Abstract
Outer membrane protein A (OmpA) is a major outer membrane protein of Escherichia coli and other Enterobacteriaeae. Although the structural features of OmpA have been well studied, its roles in the pathogenesis of various bacterial infections have not been fully elucidated. Here, we report the generation of mouse monoclonal antibody (MAb) 49.4-15, which specifically recognizes OmpA of E. coli, using immunoblot and confocal microscopic examinations. MAb 49.4-15 might be a useful tool for studying the expression and function of E. coli OmpA.
Introduction
Outer membrane protein A (OmpA) in bacteria has multiple structural and physiological functions including the maintenance of cell shape and stability, biofilm formation, adhesion/invasion, and evasion.(1–3) OmpA also plays an important role in anchoring the outer membrane to the bacterial wall.(4) OmpA has been highly conserved among Gram-negative bacteria throughout evolution.(2) Although the structural features and porin function of OmpA have been well studied,(5) its role in the pathogenesis of various bacterial infections has not been fully elucidated. OmpA of Escherichia coli is a major integral protein of the outer membrane, present at ∼100,000 copies per cell.(1) E. coli OmpA also exhibits pore-forming activity.(6)
Neutrophils or polymorphonuclear leukocytes provide the first line of defense against invading microorganisms. The interaction of OmpA with the heat shock protein gp96, which is expressed on the surface of neutrophils, reportedly increases the expression of toll-like receptor (TLR) 2 and suppresses the expression of TLR4 and complement receptor 3 in neutrophils.(2) Both TLRs and complements play important roles in the innate immune system,(7) and studies examining the interaction between OmpA of E. coli and monocytes/macrophages will likely provide meaningful clues to understanding the innate immune system.
Here, we report the production and characterization of a mouse monoclonal antibody (MAb) that specifically recognizes E. coli OmpA. This antibody might be useful for studying the physiological functions of this protein.
Materials and Methods
Construction and purification of recombinant OmpA protein
Recombinant OmpA22-350 (rOmpA) protein was expressed with hexahistidine affinity tags at their N termini using the expression vector TAGZyme pQE2 (Qiagen Sciences, Germantown, MD). The gene fragment was amplified using PCR and Pfu polymerase (Promega KK, Tokyo, Japan), E. coli strain ATCC 25922T genomic DNA as the template, and the primers Fw_rOmpA22 and Rv_rOmpA350. The PCR fragments were cloned into pQE2 at the SalI and XhoI sites, and the resulting plasmids were transformed into E. coli BL21 (DE3) (GE Healthcare, Tokyo, Japan) for protein expression. The recombinant proteins were purified using Ni2+-chelate chromatography.
Production of monoclonal antibody
Six-week-old female C57BL/6 mice (Sankyo Labo Service Tokyo, Japan) were intraperitoneally injected with 5 μg of rOmpA in 200 μL of phosphate-buffered saline (PBS) per mouse once a week for 8 weeks. Ten months after the final inoculation, the spleen cells of the immunized mouse were fused with mouse myeloma SP2/0 cells at a ratio of 2:1 in polyethylene glycol 1500 (Roche Diagnostics, Indianapolis, IN). All the experiments were performed in accordance with the guidelines of the ethics review committee for animal experiments at Tokyo Women's Medical University.
The resulting hybridoma cells were plated onto 96-well plates and were cultured in RPMI1640 containing 10% fetal bovine serum and HAT selection medium (Life Technologies Japan, Tokyo, Japan). The hybridoma supernatants were screened using an enzyme-linked immunoadsorbent assay (ELISA) against rOmpA. Positive clones were subcloned and rescreened using an ELISA.
ELISA
The rOmpA protein in PBS was adsorbed on the surface of 96-well immunoplates (Nunc, Roskilde, Denmark) by incubating overnight at 4°C. The plates were then blocked with 2% nonfat milk in PBS containing 0.05% Tween-20 (PBS-T) for 2 h at 37°C to limit non-specific binding. The hybridoma supernatants were incubated for 2 h at 37°C and then washed three times with PBS-T. The plates were incubated with HRP-conjugated anti-mouse immunoglobulins (Biosource, Camarillo, CA). After washing three times with PBS-T, the immunoreactivity was visualized using TMB Substrate Chromogen solution (DACO, Tokyo, Japan), and the OD value was read at 450 nm.
The MAb isotypes were detected using the IsoStrip antibody isotyping kit (Roche Diagnostics, Mannheim, Germany).
Western blot analysis
The antigen (rOmpA, 50 μg/gel or E. coli 25922 suspension in ddH2O) was boiled (98°C, 5 min) in Laemmli buffer (0.5 M Tris-HCl [pH 6.8], 0.5% bromophenol blue, 8% glycerol, 4% SDS, and 4% 2-mercaptoethanol), electrophoresed on a 10% SDS-PAGE gel, and transferred to a PVDF membrane using the wet transfer method. The membrane was blocked in TSB-TM buffer (10 mM Tris-HCl [pH 7.4], 0.9% NaCl, 0.05% Tween-20, 10% nonfat milk) and was cut into strips. The strips were then incubated with anti-E. coli OmpA antibody (clone 49.4-15, 18.4 μg/mL), which was purified using a protein G column (GE Healthcare). Bound antibodies were recognized using horseradish peroxidase labeled anti-mouse Ig antibodies (Abcam, Tokyo, Japan) and 50 mM of sodium acetate buffer containing 0.04% 3-amino-9-ethylcarbazole (Sigma Chemical, St. Louis, MO) and 0.015% H2O2.
Confocal microscopic examination
E. coli strain ATCC 25922T was cultured in Brain Heart Infusion (BHI) broth (BD, Franklin Lakes, NJ) aerobically for 18 h at 37°C, with vigorous shaking. The bacteria were harvested and washed twice with PBS. The bacterial suspensions were then heated at 80°C for 30 min, then resuspended in PBS, followed by labeling with CSFE (Sigma-Aldrich, Tokyo, Japan).
Mouse macrophage cell line RAW264.7 cells (Riken Cell Bank, Ibaraki, Japan) were cultured in DMEM (Life Technologies Japan, Tokyo, Japan) supplemented with 10% FCS in glass-bottom dishes (Matsumani, Tokyo, Japan) for 0.5 h.(8) The RAW 264.7 cells were then incubated for an additional 2.5 h in the presence or absence of CFSE-labeled E. coli (2×107 CFU/mL), washed three times with PBS, and subjected to immunohistochemical staining. Briefly, the cells were fixed with 95% ethanol, blocked with 3% nonfat milk containing PBS, incubated with anti-mouse CD16/32 (eBioscience, Burlingame, CA) for Fc blocking, and then stained with anti-OmpA antibody (clone 49.4-15, 50 μg/mL). The reaction was then stained with PE-conjugated anti-mouse IgG (DACO) as a secondary antibody. Nuclear staining was performed using DAPI (Vector). The analysis was performed using a laser-scanning microscope (LSM 710, Carl Zeiss, Jena, Germany).
Results
Monoclonal antibodies
Using rOmpA as an antigen, three positive MAbs were obtained (clones 49.4-15, 6.2-3, and 83.23-9) using an ELISA. The isotype of clone 49.4-15 was IgG2b, while those of clones 6.2-3 and 83.23-9 were IgM. Out of these three clones, we selected the IgG class clone 49.4-15 for use in subsequent studies. To study the specificity of the anti-OmpA antibody, a Western blot analysis of rOmpA protein was performed. The results indicated that MAb 49.4-15 clearly detected the 35 kDa rOmpA protein. Using boiled E. coli 25922, MAb 49.4-15 also recognized the 35kDa OmpA of E. coli (Fig. 1).
FIG. 1.
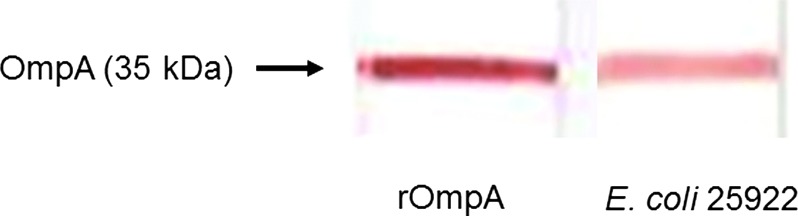
Immunoblotting identification of antigen using MAb against rOmpA protein. MAb 49.4-15 recognized a 35 kDa rOmpA protein. Using boiled E. coli 25922, MAb 49.4-15 also recognized a 35 kDa OmpA of E. coli.
Confocal microscopic examination
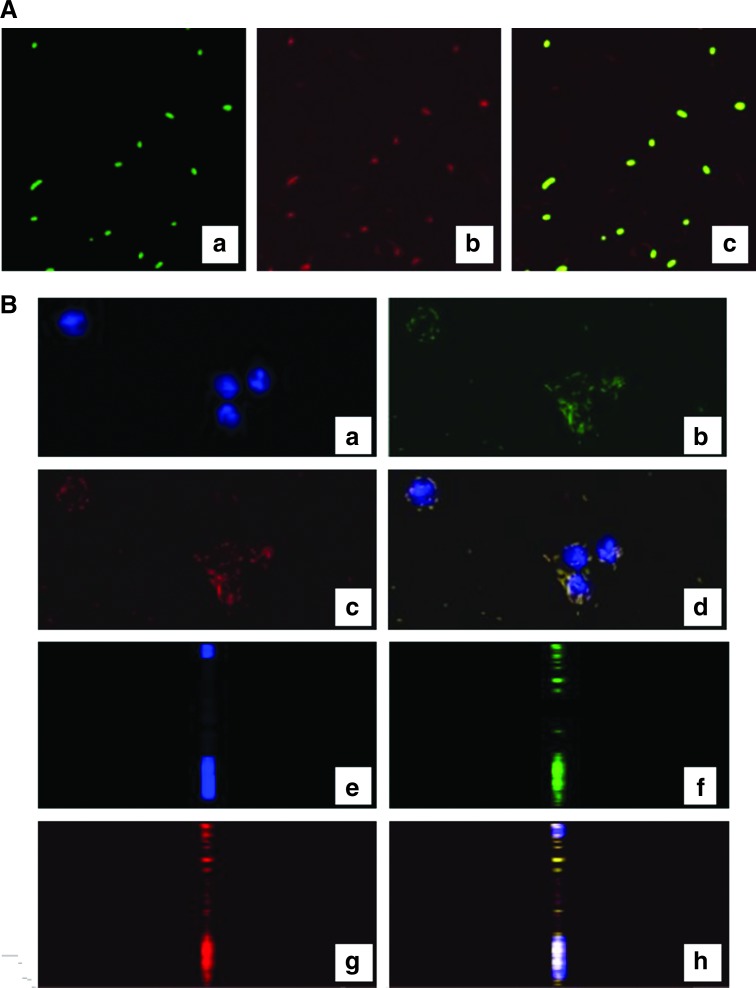
We investigated the reactivity of MAb 49.4-15, and found that CFFE-labeled E. coli were stained with MAb 49.4-15 whereas CFFE-labeled E. coli were not stained with control PE-labeled IgG antibody (data not shown). The results indicated that MAb 49.4-15 reacted specifically to CFFE-labeled E. coli (Fig. 2A). Furthermore, when CFFE-labeled E. coli were co-cultured with the macrophage cell line RAW264.7, MAb 49.4-15 was phagocytized and internalized CFFE-labeled E. coli within 3 h reaction (Fig. 2B).
FIG. 2.
(A) Detection of E. coli using MAb 49.4-15. CSFE-labeled E. coli were stained with MAb 49.4-15 followed by incubation with PE-labeled anti-mouse IgG. (a) CSFE-labeled E. coli; (b) MAb 49.4-15 staining of E. coli; (c) merged image. (B) Detection of phagocytized and internalized E. coli using MAb 49.4-15. RAW264.7 cells were incubated with CSFE-labeled E. coli and further stained with MAb 49.4-15 followed by incubation with PE-labeled anti-mouse IgG. (a,e) DAPI staining; (b,f) treatment with CSFE-labeled E. coli alone; (c,g) staining with MAb 49.4-15 followed by staining with PE-labeled anti-mouse IgG; (d,h) merged images; (a–d) horizontal image; (e–h) ortho view of z stack.
Discussion
OmpA, a 35 kDa protein, is thought to play pathological roles in many diseases. For example, OmpA of E. coli R218 has been shown to contribute to the pathogenesis of neonatal meningitis by transmigrating across the blood-brain barrier.(9) Krishnan and Prasadarao reported that the expression of OmpA influences the entry and survival of E. coli K1 (a prominent pathogen causing meningitis in neonates) in macrophages.(2) Not only is the interaction of OmpA with a specific receptor critical for the entry of bacteria into macrophages, but OmpA+ E. coli K1 also takes over the control of macrophage function. In addition, OmpA+ E. coli promotes its survival in dendritic cells (DC) and induces a negative regulatory effect on DC maturation.(2) Torres and colleagues reported that on the outer membrane of enterohemorrhagic E. coli O157:H7, OmpA acts as a critical adhesion factor for binding.(10) These reports strongly suggest that OmpA affects innate immunity. We previously reported that repeated injections of E. coli 25922 in mice caused autoimmune pancreatitis (AIP)-like pathological alterations in C57BL/6 mice.(11) However, the mechanism by which E. coli 25922 is involved in the pathogenesis of AIP has not been clarified. Studies of the interaction between OmpA of E. coli and the innate immune system would likely provide clues for understanding the pathogenesis of AIP, and additional studies are thus required.
In summary, we have reported the production and characterization of an MAb that specifically recognizes E. coli OmpA. MAb 49.4-15 is expected to be useful for studies examining the localization and function of E. coli OmpA during innate immune system processes.
Acknowledgments
We thank Ms. Naoko Kodama, Mr. Sho Takei, and Mr. Yasuhide Shigematsu for their skillful technical assistance and Mr. Masamichi Yoshikawa for intensive animal care. This work was financially supported in part by a Grant-in-Aid for Scientific Research (C23590522 to I.H.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author Disclosure Statement
The authors have no financial interests to disclose.
References
- 1.Reusch RN. Insights into the structure and assembly of Escherichia coli outer membrane protein A. FEBS J. 2012;279:894–909. doi: 10.1111/j.1742-4658.2012.08484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishnan S. Prasadarao NV. Outer membrane protein A and OmF: versatile roles in Gram-negative bacterial infections. FEBS J. 2012;279:919–931. doi: 10.1111/j.1742-4658.2012.08482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveira R. de Morais ZM. Goncales AP. Romero E. C. Vasconcellos SA. Nascimento AL. Characterization of novel OmpA-like protein of Lepospira interrogans that binds extracellular matrix molecules and plasminogen. PLos One. 2011;6(7):321962. doi: 10.1371/journal.pone.0021962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JS. Lee WC. Yeo KJ. Ryu K. Kumarasiri M. Hesek D. Lee M. Mobashery S. Song JH. Kim SI. Lee JC. Cheong C. Jeon YH. Kim HY. Mechanism of anchoring of OmpA protein to the cell wall peptidoglycan of the gram-negative bacterial outer membrane. FASEB J. 2012;26:219–228. doi: 10.1096/fj.11-188425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danoff EJ. Fleming KG. The soluble, periplasmic domain of OmpA folds as an independent unit and displays chaperone activity by reducing the self-association propensity of the unfolded OmpA transmembrane β-barrel. Biophys Chem. 2011;159:194–204. doi: 10.1016/j.bpc.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugawara E. Nikaido H. Pore-forming activity of OmpA protein of Escherichia coli. J Biol Chem. 1992;267:2507–2511. [PubMed] [Google Scholar]
- 7.Bacterial pathogenesis. In: Wilson BA, editor; Salyers AA, editor; Whitt DD, editor; Winkler ME, editor. A molecular approach. 3rd. ASM Press; Washington DC: 2011. p. 28. [Google Scholar]
- 8.Haruta I. Kikuchi K. Hashimoto E. Nakamura M. Miyakawa H. Hirota K. Shibata N. Kato H. Arimura Y. Kato Y. Uchiyama T. Nagamune H. Kobayashi M. Miyake Y. Shiratori K. Yagi J. Long-term bacterial exposure can trigger nonsuppurative destructive cholangitis associated with multifocal epithelial inflammation. Lab Invest. 2010;90(4):577–588. doi: 10.1038/labinvest.2010.40. [DOI] [PubMed] [Google Scholar]
- 9.Maruvada R. Kim KS. Extracellular loops of the Escherichia coli outer membrane protein A contribute to the pathogenesis of meningitis. J Infect Dis. 2011;203:131–140. doi: 10.1093/infdis/jiq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres AG. Jeter C. Langley W. Matthysse AG. Differential binding of Escherichia coli O157:H7 to alfalfa, human epithelial cells, and plastic is mediated by a variety of surface structures. Appl Environ Microbiol. 2005;71:8008–8015. doi: 10.1128/AEM.71.12.8008-8015.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haruta I. Yanagisawa N. Kawamura S. Furukawa T. Shimizu K. Kato H. Kobayashi M. Shiratori K. Yagi J. A mouse model of autoimmune pancreatitis with salivary gland involvement triggered by innate immunity via persistent exposure to avirulent bacteria. Lab Invest. 2010;90(12):1757–1769. doi: 10.1038/labinvest.2010.153. [DOI] [PubMed] [Google Scholar]