Abstract
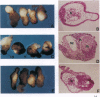
Previously, we elucidated the role of bone morphogenetic protein 4 (BMP-4) in the dorsal-ventral patterning of the Xenopus embryo by using a dominant negative mutant of the BMP-4 receptor (DN-BR). The present paper describes the involvement of Ras, Raf, and activator protein 1 (AP-1) in BMP-4 signaling during Xenopus embryonic development. The AP-1 activity was determined by injecting an AP-1-dependent luciferase reporter gene into two-cell-stage Xenopus embryos and measuring the luciferase activity at various developmental stages. We found that injection of BMP-4 mRNA increased AP-1 activity, whereas injection of DN-BR mRNA inhibited AP-1 activity. Similar inhibitory effects were seen with injection of mRNAs encoding dominant negative mutants of c-Ha-Ras, c-Raf, or c-Jun. These results suggest that the endogenous AP-1 activity is regulated by BMP-4/Ras/Raf/Jun signals. We next investigated the effects of Ras/Raf/AP-1 signals on the biological functions of BMP-4. DN-BR-induced dorsalization of the embryo, revealed by the formation of a secondary body axis or dorsalization of the ventral mesoderm explant analyzed by histological and molecular criteria, was significantly reversed by coinjection of [Val12]Ha-Ras, c-Raf, or c-Jun mRNA. Furthermore, the BMP-4-stimulated erythroid differentiation in the ventral mesoderm was substantially inhibited by coinjection with the dominant negative c-Ha-Ras, c-Raf, or c-Jun mutant. Our results suggest the involvement of Ras/Raf/AP-1 in the BMP-4 signaling pathway.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alani R., Brown P., Binétruy B., Dosaka H., Rosenberg R. K., Angel P., Karin M., Birrer M. J. The transactivating domain of the c-Jun proto-oncoprotein is required for cotransformation of rat embryo cells. Mol Cell Biol. 1991 Dec;11(12):6286–6295. doi: 10.1128/mcb.11.12.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya E., Musci T. J., Kirschner M. W. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991 Jul 26;66(2):257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Bernstein L. R., Colburn N. H. AP1/jun function is differentially induced in promotion-sensitive and resistant JB6 cells. Science. 1989 May 5;244(4904):566–569. doi: 10.1126/science.2541502. [DOI] [PubMed] [Google Scholar]
- Brown P. H., Alani R., Preis L. H., Szabo E., Birrer M. J. Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene. 1993 Apr;8(4):877–886. [PubMed] [Google Scholar]
- Chang E. H., Furth M. E., Scolnick E. M., Lowy D. R. Tumorigenic transformation of mammalian cells induced by a normal human gene homologous to the oncogene of Harvey murine sarcoma virus. Nature. 1982 Jun 10;297(5866):479–483. doi: 10.1038/297479a0. [DOI] [PubMed] [Google Scholar]
- Conrad K. E., Oberwetter J. M., Vaillancourt R., Johnson G. L., Gutierrez-Hartmann A. Identification of the functional components of the Ras signaling pathway regulating pituitary cell-specific gene expression. Mol Cell Biol. 1994 Mar;14(3):1553–1565. doi: 10.1128/mcb.14.3.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale L., Howes G., Price B. M., Smith J. C. Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development. 1992 Jun;115(2):573–585. doi: 10.1242/dev.115.2.573. [DOI] [PubMed] [Google Scholar]
- Diaz-Meco M. T., Lozano J., Municio M. M., Berra E., Frutos S., Sanz L., Moscat J. Evidence for the in vitro and in vivo interaction of Ras with protein kinase C zeta. J Biol Chem. 1994 Dec 16;269(50):31706–31710. [PubMed] [Google Scholar]
- Dong Z., Birrer M. J., Watts R. G., Matrisian L. M., Colburn N. H. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):609–613. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z., Lavrovsky V., Colburn N. H. Transformation reversion induced in JB6 RT101 cells by AP-1 inhibitors. Carcinogenesis. 1995 Apr;16(4):749–756. doi: 10.1093/carcin/16.4.749. [DOI] [PubMed] [Google Scholar]
- Fainsod A., Steinbeisser H., De Robertis E. M. On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. EMBO J. 1994 Nov 1;13(21):5015–5025. doi: 10.1002/j.1460-2075.1994.tb06830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q., Dean A. Enhancer-dependent transcription of the epsilon-globin promoter requires promoter-bound GATA-1 and enhancer-bound AP-1/NF-E2. Mol Cell Biol. 1993 Feb;13(2):911–917. doi: 10.1128/mcb.13.2.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J. M., Thies R. S., Song J. J., Celeste A. J., Melton D. A. Studies with a Xenopus BMP receptor suggest that ventral mesoderm-inducing signals override dorsal signals in vivo. Cell. 1994 Oct 7;79(1):169–179. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., Melton D. A. A truncated activin receptor inhibits mesoderm induction and formation of axial structures in Xenopus embryos. Nature. 1992 Oct 15;359(6396):609–614. doi: 10.1038/359609a0. [DOI] [PubMed] [Google Scholar]
- Jones C. M., Lyons K. M., Lapan P. M., Wright C. V., Hogan B. L. DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992 Jun;115(2):639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- Lazarus P., Calcagnotto A. Identification and partial characterization of the c-jun oncogene in Xenopus laevis. Cancer Lett. 1994 Nov 11;86(2):201–208. doi: 10.1016/0304-3835(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Li L., Hu J. S., Olson E. N. Different members of the jun proto-oncogene family exhibit distinct patterns of expression in response to type beta transforming growth factor. J Biol Chem. 1990 Jan 25;265(3):1556–1562. [PubMed] [Google Scholar]
- MacNicol A. M., Muslin A. J., Williams L. T. Raf-1 kinase is essential for early Xenopus development and mediates the induction of mesoderm by FGF. Cell. 1993 May 7;73(3):571–583. doi: 10.1016/0092-8674(93)90143-e. [DOI] [PubMed] [Google Scholar]
- Massagué J. Receptors for the TGF-beta family. Cell. 1992 Jun 26;69(7):1067–1070. doi: 10.1016/0092-8674(92)90627-o. [DOI] [PubMed] [Google Scholar]
- Maéno M., Ong R. C., Suzuki A., Ueno N., Kung H. F. A truncated bone morphogenetic protein 4 receptor alters the fate of ventral mesoderm to dorsal mesoderm: roles of animal pole tissue in the development of ventral mesoderm. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10260–10264. doi: 10.1073/pnas.91.22.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maéno M., Ong R. C., Xue Y., Nishimatsu S., Ueno N., Kung H. F. Regulation of primary erythropoiesis in the ventral mesoderm of Xenopus gastrula embryo: evidence for the expression of a stimulatory factor(s) in animal pole tissue. Dev Biol. 1994 Feb;161(2):522–529. doi: 10.1006/dbio.1994.1050. [DOI] [PubMed] [Google Scholar]
- Pumiglia K., Chow Y. H., Fabian J., Morrison D., Decker S., Jove R. Raf-1 N-terminal sequences necessary for Ras-Raf interaction and signal transduction. Mol Cell Biol. 1995 Jan;15(1):398–406. doi: 10.1128/mcb.15.1.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau J., MacLeod A. R., Szyf M. Regulation of the DNA methyltransferase by the Ras-AP-1 signaling pathway. J Biol Chem. 1995 Jan 27;270(4):1595–1601. doi: 10.1074/jbc.270.4.1595. [DOI] [PubMed] [Google Scholar]
- Ryder K., Nathans D. Induction of protooncogene c-jun by serum growth factors. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8464–8467. doi: 10.1073/pnas.85.22.8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachsenmaier C., Radler-Pohl A., Zinck R., Nordheim A., Herrlich P., Rahmsdorf H. J. Involvement of growth factor receptors in the mammalian UVC response. Cell. 1994 Sep 23;78(6):963–972. doi: 10.1016/0092-8674(94)90272-0. [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., De Robertis E. M. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995 Jul 27;376(6538):333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Thies R. S., Yamaji N., Song J. J., Wozney J. M., Murakami K., Ueno N. A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10255–10259. doi: 10.1073/pnas.91.22.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai F. Y., Keller G., Kuo F. C., Weiss M., Chen J., Rosenblatt M., Alt F. W., Orkin S. H. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994 Sep 15;371(6494):221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- Walters M., Martin D. I. Functional erythroid promoters created by interaction of the transcription factor GATA-1 with CACCC and AP-1/NFE-2 elements. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10444–10448. doi: 10.1073/pnas.89.21.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M., Melton D. A. Involvement of p21ras in Xenopus mesoderm induction. Nature. 1992 May 21;357(6375):252–254. doi: 10.1038/357252a0. [DOI] [PubMed] [Google Scholar]
- Wilson P. A., Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995 Jul 27;376(6538):331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. Novel regulators of bone formation: molecular clones and activities. Science. 1988 Dec 16;242(4885):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Xu R. H., Kim J., Taira M., Zhan S., Sredni D., Kung H. F. A dominant negative bone morphogenetic protein 4 receptor causes neuralization in Xenopus ectoderm. Biochem Biophys Res Commun. 1995 Jul 6;212(1):212–219. doi: 10.1006/bbrc.1995.1958. [DOI] [PubMed] [Google Scholar]