Abstract
The variable regions of the heavy chain (VH) and light chain (VL) were amplified by RT-PCR from the hybridoma 6E6, which secretes the monoclonal antibody against PEDV S protein. The VL and VH amplicons were combined using SOE-PCR by a 12 amino acid flexible linker (SSGGGGSGGGGS), which produced the scFv gene (named scFv/6E6). After sequence analysis, the scFv/6E6 gene was cloned into the prokaryotic expression vector pGEX-6p-1 with a GST-tag. The recombinant scFv/6E6 protein was successfully expressed in recombinant Escherichia coli by IPTG induction. Moreover, the recombinant scFv/6E6 protein was purified from the inclusion body form by the gel-cutting measure followed by electroelution and dialysis. The recombinant scFv/6E6 protein reported here will provide some basis for further antiviral drug research based on the scFv molecule.
Introduction
Porcine epidemic diarrhea (PED) is a highly contagious, enteric disease of swine caused by porcine epidemic diarrhea virus (PEDV), which is characterized by severe enteritis, vomiting, watery diarrhea, and weight loss. PEDV has a substantial detrimental effect on the swine industry due to its high mortality rates among suckling piglets.(1–3) Since PED was first reported in Belgium and the United Kingdom in 1978, many studies regarding PEDV have been carried out for the prevention of PED.(4–10) However, in recent years PEDV infections still exist and cause frequent occurrences of piglet diarrhea in many swine-raising provinces in China, leading to severe economic losses.(11–13) PEDV is a member of group 1a, genus Alphacoronavirus, family Coronaviridae, order Nidovirales. The size of its genome is approximately 28 kb, encoding three major structural proteins—spike protein (S), membrane protein (M), and nucleocapsid protein (N). Of these, the S protein plays an important role in induction of neutralizing antibodies, receptor binding, and cell membrane fusion.(14,15) These properties make the S protein a suitable target for the development of antiviral agents.
In a previous study, the monoclonal antibody (MAb) 6E6 was prepared by hybridoma technique using a truncated recombinant S protein of PEDV as an immunogen.(7) Further research revealed that the MAb 6E6 was capable of recognizing the native S protein of PEDV with a high affinity. The single-chain fragment variable (scFv) is a small, engineered antibody in which the variable light chain (VL) and heavy chain (VH) of the antibody are connected by a short polypeptide linker. At present, the scFv molecule is considered a powerful tool for therapeutic antigen targeting in vivo.(16,17)
In the current study, the scFv gene was obtained from the hybridoma cell 6E6 secreting the monoclonal antibody against S protein of PEDV. After cloning, the framework regions and complementarity determining regions of the scFv gene were analyzed on the basis of the deduced amino acid sequences. The recombinant scFv protein was successfully expressed in Escherichia coli using the prokaryotic expression vector pGEX-6p-1 with a GST-tag. Moreover, the high-purity recombinant scFv protein was produced through a simple gel-cutting procedure followed by electroelution and dialysis. Our aim is to reveal the basic sequence information of the MAb 6E6 and develop a practical procedure for the preparation of scFv to facilitate further antiviral research.
Materials and Methods
Strains and hybridoma cells
The hybridoma cell of the MAb 6E6 against PEDV S protein was kindly provided by the Division of Swine Infectious Diseases, National Key Laboratory of Veterinary Biotechnology (Harbin Veterinary Research Institute of the Chinese Academy of Agricultural Sciences, Harbin, China).(7) Escherichia coli Rosetta™ (DE3) plysS cells were obtained from Novagen (Madison, WI). Escherichia coli DH5α cells were prepared using the calcium chloride method and stored at −80°C.
Primers for scFv gene
The primers for PCR amplification of the VL and VH genes of the MAb 6E6 were designed according to the degenerate primers described by Wang and associates.(18) The 5′ ends of the VH forward primers and VL reverse primers were modified to include BamH I and Xho I sites, respectively. In order to splice the genes VL and VH, a complementary overlapping sequence encoding a flexible linker of 12 amino acids (SSGGGGSGGGGS) was added to the 5′ ends of the VH reverse primers and VL forward primers, respectively. Detailed primer information is shown in Table 1.
Table 1.
Primers for VH and VL Genes of MAb 6E6
| Ig chains | Primer names | Primer sequences |
|---|---|---|
| VH | 6E6VH-F | 5' cgcGGATCCsaggtsmagctgcagsagtcwgg 3' |
| 6E6VH-R | 5' cgagccgccacctccagagccacctccgcctgaggagactatgagagtgg 3' | |
| VL | 6E6VL-F | 5' ggcggaggtggctctggaggtggcggctcggayattgtgmtsacmcarwctmca 3' |
| 6E6VL-R | 5' ccgCTCGAGttatttgatttccagcttgg 3' |
S=G/C, R=G/A, K=G/T, M=A/C, Y=C/T, W=A/T, H=A/C/T, B=C/G/T, V=A/C/G, D=A/G/T, N=A/T/G/C.
RNA extraction and cDNA synthesis
Total RNA was prepared from the hybridoma cells of the MAb 6E6 against S protein of PEDV using the reagent Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Using the extracted RNA as templates, the first strand cDNA synthesis was carried out using the OrientExpress Oligo (dT) cDNA Synthesis Kit (Novagen, San Diego, CA) according to the manufacturer's instructions.
Amplification of VL and VH genes
The VL and VH genes of the MAb 6E6 were respectively amplified by conventional PCR using synthesized cDNA as a common template. Briefly, the PCR reaction was performed in a 25 μL mixture containing 400 nmol of each forward and reverse primer, 1 μL cDNA template, 0.25 mmol each dNTP mixture (Takara, Dalian, China), 10× buffer, and 0.5 U ExTaq DNA polymerase (Takara). The amplification conditions comprised an initial denaturation at 95°C for 5 min, followed by 30 PCR cycles of 95°C for 30 s, 52°C for 30 s, and 72°C for 30 s, and a final extension step of 72°C for 10 min. The amplified products of the VL and VH genes were purified using the Qiaquick Gel Extraction Kit (Qiagen, Hilden, Germany).
Generation of scFv/6E6 gene
Using the purified VL and VH genes as templates, an overlap extension PCR (SOE-PCR) was performed for generation of the scFv gene of the MAb 6E6 (scFv/6E6). Briefly, the SOE-PCR reaction contained 100 ng of purified VL products and 100 ng of purified VH products, 0.25 mmol each dNTP mixture, 10× buffer, and 0.5 U ExTaq DNA polymerase. 35 PCR cycles were performed as follows: 95°C for 1 min, 50°C for 1 min, and 72°C for 1 min, and a final extension step of 72°C for 10 min.
Prokaryotic expression of scFv/6E6 gene
After the scFv/6E6 gene was digested using the restriction endonucleases BamH I and Xho I, the cohesive ligation of the scFv/6E6 gene with the vector pGEX-6p-1 was carried out using T4 DNA Ligase (Novagen, San Diego, CA) at 16°C for 12 h. The ligation products were transformed into E. coli DH5α cells, and the positive plasmids were selected to determine the sequence of the scFv/6E6 gene. Sequence analysis of the framework regions (FR) and complementarity determining regions (CDR) of the scFv/6E6 gene was carried out using the IMGT/V-QUEST program v3.2.20 of the international immunogenetics information system (available at www.imgt.org/IMGT_vquest/share/textes). The recombinant plasmids were transformed into E. coli Rosetta (DE3) plysS cells, and the recombinant bacteria were induced using 1.0 mM isopropyl β-D-thiogalactoside (IPTG) at 37°C for 4 h. The induced recombinant bacteria were analyzed by 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE).
Purification of recombinant scFv/6E6 protein
The inclusion body of the recombinant scFv/6E6 protein was extracted from the lysate of the IPTG-induced host bacteria processed by supersonic waves. The extracted inclusion body proteins were treated with 2× SDS loading buffer and then separated by 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, the gel was stained with the reagent RAPIDstain™ (Merck, Darmstadt, Germany). The protein band of the recombinant scFv/6E6 protein was cut from the stained gel. The gel-cutting band containing the recombinant scFv/6E6 protein was loaded into the D-Tube™ Dialyzer Mini with MWCO 12–14 kDa (Merck), and 2.5 mL of tris-glycine SDS running buffer was added to the loaded tube. The electroelution of the loaded tube was run in a horizontal electrophoresis chamber (Beijing Liuyi Instrument Factory, Beijing, China) at 100 V for 1.5 h at room temperature in the tris-glycine SDS running buffer. After the electroelution, the dialysis of the loaded tube containing the recombinant scFv/6E6 protein was performed in 1 L of phosphate-buffered saline (PBS, pH 7.4) for 3 h. The purified recombinant scFv/6E6 protein was collected from the dialysis tube and stored at −80°C.
Western blot analysis
To verify the recombinant scFv/6E6 protein expressed in Escherichia coli Rosetta (DE3) plysS cells, Western blot analysis was performed using the following procedures. The purified scFv/6E6 protein was subjected to 12% SDS-PAGE and then transferred to a nitrocellulose membrane (NC) using a semi-dry transfer apparatus (Bio-Rad, Hercules, CA). The NC membrane was blocked using 5% (W/V) non-fat dried milk in PBS at 37°C for 1 h, and incubated with mouse monoclonal antibody against the GST-tag (1:1000 dilution in PBS) at 37°C for 1 h. After washing three times with PBST, the NC membrane was incubated with IRDye™ 700DX-conjugated affinity purified anti-mouse IgG (H&L, goat, 1:8000 dilution in PBS) at 37°C for 1 h. After washing three times with PBST, the results of the Western blot were analyzed using the ODYSSEY™ Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE).
Results
Amplification and analysis of scFv/6E6 gene
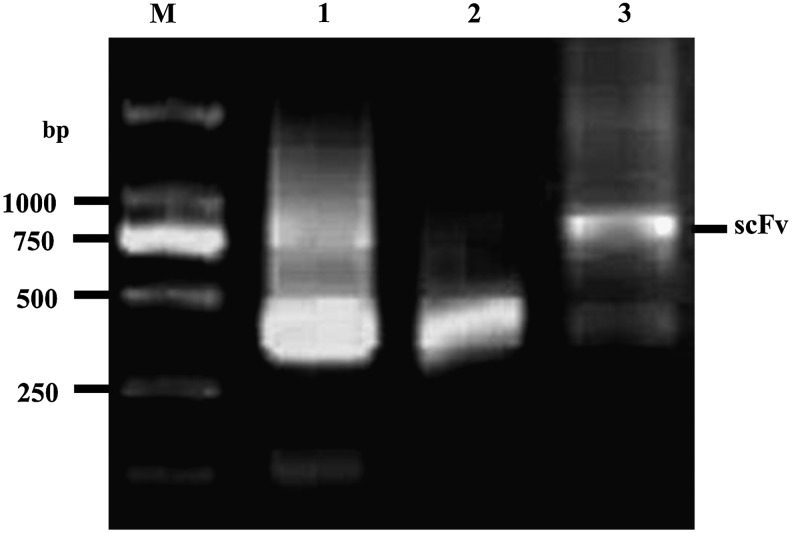
The VH and VL genes were amplified at about 360 and 400 bp by PCR using the cDNA from the hybridoma cells 6E6 as a template, and then ∼750 bp size of the scFv/6E6 gene was generated by SOE-PCR (Fig. 1). The nucleotide sequences and deduced amino acid sequences of four FRs and three CDRs of the H and L chains in the scFv/6E6 gene are shown in Table 2. Sequence analysis indicated that the VH gene of the scFv/6E6 gene showed 88.41% nucleotide identity with mouse immunoglobulin heavy chain variable region gene (Genbank accession no. AF305910), and the VL gene of the scFv/6E6 gene showed 85.67% nucleotide identity with mouse immunoglobulin kappa light chain variable region gene (Genbank accession no. AF003294).
FIG. 1.
Amplification of the scFv/6E6 gene. Lane M, DNA marker DL2000; lane 1, PCR products of the VH gene; lane 2, PCR products of the VL gene; lane 3, amplified products of the scFv/6E6 gene by SOE-PCR.
Table 2.
Sequence Analysis of Genes VL and VH of MAb 6E6
| FRI | CDRI | FR2 | CDR2 | FR3 | CDR3 | FR4 | ||
|---|---|---|---|---|---|---|---|---|
| VH | nt | CAGGTCAAGCTGCAGCAGTCAGGACCTGAGCTGGTGAAGCCTGGGGCTTCAGTGAAGATATCCTGCAAGGCTTCT | GGCTACACCTTCACTGACTACTAT | ATAAACTGGGTGAAGCAGAAGCCTGGACAGGGACTTGAGTGGATTGGATGG | ATTTATCCTGGAAGCGGTAATACT | AAGTACAATGAGAAGTTCAAGGACAAGGCCACATTGACTGTAGACACATCCTCCAGCACAGCCTACATGCAGCTCAGCAGCCTGACATCTGAGGACACTGCTGTCTATTTCTGT | GCAAGATGGAGTACTACGGTAGTAGACTAC | TGGGGCCAAGGCGCCACTCTCATAGTC |
| aa | QVKLQQSGPELVKPGASVKISCKAS | GYTFTDYY | INWVKQKPGQGLEWIGW | IYPGSGNT | KYNEKFKDKATLTVDTSSSTAYMQLSSLTSEDTAVYFC | ARWSTTVVDY | WGQGATLIV | |
| Link | nt | TCCTCAGGCGGAGGTGGCTCTGGAGGTGGCGGCTCG | ||||||
| aa | SSGGGGSGGGGS |
| FRI | CDRI | FR2 | CDR2 | FR3 | CDR3 | FR4 | ||
|---|---|---|---|---|---|---|---|---|
| VL | nt | GACATTGTGCTGACCCAGACTACATCCTCCTTATCTGCCTCTCTGGGAGAAAGAGTCAGTCTCACTTGTCGGGCAAGT | CAGGAAATTAGTGGTTAC | TTAAGCTGGCTTCAGCAGAAACCAGATGGAACTATTAAACGCCTGATGTAC | GCCGCATCC | ACTTTAGATTCTGGTGTCCCAAAAAGGTTCAGTGGCAGCAGGTCTGGGTCAGATTATTCTCTCACCATCAGCAGCCTTGAGTCTGAAGATTTTGCAGACTATTACTGT | CTAAAATATGCTAGTTATCCGTACACG | TTCGGAGGGGGGACCAAGCTGGAAATCAAA |
| aa | DIVLTQTTSSLSASLGERVSLTCRAS | QEISGY | LSWLQQKPDGTIKRLMY | AAS | TLDSGVPKRFSGSRSGSDYSLTISSLESEDFADYYC | LKYASYPYT | FGGGTKLEIK |
Expression and purification of scFv/6E6 gene
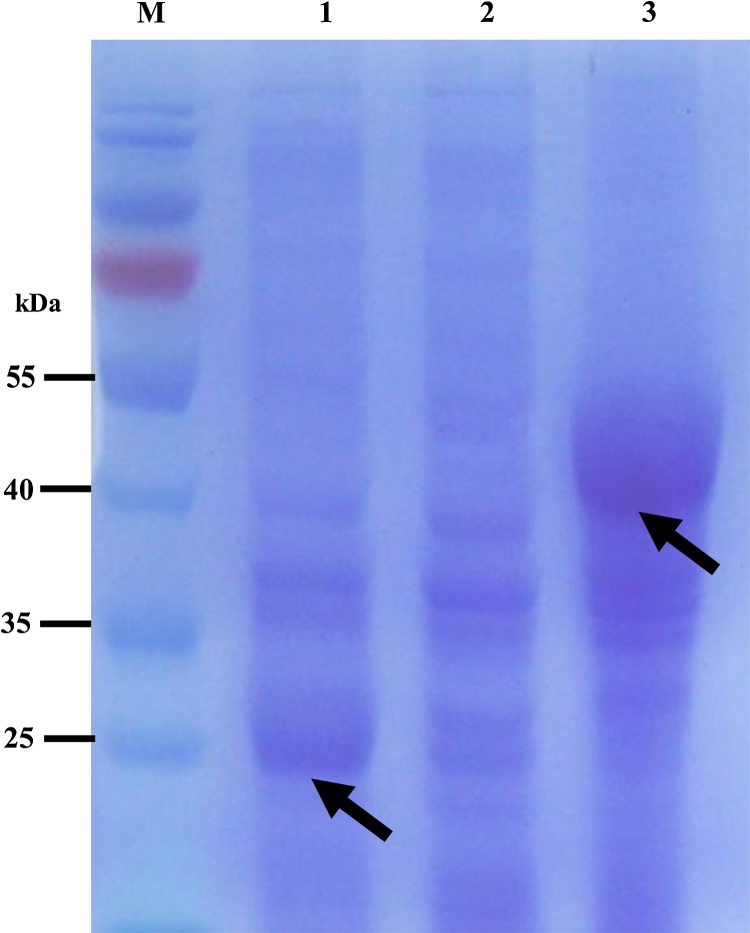
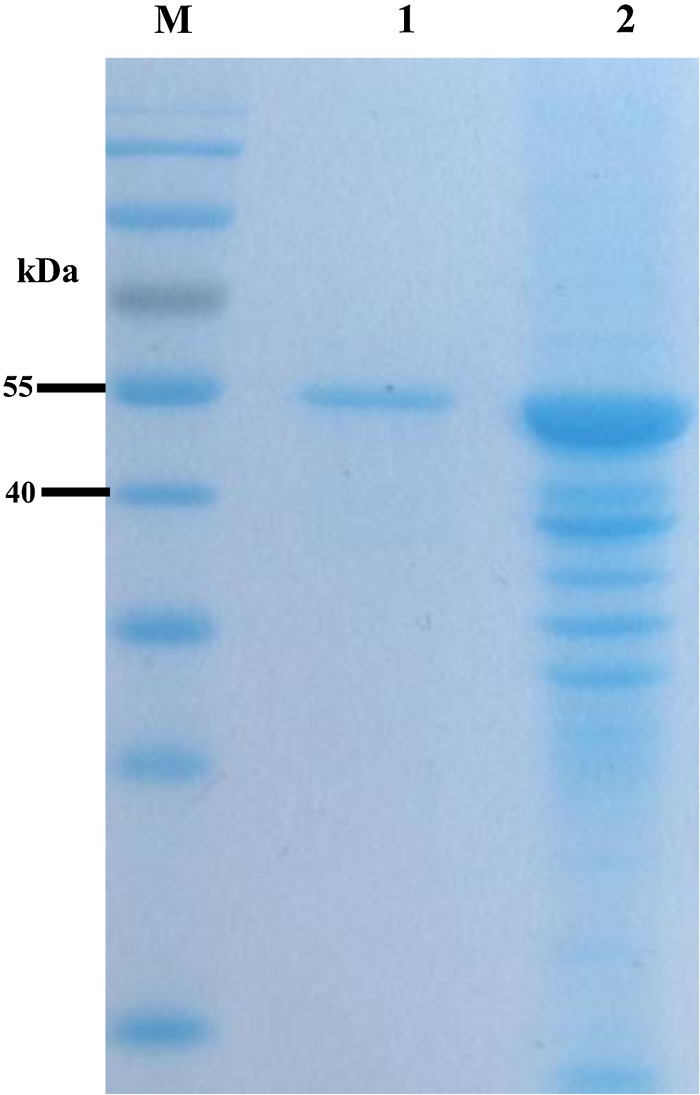
After cloning the scFv/6E6 gene into prokaryotic expression vector pGEX-6p-1, the recombinant scFv/6E6 protein was successfully expressed at a high level in Escherichia coli; its molecular weight was approximately 50 kDa (Fig. 2). The high-purity recombinant scFv/6E6 protein was obtained by simple gel-cutting purification followed by electroelution and dialysis (Fig. 3).
FIG. 2.
Expression of the scFv/6E6 gene. Lane M, PageRuler Prestained Protein Ladder (10–170 kDa); lane 1, empty vector control bacteria induced by IPTG; lane 2, uninduced recombinant bacteria of scFv/6E6 gene; lane 3, induced recombinant bacteria of scFv/6E6 gene.
FIG. 3.
Purification of the scFv/6E6 gene. Lane M, PageRuler Prestained Protein Ladder (10–170kDa); lane 1, purified recombinant scFv/6E6 protein; lane 2, precipitation of induced recombinant bacteria of scFv/6E6 gene lysed by supersonic wave treatment.
Western blot analysis of scFv/6E6 gene
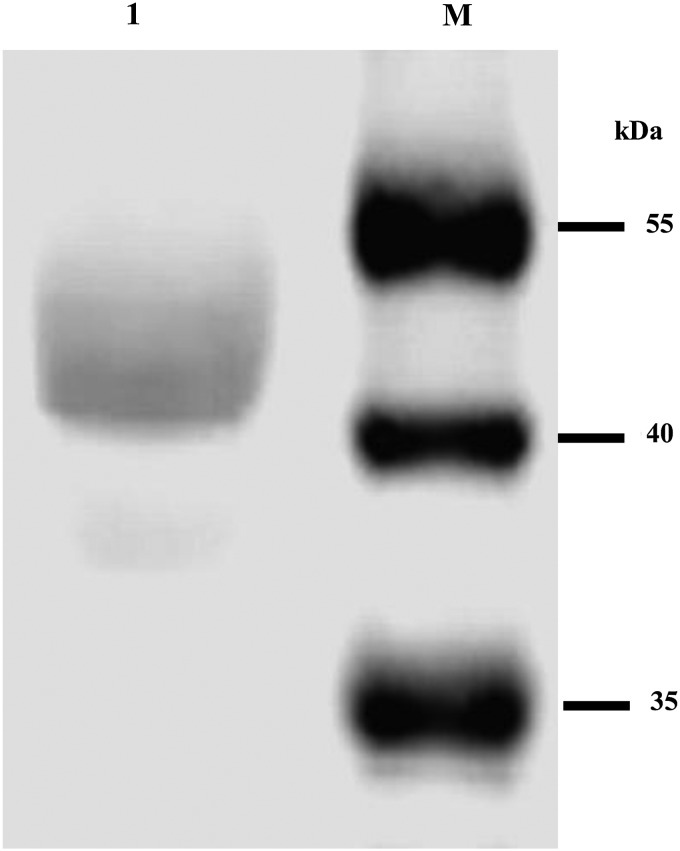
To further verify the recombinant scFv/6E6 protein, the Western blot analysis was performed using the monoclonal antibody against GST-tag. The result revealed that the recombinant scFv/6E6 protein fused with GST-tag showed a strong reaction with the monoclonal antibody against GST-tag, and the specific reaction band was found to be about 50 kDa in size (Fig. 4).
FIG. 4.
Western blot analysis of the recombinant scFv/6E6 protein. Lane 1, recombinant scFv/6E6 protein; lane M, PageRuler Prestained Protein Ladder (10–170 kDa).
Discussion
The use of antibody is a powerful therapeutic tool against viral infections.(19,20) During the past decade, advances in recombinant antibody technology have led to the development of a large variety of engineered antibody molecules for research, diagnosis, and therapy with specificities out of reach of conventional antibody technology. Of these, scFv is the smallest unit of immunoglobulin molecule functioning in antigen-binding activities. In comparison to the parental antibody, scFv molecules have several advantages in clinical practices, including better penetration, lower immunogenicity, and therapeutic antigen targeting in vivo, as well as the ability to facilitate mass production in vitro. At present, numerous scFvs have been constructed against hapten, protein, carbohydrate, receptor, tumor antigen, and viruses. All these scFvs demonstrate good potential for use in several fields, including medical therapies and diagnostic applications.(21–24)
In our study, the scFv gene of the MAb 6E6 against PEDV S protein was cloned from its hybridoma cells. Sequence analysis demonstrated that the VH and VL genes of the scFv/6E6 gene were composed of four framework regions (FRs) and three complementarity determining regions (CDRs); they showed high identity with the VH and VL (kappa) genes of mouse immunoglobulin published in GenBank. Many studies have proven that the scFv genes from different antibodies were successfully expressed in prokaryotic expression system using Escherichia coli as host cells.(25) At the beginning of this study, the prokaryotic expression vectors pET-32a with His-tag had been selected to express the scFv/6E6 gene. However, we did not obtain a positive expression of the scFv gene. Moreover, the vector pGEX-6p-1 was used in an attempt to generate the recombinant protein of the scFv/6E6 gene. The results demonstrated that the scFv/6E6 gene was expressed in Escherichia coli through the fusion effect of the GST-tag. It had been proved that the vector pGEX-6p-1 showed significant efficacy in the expression of leukotoxin of Fusobacterium necrophorum.(26)
The prokaryotic expression system has several advantages, including easy culture, rapid cell growth, low cost, high yield, and relatively short expression time. However, if the expressed protein is required for functional studies, the prokaryotic system shows a problem as most proteins become insoluble in inclusion bodies, which are very difficult to recover as functional proteins. Thus, purification of the inclusion bodies protein is a key step for prokaryotic expression system. Gel-cutting purification of the inclusion body protein has been proven to be a simple, time-saving, and much less expensive means in several studies.(9,10) Additionally, gel-cutting purification is capable of facilitating the preparation of high-purity recombinant proteins in vitro. However, it is difficult to remove the sodium dodecyl sulfate (SDS) completely from the cutting-gel purified proteins through the conventional protein dialysis. In our study, the D-Tube Dialyzer was used for electroelution and dialysis of the recombinant scFv/6E6 protein from polyacrylamide gels. The procedure efficiently recovers proteins and simultaneously removes ampholytes from proteins. The purified proteins are compatible with most downstream applications such as animal immunization for antibody production and functional assays. In addition, the RAPIDstain reagent, instead of potassium chloride staining, improved the sensitivity of the gel staining and simplified the experimental operation of the cutting-gel purification. In Western blot analysis, the recombinant scFv/6E6 protein was conformed using the monoclonal antibody against GST-tag, and the specific reaction band was found in ∼50 kDa. This result provided further evidence that the scFv protein (∼25 kDa) was correctly fused to the GST-tag (∼26 kDa) of the vector pGEX-6p-1.
In conclusion, we cloned the scFv gene from the hybridoma cells of the MAb 6E6 against PEDV S protein and expressed the recombinant scFv/6E6 protein in E. coli using the vector pGEX-6p-1 with GST-tag. Moreover, a simple purification method, combining cutting-gel purification with electroelution and dialysis, was used for purification of the recombinant scFv/6E6 protein. This study will provide a basis for further antiviral research of PEDV.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 31001081), the Program for New Century Excellent Talents in Heilongjiang Provincial University (grant no. 1252-NCET-016), and the Technological Innovation Team Building Program of University of Heilongjiang Province (grant no. 2010td05).
Author Disclosure Statement
The authors have no financial conflicts to disclose.
References
- 1.Pensaert MB. Debouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch Virol. 1978;58:243–247. doi: 10.1007/BF01317606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chasey D. Cartwright SF. Virus-like particles associated with porcine epidemic diarrhea. Res Vet Sci. 1978;25:255–256. doi: 10.1016/S0034-5288(18)32994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi K. Okada K. Ohshima K. An outbreak of swine diarrhea of a new-type associated with coronavirus-like particles in Japan. Jpn J Vet Sci. 1983;45:829–832. doi: 10.1292/jvms1939.45.829. [DOI] [PubMed] [Google Scholar]
- 4.Suo S. Ren Y. Li G. Zarlenga D. Bu RE. Su D. Li X. Li P. Meng F. Wang C. Ren X. Immune responses induced by DNA vaccines bearing Spike gene of PEDV combined with porcine IL-18. Virus Res. 2012;167:259–266. doi: 10.1016/j.virusres.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun D. Shi H. Chen J. Shi D. Zhu Q. Zhang H. Liu S. Wang Y. Qiu H. Feng L. Generation of a mouse scFv library specific for porcine aminopeptidase N using the T7 phage display system. J Virol Methods. 2012;182:99–103. doi: 10.1016/j.jviromet.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J. Liu X. Shi D. Shi H. Zhang X. Feng L. Complete genome sequence of a porcine epidemic diarrhea virus variant. J Virol. 2012;86:3408. doi: 10.1128/JVI.07150-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun D. Feng L. Shi H. Chen J. Cui X. Chen H. Liu S. Tong Y. Wang Y. Tong G. Identification of two novel B cell epitopes on porcine epidemic diarrhea virus spike protein. Vet Microbiol. 2008;131:73–81. doi: 10.1016/j.vetmic.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi HJ. Kim JH. Lee CH. Ahn YJ. Song JH. Baek SH. Kwon DH. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res. 2009;81:77–81. doi: 10.1016/j.antiviral.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun D. Shi H. Chen J. Guo D. Liu Q. He X. Bao J. Wang Y. Qiu H. Feng L. Virus-binding activity of the truncated C subunit of porcine aminopeptidase N expressed in Escherichia coli. Biotechnol Lett. 2012;34:533–539. doi: 10.1007/s10529-011-0795-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun D. Wang Y. Wu G. Zhang H. Zhu Q. He X. Guo D. Wu R. A polyclonal antibody against the C subunit of porcine aminopeptidase N expressed in Escherichia coli. Hybridoma. 2011;30:457–462. doi: 10.1089/hyb.2011.0042. [DOI] [PubMed] [Google Scholar]
- 11.Fan H. Zhang J. Ye Y. Tong T. Xie K. Liao M. Complete genome sequence of a novel porcine epidemic diarrhea virus in South china. J Virol. 2012;86:10248–10249. doi: 10.1128/JVI.01589-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Y. Zhang J. Deng X. Ye Y. Liao M. Fan H. Complete genome sequence of a highly prevalent isolate of porcine epidemic diarrhea virus in South China. J Virol. 2012;86:9551. doi: 10.1128/JVI.01455-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W. Li H. Liu Y. Pan Y. Deng F. Song Y. Tang X. He Q. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg Infect Dis. 2012;18:1350–1353. doi: 10.3201/eid1808.120002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egberink HF. Ederveen J. Callebaut P. Horzinek MC. Characterization of the structural proteins of porcine epizootic diarrhea virus, strain CV777. Am J Vet Res. 1988;49:1320–1324. [PubMed] [Google Scholar]
- 15.Brian DA. Baric RS. Coronavirus genome structure, replication. In: Enjuanes L, editor. Coronavirus Replication and Reverse Genetics. Springer-Verlag; Berlin: 2005. pp. 2–22. [Google Scholar]
- 16.Haidaris CG. Malone J. Sherrill LA. Bliss JM. Gaspari AA. Insel RA. Sullivan MA. Recombinant human antibody single chain variable fragments reactive with Candida albicans surface antigens. J Immunol Methods. 2001;257:185–202. doi: 10.1016/s0022-1759(01)00463-x. [DOI] [PubMed] [Google Scholar]
- 17.Pansri P. Jaruseranee N. Rangnoi K. Kristensen P. Yamabhai M. A compact phage display human scFv library for selection of antibodies to a wide variety of antigens. BMC Biotechnol. 2009;9:6. doi: 10.1186/1472-6750-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z. Raifu M. Howard M. Smith L. Hansen D. Goldsby R. Ratner D. Universal PCR amplification of mouse immunoglobulin gene variable regions: the design of degenerate primers and an assessment of the effect of DNA polymerase 3′ to 5′ exonuclease activity. J Immunol Methods. 2000;233:167–177. doi: 10.1016/s0022-1759(99)00184-2. [DOI] [PubMed] [Google Scholar]
- 19.Yang J. Wang S. Tian L. Zhang L. Li B. Dong C. Liu Z. Qi C. Development of neutralizing monoclonal antibodies against VP4 of rotavirus CC0812–1. Hybridoma. 2012;31:279–283. doi: 10.1089/hyb.2012.0016. [DOI] [PubMed] [Google Scholar]
- 20.Li N. Yang Y. Therapeutic use of an anti-ErbB3 monoclonal antibody. Hybridoma. 2012;31:149–54. doi: 10.1089/hyb.2011.0110. [DOI] [PubMed] [Google Scholar]
- 21.Guo JQ. You SY. Li L. Zhang YZ. Huang JN. Zhang CY. Construction and high-level expression of a single chain Fv antibody fragment specific for acidic isoferritin in Escherichia coli. J Biotechnol. 2003;102:177–189. doi: 10.1016/s0168-1656(03)00020-8. [DOI] [PubMed] [Google Scholar]
- 22.Shadidi M. Sioud M. An anti-leukemic single chain fv antibody selected from a synthetic human phage antibody library. Biochem Biophys Res Commun. 2001;280:548–552. doi: 10.1006/bbrc.2000.4158. [DOI] [PubMed] [Google Scholar]
- 23.Rajawat R. Narkar A. Damle A. Kumar GB. Mishra KP. A single-chain antibody fragment against human thyroglobulin: construction and evaluation of immunoreactivity. Hybridoma. 2011;30:253–259. doi: 10.1089/hyb.2010.0114. [DOI] [PubMed] [Google Scholar]
- 24.Salavatifar M. Amin S. Jahromi ZM. Rasgoo N. Rastgoo N. Arbabi M. Green fluorescent-conjugated anti-CEA single chain antibody for the detection of CEA-positive cancer cells. Hybridoma. 2011;30:229–238. doi: 10.1089/hyb.2011.0009. [DOI] [PubMed] [Google Scholar]
- 25.Yang H. Wang H. Xue T. Xue XP. Huyan T. Wang W. Song K. Single-chain variable fragment antibody against human aspartyl/asparaginyl beta-hydroxylase expressed in recombinant Escherichia coli. Hybridoma. 2011;30:69–79. doi: 10.1089/hyb.2010.0070. [DOI] [PubMed] [Google Scholar]
- 26.Sun DB. Wu R. Li GL. Zheng JS. Liu XP. Lin YC. Guo DH. Identification of three immunodominant regions on leukotoxin protein of Fusobacterium necrophorum. Vet Res Commun. 2009;33:749–755. doi: 10.1007/s11259-009-9223-6. [DOI] [PubMed] [Google Scholar]