protein | pilC/NGO0055 | 1.13 ± 0.04 | 1.14 ± 0.1 | 1.21 ± 0.14 | N | Y |
| Q5FAC9 | Putative pilus assembly protein | pilN/NGO0097 | 1.01 ± 0.05 | 1.13 ± 0.06 | 1.31 ± 0.03 | N | N |
| Q5F7H3 | Putative TonB-dependent receptor protein | NGO1205 | 0.88 ± 0.04 | 1.11 ± 0.03 | 0.67 ± 0.13 | Y | N |
| Q5F518 | Putative uncharacterized protein | NGO2121 | 0.69 ± 0.05 | 1.14 ± 0.09 | 0.83 ± 0.06 | Y | N |
| Q5F526 | Putative uncharacterized protein | NGO2111 | 1.08 ± 0.18 | 0.82 ± 0.04 | 0.81 ± 0.01 | N | Y |
| Q5F5E4 | Putative uncharacterized protein | NGO1985 | 0.88 ± 0.06 | 1.17 ± 0.01 | 0.83 ± 0.04 | N | N |
| Q5F5H0 | Putative uncharacterized protein | NGO1956 | 1.18 ± 0.08 | 1.4 ± 0.17 | 1.41 ± 0.02 | N | N |
| Q5F5H1 | Putative uncharacterized protein | NGO1955 | 1.2 ± 0.1 | 1.15 ± 0.24 | 1.15 ± 0.1 | N | N |
| Q5F743 | Putative uncharacterized protein | NGO1344 | 1.01 ± 0.05 | 1.05 ± 0.14 | 0.9 ± 0.11 | N | N |
| Q5FAD2 | Type IV pilus biogenesis and competence protein pilQ | pilQ, omc/NGO0094 | 1.12 ± 0.19 | 1.15 ± 0.09 | 0.89 ± 0.13 | Y | N |
| Periplasmic | |||||||
| Q5F601 | Catalase | katA/NGO1767 | 0.92 ± 0.05 | 1.09 ± 0.39 | 0.97 ± 0.05 | N | N |
| Q5F544 | Ferric enterobactin periplasmic binding protein | fetB/NGO2092 | 1.06 ± 0.06 | 0.99 ± 0.33 | 1.27 ± 0.09 | N | N |
| Q5F581 | Genome-derived Neisserial antigen 33 | NGO2048 | 1.38 ± 0.21 | 1.24 ± 0.01 | 1.28 ± 0.04 | Y | N |
| Q5F809 | Lipid modified azurin protein | azu/NGO0994 | 1.09 ± 0.24 | 1.07 ± 0.04 | 1.15 ± 0.01 | Y | Y |
| Q5F765 | Lipoprotein | NGO1321 | 1.14 ± 0.25 | 1 ± 0.32 | 1.1 ± 0.08 | N | N |
| Q5F848 | Lipoprotein | NGO0948 | 1.47 ± 0.21 | 1.34 ± 0 | 1.05 ± 0.02 | N | N |
| Q5F8T2 | Lipoprotein | NGO0678 | 1.08 ± 0.22 | 0.99 ± 0.05 | 0.78 ± 0.09 | Y | N |
| Q5F8W2 | Membrane lipoprotein | NGO0648 | 1.47 ± 0.12 | 1.02 ± 0 | 1.43 ± 0.21 | N | N |
| Q5FAC7 | Penicillin-binding protein 1A | mrcA/NGO0099 | 1.46 ± 0.14 | 0.7 ± 0 | 0.82 ± 0.01 | N | N |
| Q5F9Z6 | Periplasmic protein | NGO0238 | 1.1 ± 0.04 | 1.23 ± 0.07 | 1.31 ± 0.15 | Y | N |
| Q5F9M1 | Putative ABC transporter, periplasmic binding protein, amino acid | NGO0372 | 0.85 ± 0.05 | 0.97 ± 0.1 | 1.04 ± 0 | Y | Y |
| Q5F6Q5 | Putative ABC transporter, periplasmic binding protein, polyamine | NGO1494 | 0.79 ± 0.02 | 0.74 ± 0 | 1.24 ± 0.03 | Y | N |
| Q5F7C5 | Putative ABC transporter, periplasmic binding protein, polyamine | NGO1253 | 1.14 ± 0.02 | 0.93 ± 0.2 | 1.79 ± 0.01 | N | N |
| Q5FA28 | Putative ABC transporter, periplasmic binding protein, polyamine | NGO0206 | 0.64 ± 0 | 0.83 ± 0.01 | 1.5 ± 0.12 | Y | N |
| Q5F574 | Putative ABC transporter, thiamine-binding periplasmic protein | NGO2056 | 1.43 ± 0.29 | 0.84 ± 0.23 | 1.41 ± 0 | Y | N |
| Q5FAB9 | Putative carboxypeptidase, penicillin binding protein | pbp3/NGO0107 | 1.05 ± 0.28 | 1.43 ± 0.18 | 0.83 ± 0.17 | Y | N |
| Q5F718 | Putative cytochrome c oxidase subunit | NGO1371 | 1.15 ± 0.17 | 1.1 ± 0.46 | 1.01 ± 0.12 | N | N |
| Q5F598 | Putative cytochrome C1 | NGO2031 | 1.16 ± 0.01 | 1.3 ± 0.01 | 1.66 ± 0.04 | Y | N |
| Q5F8Y3 | Putative murein hydrolase | mltB/NGO0626 | 1.4 ± 0.06 | 1.43 ± 0.01 | 1.05 ± 0.16 | Y | N |
| Q5FA91 | Putative serine protease | NGO0138 | 1.06 ± 0.11 | 0.98 ± 0.4 | 1.11 ± 0.06 | Y | N |
| Q5F515 | Putative thioredoxin | NGO2124 | 0.9 ± 0.05 | 1.02 ± 0.18 | 1.12 ± 0.04 | N | N |
| Q5F5C7 | Putative uncharacterized protein | NGO2002 | 1.29 ± 0 | 1.06 ± 0.07 | 1.34 ± 0.23 | N | N |
| Q5F6P4 | Putative uncharacterized protein | NGO1505 | 1.18 ± 0.11 | 1.28 ± 0.14 | 1.71 ± 0.08 | N | N |
| Q5F7W0 | Putative uncharacterized protein | NGO1056 | 1.17 ± 0.15 | 1.18 ± 0.45 | 0.95 ± 0.07 | N | Y |
| Q5F7X1 | Putative uncharacterized protein | NGO1044 | 0.94 ± 0.41 | 1.02 ± 0.11 | 1.27 ± 0.1 | N | N |
| Q5F7X2 | Putative uncharacterized protein | NGO1043 | 1.07 ± 0.01 | 1.06 ± 0.2 | 0.84 ± 0.11 | Y | N |
| Q5F8C4 | Putative uncharacterized protein | NGO0861 | 1.17 ± 0.19 | 1.4 ± 0.42 | 1.23 ± 0.16 | Y | N |
| Q5F571 | Putativeptide methionine sulfoxide reductase | msrAB, pilB/NGO2059 | 1.36 ± 0.31 | 1.19 ± 0.36 | 1.29 ± 0.33 | Y | N |
| Q5F649 | Thiol:disulphide interchange protein | dsbA/NGO1717 | 0.98 ± 0.02 | 1.14 ± 0.13 | 1.28 ± 0.02 | Y | Y |
| Q5F6V7 | Thiol:disulphide interchange protein | dsbC/NGO1438 | 1.37 ± 0.03 | 1.01 ± 0.1 | 1.03 ± 0.1 | Y | N |
| Q5F505 | Transglycosylase | NGO2135 | 1.62 ± 0.22 | 1.29 ± 0.17 | 0.98 ± 0.12 | Y | N |
| Q5F912 | Type IV pilus assembly protein | pilF/NGO0595 | 1.3 ± 0.1 | 1.01 ± 0.09 | 1.07 ± 0.08 | N | N |
| Inner membrane | |||||||
| Q5F4Z4 | ATP synthase subunit b | atpF/NGO2146 | 1.23 ± 0.03 | 1.04 ± 0 | 1.17 ± 0.18 | N | N |
| Q5F9L1 | ATP-dependent zinc metalloprotease FtsH | ftsH/NGO0382 | 1.04 ± 0.05 | 1.14 ± 0.42 | 1.29 ± 0.03 | N | N |
| Q5F6M1 | Cell division protein FtsQ | ftsQ/NGO1530 | 0.95 ± 0.05 | 0.7 ± 0.3 | 1.01 ± 0.09 | N | N |
| Q5F6L1 | Division cell wall protein | dcaA/NGO1540 | 0.97 ± 0.09 | 0.88 ± 0.16 | 1 ± 0.06 | N | N |
| Q5F8I5 | Genome-derived Neisseria antigen 1220 | NGO0788 | 1.43 ± 0.03 | 1.27 ± 0 | 1.52 ± 0 | N | N |
| Q5F573 | Integral membrane protein | NGO2057 | 1.28 ± 0.09 | 1.32 ± 0.02 | 1.26 ± 0.02 | N | N |
| Q5F5L3 | Integral membrane protein | NGO1910 | 0.82 ± 0.06 | 1.1 ± 0.14 | 0.94 ± 0.03 | Y | N |
| Q5F795 | Integral membrane protein | NGO1288 | 1.15 ± 0.02 | 1.06 ± 0.02 | 1.27 ± 0.03 | N | N |
| Q5F4X8 | Lipid A export ATP-binding/permease protein MsbA | msbA/NGO2165 | 1.07 ± 0.02 | 1.05 ± 0.16 | 1.13 ± 0.11 | N | N |
| Q5F6V6 | Macrolide export ATP-binding/permease protein MacB | macB/NGO1439 | 1.25 ± 0.14 | 0.94 ± 0.4 | 0.85 ± 0.09 | N | N |
| Q5F4W6 | Membrane protein insertase YidC | yidC/NGO2178 | 1.2 ± 0.07 | 0.99 ± 0.05 | 1.2 ± 0.03 | N | N |
| Q5F6K9 | Penicillin-binding protein 2 | penA/NGO1542 | 1.15 ± 0 | 0.79 ± 0.21 | 1 ± 0.1 | N | N |
| Q5F7H2 | Phosphatidylserine decarboxylase proenzyme | psd/NGO1206 | 1.09 ± 0.02 | 1.31 ± 0.3 | 1.32 ± 0.04 | N | N |
| Q5F693 | Pilus assembly protein | pilG/NGO1669 | 1.33 ± 0.05 | 1.17 ± 0 | 1.44 ± 0.01 | N | N |
| Q5FAD0 | Pilus assembly protein | pilO/NGO0096 | 1.28 ± 0.15 | 1.03 ± 0.21 | 1.35 ± 0.11 | N | N |
| Q5F6W9 | Probable ubiquinone biosynthesis protein UbiB | ubiB/NGO1424 | 1.26 ± 0.1 | 1.18 ± 0.13 | 1.39 ± 0.18 | N | Y |
| Q5FA43 | Protein translocase subunit SecD | secD/NGO0189 | 1.24 ± 0.07 | 1.08 ± 0 | 1.21 ± 0.11 | N | N |
| Q5F567 | Putative 1-acyl-SN-glycerol-3-phosphate acyltransferase | nlaB/NGO2069 | 1.03 ± 0.08 | 1.06 ± 0.2 | 1.48 ± 0.06 | N | N |
| Q5F523 | Putative ABC transporter, ATP-binding protein | NGO2116 | 0.98 ± 0.03 | 1.12 ± 0.24 | 1.27 ± 0.05 | N | N |
| Q5F634 | Putative ABC transporter, ATP-binding protein | NGO1732 | 1.22 ± 0.06 | 1.23 ± 0.03 | 1.5 ± 0.05 | N | N |
| Q5F969 | Putative acyl-CoA ligase | NGO0530 | 1.13 ± 0.11 | 1 ± 0.39 | 1.02 ± 0.06 | N | N |
| Q5F7V3 | Putative carbon starvation protein | cstA/NGO1064 | 1.01 ± 0.04 | 1.44 ± 0.02 | 1.55 ± 0.02 | N | N |
| Q5F823 | Thiol:disulfide interchange protein DsbD | dsbD/NGO0978 | 1.11 ± 0.04 | 1.1 ± 0.21 | 1.06 ± 0.12 | N | N |
| Q5F952 | Putative dnaJ-family protein | NGO0551 | 1.29 ± 0.23 | 0.93 ± 0.35 | 1 ± 0.12 | N | N |
| Q5F7Y5 | Putative ferredoxin | NGO1026 | 0.99 ± 0 | 0.89 ± 0.03 | 1.02 ± 0.04 | N | Y |
| Q5F7V7 | Putative fimbrial assembly protein | fimB/NGO1060 | 1.14 ± 0.03 | 1.01 ± 0.04 | 1.35 ± 0.09 | N | Y |
| Q5F916 | Putative ftsK-like cell division/stress response protein | NGO0590 | 1.07 ± 0.02 | 0.98 ± 0.19 | 1.04 ± 0.08 | N | N |
| Q5F8K3 | Putative lipoprotein releasing system transmembrane protein | NGO0769 | 1.19 ± 0.11 | 1.17 ± 0.12 | 1.43 ± 0.05 | N | N |
| Q5F9U8 | Putative Na+/H+ antiporter | NGO0291 | 1.29 ± 0.2 | 1.2 ± 0.29 | 1.53 ± 0.02 | N | N |
| Q5F6S7 | Putative NADP transhydrogenase, alpha subunit | NGO1470 | 1.38 ± 0.01 | 1.14 ± 0.03 | 1.26 ± 0.02 | N | N |
| Q5F7A5 | Putative nitric oxide reductase | NGO1275 | 1.5 ± 0.16 | 1.03 ± 0.05 | 1 ± 0.19 | N | N |
| Q5F8S5 | Putative P-type cation-transporting ATPase | NGO0685 | 1.03 ± 0 | 1.22 ± 0.03 | 1.39 ± 0.02 | N | N |
| Q5F7D2 | Putative serine protease | NGO1246 | 1.46 ± 0.29 | 0.85 ± 0.17 | 0.87 ± 0 | N | N |
| Q5FAG5 | Putative thioredoxin | NGO0057 | 1.26 ± 0.09 | 1.13 ± 0.24 | 1.12 ± 0.11 | N | N |
| Q5F614 | Putative uncharacterized protein | NGO1752 | 1.14 ± 0.11 | 1.15 ± 0.34 | 0.73 ± 0.1 | N | N |
| Q5FA44 | Putative uncharacterized protein | NGO0188 | 1.04 ± 0.08 | 1.45 ± 0.35 | 1.32 ± 0.25 | N | N |
| Q5F5W9 | RIP metalloprotease RseP | rseP/NGO1800 | 1.02 ± 0.03 | 0.95 ± 0.12 | 1.03 ± 0.1 | N | N |
| Q5F5K7 | Transmembrane transporter | NGO1917 | 0.91 ± 0.12 | 1.25 ± 0.21 | 1.28 ± 0.59 | N | N |
| Q5F711 | Transport protein | exbB/NGO1378 | 1.05 ± 0.03 | 0.82 ± 0.2 | 0.99 ± 0 | N | N |
| Q5F5Q0 | Two-component system sensor kinase | NGO1867 | 1.12 ± 0.01 | 1 ± 0.14 | 1.23 ± 0.03 | N | N |
| Q5FA56 | Two-component system sensor kinase | NGO0176 | 1.14 ± 0.01 | 1.13 ± 0.08 | 1.26 ± 0.02 | N | N |
| Q5FAA1 | UPF0761 membrane protein NGO0127 | NGO0127 | 1.26 ± 0.14 | 1.14 ± 0.1 | 1.07 ± 0.2 | N | N |
| Q5F9N3 | Uroporphyrin-III C-methyltransferase | hemX/NGO0360 | 0.9 ± 0.02 | 0.86 ± 0.15 | 1.22 ± 0.1 | N | N |
| Q5F7H9 | YhbX/YhjW/YijP/YjdB family protein | NGO1198 | 1.06 ± 0.09 | 1.18 ± 0.29 | 1.12 ± 0 | N | N |
| Unknown | |||||||
| Q5F8Z8 | 1-acyl-SN-glycerol-3-phosphate acyltransferase | plsC/NGO0611 | 1.36 ± 0.11 | 1.32 ± 0.02 | 1.5 ± 0.22 | N | Y |
| Q5F5V0 | 30S ribosomal protein S11 | rpsK/NGO1820 | 1.14 ± 0.03 | 0.99 ± 0.06 | 0.92 ± 0.06 | N | Y |
| Q5F913 | 4-hydroxy-3-methylbut-2-en-1-yl diphosphate synthase | ispG/NGO0594 | 0.82 ± 0.08 | 0.67 ± 0.08 | 0.63 ± 0.09 | N | N |
| Q5F9Z8 | Cell division protein zipA | zipA/NGO0236 | 0.99 ± 0.01 | 1.11 ± 0.05 | 1.25 ± 0.01 | N | N |
| Q5F501 | Genome-derived Neisserial antigen 1946 | NGO2139 | 1.21 ± 0.04 | 1.23 ± 0.05 | 1.74 ± 0.03 | Y | Y |
| Q5F9N2 | HemY protein | hemY/NGO0361 | 1.12 ± 0.03 | 1.15 ± 0.06 | 1.29 ± 0.03 | N | N |
| Q5F9W2 | IgA-specific metalloendopeptidase | iga/NGO0275 | 0.93 ± 0.03 | 1.39 ± 0.1 | 1.05 ± 0 | Y | N |
| Q5F5I3 | Lipoprotein | NGO1942 | 0.99 ± 0.05 | 1.24 ± 0.06 | 1 ± 0.02 | N | N |
| Q5F8J5 | Membrane protein | NGO0778 | 1.12 ± 0.01 | 0.89 ± 0.27 | 1.01 ± 0.06 | N | N |
| Q5F7J8 | Minor pilin ComP | comP/NGO1177 | 1.17 ± 0.2 | 0.62 ± 0.33 | 1.54 ± 0.01 | N | N |
| Q5F521 | Outer membrane transporter | NGO2118 | 0.96 ± 0.2 | 1.07 ± 0.21 | 1.05 ± 0.15 | N | N |
| Q5F8S7 | Periplasmic protein | NGO0683 | 1.04 ± 0.4 | 0.84 ± 0.08 | 0.87 ± 0.28 | Y | N |
| Q5FAD1 | Pilin assembly protein | pilP/NGO0095 | 1.19 ± 0.04 | 1.08 ± 0.07 | 1.45 ± 0.16 | Y | N |
| Q5F4Y0 | Putative 3-oxoacyl-[acyl-carrier protein] reductase | fabG/NGO2163 | 0.62 ± 0.11 | 0.8 ± 0.21 | 0.76 ± 0.02 | N | Y |
| Q5FA61 | Putative ABC transporter, ATP-binding protein | znuC, troB/NGO0170 | 0.87 ± 0.2 | 0.77 ± 0.23 | 0.7 ± 0.13 | N | N |
| Q5F531 | Putative adhesion and penetration protein | NGO2105 | 1.04 ± 0.27 | 0.96 ± 0.13 | 0.94 ± 0.12 | N | Y |
| Q5F6P7 | Putative amidase | amiC/NGO1502 | 1.56 ± 0.09 | 1.47 ± 0 | 1.32 ± 0.14 | N | Y |
| Q5F932 | Putative carboxy-terminal processing protease | NGO0572 | 0.82 ± 0.06 | 0.98 ± 0.36 | 0.84 ± 0.04 | Y | N |
| Q5F759 | Putative cytochrome | NGO1328 | 1.16 ± 0.01 | 1.2 ± 0.12 | 1.28 ± 0.23 | N | Y |
| Q5FA09 | Putative MafB-like protein | NGO0225 | 1.18 ± 0.17 | 1.2 ± 0.46 | 1.02 ± 0.01 | Y | N |
| Q5F6X8 | Putative Na(+)-translocating NADH-ubiquinone reductase subunit C | nqrC/NGO1415 | 1.1 ± 0.19 | 1.04 ± 0.16 | 1.11 ± 0.19 | N | N |
| Q5F674 | Putative outer membrane protein OmpU | ompU/NGO1688 | 1.73 ± 0.17 | 1.44 ± 0 | 1.54 ± 0.31 | Y | N |
| Q5F8E6 | Putative oxidoreductase | NGO0831 | 1.17 ± 0.1 | 1.33 ± 0.16 | 1.28 ± 0.25 | N | N |
| Q5F766 | Putative paraquat-inducible protein B | NGO1320 | 1.01 ± 0.02 | 0.98 ± 0.13 | 1 ± 0.01 | N | N |
| Q5FAC8 | Putative pilus assembly protein | pilM/NGO0098 | 1.11 ± 0.04 | 1.34 ± 0.17 | 1.48 ± 0.09 | N | N |
| Q5F520 | Putative uncharacterized protein | NGO2119 | 1.27 ± 0.05 | 1.03 ± 0.05 | 0.7 ± 0.18 | Y | N |
| Q5F5E5 | Putative uncharacterized protein | NGO1984 | 0.9 ± 0.04 | 0.98 ± 0.14 | 1.04 ± 0.04 | N | N |
| Q5F5P4 | Putative uncharacterized protein | NGO1873 | 1.34 ± 0.28 | 1.34 ± 0.1 | 0.94 ± 0.08 | N | N |
| Q5F5W7 | Putative uncharacterized protein | NGO1802 | 1.06 ± 0.18 | 1.45 ± 0.02 | 1.06 ± 0.19 | Y | Y |
| Q5F657 | Putative uncharacterized protein | NGO1709 | 1.22 ± 0.03 | 1.3 ± 0.07 | 1.44 ± 0.01 | N | Y |
| Q5F676 | Putative uncharacterized protein | NGO1686 | 1.11 ± 0.01 | 1.22 ± 0.03 | 1.29 ± 0 | N | N |
| Q5F6K2 | Putative uncharacterized protein | NGO1549 | 1.15 ± 0.09 | 1.1 ± 0.29 | 1.31 ± 0.03 | N | Y |
| Q5F6P5 | Putative uncharacterized protein | NGO1504 | 0.95 ± 0.08 | 1.38 ± 0.22 | 1.45 ± 0.09 | N | N |
| Q5F7A6 | Putative uncharacterized protein | NGO1274 | 1.41 ± 0.02 | 1.62 ± 0.1 | 1.46 ± 0.01 | N | Y |
| Q5F7C7 | Putative uncharacterized protein | NGO1251 | 0.94 ± 0.06 | 0.88 ± 0.39 | 0.62 ± 0.05 | N | N |
| Q5FAK6 | Putative uncharacterized protein | NGO0028 | 1.07 ± 0.17 | 1.44 ± 0.09 | 1.31 ± 0.36 | N | N |
| Q5F9Q0 | Signal peptidase I | lepB/NGO0343 | 1.01 ± 0.09 | 1.07 ± 0.12 | 1.21 ± 0.1 | N | N |
| Q5F9E3 | Type IV pilus assembly protein PilX | pilX/NGO0455 | 0.57 ± 0.06 | 0.74 ± 0.03 | 0.67 ± 0.12 | N | N |
| O87406 | UPF0070 protein NGO0425 | NGO0425 | 1.11 ± 0.07 | 1.12 ± 0.22 | 0.98 ± 0.01 | N | N |
a Average ratios and standard deviations were calculated for proteins ubiquitously expressed in biological replica experiments.
b Predictions of signal peptide cleavage site using SignalP (47).
c Presence of twin-arginine signal peptide as determined by TatP (48).
d The surface localization of AniA was verified experimentally (97).
e The outer membrane localization of Ng-MIP was verified experimentally (78).
Table III. Ubiquitous proteins identified by iTRAQ in the GC MVs.
| Accession | Name | Gene | Average ratio ± S.D.a |
Sb | Tc | ||
|---|---|---|---|---|---|---|---|
| F62:FA1090 | MS11:FA1090 | 1291:FA1090 | |||||
| Outer membrane | |||||||
| Q5F5F6 | Adhesin mafA 1/4 | mafA1/NGO1067 mafA4/NGO1972 | 0.97 ± 0.24 | 1.34 ± 0.36 | 1.25 ± 0.26 | N | N |
| Q5F651 | LPS-assembly protein lptD | lptD, imp, ostA/NGO1715 | 1.13 ± 0.25 | 1.5 ± 0.04 | 1.39 ± 0.27 | Y | N |
| Q5F5V7 | Major outer membrane protein porin P.IB | porB/NGO1812 | 1.35 ± 0.33 | 1.6 ± 0.26 | 1.1 ± 0.02 | Y | N |
| Q5FAG7 | Pilus-associated protein | pilC/NGO0055 | 1.38 ± 0.13 | 0.55 ± 0.03 | 0.97 ± 0.05 | N | Y |
| Q5F6Q7 | Phospholipase A1 | pldA/NGO1492 | 0.97 ± 0.12 | 1.34 ± 0.22 | 1.29 ± 0.17 | N | N |
| Q5F5W8 | Putative uncharacterized protein | bamA/NGO1801 | 0.91 ± 0.07 | 1.21 ± 0.06 | 1.21 ± 0.04 | Y | N |
| Q5FAD2 | Type IV pilus biogenesis and competence protein pilQ | pilQ, omc/NGO0094 | 0.94 ± 0.1 | 1.34 ± 0.31 | 0.89 ± 0.15 | Y | N |
| Periplasmic | |||||||
| Q5F601 | Catalase | katA/NGO1767 | 0.98 ± 0.45 | 0.93 ± 0.1 | 0.82 ± 0.25 | N | N |
| Inner membrane | |||||||
| Q5F823 | Thiol:disulfide interchange protein DsbD | dsbD/NGO0978 | 1.24 ± 0.54 | 1 ± 0.34 | 1.44 ± 0.41 | N | N |
| Unknown | |||||||
| Q5F5U9 | 30S ribosomal protein S13 | rpsM/NGO1821 | 0.97 ± 0.16 | 0.88 ± 0.13 | 0.91 ± 0.03 | N | N |
| Q5F6H4 | Adhesin mafA 2/3 | mafA2/NGO1393;mafA3/NGO1584 | 1.22 ± 0.2 | 0.84 ± 0.04 | 0.92 ± 0.02 | N | N |
| Q5F501 | Lipoprotein | NGO2139 | 0.6 ± 0.09 | 0.81 ± 0.27 | 1 ± 0.05 | Y | Y |
| Q5F9W2 | IgA-specific metalloendopeptidase | iga/NGO0275 | 1.28 ± 0.16 | 1.35 ± 0.24 | 1.14 ± 0.37 | Y | N |
| Q5F932 | Putative carboxy-terminal processing protease | NGO0572 | 0.8 ± 0.1 | 1.54 ± 0.05 | 1.32 ± 0.4 | Y | N |
| Q5FA09 | Putative MafB-like protein | NGO0225 | 1.23 ± 0.37 | 0.94 ± 0.24 | 1.11 ± 0.05 | Y | N |
| Q5FAC8 | Putative pilus assembly protein | pilM/NGO0098 | 0.92 ± 0.13 | 0.76 ± 0.15 | 0.68 ± 0.17 | N | N |
| Q5F576 | Putative uncharacterized protein | NGO2054 | 0.97 ± 0.22 | 0.69 ± 0.07 | 1.48 ± 0.18 | Y | N |
Table IV. Differentially expressed GC cell envelope proteins identified by iTRAQ.
| Accession | Name | Gene | Average ratio ± S.D.a |
Sb | Tc | ||
|---|---|---|---|---|---|---|---|
| F62:FA1090 | MS11:FA1090 | 1291:FA1090 | |||||
| Outer membrane | |||||||
| Q5F726 | Multidrug efflux pump channel protein | mtrE/NGO1363 | 1.08 ± 0.04 | 2.65 ± 0.02 | 0.93 ± 0.15 | Y | N |
| Q5F6Q4 | Transferrin-binding protein A | tbpA/NGO1495 | 2.93 ± 0.25 | 0.83 ± 0.09 | 0.67 ± 0.07 | Y | N |
| Periplasmic | |||||||
| Q5F9E2 | Putative type IV pilin-like protein | NGO0456 | 0.14 ± 0.04 | 1.11 ± 0.11 | 0.98 ± 0.05 | N | N |
| Q5F724* | Antibiotic resistance efflux pump component MtrCd | mtrC/NGO1365 | 0.93 ± 0 | 2.98 ± 0.39 | 0.28 ± 0.06 | Y | Y |
| Inner membrane | |||||||
| Q5F725 | Antibiotic resistance efflux pump component | mtrD/NGO1364 | 1.08 ± 0.01 | 3.21 ± 0.51 | 0.69 ± 0.02 | N | N |
| Unknown | |||||||
| Q5F5N1 | Lipoprotein | NGO1889 | 2.56 ± 0.09 | 2.4 ± 0.45 | 3.35 ± 0.02 | N | N |
| Q5F865 | Putative peroxiredoxin family protein/glutaredoxin | NGO0926 | 0.39 ± 0.1 | 0.35 ± 0.11 | 0.27 ± 0.07 | N | N |
| Q5F9E4 | Type IV pilus assembly protein PilW | pilW/NGO0454 | 0.34 ± 0.09 | 0.24 ± 0.02 | 0.26 ± 0.06 | N | N |
| Cytoplasmic | |||||||
| Q5F5Q8 | Elongation factor Tu | tuf1/NGO1842 | 0.41 ± 0.1 | 0.24 ± 0.03 | 0.3 ± 0.03 | N | N |
| Q5F5T6 | 30S ribosomal protein S17 | rpsQ/NGO1830 | 0.64 ± 0.23 | 0.41 ± 0.04 | 0.45 ± 0.02 | N | N |
| Q5F5V3 | 50S ribosomal protein L17 | rplQ/NGO1817 | 3.65 ± 0.07 | 1.7 ± 0.16 | 1.4 ± 0.05 | N | Y |
a Average ratio and standard deviation were calculated for differentially expressed proteins identified in both biological replica experiments with associated p < 0.05.
b Predictions of signal peptide cleavage site using SignalP server (47).
c Presence of twin-arginine signal peptide as determined by TatP (48).
d MtrC protein has been shown to be localized to the periplasm (111).
Table V. Differentially expressed proteins identified in naturally released MVs.
| Accession | Name | Gene | Average ratio ± S.D.a |
Sb | Tc | ||
|---|---|---|---|---|---|---|---|
| F62:FA1090 | MS11:FA1090 | 1291:FA1090 | |||||
| Outer membrane | |||||||
| Q5F726 | Multidrug efflux pump channel protein | mtrE/NGO1363 | 0.9 ± 0.07 | 2.73 ± 0.22 | 1.82 ± 0.15 | Y | N |
| Q5F6Q4 | Transferrin-binding protein A | tbpA/NGO1495 | 2.66 ± 0.42 | 1.2 ± 0.17 | 1.11 ± 0.22 | Y | N |
| Periplasmic | |||||||
| Q5FA17 | ABC transporter, periplasmic binding protein, iron related | fbpA/NGO0217 | 2.58 ± 0.12 | 1.75 ± 0.03 | 4.8 ± 3.11 | Y | N |
| Q5F6V7 | Putative uncharacterized protein | dsbC/NGO1438 | 1.12 ± 0.04 | 2.38 ± 0.27 | 1.92 ± 0.56 | Y | N |
| Q5F7W0 | Putative uncharacterized protein | NGO1056 | 1.63 ± 0.18 | 1.41 ± 0.13 | 2.42 ± 0.32 | N | Y |
| Q5F8C4 | Putative uncharacterized protein | NGO0861 | 1.6 ± 0.46 | 2.2 ± 0.58 | 5.43 ± 3.25 | Y | N |
| Unknown | |||||||
| Q5F930 | Carbonic anhydrase | cah/NGO0574 | 2.14 ± 0.33 | 2.27 ± 0.08 | 6.6 ± 4.16 | Y | N |
| Q5F5H6 | Putative uncharacterized protein | NGO1949 | 2.73 ± 0 | 1.22 ± 0.06 | 2.1 ± 0.31 | Y | Y |
| Cytoplasmic | |||||||
| Q5F905 | Putative 30S ribosomal protein S1 | rpsA/NGO0604 | 0.76 ± 0.15 | 0.5 ± 0 | 0.57 ± 0.17 | N | N |
| Q5F5R4 | 50S ribosomal protein L7/L12 | rplL/NGO1852 | 0.48 ± 0.01 | 0.31 ± 0.15 | 0.63 ± 0.03 | N | N |
| Q5F9V6 | Putative uncharacterized protein | NGO0282 | 1.06 ± 0.03 | 1.71 ± 0.08 | 2.33 ± 0.21 | N | N |
a Average ratio and standard deviation were calculated for differentially expressed proteins identified in both biological replica experiments with associated p < 0.05.
b Predictions of signal peptide cleavage site using SignalP (47).
c Presence of twin-arginine signal peptide as determined by TatP (48).
Bioinformatic Analyses
Coupling bioinformatics prediction with experimental subcellular proteomics is particularly important because cytoplasmic proteins are repeatedly identified as constituents of the cell envelopes and MVs in both Gram-negative and Gram-positive bacteria (21, 63, 67, 69–71). Many of the cytoplasmic proteins co-purify as components of large protein complexes that are localized to the membrane, whereas others are likely present as a function of cell lysis occurring during culture (63). We reasoned that the latter group might be higher in autolytic bacteria like GC (13). To analyze the subcellular distribution of identified proteins, a combination of bioinformatics tools was used including PSORTb, CELLO 2.5, and SOSUIGramN (43–46). A majority-votes strategy was chosen for the cellular assignment of proteins, and in these cases where the three engines assigned different localizations, a particular protein was allocated to a group of “unknown subcellular localization.” Additionally, SignalP and TatP servers were employed to analyze the predicted amino acid sequences of individual proteins for the presence of either N-terminal signal peptide (47) or specifically Twin-arginine signal peptide cleavage sites (48), respectively (Tables II–V). These analyses revealed that in the cell envelope fractions the total numbers of 1, 21, 43, 45, 171, and 43 proteins were localized either extracellularly, to the outer membrane, periplasm, inner membrane, cytoplasm, or unknown, correspondingly (Tables II and IV, and supplemental Table S4). The predicted subcellular distribution of proteins identified in the MVs fraction was as follows: 9-outer membrane, 5-periplasmic, 1-inner membrane, 32-cytoplasmic, and 10-unknown (Tables III and V, and supplemental Table S5).
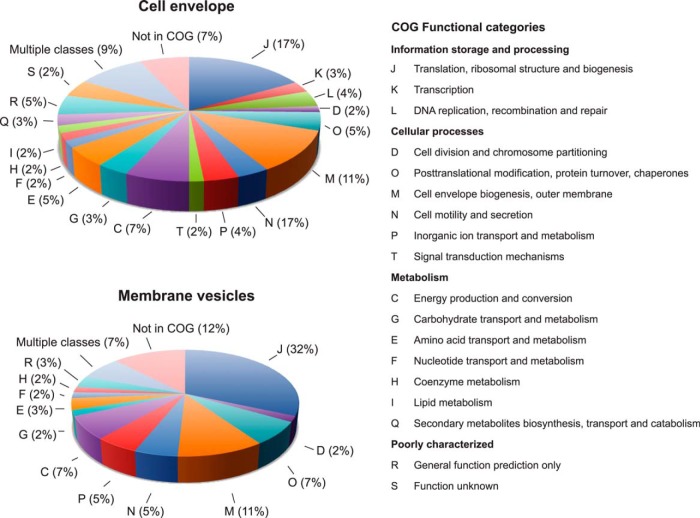
In addition, the identified proteins were subjected to classification based on predicted major cellular function (Fig. 3). All phylogenetic Clusters of Orthologous Groups (COG) of proteins were represented within the cell envelopes (Fig. 3), with the majority of proteins predicted to be involved in cell motility and secretion (17%), cell envelope biogenesis (11%), and ribosomal function (17%). The latter COG was the most represented in the MVs fraction, followed by the functional category of proteins involved in cell envelope biogenesis (Fig. 3). COGs could not be assigned for 7 and 12% of proteins associated with the cell envelopes and MVs, respectively.
Fig. 3.
Functional classification of proteins identified in GC cell envelope and MVs according to clusters of orthologous groups (COGs). The pie chart illustrates the percentages of identified proteins in each COG cluster. COG functional categories are listed on the right.
In conclusion, the subcellular distribution and biological functions of proteins identified in the GC cell envelope and MVs corroborated with previous studies assessing these proteome fractions in Gram-negative and Gram-positive bacteria (21, 63, 67, 69–76). Thus, our study adds to the growing amount of evidence that a large number of cytoplasmic proteins associates with cell envelope and MVs. Interestingly, similar observations come from proteomic profiling of membrane vesicles secreted from the plasma and endosomal membrane by various types of mammalian cells (77).
Ubiquitously Expressed Proteins Present in the GC Cell Envelopes and MVs
The quantitative analyses of relative protein abundance revealed that 305 and 46 proteins were uniformly present in the cell envelopes and MVs, respectively, in four GC strains (Tables II–III, supplemental Tables S4–S5). The large number of identified novel, and also previously described proteins precluded a detailed discussion, thus we focused only on several proteins assigned by bioinformatics analyses either to the cell envelope or with unknown localization (Tables II–III).
Among the stably expressed cell envelope proteins, several previously uncharacterized in GC have been identified including LPS-transport protein LptD (formerly OstA, Imp), a protein designated in the SwissProt database as adhesion MafA 1/4, a putative TonB-dependent receptor, and eight predicted outer membrane proteins NGO1205, NGO0834, NGO2121, NGO2111, NGO1985, NGO1956, NGO1955, and NGO1344. A plethora of predicted periplasmic and inner membrane proteins as well as proteins with unknown localization were also discovered (Table II). Moreover, our experiments revealed ubiquitous expression of a number of recognized proteins including an outer membrane-localized enzyme, Phospholipase A, and a surface-exposed lipoprotein Ng-MIP (Table II). Concurring with our data, these proteins were found invariably expressed in many different GC and meningococcal isolates (78, 79). Perhaps not surprisingly, proteins participating in the process of outer membrane biogenesis, BamA (Omp85) and BamD (ComL), were identified by iTRAQ experiments as equally present in GC strains (Table II). The pivotal role of Bam proteins comprising the β-barrel assembly machinery have been recognized in both model organisms E. coli and N. meningitidis, reviewed in (80, 81). In GC, a peptidoglycan-linked lipoprotein, BamD, although not essential for cell viability, was required for efficient transformation by species-related DNA and its depletion- caused growth defects and altered colony morphology (82). Finally, OmpA-like protein and NGO1686 quantified in our proteomic studies (Table II), could serve as additional examples of ubiquitously expressed proteins that play crucial functions in the GC biology. OmpA, characterized by Serino et al., is involved in the binding of GC to human cervical carcinoma and endometrial cells, entry into macrophages, intracellular survival in epithelial cells, and in vivo colonization (83). NGO1686 is a periplasmic, peptidoglycan-degrading peptidase and a virulence factor that protects GC against both nonoxidative polymorphonuclear leukocytes-mediated killing and oxidative-killing by hydrogen peroxide (84, 85). Together, these findings warrant further investigation of the newly discovered stably expressed proteins in the context of the GC general fitness and pathogenesis.
Our proteomic profiling of naturally shed MVs revealed a ubiquitous abundance of several outer membrane proteins including Phospholipase A, PorB, PilQ, adhesion MafA 1/4, and BamA (Table III). Sorting of these proteins into MVs was established in N. meningitidis and N. lactamica (75, 76, 78, 79, 86). One of the more fascinating features of a major GC porin, PorB (65) is its translocation from bacterial outer membrane and insertion into the membranes of eukaryotic cell organelles (87). It remains to be determined whether MVs play a role in this process, however, experimental data from different pathogenic bacteria suggest that such a mechanism could be mediated via a direct adherence and internalization of MVs to host cells (16). Further, we discovered that LptD was also uniformly distributed into the MVs (Table III). Consistent with these results, sorting of LptD into the naturally released MVs was observed in other examined Neisseriaceae, E. coli, and Pseudoaltermonas antarctica (21, 75). The localization of this organic solvent tolerance protein into MVs might contribute to the bacterial survival under hostile conditions (21). Among other proteins present at similar levels in GC MVs were catalase, DsbD, and newly identified proteins including a predicted lipoprotein NGO2139, a putative carboxy-terminal processing protease NGO0572, and an uncharacterized protein NGO2054 (Table III). The intriguing location of catalase in MVs has also been reported in Helicobacter pylori (88). Further studies will be required to determine whether catalase is enclosed within the MVs or decorates their surface, perhaps because of the release of the cell contents after bacterial cell death. However, it is tempting to speculate that the MVs localization of this potent enzyme contributes to the remarkable resistance of GC against the oxygen-dependent mechanisms of bacterial killing within the human host. In addition, our experiments suggested that a virulence factor, which specifically cleaves human IgA1, extracellular metalloprotease IgA1 (89), was uniformly present in MVs isolated from GC strains (Table III). Although protease IgA1 was the first autotransporter described in Neisseriaceae (89), its presence in MVs has also been observed in N. meningitidis (86, 90, 91), which further suggests that IgA1 might be associated with the MVs.
Differentially Abundant Proteins in the GC Cell Envelopes and MVs
Considering the recognized differences between GC strains (Table I), it was not surprising that the iTRAQ experiments revealed 22 differentially abundant proteins in the cell envelopes and MVs (Fig. 2E, Tables IV–V). Worth noting, among the significantly altered proteins, were well-characterized components of the multidrug efflux-pump complex, MtrC-MtrD-MtrE, where “Mtr” corresponds to multiple transferable resistance. The orchestrated work of this three-component machinery provides an increased resistance to macrolide antibiotics, and antimicrobial compounds that GC might encounter in the human host including bile salts, progesterone, and antimicrobial peptide, LL37 (92–94). The relative abundance of the channel protein MtrE, the periplasmic accessory lipoprotein MtrC, and the inner membrane component MtrD was about threefold higher in the strain MS11 in comparison to that in FA1090 (Table IV). In contrast, strains F62 and 1291 displayed similar relative levels of Mtr constituents compared with FA1090. Consistently, the increased abundance of MtrE was observed in the MVs fraction isolated from strain MS11 (Table V). Warner et al. (94) revealed that the elevated expression of mtr operon in strain MS11 is because of a novel mutation, mtr120, which yields the highest reported levels of Mtr-based resistance. In the same report, the authors provide experimental evidence for the correlation between an increased gradient of antimicrobial resistance and the levels of in vivo fitness. Indeed, studies in both the male urethritis model and in the murine model for GC infection clearly demonstrated that strain MS11 was more infectious than FA1090 (61). Interestingly, however, introducing the mtr mutation into strain F62 did not increase its infectivity (93), suggesting that another strain-dependent factor(s) might contribute to the levels of pathogenicity. Our iTRAQ experiments revealed previously unrecognized discrepancies between compared GC strains including differential abundance of NGO1889 encoding a putative lipoprotein, NGO0926 coding for a putative peroxiredoxin family protein, as well as putative uncharacterized proteins NGO1438, NGO1056, NGO0861, and NGO1949 (Tables IV–V). Future studies will establish whether these proteins represent virulence determinants contributing to the differential fitness observed between GC isolates.
Immunoblotting Analyses Confirm the iTRAQ Data
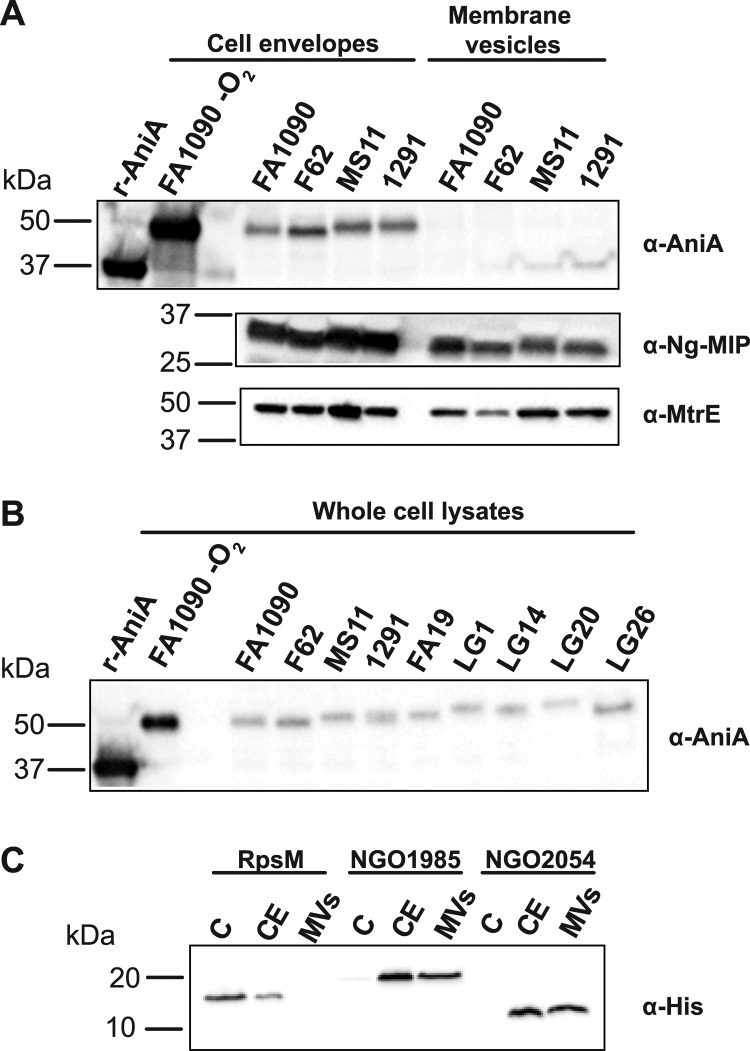
We were surprised that iTRAQ analyses indicated that AniA, which is an anaerobically induced surface-exposed outer membrane glycoprotein (95–97), was also ubiquitous in membrane fractions isolated from GC strains cultured aerobically (Table II). Therefore, we have chosen this protein as a first candidate for verification of our proteomic experiments. The gene encoding AniA (NGO1276) was cloned, overexpressed and a truncated soluble domain of AniA was purified to 95% purity as described under “Experimental Procedures.” This purified antigen was used to immunize rabbits and obtain anti-AniA antibodies. Subsequently, the same amounts of cell envelope and MVs proteins (15 μg) extracted from GC strains FA1090, F62, MS11, and 1291 were separated by 4–20% gradient SDS-PAGE, transferred, and probed with anti-AniA antiserum. An example of such immunoblotting analysis is shown in Fig. 4A. As expected, there was a shift in migration of the purified truncated AniA (rAniA) in comparison to the native protein, which migrated at about 50 kDa. In agreement with the iTRAQ experiments, similar levels of AniA were present in the cell envelope fractions isolated from these four GC strains, and the protein was not detected in the MVs. Additionally tested GC strains including FA19 and a few clinical isolates (LG1, LG14, LG20, and LG26), expressed AniA at comparable levels during aerobic growth (Fig. 4B). As anticipated, the amounts of AniA were significantly decreased in comparison to that detected in bacteria cultured anaerobically in the presence of nitrite (Fig. 4B, FA1090 -O2) (98).
Fig. 4.
Immunoblotting analyses confirmed iTRAQ data. A, Immunoblots, probed with either anti-AniA, anti-Ng-MIP, or anti-MtrE antiserum, of the cell envelopes and MVs extracted from different GC strains (as indicated above the panel). The total amounts of 15 μg of cell envelope and MVs proteins were loaded into individual wells and separated by SDS-PAGE on a 4–20% Tris-glycine gel. B, Whole-cell lysates derived from different GC isolates were resolved in a 4–20% Tris-glycine gel, transferred, and immunoblotting analysis was performed using rabbit antiserum against recombinant AniA. Samples containing whole-cell lysates were matched by equivalent OD600 units. Purified recombinant truncated version of AniA (250 ng, rAniA) and either cell envelope proteins (15 μg) or whole cell lysate from strain FA1090 grown anaerobically (-O2) were used as positive controls. The designations of the strains are indicated at the top of the immunoblot. C, The subcellular distribution of RpsM, NGO1985, and NGO2054. Liquid cultures of GC FA1090 carrying either chromosomally expressed C-terminal-6×His-tagged RpsM, NGO1985, or NGO2054, were harvested, and subjected to the fractionation procedures. The total amounts of 15 μg of cytoplasmic (C), cell envelopes (CE), and MVs proteins were separated by SDS-PAGE on a 4–20% Tris-glycine gel, and probed with monoclonal anti-His antisera. The migration of molecular weight markers and their masses in kilodaltons (kDa) are indicated on the left.
To further validate iTRAQ experiments, the immunoblotting analyses of cell envelopes and MVs fractions isolated from FA1090, F62, MS11, and 1291 were performed with anti-Ng-MIP and anti-MtrE antisera (Fig. 4A). Overall, these studies confirmed iTRAQ quantifications. Similar levels of Ng-MIP were detected in all GC cell envelopes, whereas the level of MtrE was increased in strain MS11 (Table II and Fig. 4A). In addition, Ng-MIP was detected in the MVs fractions (Fig. 2A). This protein was identified by MS/MS but failed our rigorous criteria for protein quantification and thus was not included in the final list of 57 MVs proteins (Fig. 2E and supplemental Table S3).
Lastly, we aimed to evaluate the subcellular localization of several newly identified proteins. We have chosen predicted outer membrane protein NGO1985, and proteins with unknown localization NGO2139 and NGO2054. A homolog of ribosomal protein RpsM, NGO1821, and a periplasmic catalase, NGO1767, were also selected. We engineered an epitope-encoding tail, 6×His-tag, at the 3′ end of individual genes in their respective loci of FA1090 chromosome. Subsequently, liquid cultures of GC FA1090 carrying C-terminal-tagged proteins were harvested, subjected to the fractionation procedures, and processed for the detection of His epitope. The monoclonal anti-His antisera recognized recombinant RpsM, NGO1985, and NGO2054, which migrated according to their predicted molecular weight of 13.4-, 21.7-, and 10.5-kDa (Fig. 4C). The analysis failed to detect NGO1767 and NGO2139 (data not shown). Furthermore, these experiments showed that RpsM localized predominantly to the soluble fraction containing cytoplasmic proteins and was also present in the GC cell envelope, whereas both NGO1985 and NGO2054 were detected in the cell envelope and MVs fractions (Fig. 4C). Together, these results provided additional validation of our proteomic studies.
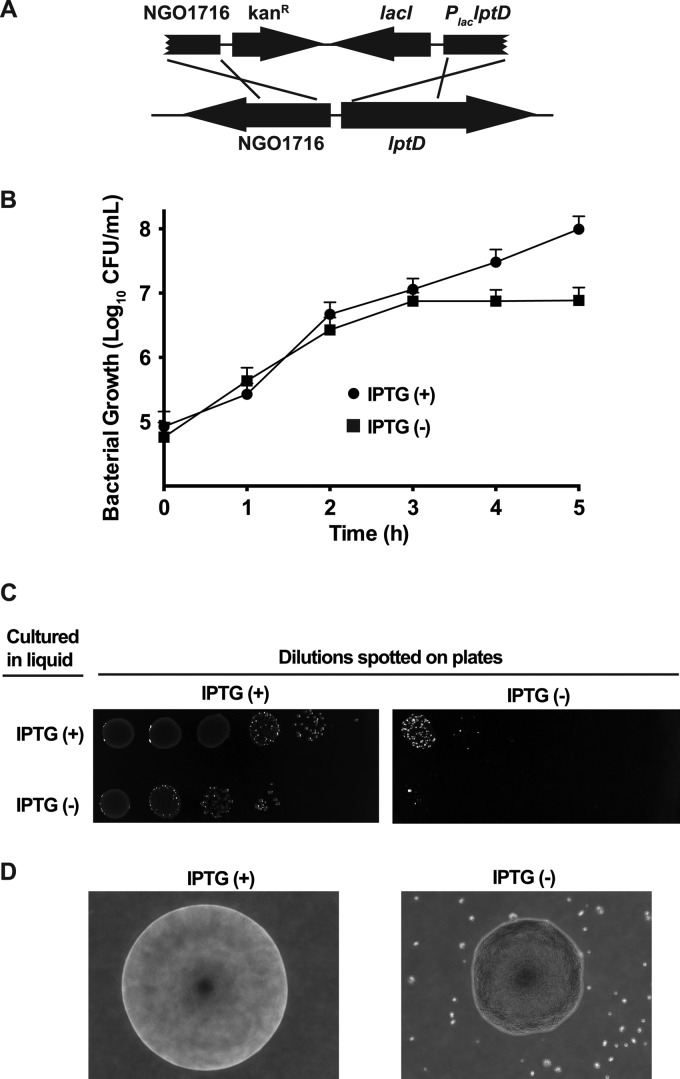
A Minor Outer Membrane Protein, LptD, is Essential in GC
The iTRAQ approaches led to the identification of 34 proteins that were ubiquitously expressed in four GC strains and identified in both cell envelopes and MVs fractions (Tables II–III). We propose that these proteins may represent attractive therapeutic targets, particularly if they are conserved throughout different GC strains and play an important biological function. As proof of principle, we focused on LptD, which was uniformly present in the cell envelopes and MVs in analyzed GC strains. This protein has not been previously characterized in GC, whereas in E. coli, LptD is an essential low-abundant outer membrane β-barrel protein involved in the cell envelope biogenesis (99). LptD is highly conserved among Gram-negative bacteria and plays a crucial function in transporting lipopolysaccharide into the outer leaflet of the outer membrane (100). Its role in the transport of lipopolysaccharide was first discovered in N. meningitidis but interestingly LptD was not found essential for the survival of this bacterium (101). To verify the notion that this gene is essential in GC (102), we engineered a strain in which chromosomal expression of lptD was placed under the control of IPTG-regulatable promoter, PlaclptD (Fig. 5A). This strain grew robustly on GCB agar and in liquid media supplemented with 100 μm IPTG, whereas it was unable to form colonies upon direct plating from freezer stocks onto plates without IPTG (Fig. 5B and data not shown). Moreover, within three hours of culture in GCBL broth in the absence of IPTG there was a cessation of growth and at 5 hours the cell viability decreased almost 13-fold as compared with the strain expressing LptD (Fig. 5B and 5C). Consequently, upon depletion of LptD, the FA1090 PlaclptD failed to form colonies when plated on solid media lacking IPTG (Fig. 5D). We noted, however, that sometimes a few colonies, likely carrying suppressor(s) mutations, were able to arise on this media (Fig. 5C). These colonies had drastically changed morphology as compared with that producing LptD. Individual colonies were characterized by an irregular edge; increase opacity, and a granular, rugose appearance (Fig. 5D). Growth defect and increase in colony opacity were also observed in N. meningitidis lptD mutant strain (101). Together, our observations support that LptD plays a pivotal function in GC.
Fig. 5.
Effect of LptD depletion in GC. A, Construction of an lptD conditional knockout in GC strain FA1090 was achieved by allelic replacement of the native lptD promoter with a DNA fragment containing IPTG-inducible promoter (Plac), lacI gene, and a nonpolar antibiotic resistance cassette apha3 (kanR). B, FA1090 cells carrying chromosomal PlaclptD were grown in the presence of 100 μm IPTG on GCB agar plates for 18 h. The bacteria were collected from plates, washed, divided, and growth was continued in the presence or absence of IPTG as indicated. Cell viability was monitored every hour by spotting serial dilutions onto GCB agar plates with (+) or without (-) IPTG, as indicated. Experiments were performed in biological quadruplicates and means and S.E. of colony forming units calculated per ml of bacterial cultures (CFU/ml) are presented. C, Representative experiments showing CFU's of strains spotted after 5 h onto GCB agar plates with or without IPTG. D, Representative micrographs illustrating the colony morphology of FA1090 PlaclptD expressing LptD (+ IPTG) and upon depletion of LptD (− IPTG). GC colonies were visualized with a Zeiss AxioObserver.D1 microscope at A-Plan 10× magnification 0.25 Phase Contrast 1.
A Lack of NGO1985 Dramatically Affects GC Cell Envelope Permeability Barrier
Finally, we have begun to address whether the newly identified proteins are important for GC physiology, and thus could serve as potential pharmacological targets. Six predicted outer membrane proteins with unknown function were selected for initial characterization including NGO1985, NGO1344, NGO1955, NGO2111, NGO2121, and NGO2139. These proteins were identified by iTRAQ as uniformly present in the cell envelopes derived from examined GC isolates (Table II). In addition, our fractionation experiments coupled with immunoblotting analyses showed that NGO1985 was localized to both cell envelope and MVs (Fig. 4C). The BLAST searches of the predicted amino acid sequences against NCBI and JCVI CMR (103) databases, revealed that all ORFs encode conserved hypothetical proteins with up to 50% identity throughout different species belonging to Neisseriaceae. Further, a presence of a putative membrane binding BON domain, bacterial OsmY and nodulation, was identified in NGO1985. This domain is found in a family of osmotic shock protection proteins, a family of hemolysins, and a group of nodulation specificity proteins and secretory channels (104). The Omp85- and the AsmA-family domains, which are characteristics of proteins involved in the assembly of outer membrane, were identified in NGO1955 and NGO1344, respectively (105, 106).
The presence of these conserved domains suggested that these proteins function in the GC cell envelope homeostasis. To test this hypothesis, we first created in-frame deletions of individual genes. Subsequently the isogenic wild-type, FA1090, as well as Δngo1985, Δngo1344, Δngo1955, Δngo2111, Δngo2121, and Δngo2139 were examined for their susceptibility toward a variety of compounds. All strains were suspended to 5 × 105 CFUs/ml, serially diluted, and plated on GCB agar either with or without different membrane-perturbing agents as well as chloramphenicol. An absence of individual proteins had no effect on GC growth under permissive conditions; however, loss of NGO1985 and NGO2121 resulted in significant inhibition of bacterial growth in the presence of bile salts and antimicrobial peptide, polymyxin B (Fig. 6A). Additionally, when compared with the parent strain, Δngo1985 displayed dramatically decreased survival upon plating on media supplemented with detergents including Tween 20, SDS, and urea as well as chloramphenicol (Fig. 6B). This strong sensitivity phenotype was reversed in the complemented strain, Δngo1985/Plac1985, to the level observed in the wild-type bacteria (Fig. 6A and 6B). We concluded that loss of NGO1985, in particular, dramatically affected the general GC cell envelope permeability barrier. These studies further underscore the concept of ubiquitous cell envelope and MVs proteins as potential targets for new therapeutic interventions.
Fig. 6.
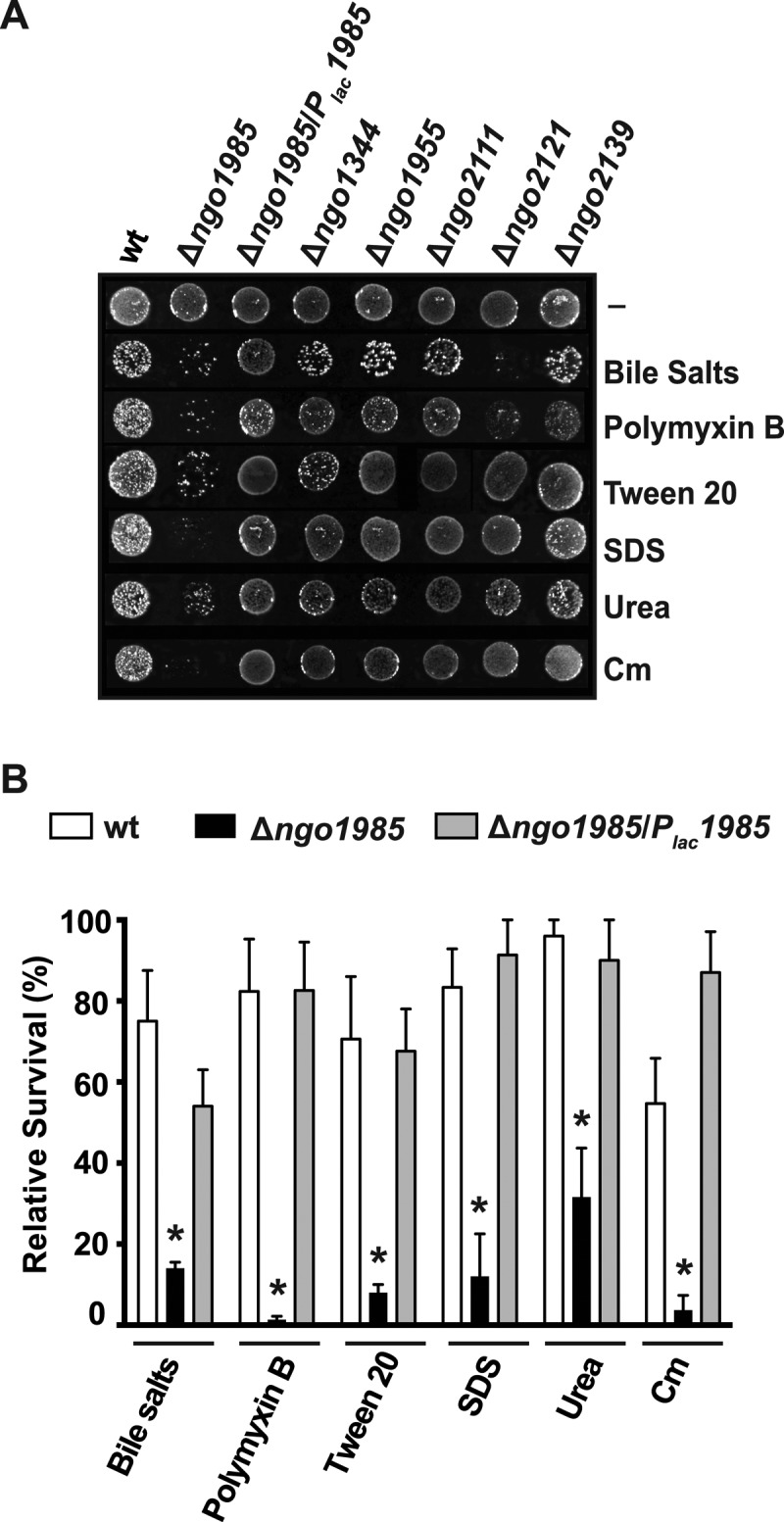
The GC cell envelope permeability barrier is compromised upon lack of NGO1985. A, GC wild-type strain, FA1090 (wt), isogenic knockouts Δngo1985, Δngo1344, Δngo1955, Δngo2111, Δngo2121, Δngo2139, and complemented strain Δngo1985/Plac1985 were tested for sensitivity phenotypes to various compounds. All strains were suspended to 5 × 105 CFUs/ml, serially diluted, and plated on GCB agar either without (-) or with different compounds including bile salts (0.05%), polymyxin B (800 U), Tween 20 (0.005%), SDS (0.001%), urea (200 mm), chloramphenicol (0.2 μg/ml). Plates were inspected after 22 h of incubation in 5% CO2 atmosphere at 37 °C. B, Experiments were performed as described above. The relative survival of parent strain, FA1090, Δngo1985, and complemented mutant, Δngo1985/Plac1985, was calculated by comparing CFUs/ml on solid media containing the tested compound (as indicated) to the CFUs/ml on GCB agar. Experiments were performed on three separate occasions and the bars represent means with calculated S.E.. Statistically significant differences (p < 0.05) are indicated (*).
Final Remarks
Significant research in the GC field has been dedicated to elucidating the function and structure of several important virulence factors that are not stable because of both phase and antigenic variation. As such these proteins are not good candidates for developing therapeutic approaches. An example of this challenge was the monovalent pilus vaccine, which failed in a clinical trial (10). Our study began to establish a map of proteins in the cell envelope and MVs of a clinically relevant human pathogen. To the best of our knowledge, this is also the first time iTRAQ technology was used to elucidate the composition of bacterial MVs. Substantial research effort will be required to establish whether the proteins identified herein play important pathophysiological functions, to examine their phase and antigenic stability, and conservation throughout current GC clinical isolates, as well as their antigenic potency. Nevertheless, this study provides a framework for future research to elucidate the potential of the identified proteins as novel drug and vaccine targets.
Supplementary Material
Acknowledgments
We thank Dr. Josephine Bonventre for her excellent assistance, Dr. Stacey Harper (Department of Toxicology, OSU) for the use of the microscope, and Lisa Jones (Proteomics Facility, FHCRC) for assistance with performing proteomics experiments. We are grateful to Dr. Ann Jerse (Uniformed Services University, Bethesda, MD) for a generous gift of GC strains and anti-MtrE antisera. We thank Dr. Hank Seifert and Dr. Elizabeth Stohl (Northwestern University, Chicago, IL) for helpful discussion regarding GC complementation and plasmid pGGC4. We are thankful to Dr. Mariagrazia Pizza (Novartis Vaccines and Diagnostics, Siena, Italy) for providing the anti-Ng-MIP antisera. We also thank Dr. Robert Chalkley (University of California, San Francisco) and the PRIDE Team for their assistance in the mass spectrometry data deposition.
Footnotes
Author contributions: R.A.Z. and A.E.S. designed research; R.A.Z., I.H.W., J.V.W., P.R.G., and A.E.S. performed research; R.A.Z., P.R.G., and A.E.S. analyzed data; R.A.Z., P.R.G., and A.E.S. wrote the paper.
* This work was supported by the Medical Research Foundation of Oregon seed grant F0731A and the General Research Fund (OSU), as well as startup funds from OSU to AES. Additionally, we would like to acknowledge Undergraduate Research, Innovation, Scholarship and Creativity (OSU) for funding to J.V. Weber.
 This article contains supplemental Tables S1 to S5 and supplemental Material S1 to S4.
This article contains supplemental Tables S1 to S5 and supplemental Material S1 to S4.
1 The abbreviations used are:
- GC
- Neisseria gonorrhoeae
- ACN
- acetonitrile
- CE
- cell envelopes
- CFU
- colony-forming units
- COG
- Clusters of Orthologous Groups
- GCB
- GC agar plates
- GCBL
- GC broth liquid medium
- IPTG
- isopropyl-β-D-galactopyranoside
- iTRAQ
- isotope tagging for relative and absolute quantification
- LPS
- lipopolysaccharide
- MVs
- membrane vesicles
- 2D-LC
- two-dimensional liquid chromatography
- SCX
- strong cation exchange
- TFA
- trifluoroacetic acid.
REFERENCES
- 1. Lusti-Narasimhan M., Ndowa F. (2011) Emergence and spread of multi-drug resistant Neisseria gonorrhoeae. J. Sex. Med. 8, 253 [Google Scholar]
- 2. McNabb S. J., Jajosky R. A., Hall-Baker P. A., Adams D. A., Sharp P., Worshams C., Anderson W. J., Javier A. J., Jones G. J., Nitschke D. A., Rey A., Wodajo M. S. (2008) Summary of notifiable diseases–United States, 2006. MMWR Morb. Mortal. Wkly Rep. 55, 1–92 [PubMed] [Google Scholar]
- 3. Edwards J. L., Apicella M. A. (2004) The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin. Microbiol. Rev. 17, 965–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fleming D. T., Wasserheit J. N. (1999) From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex. Transm. Infect. 75, 3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Workowski K. A., Berman S. (2010) Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm. Rep. 59, 1–110 [PubMed] [Google Scholar]
- 6. Yokoi S., Deguchi T., Ozawa T., Yasuda M., Ito S., Kubota Y., Tamaki M., Maeda S. (2007) Threat to cefixime treatment for gonorrhea. Emerg. Infect. Dis. 13, 1275–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Unemo M., Shipitsyna E., Domeika M. (2010) Recommended antimicrobial treatment of uncomplicated gonorrhoea in 2009 in 11 East European countries: implementation of a Neisseria gonorrhoeae antimicrobial susceptibility programme in this region is crucial. Sex. Transm. Infect. 86, 442–444 [DOI] [PubMed] [Google Scholar]
- 8. Ison C. A., Hussey J., Sankar K. N., Evans J., Alexander S. (2011) Gonorrhoea treatment failures to cefixime and azithromycin in England, 2010. Euro. Surveill. 16 [PubMed] [Google Scholar]
- 9. Stoltey J. E., Barry P. M. (2012) The use of cephalosporins for gonorrhea: an update on the rising problem of resistance. Expert opin. Pharmaco. 13, 1411–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu W., Chen C. J., Thomas C. E., Anderson J. E., Jerse A. E., Sparling P. F. (2011) Vaccines for gonorrhea: can we rise to the challenge? Front. Microbiol. 2, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Organization W. H. (2012) Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. p. 32, World Health Organization, Geneva [Google Scholar]
- 12. Prevention C. f. D. C. a. (2013) Gonorrhea Treatment Guildlines. PMCID [Google Scholar]
- 13. Chan Y. A., Hackett K. T., Dillard J. P. (2012) The lytic transglycosylases of Neisseria gonorrhoeae. Microb. Drug. Resist. 18, 271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Virji M. (2009) Pathogenic Neisseriae: surface modulation, pathogenesis and infection control. Nat. Rev. Microbiol. 7, 274–286 [DOI] [PubMed] [Google Scholar]
- 15. Cornelissen C. N. (2008) Identification and characterization of gonococcal iron transport systems as potential vaccine antigens. Future Microbiol. 3, 287–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ellis T. N., Kuehn M. J. (2010) Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 74, 81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pettit R. K., Judd R. C. (1992) The interaction of naturally elaborated blebs from serum-susceptible and serum-resistant strains of Neisseria gonorrhoeae with normal human serum. Mol. Microbiol. 6, 729–734 [DOI] [PubMed] [Google Scholar]
- 18. Pettit R. K., Judd R. C. (1992) Characterization of naturally elaborated blebs from serum-susceptible and serum-resistant strains of Neisseria gonorrhoeae. Mol. Microbiol. 6, 723–728 [DOI] [PubMed] [Google Scholar]
- 19. Kulp A., Kuehn M. J. (2010) Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 64, 163–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Britigan B. E., Cohen M. S., Sparling P. F. (1985) Gonococcal infection: a model of molecular pathogenesis. N. Engl. J. Med. 312, 1683–1694 [DOI] [PubMed] [Google Scholar]
- 21. Lee E. Y., Choi D. S., Kim K. P., Gho Y. S. (2008) Proteomics in gram-negative bacterial outer membrane vesicles. Mass Spectrom. Rev. 27, 535–555 [DOI] [PubMed] [Google Scholar]
- 22. Greiner L. L., Edwards J. L., Shao J., Rabinak C., Entz D., Apicella M. A. (2005) Biofilm Formation by Neisseria gonorrhoeae. Infect. Immun. 73, 1964–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Steichen C. T., Shao J. Q., Ketterer M. R., Apicella M. A. (2008) Gonococcal cervicitis: a role for biofilm in pathogenesis. J. Infect. Dis. 198, 1856–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Falsetta M. L., Steichen C. T., McEwan A. G., Cho C., Ketterer M., Shao J., Hunt J., Jennings M. P., Apicella M. A. (2011) The composition and metabolic phenotype of Neisseria gonorrhoeae biofilms. Front. Microbiol. 2, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clark V. L., Campbell L. A., Palermo D. A., Evans T. M., Klimpel K. W. (1987) Induction and repression of outer membrane proteins by anaerobic growth of Neisseria gonorrhoeae. Infect. Immun. 55, 1359–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keevil C. W., Davies D. B., Spillane B. J., Mahenthiralingam E. (1989) Influence of iron-limited and replete continuous culture on the physiology and virulence of Neisseria gonorrhoeae. J. Gen. Microbiol. 135, 851–863 [DOI] [PubMed] [Google Scholar]
- 27. Yoo J. S., Seong W. K., Kim T. S., Park Y. K., Oh H. B., Yoo C. K. (2007) Comparative proteome analysis of the outer membrane proteins of in vitro-induced multi-drug resistant Neisseria gonorrhoeae. Microbiol. Immunol. 51, 1171–1177 [DOI] [PubMed] [Google Scholar]
- 28. Phillips N. J., Steichen C. T., Schilling B., Post D. M., Niles R. K., Bair T. B., Falsetta M. L., Apicella M. A., Gibson B. W. (2012) Proteomic analysis of Neisseria gonorrhoeae biofilms shows shift to anaerobic respiration and changes in nutrient transport and outermembrane proteins. PLoS One 7, e38303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brodeur B. R., Johnson W. M., Johnson K. G., Diena B. B. (1977) In vitro interaction of Neisseria gonorrhoeae type 1 and type 4 with tissue culture cells. Infect. Immun. 15, 560–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen M. S., Cannon J. G., Jerse A. E., Charniga L. M., Isbey S. F., Whicker L. G. (1994) Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J. Infect. Dis. 169, 532–537 [DOI] [PubMed] [Google Scholar]
- 31. Kellogg D. S., Jr., Cohen I. R., Norins L. C., Schroeter A. L., Reising G. (1968) Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J. Bacteriol. 96, 596–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Swanson J. (1973) Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J. Exp. Med. 137, 571–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mickelsen P. A., Sparling P. F. (1981) Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect. Immun. 33, 555–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kellogg D. S., Jr., Peacock W. L., Jr., Deacon W. E., Brown L., Pirkle D. I. (1963) Neisseria gonorrhoeae. I. Virulence Genetically Linked to Clonal Variation. J. Bacteriol. 85, 1274–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spence J. M., Wright L., Clark V. L. (2008) Laboratory maintenance of Neisseria gonorrhoeae. Curr. Protoc. Microbiol. Chapter 4, Unit 4A 1 [DOI] [PubMed] [Google Scholar]
- 36. Morse S. A., Bartenstein L. (1974) Factors affecting autolysis of Neisseria gonorrhoeae. Proc. Soc. Exp. Biol. Med. 145, 1418–1421 [DOI] [PubMed] [Google Scholar]
- 37. Knapp J. S., Clark V. L. (1984) Anaerobic growth of Neisseria gonorrhoeae coupled to nitrite reduction. Infect. Immun. 46, 176–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cahoon L. A., Stohl E. A., Seifert H. S. (2011) The Neisseria gonorrhoeae photolyase orthologue phrB is required for proper DNA supercoiling but does not function in photo-reactivation. Mol. Microbiol. 79, 729–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lai E. M., Nair U., Phadke N. D., Maddock J. R. (2004) Proteomic screening and identification of differentially distributed membrane proteins in Escherichia coli. Mol. Microbiol. 52, 1029–1044 [DOI] [PubMed] [Google Scholar]
- 40. Molloy M. P., Herbert B. R., Slade M. B., Rabilloud T., Nouwens A. S., Williams K. L., Gooley A. A. (2000) Proteomic analysis of the Escherichia coli outer membrane. Eur. J. Biochem. 267, 2871–2881 [DOI] [PubMed] [Google Scholar]
- 41. Sikora A. E., Zielke R. A., Lawrence D. A., Andrews P. C., Sandkvist M. (2011) Proteomic analysis of the Vibrio cholerae type II secretome reveals new proteins, including three related serine proteases. J. Biol. Chem. 286, 16555–16566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saeed A. I., Bhagabati N. K., Braisted J. C., Liang W., Sharov V., Howe E. A., Li J., Thiagarajan M., White J. A., Quackenbush J. (2006) TM4 microarray software suite. Methods Enzymol. 411, 134–193 [DOI] [PubMed] [Google Scholar]
- 43. Gardy J. L., Laird M. R., Chen F., Rey S., Walsh C. J., Ester M., Brinkman F. S. (2005) PSORTb v. 2.0: expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics 21, 617–623 [DOI] [PubMed] [Google Scholar]
- 44. Imai K., Asakawa N., Tsuji T., Akazawa F., Ino A., Sonoyama M., Mitaku S. (2008) SOSUI-GramN: high performance prediction for sub-cellular localization of proteins in gram-negative bacteria. Bioinformation 2, 417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yu C. S., Lin C. J., Hwang J. K. (2004) Predicting subcellular localization of proteins for Gram-negative bacteria by support vector machines based on n-peptide compositions. Protein Sci. 13, 1402–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu C. S., Chen Y. C., Lu C. H., Hwang J. K. (2006) Prediction of protein subcellular localization. Proteins 64, 643–651 [DOI] [PubMed] [Google Scholar]
- 47. Petersen T. N., Brunak S., von Heijne G., Nielsen H. (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8, 785–786 [DOI] [PubMed] [Google Scholar]
- 48. Bendtsen J. D., Nielsen H., Widdick D., Palmer T., Brunak S. (2005) Prediction of twin-arginine signal peptides. BMC Bioinformatics 6, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tatusov R. L., Koonin E. V., Lipman D. J. (1997) A genomic perspective on protein families. Science 278, 631–637 [DOI] [PubMed] [Google Scholar]
- 50. Wu S., Zhu Z., Fu L., Niu B., Li W. (2011) WebMGA: a customizable web server for fast metagenomic sequence analysis. BMC Genomics 12, 444 3180703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. (1989) Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77, 61–68 [DOI] [PubMed] [Google Scholar]
- 52. Long C. D., Tobiason D. M., Lazio M. P., Kline K. A., Seifert H. S. (2003) Low-level pilin expression allows for substantial DNA transformation competence in Neisseria gonorrhoeae. Infect. Immun. 71, 6279–6291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Skaar E. P., Lazio M. P., Seifert H. S. (2002) Roles of the recJ and recN genes in homologous recombination and DNA repair pathways of Neisseria gonorrhoeae. J. Bacteriol. 184, 919–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Menard R., Sansonetti P. J., Parsot C. (1993) Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175, 5899–5906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dillard J. P. (2011) Genetic manipulation of Neisseria gonorrhoeae. Curr. Protoc. Microbiol. Chapter 4, Unit4A 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Boulanger M. J., Murphy M. E. (2002) Crystal structure of the soluble domain of the major anaerobically induced outer membrane protein (AniA) from pathogenic Neisseria: a new class of copper-containing nitrite reductases. J. Mol. Biol. 315, 1111–1127 [DOI] [PubMed] [Google Scholar]
- 57. Dudas K. C., Apicella M. A. (1988) Selection and immunochemical analysis of lipooligosaccharide mutants of Neisseria gonorrhoeae. Infect. Immun. 56, 499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schneider H., Griffiss J. M., Williams G. D., Pier G. B. (1982) Immunological basis of serum resistance of Neisseria gonorrhoeae. J. Gen. Microbiol. 128, 13–22 [DOI] [PubMed] [Google Scholar]
- 59. Swanson J., Robbins K., Barrera O., Corwin D., Boslego J., Ciak J., Blake M., Koomey J. M. (1987) Gonococcal pilin variants in experimental gonorrhea. J. Exp. Med. 165, 1344–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cornelissen C. N., Kelley M., Hobbs M. M., Anderson J. E., Cannon J. G., Cohen M. S., Sparling P. F. (1998) The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol. Microbiol. 27, 611–616 [DOI] [PubMed] [Google Scholar]
- 61. Hobbs M. M., Sparling P. F., Cohen M. S., Shafer W. M., Deal C. D., Jerse A. E. (2011) Experimental gonococcal infection in male volunteers: cumulative experience with Neisseria gonorrhoeae strains FA1090 and MS11mkC. Front. Microbiol. 2, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neisseria gonorrhoeae group Sequencing Project, Broad Institute of Harvard and MIT. ( http://www.broadinstitute.org/)
- 63. Papanastasiou M., Orfanoudaki G., Koukaki M., Kountourakis N., Sardis M. F., Aivaliotis M., Karamanou S., Economou A. (2013) The Escherichia coli peripheral inner membrane proteome. Mol. Cell. Proteomics. 12, 599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dorward D. W., Garon C. F., Judd R. C. (1989) Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J. Bacteriol. 171, 2499–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Johnston K. H., Gotschlich E. C. (1974) Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J. Bacteriol. 119, 250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Blake M. S., Gotschlich E. C. (1982) Purification and partial characterization of the major outer membrane protein of Neisseria gonorrhoeae. Infect. Immun. 36, 277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Poetsch A., Wolters D. (2008) Bacterial membrane proteomics. Proteomics 8, 4100–4122 [DOI] [PubMed] [Google Scholar]
- 68. Ross P. L., Huang Y. N., Marchese J. N., Williamson B., Parker K., Hattan S., Khainovski N., Pillai S., Dey S., Daniels S., Purkayastha S., Juhasz P., Martin S., Bartlet-Jones M., He F., Jacobson A., Pappin D. J. (2004) Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol. Cell. Proteomics 3, 1154–1169 [DOI] [PubMed] [Google Scholar]
- 69. Tjalsma H., Antelmann H., Jongbloed J. D., Braun P. G., Darmon E., Dorenbos R., Dubois J. Y., Westers H., Zanen G., Quax W. J., Kuipers O. P., Bron S., Hecker M., van Dijl J. M. (2004) Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 68, 207–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dreisbach A., van Dijl J. M., Buist G. (2011) The cell surface proteome of Staphylococcus aureus. Proteomics 11, 3154–3168 [DOI] [PubMed] [Google Scholar]
- 71. Lee E. Y., Choi D. Y., Kim D. K., Kim J. W., Park J. O., Kim S., Kim S. H., Desiderio D. M., Kim Y. K., Kim K. P., Gho Y. S. (2009) Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 9, 5425–5436 [DOI] [PubMed] [Google Scholar]
- 72. Choi D. S., Kim D. K., Choi S. J., Lee J., Choi J. P., Rho S., Park S. H., Kim Y. K., Hwang D., Gho Y. S. (2011) Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa. Proteomics 11, 3424–3429 [DOI] [PubMed] [Google Scholar]
- 73. Lee E. Y., Bang J. Y., Park G. W., Choi D. S., Kang J. S., Kim H. J., Park K. S., Lee J. O., Kim Y. K., Kwon K. H., Kim K. P., Gho Y. S. (2007) Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics 7, 3143–3153 [DOI] [PubMed] [Google Scholar]
- 74. Berlanda Scorza F., Doro F., Rodriguez-Ortega M. J., Stella M., Liberatori S., Taddei A. R., Serino L., Gomes Moriel D., Nesta B., Fontana M. R., Spagnuolo A., Pizza M., Norais N., Grandi G. (2008) Proteomics characterization of outer membrane vesicles from the extraintestinal pathogenic Escherichia coli ΔtolR IHE3034 mutant. Mol. Cell. Proteomics 7, 473–485 [DOI] [PubMed] [Google Scholar]
- 75. Vaughan T. E., Skipp P. J., O'Connor C. D., Hudson M. J., Vipond R., Elmore M. J., Gorringe A. R. (2006) Proteomic analysis of Neisseria lactamica and Neisseria meningitidis outer membrane vesicle vaccine antigens. Vaccine 24, 5277–5293 [DOI] [PubMed] [Google Scholar]
- 76. Gil J., Betancourt L. Z., Sardinas G., Yero D., Niebla O., Delgado M., Garcia D., Pajon R., Sanchez A., Gonzalez L. J., Padron G., Campa C., Sotolongo F., Barbero R., Guillen G., Herrera L., Besada V. (2009) Proteomic study via a non-gel based approach of meningococcal outer membrane vesicle vaccine obtained from strain CU385: a road map for discovering new antigens. Hum. Vaccines 5, 347–356 [DOI] [PubMed] [Google Scholar]
- 77. Choi D. S., Lee J. M., Park G. W., Lim H. W., Bang J. Y., Kim Y. K., Kwon K. H., Kwon H. J., Kim K. P., Gho Y. S. (2007) Proteomic analysis of microvesicles derived from human colorectal cancer cells. J. Proteome Res. 6, 4646–4655 [DOI] [PubMed] [Google Scholar]
- 78. Leuzzi R., Serino L., Scarselli M., Savino S., Fontana M. R., Monaci E., Taddei A., Fischer G., Rappuoli R., Pizza M. (2005) Ng-MIP, a surface-exposed lipoprotein of Neisseria gonorrhoeae, has a peptidyl-prolyl cis/trans isomerase (PPIase) activity and is involved in persistence in macrophages. Mol. Microbiol. 58, 669–681 [DOI] [PubMed] [Google Scholar]
- 79. Bos M. P., Tefsen B., Voet P., Weynants V., van Putten J. P., Tommassen J. (2005) Function of neisserial outer membrane phospholipase a in autolysis and assessment of its vaccine potential. Infect. Immun. 73, 2222–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hagan C. L., Silhavy T. J., Kahne D. (2011) β-barrel membrane protein assembly by the Bam complex. Annu. Rev. Biochem. 80, 189–210 [DOI] [PubMed] [Google Scholar]
- 81. Bos M. P., Robert V., Tommassen J. (2007) Biogenesis of the gram-negative bacterial outer membrane. Annu. Rev. Microbiol. 61, 191–214 [DOI] [PubMed] [Google Scholar]
- 82. Fussenegger M., Facius D., Meier J., Meyer T. F. (1996) A novel peptidoglycan-linked lipoprotein (ComL) that functions in natural transformation competence of Neisseria gonorrhoeae. Mol. Microbiol. 19, 1095–1105 [DOI] [PubMed] [Google Scholar]
- 83. Serino L., Nesta B., Leuzzi R., Fontana M. R., Monaci E., Mocca B. T., Cartocci E., Masignani V., Jerse A. E., Rappuoli R., Pizza M. (2007) Identification of a new OmpA-like protein in Neisseria gonorrhoeae involved in the binding to human epithelial cells and in vivo colonization. Mol. Microbiol. 64, 1391–1403 [DOI] [PubMed] [Google Scholar]
- 84. Stohl E. A., Chan Y. A., Hackett K. T., Kohler P. L., Dillard J. P., Seifert H. S. (2012) Neisseria gonorrhoeae virulence factor NG1686 is a bifunctional M23B family metallopeptidase that influences resistance to hydrogen peroxide and colony morphology. J. Biol. Chem. 287, 11222–11233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Stohl E. A., Dale E. M., Criss A. K., Seifert H. S. (2013) Neisseria gonorrhoeae metalloprotease NGO1686 is required for full piliation, and piliation is required for resistance to H2O2- and neutrophil-mediated killing. MBio 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lappann M., Otto A., Becher D., Vogel U. (2013) Comparative proteome analysis of spontaneous outer membrane vesicles and purified outer membranes of Neisseria meningitidis. J. Bacteriol. 195, 4425–4435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Muller A., Gunther D., Brinkmann V., Hurwitz R., Meyer T. F., Rudel T. (2000) Targeting of the pro-apoptotic VDAC-like porin (PorB) of Neisseria gonorrhoeae to mitochondria of infected cells. EMBO J. 19, 5332–5343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Olofsson A., Vallstrom A., Petzold K., Tegtmeyer N., Schleucher J., Carlsson S., Haas R., Backert S., Wai S. N., Grobner G., Arnqvist A. (2010) Biochemical and functional characterization of Helicobacter pylori vesicles. Mol. Microbiol. 77, 1539–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pohlner J., Halter R., Beyreuther K., Meyer T. F. (1987) Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature 325, 458–462 [DOI] [PubMed] [Google Scholar]
- 90. Post D. M., Zhang D., Eastvold J. S., Teghanemt A., Gibson B. W., Weiss J. P. (2005) Biochemical and functional characterization of membrane blebs purified from Neisseria meningitidis serogroup B. J. Biol. Chem. 280, 38383–38394 [DOI] [PubMed] [Google Scholar]
- 91. Ferrari G., Garaguso I., Adu-Bobie J., Doro F., Taddei A. R., Biolchi A., Brunelli B., Giuliani M. M., Pizza M., Norais N., Grandi G. (2006) Outer membrane vesicles from group B Neisseria meningitidis Δgna33 mutant: proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics 6, 1856–1866 [DOI] [PubMed] [Google Scholar]
- 92. Jerse A. E., Sharma N. D., Simms A. N., Crow E. T., Snyder L. A., Shafer W. M. (2003) A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect. Immun. 71, 5576–5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Warner D. M., Folster J. P., Shafer W. M., Jerse A. E. (2007) Regulation of the MtrC-MtrD-MtrE efflux-pump system modulates the in vivo fitness of Neisseria gonorrhoeae. J. Infect. Dis. 196, 1804–1812 [DOI] [PubMed] [Google Scholar]
- 94. Warner D. M., Shafer W. M., Jerse A. E. (2008) Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC-MtrD-MtrE Efflux pump system confer different levels of antimicrobial resistance and in vivo fitness. Mol. Microbiol. 70, 462–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hoehn G. T., Clark V. L. (1992) Isolation and nucleotide sequence of the gene (aniA) encoding the major anaerobically induced outer membrane protein of Neisseria gonorrhoeae. Infect. Immun. 60, 4695–4703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hoehn G. T., Clark V. L. (1992) The major anaerobically induced outer membrane protein of Neisseria gonorrhoeae, Pan 1, is a lipoprotein. Infect. Immun. 60, 4704–4708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Shewell L. K., Ku S. C., Schulz B. L., Jen F. E., Mubaiwa T. D., Ketterer M. R., Apicella M. A., Jennings M. P. (2013) Recombinant truncated AniA of pathogenic Neisseria elicits a non-native immune response and functional blocking antibodies. Biochem. Biophys. Res. Commun. 431, 215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Householder T. C., Belli W. A., Lissenden S., Cole J. A., Clark V. L. (1999) cis- and trans-acting elements involved in regulation of aniA, the gene encoding the major anaerobically induced outer membrane protein in Neisseria gonorrhoeae. J. Bacteriol. 181, 541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Braun M., Silhavy T. J. (2002) Imp/OstA is required for cell envelope biogenesis in Escherichia coli. Mol. Microbiol. 45, 1289–1302 [DOI] [PubMed] [Google Scholar]
- 100. Ruiz N., Kahne D., Silhavy T. J. (2009) Transport of lipopolysaccharide across the cell envelope: the long road of discovery. Nat. Rev. Microbiol. 7, 677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bos M. P., Tefsen B., Geurtsen J., Tommassen J. (2004) Identification of an outer membrane protein required for the transport of lipopolysaccharide to the bacterial cell surface. Proc. Natl. Acad. Sci. U.S.A. 101, 9417–9422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tobiason D. M., Seifert H. S. (2010) Genomic content of Neisseria species. J. Bacteriol. 192, 2160–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Peterson J. D., Umayam L. A., Dickinson T., Hickey E. K., White O. (2001) The comprehensive microbial resource. Nucleic Acids Res. 29, 123–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yeats C., Bateman A. (2003) The BON domain: a putative membrane-binding domain. Trends Biochem. Sci. 28, 352–355 [DOI] [PubMed] [Google Scholar]
- 105. Gentle I. E., Burri L., Lithgow T. (2005) Molecular architecture and function of the Omp85 family of proteins. Mol. Microbiol. 58, 1216–1225 [DOI] [PubMed] [Google Scholar]
- 106. Deng M., Misra R. (1996) Examination of AsmA and its effect on the assembly of Escherichia coli outer membrane proteins. Mol. Microbiol. 21, 605–612 [DOI] [PubMed] [Google Scholar]
- 107. Cannon J. G., Lee T. J., Guymon L. F., Sparling P. F. (1981) Genetics of serum resistance in Neisseria gonorrhoeae: the sac-1 genetic locus. Infect. Immun. 32, 547–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Carbonetti N., Simnad V., Elkins C., Sparling P. F. (1990) Construction of isogenic gonococci with variable porin structure: effects on susceptibility to human serum and antibiotics. Mol. Microbiol. 4, 1009–1018 [DOI] [PubMed] [Google Scholar]
- 109. Dillard J. P., Seifert H. S. (2001) A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol. Microbiol. 41, 263–277 [DOI] [PubMed] [Google Scholar]
- 110. Anderson J. E., Hobbs M. M., Biswas G. D., Sparling P. F. (2003) Opposing selective forces for expression of the gonococcal lactoferrin receptor. Mol. Microbiol. 48, 1325–1337 [DOI] [PubMed] [Google Scholar]
- 111. Hagman K. E., Pan W., Spratt B. G., Balthazar J. T., Judd R. C., Shafer W. M. (1995) Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 141, 611–622 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.