Abstract
Immune and inflammatory responses actively modulate the pathophysiological processes of acute brain injuries such as stroke. Soon after the onset of stroke, signals such as brain-derived antigens, danger-associated molecular patterns (DAMPs), cytokines, and chemokines are released from the injured brain into the systemic circulation. The injured brain also communicates with peripheral organs through the parasympathetic and sympathetic branches of the autonomic nervous system. Many of these diverse signals not only activate resident immune cells in the brain, but also trigger robust immune responses in the periphery. Peripheral immune cells then migrate toward the site of injury and release additional cytokines, chemokines, and other molecules, causing further disruptive or protective effects in the ischemic brain. Bidirectional communication between the injured brain and the peripheral immune system is now known to regulate the progression of stroke pathology as well as tissue repair. In the end, this exquisitely coordinated crosstalk helps determine the fate of animals after stroke. This article reviews the literature on ischemic brain-derived signals through which peripheral immune responses are triggered, and the potential impact of these peripheral responses on brain injury and repair. Pharmacological strategies and cell-based therapies that target the dialogue between the brain and peripheral immune system show promise as potential novel treatments for stroke.
Keywords: cerebral ischemic injury, innate immunity, inflammation, brain repair
1. Introduction
A pivotal role for immune responses in the pathogenesis of ischemic stroke has emerged in recent years. Upon reduction of the blood supply, injured neurons and other cells within the central nervous system (CNS) release ATP and neuronal antigens, which elicit activation of the immune system. Other small molecules, such as cytokines, chemokines, and adhesion molecules also activate the immune system. In addition, the autonomic nervous system (ANS) releases neurotransmitters such as acetylcholine (ACh) and norepinephrine from the compromised brain. Once an injury occurs, the brain and the immune system influence each other in specific and profound ways. Bidirectional or reciprocal neuroimmune communication presumably evolved to clear the brain of infections and dead cellular debris. In addition, some of the activated immune cells and the trophic factors that they release stimulate neurovascular remodeling and neuroregeneration in the late-phase of ischemia (Hanisch and Kettenmann, 2007;Lalancette-Hebert et al., 2007). Thus, the immune system serves to protect the brain from the necrotic spillage of intracellular contents and promotes brain repair. However, when overactivated, the immune system can propel and propagate neuronal cell death. Therefore, revealing the paths of communication between the brain and the immune system after stroke promises to unveil effective immunomodulatory therapies that trigger beneficial neuroimmune responses and dampen harmful neuroimmune responses. In this review, we discuss the intricate molecular dialogues between the brain and the immune system after ischemic stroke. We discuss in detail how immune cells are activated and how immune responses contribute to neuroinflammatory damage and brain tissue repair.
1.1. Different modalities of the immunerespon ses
1.1.1. Innate versusadaptive immune responses
The innate and adaptive immune systems promptly respond to stress and contribute to the development of brain injury as well as tissue repair. As the first line of defense in the CNS, the innate immune system provides the earliest responses against acute brain injury, consisting of both physical and chemical barriers as well as innate immune cells (microglia/macrophages, neutrophils, and natural killer cells (NK cells)) and the complement system. Through pattern recognition receptors, such as the Toll-like receptors (TLRs) and Nod-like receptors (NLRs) (Chamorro et al., 2012), the innate immune system senses exogenous pathogens as well as endogenous injury. The sensing of injury markers leads to activation of downstream pathways, such as nuclear factor-κB and mitogen-activated protein kinase (MAPK) pathways, as well as production of reactive oxygen species (ROS), pro-inflammatory cytokines, and chemokines. Although the activation of the innate immune system may initiate a systemic response to brain injury, the activation of downstream pathways may compound the post-ischemic production of ROS and inflammation and inadvertently contribute to brain injury.
In contrast to the generalized and acute response elicited by the innate immune system, the targeted nature of the adaptive immune response occurs primarily via the generation of specific antibodies against specific brain-derived molecules and is largely coordinated by B and T lymphocytes and antigen-presenting cells. Although the adaptive immune response is delayed relative to the innate immune response, the specific nature of adaptive immune responses may serve to build a defense system in the brain by programming autoimmunity or tolerance, thereby resulting in less gross inflammation and limiting the potential exacerbation of ischemic pathology. In addition to these functions, emerging evidence indicates that adaptive immune responses may also underlie reparative efforts following ischemic recovery.
1.1.2. Intrinsic versus peripheral immune responses
Another factor in the immune response to brain injury lies in the origin of the immune responses. Traditionally, the focus of the immune response to brain injury has rested mainly on an intrinsic (i.e., localized) response derived from cells or structures housed within the brain itself. In this scenario, resident microglia and barrier defenses (e.g., the blood-brain barrier) are considered to be the major elements of an intrinsic immune response. However, given more recent findings, we now know that immune cells not normally found in cerebral tissue can also mount a robust response to brain injury. Thus, brain injury can trigger both central as well as peripheral immune responses. The peripheral cells that infiltrate the brain include macrophages, neutrophils, and lymphocytes. The mechanism of activation of the peripheral immune response is still unclear, but may occur either at the junction of the periphery with cerebral tissue or via circulating factors acting completely within the periphery. Both of these mechanisms may impact ischemic injury and recovery in the brain. However, recent findings also implicate peripheral processes in determining brain injury outcomes. Therefore, we will focus much of the present review on the novel role of these immune actions in cerebral ischemic injury.
2. How does the injured brain activate the immune system?
2.1. Brain-derived antigens
The brain has long been thought to be an immune privileged organdue to the presence of the tight blood-brain barrier (BBB), which effectively inhibits neuronal peptides from making contact with immune cells and eliciting autoimmune responses. However, this notion has now been challenged by a wide number of studies that indicate that, under many circumstances, the brain and the immune system may communicate in a bidirectional manner. For example, in some autoimmune diseases, such as multiple sclerosis, the immune system is exposed to myelin antigens (Dittel et al., 1999;de Vos et al., 2002;Mecha et al., 2013). These antigens are presented by dendritic cells (DCs) to antigen-specific T cells, resulting in adaptive immune responses and consequent demyelination of the CNS (Dittel et al., 1999). Although brain injuries are not autoimmune diseases, breakdown of the BBB is a common pathological change in these disorders, and allows antigens from the CNS to encounter and activate the peripheral immune system (Schroeter et al., 1994;Campanella et al., 2002;Jauch et al., 2006). The interactions between CNS antigens and immune cells may occur in both the injured brain and in the periphery, leading to specific immune responses.
Evidence for an adaptive immune response following stroke has been found in human patients. For example, patients with a history of stroke have increased titers of circulating antibodies to neurofilaments and to a portion of N-methyl-D-aspartic acid (NMDA) receptors (Bornstein et al., 2001;Dambinova et al., 2003). Using immunohistochemistry, neuron-derived antigens, such as microtubule-associated protein 2 (MAP2) and NMDA receptor subunit NR-2A, and myelin-derived antigens, such as myelin basic protein (MBP) and myelin oligodendrocyte glycoprotein (MOG), were all found in palatine tonsils and cervical lymph nodes of patients with acute stroke (Planas et al., 2012). These antigens are processed by antigen presenting cells (APCs), such as DCs and macrophages (Planas et al., 2012), and are able to induce auto-reactive T cell responses in T cell zones of lymph nodes (Tsuchida et al., 1994). Consistently, increased numbers of CD69+ T cells (indicative of T cell activation) are present in stroke patients compared to control patients (Planas et al., 2012). These studies suggest that brain-derived antigens may initiate the activation of the adaptive immune response in human populations.
2.2. Signaling via extracellular release of molecules: Danger associated molecular patterns (DAMPs)
DAMPs are a set of molecular determinants derived from cellular debris, intracellular proteins/enzymes or nuclear DNA/RNA that are released from injured cells following catastrophic events (Seong and Matzinger, 2004). In contrast to the pathogen-associated molecular patterns (PAMPs) that trigger infectious inflammatory responses, DAMPs initiate non-infectious immune responses toward injured tissues. There are a variety of DAMPs in different types of cells in injured tissues. Several DAMPs, including high-mobility group box 1 (HMGB1), ATP and S100 are known to be critical for the initiation of immune responses following CNS injuries.
2.2.1. ATP, the early signal that activates the immune system
Over the course of ischemic injury, neurons undergo rapid energy failure, leading to the loss of ionic gradients, consequent depolarization, depletion of intracellular ATP and outflow of ATP into the interstitial space (Juranyi et al., 1999;Latini and Pedata, 2001). In a model of permanent focal cerebral ischemia, outflow of ATP from the rat striatum increased significantly and remained high during the 220 minutes of ischemia (Melani et al., 2005). In addition to neuronal release of ATP, ATP can also be released from damaged vascular cells and from blood cells (Bune et al., 2010).
Elevated parenchymal levels of ATP act on purinergic receptors such as P2X7 or P2X4, which leads to activation of immune cells and the release of pro-inflammatory cytokines. Microglial P2X7 receptors are upregulated in response to cerebral ischemia, presumably in anticipation of the release of ATP from injured cells (Franke et al., 2004). Such receptor upregulation enhances microglial activation. P2X7 receptors also activate the inflammasome in neurons and astrocytes via pannexin-1 (Silverman et al., 2009). Inflammasomes are NLR- and caspase-1-containing cytoplasmic multiprotein complexes that, when activated, cause the processing and release of the cytokines interleukin (IL)-1β and IL-18. Accordingly, P2X7 receptor antagonists have been shown to protect against transient global cerebral ischemia reperfusion injury by reducing inflammatory responses (Chu et al., 2012), indicating that ATP outflow from injured neurons may contribute to ischemic injury through the immune system. A role for extracellular ATP in ischemic injury has been verified by the recent finding that systemic administration of ATP worsens stroke outcomes (Zhang et al., 2013).
Activation of the microglial P2X4 receptor occurs in models of CNS diseases that involve inflammatory responses, such as in spinal cord injury, cerebral ischemia, preterm hypoxia ischemia, and experimental autoimmune encephalomyelitis (EAE) (Wixey et al., 2009;Schwab et al., 2005;Tsuda et al., 2003;Li et al., 2011;Guo and Schluesener, 2005;Cavaliere et al., 2003;Ulmann et al., 2008). In a rat model of preterm hypoxia-ischemia, the expression of P2X4 receptors was significantly increased and was associated with an increase in ionized calcium binding adapter molecule 1 (Iba1) protein, which is indicative of microglial activation (Wixey et al., 2009). Administration of minocycline, a potent inhibitor of microglia, attenuated the upregulation of P2X4 receptors induced by hypoxia-ischemia (Wixey et al., 2009). In addition, increased expression of P2X4 was also observed in the hippocampus of gerbils subjected to bilateral common carotid occlusion (Cavaliere et al., 2003).
2.2.2. High-mobility group box 1 (HMGB1)
HMGB1 is a nuclear protein that binds to DNA and regulates gene transcription and is expressed widely in neurons and oligodendrocytes (Kim et al., 2008). HMGB1 also functions as an alarm signal in the activation of microglia/macrophages (Muhammad et al., 2008). In in vitro and in vivo models of ischemic stroke, HMGB1 was shown to be rapidly released from injured neurons (Kim et al., 2008;Kim et al., 2006), Extracellular HMGB1 can be recognized by several microglia/macrophage receptors, including TLRs and the receptor for advanced glycation endproducts (RAGE). Indeed, HMGB1-induced toxicity in neuron-glial co-cultures requires glial expression of RAGE(Muhammad et al., 2008), and chimeric mice generated by transplanting RAGE−/− bone marrow into wild-type mice further indicate that RAGE expression on immigrant macrophages mediates post-stroke cerebral inflammation and brain damage (Muhammad et al., 2008). Similar to RAGE, TLR4 expressed by infiltrating macrophages may be involved in the development of ischemic brain damage (Yang et al., 2011), suggesting that HMGB1 and its receptors link neuronal necrosis with microglia/macrophage activation. Thus, the interaction between HMGB1 and immune cells may be a rational target for the treatment of cerebral ischemia. Consistently, blocking HMGB1 by either shRNA or neutralizing antibodies has been shown to inhibit cerebral inflammation and provide protection against ischemic brain injury, revealing the importance of this protein in neuron-glia crosstalk and subsequent deleterious inflammation (Kim et al., 2006).
2.2.3. S100B
Another DAMP that has been postulated to be released following brain injury and activate the immune system is S100B. Mainly expressed in astrocytes in the CNS, S100B has been shown to be a surrogate marker for the severity of brain damage and to be predictive of stroke prognosis (Foerch et al., 2005). The effect of S100B in triggering post-ischemia immune responses has been shown in transgenic mice overexpressing human S100B. These mice exhibit increases in peri-infarct gliosis and brain infarct size after permanent middle cerebral artery occlusion (MCAO) (Mori et al., 2008). Similar to HMGB1, S100B binds to RAGE on microglia/macrophage. In non-ischemic models, S100B stimulates nitric oxide production from microglia in a manner dependent on RAGE extracellular domains (Adami et al., 2004). In addition, the engagement of S100B with microglial RAGE upregulates IL-1β and tumor necrosis factor (TNF)-α expression and concurrently stimulates NF-κB and AP-1 transcriptional activity (Bianchi et al., 2010). S100B-engaged RAGE also contributes to microglial migration and the upregulation of several chemokines (CCL3, CCL5, and CXCL12) and chemokine receptors (CCR1 and CCR5)(Bianchi et al., 2011).
In summary, a variety of DAMPs are released from neurons or other CNS cells immediately after brain ischemic injury. The early release of such DAMPs likely serve as alarmsto alert local and peripheral immune cells and initiate their activation.
2.3. Immune signaling molecules: Pivotal roles of cytokines, chemokines, and adhesion molecules
2.3.1. Cytokines after cerebral ischemia
Cytokines are exquisitely sensitive molecules that are rapidly produced and released by microglial cells in a context- and cell-dependent fashion. The production of cytokines is determined at least in part by extended microglial processes that interdigitate with cell bodies and processes of neurons and other glial cells. Recently, studies employing two-photon microscopy indicate that resting microglial cells move their processes slowly and continuously in order to detect changes in local homoeostasis (Nimmerjahn et al., 2005). Thus, microglia appear to act as sensors that detect signals from injured neurons and other cells in the CNS. Apart from this important role, even minor changes in neural electrical transmission, the composition of extracellular electrolytes, or the release of soluble factors such as purine nucleotides, are sufficient to trigger cytokine release from microglia (Gehrmann et al., 1993;Walz et al., 1993).
Microglia can play two different roles depending on their polarization (as discussed in detail in section 3.1.1.) (Hu et al., 2012;Frieler et al., 2011). The M1 population of microglia sends out pro-inflammatory cytokines, such as IL-1β, IL-1α, IL-6, and TNF-α. IL-1 is the predominant pro-inflammatory cytokine in the CNS. Microglia secrete pro-IL-1β, which is biologically inactive, in response to injury. In order to exert pro-inflammatory effects, pro-IL-1β must be cleaved by caspase-related enzymes (Black et al., 1988) or by matrix metalloproteinases (MMPs) such as MMP-2 or MMP-9 (Schonbeck et al., 1998). Earlier studies indicated that the mRNA of IL-1β, but not IL-1α, was strongly induced after 30 min MCAO (Boutin et al., 2001). Within 16 to 24 hours after stroke, amoeboid microglia express IL-1β within the ischemic regions of the cortex (Mabuchi et al., 2000). Interestingly, more recent evidence argues that IL-1α, but not IL-1β, is upregulated soon after cerebral ischemia and might be the major form of IL-1 that contributes to post-stroke inflammation (Luheshi et al., 2011). Although single deletion of either IL-1α or IL-1β in knockout models failed to influence brain damage following stroke, double deletion knockout mice lacking both forms of IL-1 exhibited dramatically reduced infarct volume. These studies suggest that the IL-1 system plays an important role in ischemic brain injury and that the two IL-1 isoforms may compensate for each other’s absence in single deletion models (Boutin et al., 2001).
Besides its role as a pro-inflammatory messenger, IL-1β can also induce the expression of other pro-inflammatory mediators in microglial cells, such as IL-6 and TNF-α (Lee et al., 1993). Those two cytokines can also be secreted by active microglia into the circulation. In the presence of elevated levels of IL-1, IL-6 and TNF-α, peripheral immune cells are stimulated, and subsequently, the inflammatory cascade is triggered. Increased concentrations of IL-1β and TNF-α have been detected in peripheral blood in stroke patients (Sergeeva et al., 2011). Furthermore, plasma IL-6 levels in stroke patients were associated with larger infarct size, more severe neurological deficits, and increased risk of death or poor functional outcome (Shenhar-Tsarfaty et al., 2010;Dziedzic, 2008;Whiteley et al., 2009). Although the cellular sources of these cytokines in the blood have not yet been determined, it is possible that at least part of the increased levels of cytokines in the circulation were derived from active microglia.
2.3.2. Chemokines, the small signaling messengers from the injured brain
Chemokines are a group of small chemotactic cytokines capable of recruiting immune cells to the site of inflammation. Four subfamilies of chemokines have been classified based on the first two cysteines at the N-terminal end (CC, CXC, XC and CX3C) (Reaux-Le et al., 2013). The chemokines are also defined as “homeostatic” or “inflammatory” according to their function: homeostatic chemokines are involved in the control of cell migration during tissue maintenance or development, whereas inflammatory chemokines convey pro-inflammatory signals and induce leukocyte recruitment to injured tissues. These dual roles of chemokines are important to consider when considering stroke pathology (inflammatory) and recovery (tissue repair).
In response to cerebral ischemic injury, damaged CNS cells secrete a variety of chemokines, such as macrophage inflammatory protein-1α (MIP-1α or CCL3), monocyte chemotactic protein-1 (MCP-1 or CCL2) (Yamagami et al., 1999;Losy and Zaremba, 2001), CCL5, or RANTES (Regulated upon Activation, Normal T-cell Expressed, and Secreted) (Zaremba et al., 2006). The cellular sources of specific chemokines may vary in different animal models. For example, in adult stroke models, astrocytes were the predominant source of CCL2, while in neonatal hypoxia/ischemia (HI) models, injured neurons were the major source of CCL2 (Ivacko et al., 1997). CCL3 was also markedly increased after HI in the neonatal cerebral hemisphere (Cowell et al., 2002). In addition, T helper cell type 1 (Th1)-derived CCL5 was deposited on the cerebral vasculature and was associated with the recruitment of leukocytes in chronic systemic infections that exacerbate stroke in mice (Denes et al., 2010).
In cerebral ischemic injury, the CCL2/CCR2 interaction is believed to result in the recruitment of monocytes and neutrophils (Dimitrijevic et al., 2007). Increased levels of CCL2 have been found in both the cerebrospinal fluid and serum in the early hours after ischemic stroke (Losy and Zaremba, 2001;Zaremba et al., 2006). In animal models, CCL2 mRNA expression increased in the ischemic cortex at 6 hours after MCAO, peaked at 2 days, and decreased 5 days after cerebral ischemia (Wang et al., 1995;Yamagami et al., 1999). This temporal profile supports the contention that CCL2 is a potent mediator of macrophage infiltration in cerebral ischemic injury. Not surprisingly, CCL2-deficient mice had smaller infarcts and decreased infiltration of inflammatory cells (Hughes et al., 2002;Schilling et al., 2009). Furthermore, CCL2 overexpression was associated with an enlarged ischemic territory (Chen et al., 2003).
Following cerebral ischemic injury, CCL3 also acts as a pro-inflammatory chemokine by binding to CCR1 and CCR5 (Cheng et al., 2001;Ottonello et al., 2005). CCL3 upregulation has been observed in both the neonatal HI model and the adult model of cerebral ischemia (Cowell et al., 2002;Takami et al., 1997). Intracerebroventricular injection of CCL3 increased infarct volume following transient focal ischemia in rats, while intracerebroventricular injection of viral macrophage inflammatory protein (vMIP)-II, a broad-spectrum chemokine antagonist, reduced infarct volume in a dose-dependent manner (Takami et al., 2001;Takami et al., 1997). As a classical monocyte chemoattractant, CCL3 is associated with monocyte accumulation and microglial activation in injured brain tissue (Kim et al., 1995b). Additionally, CCL3 has also been recently shown to be a potent neutrophil chemoattractant in mice and humans (Reichel et al., 2009). Further studies are necessary to identify the means by which CCL3 exacerbates cerebral ischemic injury.
CCL5 is a potent pro-inflammatory chemokine involved in cerebral ischemic injury. It selectively binds to three different receptors, CCR1, CCR3, and CCR5. The activity of CCL5 is associated with leukocyte infiltration in brain injury after stroke. Compared to wild-type mice, CCL5 knockout mice exhibit significantly reduced infarct volumes and improved BBB function following focal cerebral ischemia (Terao et al., 2008). Similarly, transplantation of bone marrow from CCL5 knockout mice into wild type mice reduced brain injury after cerebral ischemia (Terao et al., 2008). CCL5-mediated pro-inflammatory effects are associated with leukocyte recruitment and platelet adhesion to the cerebral microvasculature (Terao et al., 2008). The number of leukocytes in the periphery increases during systemic inflammation, and it has been suggested that the release of CCL5 from blood leukocytes enhances MMP-9 activity, causing further breakdown of the BBB and exacerbating brain injury (Zaremba et al., 2006;Verslegers et al., 2013).
The functions of CX3CL1 in stroke have been investigated using CX3CR1-deficient mice. CX3CR1 knockout mice display reduced neuroinflammation and less neuronal damage after focal cerebral ischemia (Denes, Ferenczi et al. 2008). These results suggest that CX3CL1 propels inflammation and worsens stroke outcomes.
Cerebral ischemia results in a prompt and long-lasting elevation of CXCL12 in the ischemic penumbra, particularly in association with reactive astrocytes in perivascular areas (Hill et al., 2004). Interestingly, transplanted GFP+ bone marrow cells were recruited in proximity to these CXCL12+ vessels and displayed characteristics of activated microglial cells. These results suggest that CXCL12 is important in the homing of bone marrow-derived monocytes, which transform into microglia in the areas of ischemic injury. An in vitro study with microglial cultures suggests that exposure to hypoxia significantly enhances CXCR4 expression on microglia in a hypoxia inducible factor-1α (HIF-1α)-dependent manner, resulting in the accelerated migration toward CXCL12 (Wang et al., 2008b). This accumulation of monocyte-derived and local microglia may contribute to severe neuroinflammation after brain injury.
Other chemokines have also been implicated in cerebral ischemic brain injury. For example, CXCL8 is thought to be an important chemoattractant for neutrophils after ischemic stroke (Domac and Misirli, 2008). Inhibition of CXCL8 receptor with reparixin reduced cerebral inflammationand improved neurological deficits in stroke rats(Villa et al., 2007).
2.3.3. Endothelial adhesion molecules
In an environment with high pro-inflammatory cytokine levels, cerebral endothelial cells increase their expression of cell surface adhesion molecules that mediate the recruitment of leukocytes and platelets to the ischemic region. Several lines of evidence indicate that the expression of intercellular adhesion molecule-1 (ICAM-1) (Stanimirovic et al., 1997;Yang et al., 1999;Yilmaz and Granger, 2010), P-selectin, E-selectin (Zhang et al., 1998;Huang et al., 2000), and integrins (Kim et al., 1995a;Fiszer et al., 1998) on endothelial cells can promote the adhesion and trans-endothelial migration of leukocytes (Okada et al., 1994;Lindsberg et al., 1996), thereby contributing to neuroinflammation-mediated neuronal cell death. In the ischemic brain, the expression of specific adhesion glycoproteins on cerebral microvascular endothelial cells was significantly increased following an ischemic insult. Increased expression of P-selectin was detected as early as 15 minutes following cerebral ischemia and this increase persisted for at least 24 hours. The prolonged upregulation of P-selectin occurs on cerebral endothelial cells or on platelets expressing P-selectin which then bind to endothelial cells (Ishikawa et al., 2003).
In addition to P-selectin, increased mRNA expression of E-selectin and ICAM-1 in the ischemic brain was noted as early as after 1–2 hours of ischemia and remained elevated for several hours (Okada et al., 1994;Zhang et al., 1998). Compared to wild-type mice, ICAM-1 deficient mice exhibited smaller infarct volumes and less neutrophil infiltration after cerebral ischemia (Connolly, Jr. et al., 1996;Soriano et al., 1996). As expected, immuno-neutralization or genetic depletion of cell adhesion molecules that mediate leukocyte recruitment reduced tissue injury and brain dysfunction in animal models of focal cerebral ischemia (Okada et al., 1994;Zhang et al., 1998).
Despite the prevailing evidence from animal studies indicating that the expression of adhesion molecules on endothelial cells plays a pivotal role in the development of neuroinflammation in the ischemic brain, preclinical studies of anti-ICAM therapy with Enlimomab failed to improve outcome in stroke patients (Enlimomab Acute Stroke Trial Investigators, 2001). Nevertheless, the disappointing results from the clinical trials of anti-ICAM therapy do not indicate that animal studies of adhesion molecules have no translational value. Because soluble circulating levels of E-selectin, ICAM-1, and vascular cell adhesion molecule (VCAM) are all elevated in the plasma of patients suffering an acute ischemic stroke (Frijns et al., 1997), and are shed from the surface of activated endothelial cells, ICAM expression may serve as a useful surrogate marker in acute and chronic inflammatory states.
2.4. Signals from the autonomic nervous system (ANS)
Although stress is highly associated with brain injuries and appears to affect clinical outcomes, the involvement of the ANS following stroke has received relatively little attention. Recent studies reveal fundamental immunological changes in response to stroke-induced activation of the ANS. Considering the rapid nature of action potentials, signal transduction in the ANS has been suggested to be among the first means of communication between the brain and the peripheral immune system after stroke. The ANS is comprised of the parasympathetic nervous system (PSNS) and sympathetic nervous system (SNS), both of which mediate immune responses in the periphery (Borovikova et al., 2000;Prass et al., 2003).
2.4.1. The immunological influence of the vagus nerve
The vagus nerve, also known as cranial nerve 10 of the PSNS, derives its name from the Latin term “wandering” because of its extensive and widespread innervation of the viscera. Stimulation of the vagus nerve significantly inhibits the release of TNF-α from macrophages during systemic inflammatory responses to endotoxin (Borovikova et al., 2000), with the nicotinic acetylcholine receptor (nACh) α7 subunit acting as an essential regulator of inflammation (Wang et al., 2003). This phenomenon has been termed the brain-to-immune mechanism, the cholinergic anti-inflammatory pathway, or the inflammatory reflex.
Interestingly, several immune cells in the spleen, such as macrophages, neutrophils, and T cells are influenced, or functionally innervated, by the vagus nerve (Borovikova et al., 2000;Huston et al., 2009;Rosas-Ballina et al., 2011). Indeed, macrophages were the first type of immune cells shown to be controlled by the vagus nerve. Numerous studies have confirmed that the release of TNF-α from macrophages can be significantly inhibited by stimulation of the vagus (Borovikova et al., 2000;Bernik et al., 2002;Guarini et al., 2003;Pavlov et al., 2007). The vagus can also modulate excessive accumulation of neutrophils at inflammatory sites. Furthermore, vagal activity attenuates the levels of CD11b on the surfaces of neutrophils in the intact and innervated spleen, which subsequently prevents tissue injury during inflammation (Saeed et al., 2005).
Nerve fibers in the spleen that originate in the celiac ganglion are adrenergic instead of cholinergic, and lack the enzymatic machinery necessary for the production of ACh. However, splenic nerve fibers affect vagus nerve signaling, raising questions on how the neural circuit influences cholinergic responses. Populations of ACh-producing memory phenotype T cells have been identified as integral to the inflammatory reflex (Rosas-Ballina et al., 2011). These memory phenotype T cells were able to produce ACh by increased norepinephrine in sympathetic nerve fibers in the spleen. This effect, in turn, inhibited the production of cytokines in macrophages (Rosas-Ballina et al., 2011). These studies shed light on the inhibition of the immune system by the vegetative, or parasympathetic arm of the ANS. Because the immune system can exacerbate ischemic injury, stimulation of the vagus nerve might therefore be predicted to elicit neuroprotection, as described below.
Recently, stimulation of the vagus nerve has been found to provide neuroprotection against acute cerebral ischemic injury in rats (Ay et al., 2011). However, the underlying mechanisms remain to be determined. Given the fundamental role of TNF-α in cerebral ischemic brain injuries, the dampened secretion of TNF-α following vagal stimulation might be explored as a potential factor contributing to vagus-induced neuroprotection. In addition, stimulation of the vagus nerve significantly increases serum ghrelin levels following traumatic brain injury (TBI), suggesting that ghrelin may play an important role in the anti-inflammatory effects of vagus nerve stimulation (Bansal et al., 2012).
2.4.2. Signaling via the sympathetic nervous system and immune responses
The SNS is a branch of the ANS that mobilizes the fight-or-flight response. Stress triggered by cerebral ischemic brain injury immediately activates the SNS, leading to splenic contraction, spleen shrinkage (Ajmo, Jr. et al., 2009), and dysfunction of invariant NKT cells (iNKT) (Wong et al., 2011), all of which eventually lead to profound immune suppression.
Sympathetic innervation accounts for 98% of the nerve fibers innervating the spleen (Klein et al., 1982), and the activation of smooth muscle cells by the α1 receptor agonist phenylephrine yields dose-dependent contractions of the spleen (Hardy et al., 1994). When ischemia is induced in the brain, the SNS is immediately collared into action as a fast-acting stress response, leading to subsequent activation of α1 adrenergic receptors on smooth muscle cells and splenic contraction. Blockade of the α1 receptor with the antagonist prazosin inhibits spleen shrinkage (Ajmo, Jr. et al., 2009), and blockade with the pan adrenergic receptor carvedilol reduces infarct volume (Ajmo, Jr. et al., 2009). Splenectomy prior to MCAO or neonatal hypoxia-ischemia reduces brain infarction (Fathali et al., 2013), and is accompanied by a significant reduction in the number of infiltrating neutrophils and activated microglia in the injured brain (Ajmo, Jr. et al., 2008). Post-stroke splenic irradiation has also been shown to significantly reduce brain infarction in rats (Ostrowski et al., 2012). These three lines of evidence indicate that the sympathetic innervation of the spleen contributes to the mobilization of spleen-derived immune cells and their infiltration into the ischemic brain. Thus, in contrast to activation of the PSNS, activation of the SNS initially mobilizes the immune response and propels neuronal injury in MCAO, thereby contributing to ischemic injury. On the other hand, emerging evidence suggests that activation of the SNS may also suppress the immune system via hepatic functions.
Recently, Wong et al. reported that noradrenergic innervation of the liver signals through hepatic iNKT cells to promote systemic immune suppression after MCAO (Wong et al., 2011). Either blockade of β-adrenergic receptors with propranolol or depletion of noradrenergic nerve terminals in the liver attenuated immune suppression and bacterial infection by increasing interferon-γ (IFN-γ) secretion from iNKT cells (Wong et al., 2011). Conversely, injection of noradrenaline into the liver increased immune suppression by inhibiting the normal functions of iNKT cells (Wong et al., 2011). This study is consistent with the long-held belief that stress strongly suppresses the immune system via activation of the SNS (Webster Marketon and Glaser, 2008) and defines the neuroimmune circuitry involved in brain injuries. The discrepant actions of the SNS on immune activation versus suppression defies simplistic generalizations and may reflect the specific endpoint measured, the target organ, and the differential activation of the immune system depending upon the stage of brain injury.
Of the various influences that contribute to the seemingly opposite effects of the ANS on immune function, the stage of brain injury may play a defining role. In the earlier stages of stroke, the mobilization of immune cells by splenic contractions triggers a pro-inflammatory state and initiates an acute inflammatory response in the ischemic brain. In the meantime, parasympathetic tone, which can dampen the production of pro-inflammatory cytokines, is reduced. Consequently, the autonomic balance shifts towards increased adrenergic tone at the adrenals and enhanced noradrenergic, pro-inflammatory influences in the spleen. In later stages of stroke, the supply of spleen-derived immune cells is exhausted, spleen size is dramatically reduced, and post-stroke immunodepression develops (Dirnagl et al., 2007). Furthermore, activation of β2 receptors, the primary adrenoreceptor expressed on innate and adaptive immune cells (Kin and Sanders, 2006), is thought to suppress immune function. In support of this notion, the β receptor antagonist propranolol blocks post-stroke suppression of the immune system (Prass et al., 2006), and is associated with a dramatic reduction in mortality from infections (Prass et al., 2003). Blockade of α and β adrenergic receptors significantly inhibits the reduction in spleen size and reduces infarct volume after MCAO (Ajmo, Jr. et al., 2009). In addition, carvedilol, an α1 and β adrenergic receptor antagonist marketed for the treatment of congestive heart failure and hypertension, significantly reduces ischemic brain injury and prevents the reduction in spleen size (Ajmo, Jr. et al., 2009). In summary, these results illustrate the profound influence of parasympathetic and sympathetic tone on the activation of immune cells and the consequent progression of central ischemic injury (Figure 1). Manipulating the various stages of immune activation or suppression may therefore be a novel therapeutic treatment for stroke victims.
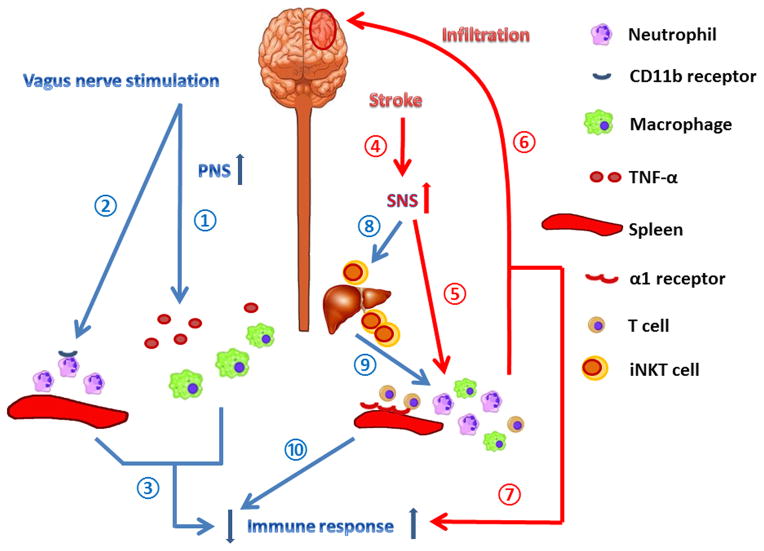
Figure 1. Immune responses mediated by the autonomic nervous system after stroke.
Stimulation of the vagus nerve provides neuroprotection against acute cerebral ischemic injury, by inhibiting the release of TNF-α from macrophages ①, reducing CD11b levels on the surface of neutrophils ②, and subsequently down-regulating the peripheral immune response ③ and preventing tissue injury during inflammation. Stroke immediately elicits a fast-acting stress response ④, which activates α1 adrenergic receptors on smooth muscle cells in the spleen ⑤, leading to splenic contraction. The contraction of the spleen results in the mobilization of immune cells that infiltrate into the ischemic brain ⑥, further exacerbating ischemic brain injury and over-activating the peripheral immune response ⑦. Stroke-induced activation of the sympathetic nervous system (SNS) inhibits the normal functions of iNKT cells ⑧, which regulate T cell activation ⑨. The malfunction of iNKT cells thus contributes to immunosuppression after stroke ⑩. The discrepant actions of the SNS on immune activation versus suppression defies simplistic generalizations and may reflect the specific endpoint measured, the target organ, and the differential activation of the immune system depending upon the stage of brain injury.
In addition to the molecules and signals mentioned above, there are other potential immune triggers that are responsible for crosstalk between the CNS and the immune system. For example, the shear stress associated with thrombosis is known to activate platelets to release microparticles (Nomura et al., 2001). These platelet-derived microparticles contain a variety of inflammatory mediators that are able to activate the immune system. In addition, free radicals have also been shown to be important in triggering post-stroke inflammation (Wang et al., 2008a). Understanding the molecules that trigger the immune system after stroke and elucidating their downstream signaling pathways may lead to early interventions to slow or halt the progressionof ischemic brain injuries.
3. The impact of immune cells on stroke pathogenesis
As summarized above, the brain and the immune system have various modalities to communicate and elicit responses to cerebral injury. Recently, the immune system has been shown to exert a profound influence on the brain in stroke and deeply affect outcome, including the progression of ischemic injuries and tissue repair during recovery. In this section, we discuss how the innate and adaptive immune responses affect the pathogenesis of stroke and describe the roles of different types of immune cells in ischemic brain injury.
3.1. Innate immune responses after stroke
Inflammation and activation of resident microglial cells have long been observed in the post-ischemic brain, as has the breakdown of physical immunological barriers, such as the BBB. These innate immune responses may exacerbate ischemic brain damage, and are often associated with TLR signaling (see below). Mice lacking TLR2 or TLR4 exhibit increases in infarct size (Lehnardt et al., 2007;Caso et al., 2007), supporting the concept that innate immune signaling via TLRs contributes to ischemic brain injury. On the other hand, emerging evidence suggests that innate immune cells may also be protective by participating in post-stroke recovery. The regulatory functions of various innate immune cells in the pathogenesis of stroke are reviewed in the following sections.
3.1.1. Microglia/macrophages
Resident microglia are the major inflammatory cells in the CNS, and their activation is the first step of the inflammatory response after acute brain injuries. For example, microglia are rapidly activated within minutes of ischemia, peaking 48–72 hours later. The microglial response can last for several weeks (Denes et al., 2007;Lalancette-Hebert et al., 2007). In contrast, the recruitment of blood-derived leukocytes to the injured brain tissue occurs over a period of hours to days (Schilling et al., 2003).
Microglia are normally ramified in appearance, but become activated and assume an amoeboid morphology in response to an insult. The expression of microglia surface markers, such as the major histocompatibility complex (MHC)-II, Iba-1, CD11, is also upregulated in the injured brain. Microglia are virtually indistinguishable from infiltrating macrophages/monocytes both in terms of morphology and surface marker expression, reflecting their common heritage (Schilling et al., 2003).
To distinguish the roles and tissue distributions of microglia and peripheral macrophages in ischemic brain injury, chimeric bone marrow mice have been generated by transplanting green fluorescent protein (GFP) transgenic bone marrow into irradiated wild-type recipients (Tanaka et al., 2003;Schilling et al., 2003;Schilling et al., 2005;Schilling et al., 2009). By exposing these chimeric mice to transient MCAO, it has been shown that the activation of resident microglia precedes the infiltration of blood-derived GFP+ macrophages (Schilling et al., 2003;Schilling et al., 2005). This finding is consistent with the notion that innate immune responses, and intrinsic cells in particular, mediate the acute phase following brain injury. In contrast to early microglial activation, peripheral macrophages were most abundant in ischemic brain tissue 3–7 days after transient focal cerebral ischemia, and decreased thereafter (Schilling et al., 2003;Breckwoldt et al., 2008). At 7 days after transient ischemia, multiple process-bearing ramified blood-derived macrophages are located in the peri-infarct area, with phagocytic cells detected in the core area of the infarction (Tanaka et al., 2003). The majority of phagocytes in the area of the infarct are derived from local microglia (Schilling et al., 2005). Taken together, these findings suggest that the activation of local microglia precedes and predominates over the infiltration of peripheral monocyte/macrophage during the first few days after cerebral ischemic injury. In contrast, blood-derived macrophages contribute to the delayed post-ischemic inflammation and brain injury.
Despite the rapidity of their response, the activation of microglia is not a uniform event, as the associated changes in morphology and gene expression vary enormously with the nature, strength, and duration of the stimulus (Ransohoff and Perry, 2009). In particular, in vitro stimulation with lipopolysaccharide (LPS) and IFN-γ promoted the differentiation of “classically activated” M1 microglia/macrophages that typically release destructive pro-inflammatory mediators, such as TNF-α, IL-1β, IL-6, and MMP-9 (Ding et al., 1988;del Zoppo et al., 2007). These events are associated in vivo with the early disruption of the BBB and facilitation of blood-derived leukocyte infiltration (del Zoppo et al., 2007;Pun et al., 2009). In contrast, conditioned medium obtained from macrophages differentiated into M2 phenotypes (by IL-4 or IL-10 treatment) improved axonal growth in cultured cortical neurons and DRG cells (Goerdt et al., 1999;Durafourt et al., 2012;Kigerl et al., 2009). The dualistic roles of distinctly polarized microglial populations have been reported in several CNS injuries, including ischemic stroke (Hu et al., 2012). Microglia assume the M2 phenotype during the early stages of ischemic stroke but gradually transform into the M1 phenotype at the site of injury. In vitro studies have demonstrated that ischemic neurons prime the polarization of microglia towards M1. This is thought to potentiate the injury as M1-polarized microglia exacerbated oxygen glucose deprivation (OGD)-induced neuronal loss. In contrast, maintaining microglia in the M2 phenotype protected neurons against OGD (Hu et al., 2012).
These findings suggest that stroke therapies should focus on adjusting the balance between beneficial and detrimental microglia/macrophage responses rather than simply suppressing them. The Janus-faced nature of microglial responses reflect the well-known shift between the short-term protective effects of early immune activation and the long-term detrimental effects of chronic immune overactivation. Furthermore, the time-dependent polarity mimics the traditional view of stress, as described by Hans Selye in the early half of the 20th century, that acute stress elicits resistance, whereas chronic stress weakens defenses (Selye, 1998). This closely intertwined relationship between degree of stress and the immune system is also reflected in other aspects of immunological responses to brain injury.
It is important to note that mononuclear phagocytes exhibit extensive heterogeneity and plasticity in response to different physiologic and pathologic stimuli. Indeed, there are likely to be additional activation states besides M1 and M2, as well as transitional stages between the two. Resident microglia/macrophages that are present during stimulation therefore do not only comprise 1 or 2 discrete subpopulations but probably exhibit a spectrum of diverse activation states (Mosser and Edwards, 2008). Thus, it should be noted that the concept of M1 and M2 microglia/macrophage polarization is a simplified framework that only represents two extremes along a continuum. This framework is nevertheless useful to partially describe the different functional states of phagocytes during injury progression and to explore therapeutic strategies targeting microglia/macrophage responses.
3.1.2. Neutrophil infiltration
Neutrophil infiltration from the bone marrow to sites of injury is a generalized response characteristic of the innate immune system. The infiltration of neutrophils into injured areas of the ischemic brain has been demonstrated not only in animal models (Tanaka et al., 2003;Breckwoldt et al., 2008) but also in stroke patients (Price et al., 2004). The accumulation of neutrophils in the injured parenchyma correlates with infarct expansion (Price et al., 2004). Higher counts of peripheral neutrophils are associated with poorer stroke outcomes (Kim et al., 2012). In rodent models of transient focal ischemia, depletion of neutrophils by treatment with anti-neutrophil monoclonal antibodies (RP3) significantly reduced the size of infarct as well as the formation of brain edema (Shiga et al., 1991;Matsuo et al., 1994). Together, these studies indicate that neutrophil infiltration may contribute to post-ischemic brain injury.
Neutrophils are among the first to infiltrate the injured brain (Jin et al., 2010). Neutrophil extravasation into damaged tissues in the periphery is usually followed by monocytes/macrophages – the second responders to inflammatory stimuli. In contrast to monocytes, which are most abundant in injured parenchyma 3–7 days after ischemia, neutrophils are found at the injury site within a few hours and their concentration peaks 1–3 days after cerebral ischemia in animal models of stroke (Jin et al., 2010). In patients with stroke, neutrophils are recruited within 24 hours of symptom onset (Price et al., 2004). Cellular adhesion molecules, including ICAM-1 and P-selectin, appear to be involved in the trans-endothelial migration of neutrophils into the brain, similar to their role in peripheral tissues. Both ICAM-1-deficient and P-selectin-deficient mice exhibit less infiltration of neutrophils as well as smaller infarct volumes following acute stroke compared with wild-type mice (Connolly, Jr. et al., 1996;Connolly, Jr. et al., 1997;Soriano et al., 1996).
Infiltrating neutrophils, as well as microglia and macrophages, are able to release toxic amounts of nitric oxide (NO) via the inducible nitric oxide synthase (iNOS) isoform. The release of this free radical gas may contribute to post-ischemic brain damage (Amantea et al., 2009) by multiple mechanisms, including the activation of MMP-9 (Gu et al., 2002). Indeed, neutrophils greatly contribute to elevations in MMP-9 following cerebral ischemia (Justicia et al., 2003), which, in turn, causes the breakdown of the BBB, promotes hematogenous leukocyte infiltration, and ultimately results in neuronal injury (Ajmo, Jr. et al., 2009). Based on these findings, suppression of neutrophils or neutrophil infiltration might be reasonable peripheral targets for stroke treatment in the clinic.
3.1.3. Dendritic cells
DCs are phagocytic cells that quickly respond to injury in a generalized fashion as part of the innate immune system. However, DCs also function as powerful antigen presenting cells, thereby linking the innate and adaptive immune responses. There are two lineages of DCs: myeloid DCs (mDCs), which respond to bacterial and fungal infections and release IL-12, and plasmacytoid DCs (pDCs), which release IFN-α upon viral infection (Liu, 2001). The precursor cells of both lineages can be detected in blood, where these immature DCs patrol the circulation and invade tissues in response to a local infection. Upon migration into tissue, DCs pick up antigens and acquire the ability to stimulate T cells, subsequently inducing adaptive immune responses against antigens not previously encountered (Banchereau et al., 2000), such as the cerebral antigens sequestered in the CNS under normal conditions.
A rapid increase in the numbers of DCs in ischemic brains was first observed in a rat model of permanent focal cerebral ischemia (Kostulas et al., 2002). A detailed study of the temporal dynamics of immune cell accumulation in the mouse brain after transient focal ischemia revealed a significant increase in DCs in the ipsilateral brain at 1 day after cerebral ischemia/reperfusion (Gelderblom et al., 2009). This was followed by a 20-fold increase on day 3 and a 12-fold increase on day 7. In order to differentiate DC invasion from a population of DCs that reside in the healthy brain, radiation chimeras (wild-type hosts restored with CD11c/EYFP transgenic bone marrow or CD11c/EYFP transgenic hosts restored with wild-type bone marrow) were used to demonstrate that the infiltration of DCs into the ischemic hemisphere started 1 day after transient focal ischemia (Felger et al., 2010). However, these studies also indicated a role for the original brain DCs in post-ischemic neuroinflammation. A significant decrease in circulating DC precursors (Yilmaz and Granger, 2010) after human or experimental stroke has been reported, likely reflecting the reduced co-stimulatory efficacy of circulating cells (Hug et al., 2011). These results suggest that peripheral DCs are recruited from the blood into the ischemic brain, possibly acting either to trigger post-ischemic cerebral immune reactions or inducing systemic immunosuppression. It is also noteworthy that DCs might be a part of the mononuclear phagocyte system and that CD11c is not only expressed on DCs but also on macrophages (Hume, 2008). Therefore, it is possible that many studies on DCs that were based on CD11c expression are examining CD11c+ microglia/macrophages instead. As the role of DCs (both peripheral DCs and those originally from brain) as mediators of inflammation in stroke has not been sufficiently investigated, they represent an intriguing new area of stroke research.
3.2. Adaptive immune responses after stroke
Following an acute stroke, the adaptive immune system is activated by brain-derived antigens that are released from the sites of injury. B cells and T cells recognize the antigens, proliferate, and then differentiate into effector cells. Effector B cells generate specific antibodies against certain brain-derived molecules, while effector T cells are actively involved in autoimmune or tolerance reactions. The field of exploration of the adaptive immune system in stroke models is fairly new. Nevertheless, we know some of the functions of specific adaptive immune cells in stroke pathogenesis. These functions are reviewed in detail in the following sections.
3.2.1. T lymphocytes
T lymphocytes play a central role in cell-mediated adaptive immunity. Accumulating evidence supports the involvement of T lymphocytes in post-ischemic neuroinflammation and brain damage. Several earlier studies indicated that the infiltration of T lymphocytes into ischemic brain occurs relatively late (3–4 days post-ischemia), following CD11b+ microglia/macrophage and Ly6G+ neutrophil infiltration (Campanella et al., 2002;Stevens et al., 2002). However, there is now mounting evidence from rodent models demonstrating that T cell accumulation in the post-ischemic brain actually begins within the first 24 hours of reperfusion (Jander et al., 1995;Yilmaz et al., 2006;Brait et al., 2010). Despite the discrepancies in temporal kinetics, the post-ischemic infiltration of T lymphocytes suggests that they help determine stroke outcomes.
Until recently, determination of the role of T cells in brain ischemia was hampered by the limitations in technical methods. Earlier studies relied on an anti-α4-integrin antibody to block T lymphocyte recruitment into the post-ischemic brain, and reported that infarct volume after transient cerebral ischemia was reduced by the antibody treatment (Becker et al., 2001;Relton et al., 2001). In addition, mice lacking RANTES (also called CCL5), which plays a critical role in recruiting and activating T lymphocytes, also exhibited smaller infarct volumes (Appay and Rowland-Jones, 2001). More recently, the development of several lymphocyte-deficient mouse strains has allowed for superior assessments of the contribution of T lymphocytes to ischemic injury. Severe combined immunodeficiency (SCID) mice (Hurn et al., 2007) or Rag1−/− mice lacking mature B and T lymphocytes (Yilmaz et al., 2006;Kleinschnitz et al., 2010) all exhibit smaller infarct volumes and improved functional outcomes after transient cerebral ischemia. Moreover, this improvement in outcomes following ischemia was almost completely abolished when wild-type CD3+ T lymphocytes were transplanted into Rag1−/− mice (Yilmaz et al., 2006;Kleinschnitz et al., 2010). These findings confirm that circulating T lymphocytes play a detrimental role in ischemia/reperfusion injury. However, the mechanism by which T cells exert such unfavorable effects remains unclear. Surprisingly, a recent study has found that the contribution of T cells to ischemic injury is independent of both antigen recognition and TCR co-stimulation in the early stages of ischemic stroke (Kleinschnitz et al., 2010). This observation suggests that mechanisms other than classic adaptive immune pathways may be involved in T cell-mediated early ischemia/reperfusion injury.
In addition to characterizing T cell involvement in brain injury, recent studies have focused on the roles of specific T cell subtypes in brain injuries, yielding unexpected results. Notably, not all T-cell subtypes appear to exert detrimental effects after ischemic stroke. Subtypes of T-cells are characterized by the unique expression profiles of specific cell-membrane proteins. including CD3+ T lymphocytes, which encompass both CD8+ cytotoxic T cells and CD4+ helper T cells in approximately equal proportions (Seder and Ahmed, 2003). Regulatory T cells (Tregs) are identified by the expression of CD25 and the transcription factor Fox P3. Based on these T-cell subclassifications, we will discuss the differential effects of the individual T cell subtypes on the development of brain injuries.
CD8+ cytotoxic T cells and CD4+ helper T cells
As their name suggests, CD8+ cytotoxic T cells are capable of directly inducing the death of pathogen-infected somatic cells or tumor cells either through the release of cytotoxins, perforin, and granzymes, or via cell-surface interactions, such as the Fas-FasL pathway (Russell and Ley, 2002). CD8+ cytotoxic T cells can also produce several cytokines, including IFN-γ and TNF-α, which serve to promote both immune and inflammatory responses (Phillips et al., 2010). In contrast, CD4+ helper T cells have no cytotoxic activity themselves, but instead help to activate and direct other immune cells, including CD8+ cytotoxic T cells (Santana and Rosenstein, 2003). Although both CD4+ and CD8+ T cells are recruited to the ischemic hemisphere following stroke, there are greater numbers of CD4+ helper T cells than CD8+ cytotoxic T cells (Gelderblom et al., 2009;Saino et al., 2010).
Importantly, mice deficient in either CD4+ or CD8+ T cells exhibit significantly smaller infarct volumes than wild-type controls (Yilmaz et al., 2006). Mice deficient in these T cell subtypes show similar reductions in neurological deficits at 24 hours after ischemia, although the difference did not reach statistical significance (Yilmaz et al., 2006). The notion that CD8+ and CD4+ helper T cells contribute to ischemic injury was recently supported by a study showing that selective antibody-mediated depletion of CD4+ or CD8+ T lymphocytes remarkably reduces infarct volumes in mice with permanent cerebral ischemia (Liesz et al., 2011). In sum, these findings demonstrate a detrimental role for both CD4+ helper and CD8+ cytotoxic T cells in the development of brain injury following stroke. In addition, a rat model of TBI found significant accumulation of CD8+ cells 3 days post-injury, distributed strictly in the pan-necrotic areas and around the perimeter (Ling et al., 2006). The accumulation of activated antigen-specific T-cells at the sites of traumatic injury, in addition to antigen-containing areas, suggest that they may serve to amplify local inflammatory processes in the CNS (Ling et al., 2006).
Regulatory T cells
Tregs are a subtype of CD4+ T cells that account for about 10% of CD4+ T cells in the periphery and express CD25 and the transcription factor FoxP3 (Sakaguchi, 2005). Tregs exert immunosuppressive functions, but the underlying mechanism is not yet clear. However, it is known that Tregs possess a diverse arsenal of inhibitory mechanisms, ranging from secretion of inhibitory cytokines, such as the transforming growth factor-β (TGF-β), IL-10 (Liesz et al., 2009) and a novel anti-inflammatory cytokine, IL-35 (Collison et al., 2007;Niedbala et al., 2007), to the expression of inhibitory surface molecules, such as CTLA-4 and GITR (Vignali et al., 2008).
With their vigorous immunosuppressive abilities, Tregs play critical roles in the maintenance of immunologic self-tolerance and the inhibition of a number of physiological and pathological immune responses (Sakaguchi et al., 2006). For example, Tregs may be a critical factor in controlling EAE and Parkinson’s disease. Expansion of Tregs by prophylactic infusion of immunoglobulins prevented the development of EAE, and the protection was associated with increases in peripheral Treg number and function (Ephrem et al., 2008). Depletion of Tregs abrogated the protection of intravenous immunoglobulins, which strongly suggested that Tregs mediated the protection (Ephrem et al., 2008). Adoptive transfer of Tregs to MPTP-intoxicated mice attenuated Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration and provided significant protection of the nigrostriatal system (Reynolds et al., 2007;Reynolds et al., 2010).
In the context of cerebral ischemia, the activation of Tregs appears to be one of the intrinsic mechanisms that the body uses to naturally restrict cerebral inflammation induced by ischemic stroke. With regard to cell function, it was recently reported that the suppressive effect of Tregs was unaltered after stroke in mice and humans (Hug et al., 2011). Thus, an increase in the number of Tregs, rather than an enhancement of the function of each cell, may be responsible for endogenous immunosuppression after stroke.
The expansion of endogenous Tregs might underlie the mechanism of protection afforded by tolerance to MBP and E-selectin (Gee et al., 2008;Ishibashi et al., 2009). Supporting this concept, rats tolerant of MBP were less likely to develop Th1 responses compared to rats tolerant to ovalbumin following experimental stroke (Gee et al., 2008). Mucosal tolerance to E-selectin protected against stroke in spontaneously hypertensive rats (Ishibashi et al., 2009). The protective effect of endogenous Tregs after stroke was mediated by a reduction in secondary cerebral inflammatory responses and has been confirmed by a recent study showing that depletion of endogenous Tregs profoundly increased delayed brain damage and neurological dysfunction after brain ischemia (Liesz et al., 2009). Our recent study also showed that delayed post-stroke transplantation of Tregs robustly reduced brain damage and improved long-term neurological outcomes (Li et al., 2012). However, controversial evidence exists showing that Tregs have no effect (Ren et al., 2011b) or even increase brain infarct volume (Kleinschnitz et al., 2013). Although some of the discrepancies between these studies may be explained by differences in animal models, experimental settings, or timing/dosage of Treg treatment, these conflicting data clearly demonstrate that research on the impact of Tregs in stroke still lies in its infancy and that more studies are warranted to advance our knowledge in this field.
3.2.2. B lymphocytes
There are few studies investigating the role of B lymphocytes in cerebral ischemia/reperfusion injury. In a wide variety of ischemia/reperfusion injury models in peripheral organs (e.g., intestines, heart, kidney, and skeletal muscles), B lymphocytes exert predominantly deleterious effects (Linfert et al., 2009). However, in cerebral ischemic injury models, several studies suggest a protective role for B cells. In mice deficient in both T- and B-cells, (Rag−/− (Yilmaz et al., 2006) or SCID (Hurn et al., 2007) mice), a significant reduction in infarct size and neurological damage was observed following transient MCAO. However, mice lacking only B cells failed to show improvement against ischemic injury (Yilmaz et al., 2006), suggesting that B cells are, at the least, not acting in a detrimental fashion following cerebral ischemia. Recently, another study demonstrated that B-cell deficiency worsened histological damage and functional outcomes after transient cerebral ischemia (Ren et al., 2011a). Consistent with these findings, adoptive transfer of B cells to B-cell deficient mice reduced the size of ischemic infarcts and decreased neurological deficits (Ren et al., 2011a). The same study further identified IL-10-secreting regulatory B cells as a major regulatory cell type in stroke, and associated the secretion of IL-10 with B-cell-mediated neuroprotection (Ren et al., 2011a). Together, these data suggest that the role of B lymphocytes in cerebral ischemia appears to be different than in peripheral organ ischemia models, and may serve a protective rather than pathogenic function in cerebral ischemia/reperfusion injury.
4. Role of immune system in post-ischemic tissue repair
4.1. Resolution of local inflammation
After ischemic injury, debris from dead cells is scattered in and around the infarct area. Necrotic cells release molecules that can strongly trigger inflammatory processes via the TLR pathway and destroy the environment of uninjured cells. Thus, clearance of cellular debris is essential for neural regeneration and recovery. Moreover, the removal of apoptotic neutrophils by phagocytosis is critical to resolve inflammation and prevent further damage to the injured site (Meszaros et al., 2000). This process is mainly executed by activated microglia/macrophages. Weston et al. reported that ED-1-positive activated microglia/macrophages engulfed neutrophils in a rat model of endothelin-1-induced cerebral ischemia (Weston et al., 2007). Besides phagocytic activity, immune cells also exert anti-inflammatory functions. M2 type microglia and Tregs can attenuate the activation of resident and invading inflammatory cells by releasing IL-10 (Strle et al., 2002;Liesz et al., 2009). M2-microglia are also able to release TGF-β to suppress local inflammation (Streit et al., 2000). In our recent study, adoptively transferred Tregs were shown to reduce inflammatory responses both intrinsic and extrinsic to the CNS in rodent models of transient focal cerebral ischemia. Moreover, Tregs provided neurovascular protection against stroke by inhibiting peripheral neutrophil-derived MMP-9. These findings suggest that Tregs may be promising candidates for cell-based therapies targeting post-stroke inflammatory dysregulation and neurovascular disruption (Li et al., 2012).
4.2. Angiogenesis and neovascularization
Angiogenesis is critical for the repair of tissue after stroke. After an ischemic insult, microvascular endothelial cells proliferate and sprout in the direction of stimulatory signals. Angiogenesis can be triggered by a large number of inflammation-associated inducers. For example, early angiogenesis after stroke is mediated by CCL2 (Goede et al., 1999). In addition, monocytes/macrophages express IL-8, vascular endothelial growth factors (VEGF), TGF-β, prostaglandin, and MMP-9 (Koch et al., 1992;Sunderkotter et al., 1994;Melter et al., 2000;Lu and Wahl, 2005;Moldovan and Moldovan, 2005), all of which enhance the angiogenic process.
How post-stroke angiogenesis is spatially and temporally regulated is not yet fully understood, but it is widely thought to be associated with activation of the MAPK pathway, which directly promotes endothelial proliferation via the transcription factor c-fos. Among the multiple factors that stimulate the MAPK pathway, VEGF is the classical promoter of angiogenesis after stroke (Dzietko et al., 2013). VEGF activates the PI3K/Akt and MAPK pathways to mediate survival, proliferation, and migration of microvascular endothelial cells. In an MCAO model, VEGF treatment significantly promoted angiogenesis in the ischemic penumbra, as reflected by an enhanced density of microvessels (Zhang et al., 2000). In addition to its impact on angiogenesis, VEGF facilitated the repair of stroke tissue through its effects on neurogenesis and glial progenitors (Bain et al., 2013). VEGF treatment also accelerated the proliferation of neural stem cells in vitro (Jin et al., 2002) and in the subventricular zone (SVZ) and dentate gyrus (DG) of the cerebrum after ischemia (Jin et al., 2002;Sun et al., 2003).
A variety of immune cells contribute to blood vessel formation by producing angiogenesis inducers. Under certain conditions, monocytes and microglia secrete VEGF, as well as stromal cell-derived factor-1 (SDF-1) (Unoki et al., 2010;Rymo et al., 2011). Microglia and monocytes also produce several other factors relevant to angiogenesis, such as MMPs (Zhao et al., 2006) and NO via iNOS (Fukumura et al., 2001). Granulocytes produce MMPs with other neovascularization-associated factors such as VEGF, basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), and epidermal growth factor (EGF) in their granules (McCourt et al., 1999;Hiromatsu and Toda, 2003;Puxeddu et al., 2005a, 2005b; Ardi et al., 2007). However, because of their short life span, almost all of these factors only facilitate angiogenesis within vessels.
Different subtypes of T cells have distinct effects on angiogenesis. CD4+ Th1 cells, especially those secreting IFN-γ, inhibit angiogenesis (Albini et al., 2000;Qin and Blankenstein, 2000). However, Tregs not only can inhibit this process (Facciabene et al., 2012), but they are also able to release VEGF and TGF-β to promote angiogenesis (Facciabene et al., 2011). Unlike Th1 cells, Th2 cells can enhance angiogenesis through TGF-β and IL-6 (Larsson et al., 2001;Wei et al., 2003). Th17 cells can also trigger angiogenesis with IL-17 (Numasaki et al., 2003). Taken together, these findings suggest that inflammation and the immune system are robust modulators of angiogenesis and may thereby help determine tissue repair after stroke.
4.3. Neurogenesis and neuronal replacement
Neurogenesis is largely dependent on the neural stem niche. An appropriate niche refers to one with a moderate redox potential (Panchision, 2009), the presence of neural trophic factors such as brain-derived neurotrophic factor (BDNF) and bFGF, and proper angiogenesis (Doetsch, 2003). After they are produced in the SVZ or SGZ, neural progenitor cells are guided to the sites of injury to replace dead neurons.
Multiple pathways are associated with the stimulation of neurogenesis, including PI3K/Akt, NF-κb, MAPK, and HIF-1α. BDNF is a robust trophic factor that enhances neurogenesis after ischemic injury by activating the prosurvival and pro-neurogenic PI3K/Akt and MAPK pathways. Direct injection of BDNF into the hippocampus has been shown to trigger the proliferation of neural stem cells (Scharfman et al., 2005). Furthermore, pretreatment with BDNF increased the tolerance of brains to ischemia (Yanamoto et al., 2000).
Immune cells have a profound impact on neurogenesis. Activated resident microglia and peripheral immunocytes infiltrating the ischemic area are able to produce ROS, including NO, which, at the right concentration, can promote the proliferation of neural stem cells (Chua et al., 1998;Le Belle et al., 2011). Microglia and monocytes can also produce neural trophic factors, such as BDNF (Czeh et al., 2011), and promote neurogenesis (Scharfman et al., 2005). CD14+ monocytes, a multi-potent stem cell, have the capability to directly differentiate into the neuronal lineage (Kodama et al., 2006). T cells also take part in the self-renewal of neural stem cells. For example, CD4+ T cells can increase the astrocytic expression of BDNF (Hall et al., 2000). B regulatory cells also have the potential to assist in neurogenesis. Monocytes and microglia in the ischemic area release molecules such as CCL2 and SDF-1 that facilitate the migration of newborn neurons into the area (Yan et al., 2007;Unoki et al., 2010). TGF-β can also enhance the proliferation of neural stem cells in a model of stroke in adults (Battista et al., 2006;Ma et al., 2008;Mathieu et al., 2010) by suppressing immune responses and increasing the expression of glial cell-derived neurotrophic factor (GDNF), ciliary neurotrophic factor (CNTF), and fibroblast growth factor (FGF)-2 in neural glia (Unsicker and Krieglstein, 2000;Krieglstein et al., 2002). These studies suggest that activation of immune cells may contribute to post-ischemic recovery via promotion of neural regeneration and migration.
4.4. Oligodendrocyte regeneration and remyelination
Oligodendrocytes are vulnerable to inflammation and other immune responses, particularly in some autoimmune diseases (Mekhail et al., 2012). Microglia and monocytes can act on oligodendrocyte precursor cells (OPCs) positively or negatively according to their phenotypes. For example, M1 cells increase OPC cell death (Mitrovic et al., 1995), whereas M2 cells mediate OPC chemotaxis and differentiation (Lalive et al., 2005). Lymphocytes also play an important role in remyelination, as demonstrated by the obstruction of remyelination in SCID mice (Bieber et al., 2003). CD4+ T cells are thought to be at the core of the pathological lesion in EAE. Although it has not yet been demonstrated experimentally, CD4+ Th1 cells may therefore have the potential to induce demyelination after stroke. In contrast, Tregs are able to limit the EAE lesion through the IL-10-mediated inhibition of CD4+ Th1 cells. Moreover, Th2 cells can improve the survival of OPCs and promote their maturation with TGF-β (Lalive et al., 2005).
In summary, components of the immune system influence recovery after stroke in multiple ways by participating in different aspects of tissue repair (Figure 2). Immune cells not only help to resolve local inflammation, but also facilitate angiogenesis, neurogenesis, and remyelination via various bioactive substances. These important functions must be taken into consideration in the development of immunomodulatory stroke therapies. Although there may be many benefits to promoting immune function, overactivation or acute inflammation may exacerbate brain injury. Mounting evidence shows that overactivated immune cells release cytotoxic molecules such as NO, ROS, and proinflammatory cytokines, which augment neuronal death (Zhang et al., 2009;Fogal et al., 2005;Hur et al., 2010;Uchida et al., 2010;Liesz et al., 2009). In addition, damaged brain cells and activated peripheral inflammatory cells produce cytokines such as IL-1β, TNF-α and IFN-γ that could further activate the immune system, thereby triggering a vicious cycle that can propel neuronal death. This traditional concept has been reviewed extensively elsewhere. Despite the emerging data supporting a beneficial role for immune activation in ischemic recovery, this previous body of knowledge on the detrimental effects must be considered during the development of stroke therapeutics. Tailoring therapeutics to the specific timeframe following ischemia should also be considered to minimize detrimental or off-target effects.
Figure 2. Role of immune cells in tissue repair after stroke.
Cellular components of the immune system participate in various aspects of post-stroke tissue repair. Microglia/macrophages engulf dead cells and debris at the site of injury ①. IL-10 and TGF-β produced by microglia/macrophages or Tregs resolve the local inflammation. Angiogenesis is enhanced by various factors released by microglia/macrophages, Tregs, and Th17 cells, such as VEGF, TGF-β, and BDNF ②. Microglia/macrophages, Tregs, and Th2 cells are also able to promote neurogenesis ③, oligodendrocyte regeneration ④, and remyelination by factors such as BDNF and TGF-β.
5. Treatments targeting post-stroke immune responses
Ischemia-induced neuronal damage is a progressive process that can continue for days after the initial injury (Clark et al., 1994). As discussed above, strong evidence now exists to suggest that neuroinflammation develops over hours or days and contributes to the secondary (delayed) injury processes that are involved in, and ultimately determine, the degree of cell survival after injury (Barinaga, 1998). This suggests that the neuroprotective anti-inflammatory treatments discussed below are feasible potential strategies for stroke intervention.
Concerns have been raised about using anti-inflammatory strategies in the treatment of stroke because, even without such treatments, patients with stroke generally experience a long-lasting suppression of immunity (Chamorro et al., 2005). Similarly, in animal models of stroke, apoptosis of B cells, T cells, and NK cells is observed the spleen and thymus and the numbers of these cells drop in the blood, thymus, and spleen (Prass et al., 2003). Levels of TNF-α, IFN-γ, and IL-4 were also reduced, leading to impaired activation of T cells (Prass et al., 2003). In addition, the functioning of iNKTs in liver was also impaired (Wong et al., 2011).
Several hypotheses have been proposed to explain this immunosuppression. Activation of the hypothalamic-pituitary-adrenal (HPA) axis is generally believed to take part in this process. Various cytokines, including TNF-α, IL-1β, and IL-6, can stimulate neurons in the paraventricular nucleus (PVN), which, in turn, releases corticotropin-releasing factor (Chrousos, 1995). Corticotropin-releasing factor stimulates the secretion of adrenocorticotropin hormone (ACTH) in the pituitary and increases the adrenal release of cortisol, which depresses inflammation and immune responses. This has been demonstrated in stroke mice as a decreased splenic response to mitogens (Offner et al., 2006b), which was mediated by adrenergic signaling (Offner et al., 2006a) and inhibited by the β-adrenoreceptor blocker propranolol (Prass et al., 2003). Neurons in the PVN also suppress the activity of macrophages (Pavlov et al., 2003). Lymph nodes supplied by sympathetic nerves also respond to this process. As a result, the pattern of cytokines is switched from a pro-inflammatory state to an anti-inflammatory state with increased production of IL-10 and decreased production of TNF-α, IL-1, IL-12, IFN-γ, and NO (Woiciechowsky et al., 1999).
In terms of outcome, stroke-induced immunodepression is a double-edged sword. On the one hand, it limits the autoimmune responses to CNS antigens by suppressing the activity of autoreactive T cells and thus minimizes further damage to the brain. On the other hand, stroke-induced immunodepression can be deleterious by increasing the risk of systemic infections after stroke, a major indicator of poor neurological function, morbidity, and mortality. Antibiotics can be administered after a stroke to prevent potential infections, and some antibiotics, such as doxycycline (Lee et al., 2009), minocycline (Fagan et al., 2010), and β-lactam (Rothstein et al., 2005), have also been shown to have neuroprotective effects. Nevertheless, special attention must be paid to the immunosuppressive state following stroke when developing immunomodulatory therapies. These therapies are discussed in the following sections.
5.1. Immune suppressive drugs
It has been established that the activation of microglia is the initial step in the neuroinflammatory response to ischemia and is followed by the infiltration of circulating inflammatory cells and by reactive astrocytosis (Zheng and Yenari, 2004). Ischemic brain injury can induce a repertoire of responses in microglia, including rapid migration, proliferation, and the release of superoxide, NO, proteases, and cytokines (Yenari et al., 2010). These responses can trigger a series of subsequent innate and adaptive immune responses that exacerbate ischemic brain injury (Yenari et al., 2010).
Currently, many studies are examining the effects of anti-inflammatory agents on ischemia-induced activation of microglia. Agents that effectively block microglial activation and effector responses have been shown to be neuroprotective in animal models of experimental stroke or in patients with stroke. For example, minocycline, a semisynthetic tetracycline antibiotic, has been reported to be neuroprotective against ischemic cerebral injury by exerting anti-inflammatory effects, including inhibition of the activation and proliferation of microglia, as well as reductions in iNOS, IL-1β converting enzyme, and cyclooxygenase-2 (Yrjanheikki et al., 1998;Yrjanheikki et al., 1999;Chu et al., 2007). The administration of minocycline reduces infarct size and enhances functional recovery following experimental stroke (Yrjanheikki et al., 1999;Chu et al., 2010), and even provides significant protection when the treatment is started 4 hours after the insult (Yrjanheikki et al., 1999). Moreover, stroke patients treated with minocycline within 6 to 24 hours after onset of stroke have been shown to have significantly better outcomes than patients on placebo (Lampl et al., 2007). In addition, several other anti-inflammatory agents, such as compound K (a ginseng saponin metabolite) (Park et al., 2012) or paeonol (a natural compound extracted from Moutan cortex), have also been reported to protect against cerebral ischemic injury by the suppression of microglial activation or inhibition of microglia-mediated inflammation in rodent models of stroke.
A variety of cytokines, including TNF-α, IL-1β, IL-6, TGF-β, and IL-10, have been associated with inflammatory responses in acute ischemic stroke. Small molecular inhibitors of pro-inflammatory cytokines such as TNF-α appear to be neuroprotective against cerebral ischemia (Tuttolomondo et al., 2009). On the other hand, administration of anti-inflammatory cytokine IL-10 has also been demonstrated to provide neuroprotection in a rat model of ischemic stroke (Spera et al., 1998). Adhesion molecules, such as ICAM-1, are necessary for the process of leukocyte-endothelial cell adhesion by which inflammatory cells infiltrate into the ischemic brain. Treatment with an anti-ICAM-1 antibody has therefore been investigated in stroke patients (Enlimomab Acute Stroke Trial Investigators, 2001). Unfortunately, there were significantly more adverse events (primarily infections and fever) in patients treated with anti-ICAM-1 antibody than with placebo, resulting in more fatalities (Tuttolomondo et al., 2009). Nevertheless, most of these results suggest that anti-inflammatory drugs targeting secondary neuroinflammatory responses provide a sufficiently wide therapeutic window, and may be neuroprotective in human stroke. Of course, the safety of any anti-inflammatory treatment must be carefully evaluated due to the possible enhancement of systemic immunosuppression after stroke.
5.2. Immune modulatory therapy
An alternative strategy for treating brain damage induced by neuroinflammation after stroke is to target immune modulator cells. Earlier studies reported a decrease in infarct size, as well as improved behavioral outcomes, after transient focal cerebral ischemia in animals that were tolerant of brain myelin antigens (Becker et al., 2003). This neuroprotective effect is thought to be associated with the induction of IL-10-producing CD4+ T cells (Frenkel et al., 2003). Adoptive transfer of CD4+ T cell from myelin antigen-tolerant wild-type mice, but not IL-10-deficient mice, significantly decreased the infarct size after cerebral ischemia/reperfusion (Frenkel et al., 2005).
As discussed above, the most well understood population of Tregs is characterized by the expression of CD25 and the transcription factor Foxp3. Depletion of Tregs with anti-CD25 antibodies increases the extent of delayed brain injury and worsens functional outcomes, suggesting that Tregs are key protective immunomodulators in stroke (Liesz et al., 2009). As mentioned above, we found that adoptive transfer of CD4+CD25+ Tregs robustly reduced brain damage and improved long-term behavioral recovery (Li et al., 2012). Importantly, our results showed that Treg treatment did not enhance systemic post-ischemic immunosuppression in a mouse model of MCAO. Our data, taken together with previous findings, suggest that Treg adoptive therapy is a novel and potent cell-based intervention against stroke.
As mentioned above, another recent study also indicates that IL-10-producing B cells may serve as cell-based therapies against stroke. B-cell-deficient mice exhibited larger infarct volumes and had more severe functional deficits after transient focal cerebral ischemia (Ren et al., 2011a). Adoptive transfer of wild-type B cells, but not IL-10-deficient B cells, led to clear improvements in those outcomes in B-cell-deficient mice after ischemia (Ren et al., 2011a). Although these findings indicate that therapies using immune modulator cells (either regulatory T cells or regulatory B cells) may be a potent strategy for stroke intervention, the translation of this approach into clinical practice is still a long way off.
5.3. Perspectives
To date, several anti-inflammatory strategies have been investigated as treatments for ischemic stroke. The advantages of anti-inflammatory therapies are manifold, including a prolonged therapeutic time window, as well as the limitation of inflammation during reperfusion. Nevertheless, despite the positive results observed in experimental models of stroke, the translation of anti-inflammatory strategies into clinical application has been largely unsuccessful. Most interventions fail to show efficacy in human stroke victims and, even more troubling, some agents actually worsened stroke outcomes and caused serious adverse effects (Table 1). Thus far, no Phase III acute interventional trial has successfully demonstrated improvements in patients with stroke.
Table 1.
Results of selected phase II/III clinical trials of therapeutic strategies targeting inflammation in ischemic stroke
| Intervention | Target | Phase | Results | References |
|---|---|---|---|---|
| Murine anti-ICAM-1 monoclonal antibody | Efficacy and safety of Enlimomab vs. placebo | III | Significantly more adverse events with Enlimomab treatment than placebo | (Enlimomab Acute Stroke Trial Investigators, 2001) |
| Human anti-CD18 monoclonal antibody | Efficacy and safety of Hu23F2G vs. placebo in acute ischemic stroke | III | Aborted and no benefit shown with Hu23F2G treatment | (Becker, 2002) |
| Recombinant neutrophil inhibitory factor | Dose-response study of UK-279,276 in acute stroke | II | UK-279,276 did not improve recovery in acute ischemic stroke patients but was devoid of serious side effects | (Krams et al., 2003) |
| Recombinant human IL-1 receptor antagonist (rhIL-1ra) | Efficacy and safety of rhIL-1ra vs. placebo in acute ischemic stroke | II | rhIL-1ra was safe and exhibited biological activity relevant to the pathophysiology and clinical outcome of ischemic stroke | (Emsley et al., 2005) |
| Minocycline | Dose-response study of minocycline in acute ischemic stroke | II | Minocycline was safe and well tolerated alone and in combination with tissue plasminogen activator | (Fagan et al., 2010;Fagan et al., 2011) |
Several reasons may account for these disappointing results: First, the numerous differences between rodent models of stroke and human stroke may confound the translation of a proper therapeutic window from rodents to humans. Second, the perfusion status of patients in some of the studies was not assessed (Krams et al., 2003), and patients with complete occlusions of a blood vessel were likely to have been included. Indeed, it has been demonstrated that anti-leukocyte strategies are less effective in models of permanent MCAO as opposed to transient MCAO (Jin et al., 2010). Third, the sophisticated modulatory roles that various components of the immune system play in the pathogenesis of stroke may be the most important reason why approaches that target a single type of cell or pathway fails to protect. Although immune and inflammatory cells cause damage in the acute stage of ischemic stroke, some of those cells contribute significantly to post-stroke tissue repair, as reviewed above. Broad suppression of immune responses may deprive patients of normal, physiological immune functions that repair brain tissue and battle systemic infections and thereby worsen stroke outcomes. Therefore, therapies targeting immune responses after stroke should change their focus from wide immunosuppressive strategies to subtler immunomodulatory approaches that adjust the balance between protective and detrimental responses. To this end, a thorough understanding of the role of the immune system in stroke is needed, particularly in regard to its different contributions at distinct temporal stages.
6. Summary and conclusions
In summary, an exquisitely coordinated crosstalk between the CNS and peripheral immune system helps determine the fate of animals after acute brain injury. In addition to the conventional idea that the brain conveys danger signals to the periphery, recent studies reveal multiple novel neural circuits through which brain injury evokes a robust immune response. Such immune responses used to be considered innate or antigen-nonspecific. However, recent work on CD8+ cytotoxic T cells, CD4+ helper T cells, regulatory T cells, and B cells reveals that adaptive immunity also plays pivotal roles in secondary brain injury and has reshaped our thinking (Figure 3). Immunocytes also take part in brain repair after ischemic injury. M2 microglia can trigger neurogenesis with neurotropic factors. However, the impact of other cell types on neurogenesis in stroke merit more investigation. In tumors, most inflammatory and immune cells are known to enhance angiogenesis. Some of these cells, such as Tregs, microglia, and granulocytes, also have been shown to elicit neovascularization in stroke. In addition, Tregs, M2 microglia, and Th2 cells also regulate oligodendrocyte genesis. With these new findings on neuroimmune communication, therapeutic candidates that can modulate immune responses and improve stroke outcome may lie on the horizon. Finally, it seems reasonable to propose that immunomodulation through the transplantation of immune cells will improve brain function more profoundly than immunosuppressive drugs, because immune cells can harness the protective power of the body’s natural defenses. Further research on immunomodulatory therapies that leverage our current knowledge of brain injuries is highly warranted.
Figure 3. Innate and adaptive immune responses after stroke.
In response to ischemic brain injury, a variety of danger signals are released from neurons, microglia, and astrocytes. These signals include ATP, cytokines, and chemokines. ATP can bind to P2X receptors on microglia and induce their activation and further release of inflammatory cytokines, such as TNF-α and IL-6. Activated microglia can also release chemokines, such as CCL2 and CCL3, to recruit leukocyte infiltration. The CCL2 and CCR2 axis is responsible for the recruitment of macrophages. The infiltration of macrophages has been suggested to be both detrimental in ischemic injury and protective against hemorrhagic transformation. In the ischemic brain, inflamed endothelial cells express adhesion molecules, such as ICAM-1, P-selectin, and E-selectin, which recruit leukocyte adhesion and infiltration. Following stimulation by IL-6 and TNF-α, neutrophils are activated and release MMP-9, which degrades the matrix proteins of the blood-brain barrier and stimulates peripheral leukocyte infiltration. Furthermore, brain-derived antigens can be processed by antigen presenting cells, such as dendritic cells, and presented by MHC molecules on the cell surface. Receptors on the surface of T cells can recognize the MHC and brain antigen complex. Subsequently, the adaptive immune system is activated. CD4+ T helper cells, CD8+ cytotoxic T cells, B cells, and Th17 cells all have been reported to contribute to the pathogenesis of neuroinflammation and neuronal cell death following stroke. Regulatory T cells (Tregs) and a special subset of regulatory B cells have been demonstrated to be neuroprotective through the release of IL-10. IL-35 and TGF-β are two other soluble anti-inflammatory factors that are also secreted by Tregs. Tregs can also inhibit MMP-9 production from neutrophils in a cell-cell contact-dependent manner.
Highlights.
Molecular interactions between brain injury and peripheral immunity
Peripheral immunity and inflammation triggering secondary injuries in the ischemic brain
Anti-inflammatory effects of specific immune components
Brain tissue repair facilitated by peripheral immunity and inflammation
Acknowledgments
This work was supported by NIH grants NS036736, NS045048, and NS056118 (to J.C.), AHA grant 13SDG14570025 (to X.H.), Chinese Natural Science Foundation grants (81271275, 81070947 and 30770759 to B-L Sun; 81020108021 and 81171149 to Y.G.; and 81228008 to J.C. and P.Z.), Natural Science Foundation of Shandong grant ZR2012HZ006 (to B-L Sun). J.C. is a recipient of the Research Career Scientist Award from Department of Veterans Affairs and the RK Mellon Endowed Chair for Cerebrovascular Disease Research from the University of Pittsburgh Medical Center. We thank Dr. Beth Fischer for editorial assistance.
Abbreviations
- ACh
acetylcholine
- ACTH
adrenocorticotropin hormone
- ANS
autonomic nervous system
- APC
antigen presenting cell
- BBB
blood brain barrier
- BDNF
brain-derived neurotrophic factor
- bFGF
basic fibroblast growth factor
- CNS
central nervous system
- CNTF
ciliary neurotrophic factor
- DAMP
danger-associated molecular pattern
- DC
dendritic cell
- DG
dentate gyrus
- EAE
experimental autoimmune encephalomyelitis
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- GDNF
glial cell-derived neurotrophic factor
- GFP
green fluorescent protein
- HI
hypoxia/ischemia
- HIF
hypoxia-inducible factor
- HMGB1
high-mobility group box 1
- HPA
hypothalamic-pituitary-adrenal
- Iba1
ionized calcium binding adapter molecule 1
- ICAM-1
intercellular adhesion molecule-1
- IFN-γ
interferon-γ
- IL
interleukin
- iNKT
invariant NKT cells
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- MAP2
microtubule-associated protein 2
- MAPK
mitogen-activated protein kinase
- MBP
myelin basic protein
- MCAO
middle cerebral artery occlusion
- MCP-1
monocyte chemotactic protein-1
- MHC
major histocompatibility complex
- MIP
macrophage inflammatory protein
- MMP
matrix metalloproteinases
- MOG
myelin oligodendrocyte glycoprotein
- mDCs
myeloid dendritic cells
- nACh
nicotinic acetylcholine receptor
- NK cells
natural killer cells
- NLR
Nod-like receptor
- NMDA
N-methyl-D-aspartic acid
- NO
nitric oxide
- OGD
oxygen glucose deprivation
- OPC
oligodendrocyte precursor cell
- PAMP
pathogen-associated molecular pattern
- pDCs
plasmacytoid dendritic cells
- PDGF
platelet-derived growth factor
- PSNS
parasympathetic nervous system
- PVN
paraventricular nucleus
- RAGE
receptor for advanced glycation endproducts
- RANTES
regulated upon activation, normal t-cell expressed, and secreted
- ROS
reactive oxygen species
- SCID
severe combined immunodeficiency
- SDF-1
stromal cell-derived factor-1
- SNS
sympathetic nervous system
- SVZ
subventricular zone
- TBI
traumatic brain injury
- TGF-β
transforming growth factor-β
- Th1
T helper cell type 1
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor-α
- Treg
regulatory T cell
- VCAM
vascular cell adhesion molecule
- VEGF
vascular endothelial growth factor
- vMIP
viral macrophage inflammatory protein
Footnotes
Conflicts of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adami C, Bianchi R, Pula G, Donato R. S100B-stimulated NO production by BV-2 microglia is independent of RAGE transducing activity but dependent on RAGE extracellular domain. Biochim Biophys Acta. 2004;1742:169–177. doi: 10.1016/j.bbamcr.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Ajmo CT, Jr, Collier LA, Leonardo CC, Hall AA, Green SM, Womble TA, Cuevas J, Willing AE, Pennypacker KR. Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp Neurol. 2009;218:47–55. doi: 10.1016/j.expneurol.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajmo CT, Jr, Vernon DO, Collier L, Hall AA, Garbuzova-Davis S, Willing A, Pennypacker KR. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008;86:2227–2234. doi: 10.1002/jnr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albini A, Marchisone C, Del GF, Benelli R, Masiello L, Tacchetti C, Bono M, Ferrantini M, Rozera C, Truini M, Belardelli F, Santi L, Noonan DM. Inhibition of angiogenesis and vascular tumor growth by interferon-producing cells: A gene therapy approach. Am J Pathol. 2000;156:1381–1393. doi: 10.1016/S0002-9440(10)65007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 2009;276:13–26. doi: 10.1111/j.1742-4658.2008.06766.x. [DOI] [PubMed] [Google Scholar]
- Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22:83–87. doi: 10.1016/s1471-4906(00)01812-3. [DOI] [PubMed] [Google Scholar]
- Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2007;104:20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ay I, Sorensen AG, Ay H. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia: an unlikely role for cerebral blood flow. Brain Res. 2011;1392:110–5. doi: 10.1016/j.brainres.2011.03.060. Epub;%2011 Mar 31.:110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain JM, Moore L, Ren Z, Simonishvili S, Levison SW. Vascular endothelial growth factors A and C are induced in the SVZ following neonatal hypoxia-ischemia and exert different effects on neonatal glial progenitors. Transl Stroke Res. 2013;4:158–170. doi: 10.1007/s12975-012-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Bansal V, Ryu SY, Lopez N, Allexan S, Krzyzaniak M, Eliceiri B, Baird A, Coimbra R. Vagal stimulation modulates inflammation through a ghrelin mediated mechanism in traumatic brain injury. Inflammation. 2012;35:214–220. doi: 10.1007/s10753-011-9307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barinaga M. Stroke-damaged neurons may commit cellular suicide. Science. 1998;281:1302–1303. doi: 10.1126/science.281.5381.1302. [DOI] [PubMed] [Google Scholar]
- Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- Becker K, Kindrick D, McCarron R, Hallenbeck J, Winn R. Adoptive transfer of myelin basic protein-tolerized splenocytes to naive animals reduces infarct size: a role for lymphocytes in ischemic brain injury? Stroke. 2003;34:1809–1815. doi: 10.1161/01.STR.0000078308.77727.EA. [DOI] [PubMed] [Google Scholar]
- Becker K, Kindrick D, Relton J, Harlan J, Winn R. Antibody to the alpha4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke. 2001;32:206–211. doi: 10.1161/01.str.32.1.206. [DOI] [PubMed] [Google Scholar]
- Becker KJ. Anti-leukocyte antibodies: LeukArrest (Hu23F2G) and Enlimomab (R6.5) in acute stroke. Curr Med Res Opin. 2002;18(Suppl 2):s18–s22. doi: 10.1185/030079902125000688. [DOI] [PubMed] [Google Scholar]
- Bernik TR, Friedman SG, Ochani M, DiRaimo R, Susarla S, Czura CJ, Tracey KJ. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg. 2002;36:1231–1236. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Giambanco I, Donato R. S100B/RAGE-dependent activation of microglia via NF-kappaB and AP-1 Co-regulation of COX-2 expression by S100B, IL-1beta and TNF-alpha. Neurobiol Aging. 2010;31:665–677. doi: 10.1016/j.neurobiolaging.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Kastrisianaki E, Giambanco I, Donato R. S100B protein stimulates microglia migration via RAGE-dependent up-regulation of chemokine expression and release. J Biol Chem. 2011;286:7214–7226. doi: 10.1074/jbc.M110.169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber AJ, Kerr S, Rodriguez M. Efficient central nervous system remyelination requires T cells. Ann Neurol. 2003;53:680–684. doi: 10.1002/ana.10578. [DOI] [PubMed] [Google Scholar]
- Black RA, Kronheim SR, Cantrell M, Deeley MC, March CJ, Prickett KS, Wignall J, Conlon PJ, Cosman D, Hopp TP. Generation of biologically active interleukin-1 beta by proteolytic cleavage of the inactive precursor. J Biol Chem. 1988;263:9437–9442. [PubMed] [Google Scholar]
- Bornstein NM, Aronovich B, Korczyn AD, Shavit S, Michaelson DM, Chapman J. Antibodies to brain antigens following stroke. Neurology. 2001;56:529–530. doi: 10.1212/wnl.56.4.529. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci. 2001;21:5528–5534. doi: 10.1523/JNEUROSCI.21-15-05528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brait VH, Jackman KA, Walduck AK, Selemidis S, Diep H, Mast AE, Guida E, Broughton BR, Drummond GR, Sobey CG. Mechanisms contributing to cerebral infarct size after stroke: gender, reperfusion, T lymphocytes, and Nox2-derived superoxide. J Cereb Blood Flow Metab. 2010;30:1306–1317. doi: 10.1038/jcbfm.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckwoldt MO, Chen JW, Stangenberg L, Aikawa E, Rodriguez E, Qiu S, Moskowitz MA, Weissleder R. Tracking the inflammatory response in stroke in vivo by sensing the enzyme myeloperoxidase. Proc Natl Acad Sci U S A. 2008;105:18584–18589. doi: 10.1073/pnas.0803945105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bune LT, Thaning P, Johansson PI, Bochsen L, Rosenmeier JB. Effects of nucleotides and nucleosides on coagulation. Blood Coagul Fibrinolysis. 2010;21:436–441. doi: 10.1097/MBC.0b013e328338db27. [DOI] [PubMed] [Google Scholar]
- Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33:586–592. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- Cavaliere F, Florenzano F, Amadio S, Fusco FR, Viscomi MT, D’Ambrosi N, Vacca F, Sancesario G, Bernardi G, Molinari M, Volonte C. Up-regulation of P2X2, P2X4 receptor and ischemic cell death: prevention by P2 antagonists. Neuroscience. 2003;120:85–98. doi: 10.1016/s0306-4522(03)00228-8. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Horcajada JP, Obach V, Vargas M, Revilla M, Torres F, Cervera A, Planas AM, Mensa J. The Early Systemic Prophylaxis of Infection After Stroke study: a randomized clinical trial. Stroke. 2005;36:1495–1500. doi: 10.1161/01.STR.0000170644.15504.49. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R. The immunology of acute stroke. Nat Rev Neurol. 2012;8:401–410. doi: 10.1038/nrneurol.2012.98. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, Berman NE, Vogel SN. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab. 2003;23:748–755. doi: 10.1097/01.WCB.0000071885.63724.20. [DOI] [PubMed] [Google Scholar]
- Cheng SS, Lai JJ, Lukacs NW, Kunkel SL. Granulocyte-macrophage colony stimulating factor up-regulates CCR1 in human neutrophils. J Immunol. 2001;166:1178–1184. doi: 10.4049/jimmunol.166.2.1178. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- Chu K, Yin B, Wang J, Peng G, Liang H, Xu Z, Du Y, Fang M, Xia Q, Luo B. Inhibition of P2X7 receptor ameliorates transient global cerebral ischemia/reperfusion injury via modulating inflammatory responses in the rat hippocampus. J Neuroinflammation. 2012;9:69. doi: 10.1186/1742-2094-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu LS, Fang SH, Zhou Y, Yin YJ, Chen WY, Li JH, Sun J, Wang ML, Zhang WP, Wei EQ. Minocycline inhibits 5-lipoxygenase expression and accelerates functional recovery in chronic phase of focal cerebral ischemia in rats. Life Sci. 2010;86:170–177. doi: 10.1016/j.lfs.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Chu LS, Fang SH, Zhou Y, Yu GL, Wang ML, Zhang WP, Wei EQ. Minocycline inhibits 5-lipoxygenase activation and brain inflammation after focal cerebral ischemia in rats. Acta Pharmacol Sin. 2007;28:763–772. doi: 10.1111/j.1745-7254.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- Chua CC, Hamdy RC, Chua BH. Upregulation of vascular endothelial growth factor by H2O2 in rat heart endothelial cells. Free Radic Biol Med. 1998;25:891–897. doi: 10.1016/s0891-5849(98)00115-4. [DOI] [PubMed] [Google Scholar]
- Clark RK, Lee EV, White RF, Jonak ZL, Feuerstein GZ, Barone FC. Reperfusion following focal stroke hastens inflammation and resolution of ischemic injured tissue. Brain Res Bull. 1994;35:387–392. doi: 10.1016/0361-9230(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- Connolly ES, Jr, Winfree CJ, Prestigiacomo CJ, Kim SC, Choudhri TF, Hoh BL, Naka Y, Solomon RA, Pinsky DJ. Exacerbation of cerebral injury in mice that express the P-selectin gene: identification of P-selectin blockade as a new target for the treatment of stroke. Circ Res. 1997;81:304–310. doi: 10.1161/01.res.81.3.304. [DOI] [PubMed] [Google Scholar]
- Connolly ES, Jr, Winfree CJ, Springer TA, Naka Y, Liao H, Yan SD, Stern DM, Solomon RA, Gutierrez-Ramos JC, Pinsky DJ. Cerebral protection in homozygous null ICAM-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest. 1996;97:209–216. doi: 10.1172/JCI118392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell RM, Xu H, Galasso JM, Silverstein FS. Hypoxic-ischemic injury induces macrophage inflammatory protein-1alpha expression in immature rat brain. Stroke. 2002;33:795–801. doi: 10.1161/hs0302.103740. [DOI] [PubMed] [Google Scholar]
- Czeh M, Gressens P, Kaindl AM. The yin and yang of microglia. Dev Neurosci. 2011;33:199–209. doi: 10.1159/000328989. [DOI] [PubMed] [Google Scholar]
- Dambinova SA, Khounteev GA, Izykenova GA, Zavolokov IG, Ilyukhina AY, Skoromets AA. Blood test detecting autoantibodies to N-methyl-D-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clin Chem. 2003;49:1752–1762. doi: 10.1373/49.10.1752. [DOI] [PubMed] [Google Scholar]
- de Vos AF, van MM, Brok HP, Boven LA, Hintzen RQ, van d V, Ravid R, Rensing S, Boon L, ‘t Hart BA, Laman JD. Transfer of central nervous system autoantigens and presentation in secondary lymphoid organs. J Immunol. 2002;169:5415–5423. doi: 10.4049/jimmunol.169.10.5415. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Milner R, Mabuchi T, Hung S, Wang X, Berg GI, Koziol JA. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke. 2007;38:646–651. doi: 10.1161/01.STR.0000254477.34231.cb. [DOI] [PubMed] [Google Scholar]
- Denes A, Humphreys N, Lane TE, Grencis R, Rothwell N. Chronic systemic infection exacerbates ischemic brain damage via a CCL5 (regulated on activation, normal T-cell expressed and secreted)-mediated proinflammatory response in mice. J Neurosci. 2010;30:10086–10095. doi: 10.1523/JNEUROSCI.1227-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes A, Vidyasagar R, Feng J, Narvainen J, McColl BW, Kauppinen RA, Allan SM. Proliferating resident microglia after focal cerebral ischaemia in mice. J Cereb Blood Flow Metab. 2007;27:1941–1953. doi: 10.1038/sj.jcbfm.9600495. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2007;38:1345–1353. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T, Prass K, Meisel A. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770–773. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- Dittel BN, Visintin I, Merchant RM, Janeway CA., Jr Presentation of the self antigen myelin basic protein by dendritic cells leads to experimental autoimmune encephalomyelitis. J Immunol. 1999;163:32–39. [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Domac FM, Misirli H. The role of neutrophils and interleukin-8 in acute ischemic stroke. Neurosciences (Riyadh) 2008;13:136–141. [PubMed] [Google Scholar]
- Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, Bar-Or A, Antel JP. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–727. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]
- Dziedzic T. Clinical significance of acute phase reaction in stroke patients. Front Biosci. 2008;13:2922–2927. doi: 10.2741/2897. [DOI] [PubMed] [Google Scholar]
- Dzietko M, Derugin N, Wendland MF, Vexler ZS, Ferriero DM. Delayed VEGF Treatment Enhances Angiogenesis and Recovery After Neonatal Focal Rodent Stroke. Transl Stroke Res. 2013;4:189–200. doi: 10.1007/s12975-012-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley HC, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, Tyrrell PJ. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76:1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enlimomab Acute Stroke Trial Investigators. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57:1428–1434. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- Ephrem A, Chamat S, Miquel C, Fisson S, Mouthon L, Caligiuri G, Delignat S, Elluru S, Bayry J, Lacroix-Desmazes S, Cohen JL, Salomon BL, Kazatchkine MD, Kaveri SV, Misra N. Expansion of CD4+CD25+ regulatory T cells by intravenous immunoglobulin: a critical factor in controlling experimental autoimmune encephalomyelitis. Blood. 2008;111:715–722. doi: 10.1182/blood-2007-03-079947. [DOI] [PubMed] [Google Scholar]
- Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162–2171. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- Fagan SC, Cronic LE, Hess DC. Minocycline Development for Acute Ischemic Stroke. Transl Stroke Res. 2011;2:202–208. doi: 10.1007/s12975-011-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan SC, Waller JL, Nichols FT, Edwards DJ, Pettigrew LC, Clark WM, Hall CE, Switzer JA, Ergul A, Hess DC. Minocycline to improve neurologic outcome in stroke (MINOS): a dose-finding study. Stroke. 2010;41:2283–2287. doi: 10.1161/STROKEAHA.110.582601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathali N, Ostrowski RP, Hasegawa Y, Lekic T, Tang J, Zhang JH. Splenic Immune Cells In Experimental Neonatal Hypoxia-Ischemia. Transl Stroke Res. 2013;4:208–219. doi: 10.1007/s12975-012-0239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Abe T, Kaunzner UW, Gottfried-Blackmore A, Gal-Toth J, McEwen BS, Iadecola C, Bulloch K. Brain dendritic cells in ischemic stroke: time course, activation state, and origin. Brain Behav Immun. 2010;24:724–737. doi: 10.1016/j.bbi.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszer U, Korczak-Kowalska G, Palasik W, Korlak J, Gorski A, Czlonkowska A. Increased expression of adhesion molecule CD18 (LFA-1beta) on the leukocytes of peripheral blood in patients with acute ischemic stroke. Acta Neurol Scand. 1998;97:221–224. doi: 10.1111/j.1600-0404.1998.tb00641.x. [DOI] [PubMed] [Google Scholar]
- Foerch C, Singer OC, Neumann-Haefelin T, du Mesnil de RR, Steinmetz H, Sitzer M. Evaluation of serum S100B as a surrogate marker for long-term outcome and infarct volume in acute middle cerebral artery infarction. Arch Neurol. 2005;62:1130–1134. doi: 10.1001/archneur.62.7.1130. [DOI] [PubMed] [Google Scholar]
- Fogal B, Hewett JA, Hewett SJ. Interleukin-1beta potentiates neuronal injury in a variety of injury models involving energy deprivation. J Neuroimmunol. 2005;161:93–100. doi: 10.1016/j.jneuroim.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Franke H, Gunther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D, Illes P. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol. 2004;63:686–699. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- Frenkel D, Huang Z, Maron R, Koldzic DN, Hancock WW, Moskowitz MA, Weiner HL. Nasal vaccination with myelin oligodendrocyte glycoprotein reduces stroke size by inducing IL-10-producing CD4+ T cells. J Immunol. 2003;171:6549–6555. doi: 10.4049/jimmunol.171.12.6549. [DOI] [PubMed] [Google Scholar]
- Frenkel D, Huang Z, Maron R, Koldzic DN, Moskowitz MA, Weiner HL. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. J Neurol Sci. 2005;233:125–132. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Frieler RA, Meng H, Duan SZ, Berger S, Schutz G, He Y, Xi G, Wang MM, Mortensen RM. Myeloid-specific deletion of the mineralocorticoid receptor reduces infarct volume and alters inflammation during cerebral ischemia. Stroke. 2011;42:179–185. doi: 10.1161/STROKEAHA.110.598441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijns CJ, Kappelle LJ, van GJ, Nieuwenhuis HK, Sixma JJ, Fijnheer R. Soluble adhesion molecules reflect endothelial cell activation in ischemic stroke and in carotid atherosclerosis. Stroke. 1997;28:2214–2218. doi: 10.1161/01.str.28.11.2214. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee JM, Kalil A, Thullbery M, Becker KJ. Induction of immunologic tolerance to myelin basic protein prevents central nervous system autoimmunity and improves outcome after stroke. Stroke. 2008;39:1575–1582. doi: 10.1161/STROKEAHA.107.501486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann J, Mies G, Bonnekoh P, Banati R, Iijima T, Kreutzberg GW, Hossmann KA. Microglial reaction in the rat cerebral cortex induced by cortical spreading depression. Brain Pathol. 1993;3:11–17. doi: 10.1111/j.1750-3639.1993.tb00720.x. [DOI] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Goede V, Brogelli L, Ziche M, Augustin HG. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer. 1999;82:765–770. doi: 10.1002/(sici)1097-0215(19990827)82:5<765::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Goerdt S, Politz O, Schledzewski K, Birk R, Gratchev A, Guillot P, Hakiy N, Klemke CD, Dippel E, Kodelja V, Orfanos CE. Alternative versus classical activation of macrophages. Pathobiology. 1999;67:222–226. doi: 10.1159/000028096. [DOI] [PubMed] [Google Scholar]
- Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- Guarini S, Altavilla D, Cainazzo MM, Giuliani D, Bigiani A, Marini H, Squadrito G, Minutoli L, Bertolini A, Marini R, Adamo EB, Venuti FS, Squadrito F. Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107:1189–1194. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- Guo LH, Schluesener HJ. Lesional accumulation of P2X(4) receptor(+) macrophages in rat CNS during experimental autoimmune encephalomyelitis. Neuroscience. 2005;134:199–205. doi: 10.1016/j.neuroscience.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hardy J, Bednarski RM, Biller DS. Effect of phenylephrine on hemodynamics and splenic dimensions in horses. Am J Vet Res. 1994;55:1570–1578. [PubMed] [Google Scholar]
- Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE, Conway SJ. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63:84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- Hiromatsu Y, Toda S. Mast cells and angiogenesis. Microsc Res Tech. 2003;60:64–69. doi: 10.1002/jemt.10244. [DOI] [PubMed] [Google Scholar]
- Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- Huang J, Choudhri TF, Winfree CJ, McTaggart RA, Kiss S, Mocco J, Kim LJ, Protopsaltis TS, Zhang Y, Pinsky DJ, Connolly ES., Jr Postischemic cerebrovascular E-selectin expression mediates tissue injury in murine stroke. Stroke. 2000;31:3047–3053. [PubMed] [Google Scholar]
- Hug A, Liesz A, Muerle B, Zhou W, Ehrenheim J, Lorenz A, Dalpke A, Veltkamp R. Reduced efficacy of circulating costimulatory cells after focal cerebral ischemia. Stroke. 2011;42:3580–3586. doi: 10.1161/STROKEAHA.111.620948. [DOI] [PubMed] [Google Scholar]
- Hughes PM, Allegrini PR, Rudin M, Perry VH, Mir AK, Wiessner C. Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. J Cereb Blood Flow Metab. 2002;22:308–317. doi: 10.1097/00004647-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Hume DA. Macrophages as APC and the dendritic cell myth. J Immunol. 2008;181:5829–5835. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- Hur J, Lee P, Kim MJ, Kim Y, Cho YW. Ischemia-activated microglia induces neuronal injury via activation of gp91phox NADPH oxidase. Biochem Biophys Res Commun. 2010;391:1526–1530. doi: 10.1016/j.bbrc.2009.12.114. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston JM, Rosas-Ballina M, Xue X, Dowling O, Ochani K, Ochani M, Yeboah MM, Chatterjee PK, Tracey KJ, Metz CN. Cholinergic neural signals to the spleen down-regulate leukocyte trafficking via CD11b. J Immunol. 2009;183:552–559. doi: 10.4049/jimmunol.0802684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi S, Maric D, Mou Y, Ohtani R, Ruetzler C, Hallenbeck JM. Mucosal tolerance to E-selectin promotes the survival of newly generated neuroblasts via regulatory T-cell induction after stroke in spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2009;29:606–620. doi: 10.1038/jcbfm.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Cooper D, Russell J, Salter JW, Zhang JH, Nanda A, Granger DN. Molecular determinants of the prothrombogenic and inflammatory phenotype assumed by the postischemic cerebral microcirculation. Stroke. 2003;34:1777–1782. doi: 10.1161/01.STR.0000074921.17767.F2. [DOI] [PubMed] [Google Scholar]
- Ivacko J, Szaflarski J, Malinak C, Flory C, Warren JS, Silverstein FS. Hypoxic-ischemic injury induces monocyte chemoattractant protein-1 expression in neonatal rat brain. J Cereb Blood Flow Metab. 1997;17:759–770. doi: 10.1097/00004647-199707000-00006. [DOI] [PubMed] [Google Scholar]
- Jander S, Kraemer M, Schroeter M, Witte OW, Stoll G. Lymphocytic infiltration and expression of intercellular adhesion molecule-1 in photochemically induced ischemia of the rat cortex. J Cereb Blood Flow Metab. 1995;15:42–51. doi: 10.1038/jcbfm.1995.5. [DOI] [PubMed] [Google Scholar]
- Jauch EC, Lindsell C, Broderick J, Fagan SC, Tilley BC, Levine SR. Association of serial biochemical markers with acute ischemic stroke: the National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke. 2006;37:2508–2513. doi: 10.1161/01.STR.0000242290.01174.9e. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranyi Z, Sperlagh B, Vizi ES. Involvement of P2 purinoceptors and the nitric oxide pathway in [3H]purine outflow evoked by short-term hypoxia and hypoglycemia in rat hippocampal slices. Brain Res. 1999;823:183–190. doi: 10.1016/s0006-8993(99)01169-5. [DOI] [PubMed] [Google Scholar]
- Justicia C, Panes J, Sole S, Cervera A, Deulofeu R, Chamorro A, Planas AM. Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J Cereb Blood Flow Metab. 2003;23:1430–1440. doi: 10.1097/01.WCB.0000090680.07515.C8. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Song TJ, Park JH, Lee HS, Nam CM, Nam HS, Kim YD, Heo JH. Different prognostic value of white blood cell subtypes in patients with acute cerebral infarction. Atherosclerosis. 2012;222:464–467. doi: 10.1016/j.atherosclerosis.2012.02.042. [DOI] [PubMed] [Google Scholar]
- Kim JB, Lim CM, Yu YM, Lee JK. Induction and subcellular localization of high-mobility group box-1 (HMGB1) in the postischemic rat brain. J Neurosci Res. 2008;86:1125–1131. doi: 10.1002/jnr.21555. [DOI] [PubMed] [Google Scholar]
- Kim JB, Sig CJ, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006;26:6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Chopp M, Chen H, Levine SR, Carey JL, Welch KM. Adhesive glycoproteins CD11a and CD18 are upregulated in the leukocytes from patients with ischemic stroke and transient ischemic attacks. J Neurol Sci. 1995a;128:45–50. doi: 10.1016/0022-510x(94)00203-z. [DOI] [PubMed] [Google Scholar]
- Kim JS, Gautam SC, Chopp M, Zaloga C, Jones ML, Ward PA, Welch KM. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 after focal cerebral ischemia in the rat. J Neuroimmunol. 1995b;56:127–134. doi: 10.1016/0165-5728(94)00138-e. [DOI] [PubMed] [Google Scholar]
- Kin NW, Sanders VM. It takes nerve to tell T and B cells what to do. J Leukoc Biol. 2006;79:1093–1104. doi: 10.1189/jlb.1105625. [DOI] [PubMed] [Google Scholar]
- Klein RL, Wilson SP, Dzielak DJ, Yang WH, Viveros OH. Opioid peptides and noradrenaline co-exist in large dense-cored vesicles from sympathetic nerve. Neuroscience. 1982;7:2255–2261. doi: 10.1016/0306-4522(82)90135-x. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2013;121:679–691. doi: 10.1182/blood-2012-04-426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschnitz C, Schwab N, Kraft P, Hagedorn I, Dreykluft A, Schwarz T, Austinat M, Nieswandt B, Wiendl H, Stoll G. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood. 2010;115:3835–3842. doi: 10.1182/blood-2009-10-249078. [DOI] [PubMed] [Google Scholar]
- Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Kodama H, Inoue T, Watanabe R, Yasutomi D, Kawakami Y, Ogawa S, Mikoshiba K, Ikeda Y, Kuwana M. Neurogenic potential of progenitors derived from human circulating CD14+ monocytes. Immunol Cell Biol. 2006;84:209–217. doi: 10.1111/j.1440-1711.2006.01424.x. [DOI] [PubMed] [Google Scholar]
- Kostulas N, Li HL, Xiao BG, Huang YM, Kostulas V, Link H. Dendritic cells are present in ischemic brain after permanent middle cerebral artery occlusion in the rat. Stroke. 2002;33:1129–1134. doi: 10.1161/hs0402.105379. [DOI] [PubMed] [Google Scholar]
- Krams M, Lees KR, Hacke W, Grieve AP, Orgogozo JM, Ford GA. Acute Stroke Therapy by Inhibition of Neutrophils (ASTIN): an adaptive dose-response study of UK-279,276 in acute ischemic stroke. Stroke. 2003;34:2543–2548. doi: 10.1161/01.STR.0000092527.33910.89. [DOI] [PubMed] [Google Scholar]
- Krieglstein K, Strelau J, Schober A, Sullivan A, Unsicker K. TGF-beta and the regulation of neuron survival and death. J Physiol Paris. 2002;96:25–30. doi: 10.1016/s0928-4257(01)00077-8. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalive PH, Paglinawan R, Biollaz G, Kappos EA, Leone DP, Malipiero U, Relvas JB, Moransard M, Suter T, Fontana A. TGF-beta-treated microglia induce oligodendrocyte precursor cell chemotaxis through the HGF-c-Met pathway. Eur J Immunol. 2005;35:727–737. doi: 10.1002/eji.200425430. [DOI] [PubMed] [Google Scholar]
- Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69:1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- Larsson J, Goumans MJ, Sjostrand LJ, van Rooijen MA, Ward D, Leveen P, Xu X, ten DP, Mummery CL, Karlsson S. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J. 2001;20:1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Park JW, Kim SP, Lo EH, Lee SR. Doxycycline inhibits matrix metalloproteinase-9 and laminin degradation after transient global cerebral ischemia. Neurobiol Dis. 2009;34:189–198. doi: 10.1016/j.nbd.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol. 1993;150:2659–2667. [PubMed] [Google Scholar]
- Lehnardt S, Lehmann S, Kaul D, Tschimmel K, Hoffmann O, Cho S, Krueger C, Nitsch R, Meisel A, Weber JR. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J Neuroimmunol. 2007;190:28–33. doi: 10.1016/j.jneuroim.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Li F, Wang L, Li JW, Gong M, He L, Feng R, Dai Z, Li SQ. Hypoxia induced amoeboid microglial cell activation in postnatal rat brain is mediated by ATP receptor P2X4. BMC Neurosci. 2011;12:111–112. doi: 10.1186/1471-2202-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Gan Y, Sun BL, Zhang F, Lu B, Gao Y, Liang W, Thomson AW, Chen J, Hu X. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann Neurol. 2012:10. doi: 10.1002/ana.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Liesz A, Zhou W, Mracsko E, Karcher S, Bauer H, Schwarting S, Sun L, Bruder D, Stegemann S, Cerwenka A, Sommer C, Dalpke AH, Veltkamp R. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain. 2011;134:704–720. doi: 10.1093/brain/awr008. [DOI] [PubMed] [Google Scholar]
- Lindsberg PJ, Carpen O, Paetau A, Karjalainen-Lindsberg ML, Kaste M. Endothelial ICAM-1 expression associated with inflammatory cell response in human ischemic stroke. Circulation. 1996;94:939–945. doi: 10.1161/01.cir.94.5.939. [DOI] [PubMed] [Google Scholar]
- Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant Rev (Orlando) 2009;23:1–10. doi: 10.1016/j.trre.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Sandor M, Suresh M, Fabry Z. Traumatic injury and the presence of antigen differentially contribute to T-cell recruitment in the CNS. J Neurosci. 2006;26:731–741. doi: 10.1523/JNEUROSCI.3502-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- Losy J, Zaremba J. Monocyte chemoattractant protein-1 is increased in the cerebrospinal fluid of patients with ischemic stroke. Stroke. 2001;32:2695–2696. doi: 10.1161/hs1101.097380. [DOI] [PubMed] [Google Scholar]
- Lu Y, Wahl LM. Production of matrix metalloproteinase-9 by activated human monocytes involves a phosphatidylinositol-3 kinase/Akt/IKKalpha/NF-kappaB pathway. J Leukoc Biol. 2005;78:259–265. doi: 10.1189/jlb.0904498. [DOI] [PubMed] [Google Scholar]
- Luheshi NM, Kovacs KJ, Lopez-Castejon G, Brough D, Denes A. Interleukin-1alpha expression precedes IL-1beta after ischemic brain injury and is localised to areas of focal neuronal loss and penumbral tissues. J Neuroinflammation. 2011;8:186–188. doi: 10.1186/1742-2094-8-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Ma Y, Yi X, Guo R, Zhu W, Fan X, Xu G, Frey WH, Liu X. Intranasal delivery of transforming growth factor-beta1 in mice after stroke reduces infarct volume and increases neurogenesis in the subventricular zone. BMC Neurosci. 2008;9:117–119. doi: 10.1186/1471-2202-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi T, Kitagawa K, Ohtsuki T, Kuwabara K, Yagita Y, Yanagihara T, Hori M, Matsumoto M. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke. 2000;31:1735–1743. doi: 10.1161/01.str.31.7.1735. [DOI] [PubMed] [Google Scholar]
- Mathieu P, Piantanida AP, Pitossi F. Chronic expression of transforming growth factor-beta enhances adult neurogenesis. Neuroimmunomodulation. 2010;17:200–201. doi: 10.1159/000258723. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Onodera H, Shiga Y, Nakamura M, Ninomiya M, Kihara T, Kogure K. Correlation between myeloperoxidase-quantified neutrophil accumulation and ischemic brain injury in the rat. Effects of neutrophil depletion. Stroke. 1994;25:1469–1475. doi: 10.1161/01.str.25.7.1469. [DOI] [PubMed] [Google Scholar]
- McCourt M, Wang JH, Sookhai S, Redmond HP. Proinflammatory mediators stimulate neutrophil-directed angiogenesis. Arch Surg. 1999;134:1325–1331. doi: 10.1001/archsurg.134.12.1325. [DOI] [PubMed] [Google Scholar]
- Mecha M, Carrillo-Salinas FJ, Mestre L, Feliu A, Guaza C. Viral models of multiple sclerosis: neurodegeneration and demyelination in mice infected with Theiler’s virus. Prog Neurobiol. 2013;101–102:46–64. doi: 10.1016/j.pneurobio.2012.11.003. Epub;%2012 Nov 29.:46–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekhail M, Almazan G, Tabrizian M. Oligodendrocyte-protection and remyelination post-spinal cord injuries: a review. Prog Neurobiol. 2012;96:322–339. doi: 10.1016/j.pneurobio.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Melani A, Turchi D, Vannucchi MG, Cipriani S, Gianfriddo M, Pedata F. ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem Int. 2005;47:442–448. doi: 10.1016/j.neuint.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Melter M, Reinders ME, Sho M, Pal S, Geehan C, Denton MD, Mukhopadhyay D, Briscoe DM. Ligation of CD40 induces the expression of vascular endothelial growth factor by endothelial cells and monocytes and promotes angiogenesis in vivo. Blood. 2000;96:3801–3808. [PubMed] [Google Scholar]
- Meszaros AJ, Reichner JS, Albina JE. Macrophage-induced neutrophil apoptosis. J Immunol. 2000;165:435–441. doi: 10.4049/jimmunol.165.1.435. [DOI] [PubMed] [Google Scholar]
- Mitrovic B, Ignarro LJ, Vinters HV, Akers MA, Schmid I, Uittenbogaart C, Merrill JE. Nitric oxide induces necrotic but not apoptotic cell death in oligodendrocytes. Neuroscience. 1995;65:531–539. doi: 10.1016/0306-4522(94)00491-m. [DOI] [PubMed] [Google Scholar]
- Moldovan L, Moldovan NI. Role of monocytes and macrophages in angiogenesis. EXS. 2005:127–146. doi: 10.1007/3-7643-7311-3_9. [DOI] [PubMed] [Google Scholar]
- Mori T, Tan J, Arendash GW, Koyama N, Nojima Y, Town T. Overexpression of human S100B exacerbates brain damage and periinfarct gliosis after permanent focal ischemia. Stroke. 2008;39:2114–2121. doi: 10.1161/STROKEAHA.107.503821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, Bendszus M, Rossetti G, Nawroth PP, Bierhaus A, Schwaninger M. The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci. 2008;28:12023–12031. doi: 10.1523/JNEUROSCI.2435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nomura S, Tandon NN, Nakamura T, Cone J, Fukuhara S, Kambayashi J. High-shear-stress-induced activation of platelets and microparticles enhances expression of cell adhesion molecules in THP-1 and endothelial cells. Atherosclerosis. 2001;158:277–287. doi: 10.1016/s0021-9150(01)00433-6. [DOI] [PubMed] [Google Scholar]
- Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006a;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006b;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- Okada Y, Copeland BR, Mori E, Tung MM, Thomas WS, del Zoppo GJ. P-selectin and intercellular adhesion molecule-1 expression after focal brain ischemia and reperfusion. Stroke. 1994;25:202–211. doi: 10.1161/01.str.25.1.202. [DOI] [PubMed] [Google Scholar]
- Ostrowski RP, Schulte RW, Nie Y, Ling T, Lee T, Manaenko A, Gridley DS, Zhang JH. Acute Splenic Irradiation Reduces Brain Injury in the Rat Focal Ischemic Stroke Model. Transl Stroke Res. 2012;3:473–481. doi: 10.1007/s12975-012-0206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottonello L, Montecucco F, Bertolotto M, Arduino N, Mancini M, Corcione A, Pistoia V, Dallegri F. CCL3 (MIP-1alpha) induces in vitro migration of GM-CSF-primed human neutrophils via CCR5-dependent activation of ERK 1/2. Cell Signal. 2005;17:355–363. doi: 10.1016/j.cellsig.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol. 2009;220:562–568. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- Park JS, Shin JA, Jung JS, Hyun JW, Van Le TK, Kim DH, Park EM, Kim HS. Anti-inflammatory mechanism of compound K in activated microglia and its neuroprotective effect on experimental stroke in mice. J Pharmacol Exp Ther. 2012;341:59–67. doi: 10.1124/jpet.111.189035. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish WR, Rosas-Ballina M, Czura CJ, Larosa GJ, Miller EJ, Tracey KJ, Al-Abed Y. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med. 2007;35:1139–1144. doi: 10.1097/01.CCM.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9:125–134. [PMC free article] [PubMed] [Google Scholar]
- Phillips S, Chokshi S, Riva A, Evans A, Williams R, Naoumov NV. CD8(+) T cell control of hepatitis B virus replication: direct comparison between cytolytic and noncytolytic functions. J Immunol. 2010;184:287–295. doi: 10.4049/jimmunol.0902761. [DOI] [PubMed] [Google Scholar]
- Planas AM, Gomez-Choco M, Urra X, Gorina R, Caballero M, Chamorro A. Brain-derived antigens in lymphoid tissue of patients with acute stroke. J Immunol. 2012;188:2156–2163. doi: 10.4049/jimmunol.1102289. [DOI] [PubMed] [Google Scholar]
- Prass K, Braun JS, Dirnagl U, Meisel C, Meisel A. Stroke propagates bacterial aspiration to pneumonia in a model of cerebral ischemia. Stroke. 2006;37:2607–2612. doi: 10.1161/01.STR.0000240409.68739.2b. [DOI] [PubMed] [Google Scholar]
- Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk HD, Meisel A. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Menon DK, Peters AM, Ballinger JR, Barber RW, Balan KK, Lynch A, Xuereb JH, Fryer T, Guadagno JV, Warburton EA. Cerebral neutrophil recruitment, histology, and outcome in acute ischemic stroke: an imaging-based study. Stroke. 2004;35:1659–1664. doi: 10.1161/01.STR.0000130592.71028.92. [DOI] [PubMed] [Google Scholar]
- Pun PB, Lu J, Moochhala S. Involvement of ROS in BBB dysfunction. Free Radic Res. 2009;43:348–364. doi: 10.1080/10715760902751902. [DOI] [PubMed] [Google Scholar]
- Puxeddu I, Alian A, Piliponsky AM, Ribatti D, Panet A, Levi-Schaffer F. Human peripheral blood eosinophils induce angiogenesis. Int J Biochem Cell Biol. 2005a;37:628–636. doi: 10.1016/j.biocel.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Puxeddu I, Ribatti D, Crivellato E, Levi-Schaffer F. Mast cells and eosinophils: a novel link between inflammation and angiogenesis in allergic diseases. J Allergy Clin Immunol. 2005b;116:531–536. doi: 10.1016/j.jaci.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Qin Z, Blankenstein T. CD4+ T cell--mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity. 2000;12:677–686. doi: 10.1016/s1074-7613(00)80218-6. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Reaux-Le GA, Van SJ, Rostene W, Melik PS. Current status of chemokines in the adult CNS. Prog Neurobiol. 2013;104:67–92. doi: 10.1016/j.pneurobio.2013.02.001. Epub;%2013 Feb 27.:67–92. [DOI] [PubMed] [Google Scholar]
- Reichel CA, Rehberg M, Lerchenberger M, Berberich N, Bihari P, Khandoga AG, Zahler S, Krombach F. Ccl2 and Ccl3 mediate neutrophil recruitment via induction of protein synthesis and generation of lipid mediators. Arterioscler Thromb Vasc Biol. 2009;29:1787–1793. doi: 10.1161/ATVBAHA.109.193268. [DOI] [PubMed] [Google Scholar]
- Relton JK, Sloan KE, Frew EM, Whalley ET, Adams SP, Lobb RR. Inhibition of alpha4 integrin protects against transient focal cerebral ischemia in normotensive and hypertensive rats. Stroke. 2001;32:199–205. doi: 10.1161/01.str.32.1.199. [DOI] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD, Offner H. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J Neurosci. 2011a;31:8556–8563. doi: 10.1523/JNEUROSCI.1623-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. CD4+FoxP3+ regulatory T-cells in cerebral ischemic stroke. Metab Brain Dis. 2011b;26:87–90. doi: 10.1007/s11011-010-9226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson’s disease. J Leukoc Biol. 2007;82:1083–1094. doi: 10.1189/jlb.0507296. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Stone DK, Hutter JA, Benner EJ, Mosley RL, Gendelman HE. Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease. J Immunol. 2010;184:2261–2271. doi: 10.4049/jimmunol.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes HM, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–70. doi: 10.1146/annurev.immunol.20.100201.131730. Epub;%2001 Oct 4.:323–370. [DOI] [PubMed] [Google Scholar]
- Rymo SF, Gerhardt H, Wolfhagen SF, Lang R, Uv A, Betsholtz C. A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures. PLoS One. 2011;6:e15846. doi: 10.1371/journal.pone.0015846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, Tracey KJ, Al-Abed Y, Metz CN. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino O, Taguchi A, Nakagomi T, Nakano-Doi A, Kashiwamura S, Doe N, Nakagomi N, Soma T, Yoshikawa H, Stern DM, Okamura H, Matsuyama T. Immunodeficiency reduces neural stem/progenitor cell apoptosis and enhances neurogenesis in the cerebral cortex after stroke. J Neurosci Res. 2010;88:2385–2397. doi: 10.1002/jnr.22410. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- Santana MA, Rosenstein Y. What it takes to become an effector T cell: the process, the cells involved, and the mechanisms. J Cell Physiol. 2003;195:392–401. doi: 10.1002/jcp.10258. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2003;183:25–33. doi: 10.1016/s0014-4886(03)00082-7. [DOI] [PubMed] [Google Scholar]
- Schilling M, Besselmann M, Muller M, Strecker JK, Ringelstein EB, Kiefer R. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2005;196:290–297. doi: 10.1016/j.expneurol.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Schilling M, Strecker JK, Ringelstein EB, Schabitz WR, Kiefer R. The role of CC chemokine receptor 2 on microglia activation and blood-borne cell recruitment after transient focal cerebral ischemia in mice. Brain Res. 2009;1289:79–84. doi: 10.1016/j.brainres.2009.06.054. Epub;%2009 Jun 24.:79–84. [DOI] [PubMed] [Google Scholar]
- Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol. 1998;161:3340–3346. [PubMed] [Google Scholar]
- Schroeter M, Jander S, Witte OW, Stoll G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J Neuroimmunol. 1994;55:195–203. doi: 10.1016/0165-5728(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Guo L, Schluesener HJ. Spinal cord injury induces early and persistent lesional P2X4 receptor expression. J Neuroimmunol. 2005;163:185–189. doi: 10.1016/j.jneuroim.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- Selye H. A syndrome produced by diverse nocuous agents. 1936. J Neuropsychiatry Clin Neurosci. 1998;10:230–231. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- Sergeeva SP, Erofeeva LM, Gul’tiaev MM. IL-1beta, IL-10, INF-gamma, TNF-alpha, S100beta, AMA-M2 and cell immune response in stroke. Patol Fiziol Eksp Ter. 2011:41–45. [PubMed] [Google Scholar]
- Shenhar-Tsarfaty S, Ben AE, Bova I, Shopin L, Fried M, Berliner S, Shapira I, Bornstein NM. Interleukin-6 as an early predictor for one-year survival following an ischaemic stroke/transient ischaemic attack. Int J Stroke. 2010;5:16–20. doi: 10.1111/j.1747-4949.2009.00396.x. [DOI] [PubMed] [Google Scholar]
- Shiga Y, Onodera H, Kogure K, Yamasaki Y, Yashima Y, Syozuhara H, Sendo F. Neutrophil as a mediator of ischemic edema formation in the brain. Neurosci Lett. 1991;125:110–112. doi: 10.1016/0304-3940(91)90003-c. [DOI] [PubMed] [Google Scholar]
- Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano SG, Lipton SA, Wang YF, Xiao M, Springer TA, Gutierrez-Ramos JC, Hickey PR. Intercellular adhesion molecule-1-deficient mice are less susceptible to cerebral ischemia-reperfusion injury. Ann Neurol. 1996;39:618–624. doi: 10.1002/ana.410390511. [DOI] [PubMed] [Google Scholar]
- Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neurosci Lett. 1998;251:189–192. doi: 10.1016/s0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- Stanimirovic DB, Wong J, Shapiro A, Durkin JP. Increase in surface expression of ICAM-1, VCAM-1 and E-selectin in human cerebromicrovascular endothelial cells subjected to ischemia-like insults. Acta Neurochir Suppl. 1997;70:12–16. doi: 10.1007/978-3-7091-6837-0_4. [DOI] [PubMed] [Google Scholar]
- Stevens SL, Bao J, Hollis J, Lessov NS, Clark WM, Stenzel-Poore MP. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res. 2002;932:110–119. doi: 10.1016/s0006-8993(02)02292-8. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Hurley SD, McGraw TS, Semple-Rowland SL. Comparative evaluation of cytokine profiles and reactive gliosis supports a critical role for interleukin-6 in neuron-glia signaling during regeneration. J Neurosci Res. 2000;61:10–20. doi: 10.1002/1097-4547(20000701)61:1<10::AID-JNR2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Strle K, Zhou JH, Broussard SR, Venters HD, Johnson RW, Freund GG, Dantzer R, Kelley KW. IL-10 promotes survival of microglia without activating Akt. J Neuroimmunol. 2002;122:9–19. doi: 10.1016/s0165-5728(01)00444-1. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- Takami S, Minami M, Nagata I, Namura S, Satoh M. Chemokine receptor antagonist peptide, viral MIP-II, protects the brain against focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2001;21:1430–1435. doi: 10.1097/00004647-200112000-00007. [DOI] [PubMed] [Google Scholar]
- Takami S, Nishikawa H, Minami M, Nishiyori A, Sato M, Akaike A, Satoh M. Induction of macrophage inflammatory protein MIP-1alpha mRNA on glial cells after focal cerebral ischemia in the rat. Neurosci Lett. 1997;227:173–176. doi: 10.1016/s0304-3940(97)00338-8. [DOI] [PubMed] [Google Scholar]
- Tanaka R, Komine-Kobayashi M, Mochizuki H, Yamada M, Furuya T, Migita M, Shimada T, Mizuno Y, Urabe T. Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience. 2003;117:531–539. doi: 10.1016/s0306-4522(02)00954-5. [DOI] [PubMed] [Google Scholar]
- Terao S, Yilmaz G, Stokes KY, Russell J, Ishikawa M, Kawase T, Granger DN. Blood cell-derived RANTES mediates cerebral microvascular dysfunction, inflammation, and tissue injury after focal ischemia-reperfusion. Stroke. 2008;39:2560–2570. doi: 10.1161/STROKEAHA.107.513150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida T, Parker KC, Turner RV, McFarland HF, Coligan JE, Biddison WE. Autoreactive CD8+ T-cell responses to human myelin protein-derived peptides. Proc Natl Acad Sci U S A. 1994;91:10859–10863. doi: 10.1073/pnas.91.23.10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Tuttolomondo A, Di SR, Di RD, Renda C, Pinto A, Licata G. Inflammation as a therapeutic target in acute ischemic stroke treatment. Curr Top Med Chem. 2009;9:1240–1260. doi: 10.2174/156802609789869619. [DOI] [PubMed] [Google Scholar]
- Uchida H, Fujita Y, Matsueda M, Umeda M, Matsuda S, Kato H, Kasahara J, Araki T. Damage to neurons and oligodendrocytes in the hippocampal CA1 sector after transient focal ischemia in rats. Cell Mol Neurobiol. 2010;30:1125–1134. doi: 10.1007/s10571-010-9545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unoki N, Murakami T, Nishijima K, Ogino K, van RN, Yoshimura N. SDF-1/CXCR4 contributes to the activation of tip cells and microglia in retinal angiogenesis. Invest Ophthalmol Vis Sci. 2010;51:3362–3371. doi: 10.1167/iovs.09-4978. [DOI] [PubMed] [Google Scholar]
- Unsicker K, Krieglstein K. Co-activation of TGF-ss and cytokine signaling pathways are required for neurotrophic functions. Cytokine Growth Factor Rev. 2000;11:97–102. doi: 10.1016/s1359-6101(99)00033-7. [DOI] [PubMed] [Google Scholar]
- Verslegers M, Lemmens K, Van HI, Moons L. Matrix metalloproteinase-2 and -9 as promising benefactors in development, plasticity and repair of the nervous system. Prog Neurobiol. 2013;105:60–78. doi: 10.1016/j.pneurobio.2013.03.004. Epub;%2013 Apr 6.:60–78. [DOI] [PubMed] [Google Scholar]
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa P, Triulzi S, Cavalieri B, Di BR, Bertini R, Barbera S, Bigini P, Mennini T, Gelosa P, Tremoli E, Sironi L, Ghezzi P. The interleukin-8 (IL-8/CXCL8) receptor inhibitor reparixin improves neurological deficits and reduces long-term inflammation in permanent and transient cerebral ischemia in rats. Mol Med. 2007;13:125–133. doi: 10.2119/2007-00008.Villa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W, Ilschner S, Ohlemeyer C, Banati R, Kettenmann H. Extracellular ATP activates a cation conductance and a K+ conductance in cultured microglial cells from mouse brain. J Neurosci. 1993;13:4403–4411. doi: 10.1523/JNEUROSCI.13-10-04403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Wang HK, Park UJ, Kim SY, Lee JH, Kim SU, Gwag BJ, Lee YB. Free radical production in CA1 neurons induces MIP-1alpha expression, microglia recruitment, and delayed neuronal death after transient forebrain ischemia. J Neurosci. 2008a;28:1721–1727. doi: 10.1523/JNEUROSCI.4973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li C, Chen Y, Hao Y, Zhou W, Chen C, Yu Z. Hypoxia enhances CXCR4 expression favoring microglia migration via HIF-1alpha activation. Biochem Biophys Res Commun. 2008b;371:283–288. doi: 10.1016/j.bbrc.2008.04.055. [DOI] [PubMed] [Google Scholar]
- Wang X, Yue TL, Barone FC, Feuerstein GZ. Monocyte chemoattractant protein-1 messenger RNA expression in rat ischemic cortex. Stroke. 1995;26:661–665. doi: 10.1161/01.str.26.4.661. [DOI] [PubMed] [Google Scholar]
- Webster Marketon JI, Glaser R. Stress hormones and immune function. Cell Immunol. 2008;252:16–26. doi: 10.1016/j.cellimm.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, Hsieh CY. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517–1527. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- Weston RM, Jones NM, Jarrott B, Callaway JK. Inflammatory cell infiltration after endothelin-1-induced cerebral ischemia: histochemical and myeloperoxidase correlation with temporal changes in brain injury. J Cereb Blood Flow Metab. 2007;27:100–114. doi: 10.1038/sj.jcbfm.9600324. [DOI] [PubMed] [Google Scholar]
- Whiteley W, Jackson C, Lewis S, Lowe G, Rumley A, Sandercock P, Wardlaw J, Dennis M, Sudlow C. Inflammatory markers and poor outcome after stroke: a prospective cohort study and systematic review of interleukin-6. PLoS Med. 2009;6:e1000145. doi: 10.1371/journal.pmed.1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixey JA, Reinebrant HE, Carty ML, Buller KM. Delayed P2X4R expression after hypoxia-ischemia is associated with microglia in the immature rat brain. J Neuroimmunol. 2009;212:35–43. doi: 10.1016/j.jneuroim.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Woiciechowsky C, Schoning B, Lanksch WR, Volk HD, Docke WD. Mechanisms of brain-mediated systemic anti-inflammatory syndrome causing immunodepression. J Mol Med (Berl) 1999;77:769–780. doi: 10.1007/s001099900051. [DOI] [PubMed] [Google Scholar]
- Wong CH, Jenne CN, Lee WY, Leger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–105. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- Yamagami S, Tamura M, Hayashi M, Endo N, Tanabe H, Katsuura Y, Komoriya K. Differential production of MCP-1 and cytokine-induced neutrophil chemoattractant in the ischemic brain after transient focal ischemia in rats. J Leukoc Biol. 1999;65:744–749. doi: 10.1002/jlb.65.6.744. [DOI] [PubMed] [Google Scholar]
- Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, Dempsey RJ. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27:1213–1224. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- Yanamoto H, Nagata I, Sakata M, Zhang Z, Tohnai N, Sakai H, Kikuchi H. Infarct tolerance induced by intra-cerebral infusion of recombinant brain-derived neurotrophic factor. Brain Res. 2000;859:240–248. doi: 10.1016/s0006-8993(00)01966-1. [DOI] [PubMed] [Google Scholar]
- Yang GY, Schielke GP, Gong C, Mao Y, Ge HL, Liu XH, Betz AL. Expression of tumor necrosis factor-alpha and intercellular adhesion molecule-1 after focal cerebral ischemia in interleukin-1beta converting enzyme deficient mice. J Cereb Blood Flow Metab. 1999;19:1109–1117. doi: 10.1097/00004647-199910000-00007. [DOI] [PubMed] [Google Scholar]
- Yang QW, Lu FL, Zhou Y, Wang L, Zhong Q, Lin S, Xiang J, Li JC, Fang CQ, Wang JZ. HMBG1 mediates ischemia-reperfusion injury by TRIF-adaptor independent Toll-like receptor 4 signaling. J Cereb Blood Flow Metab. 2011;31:593–605. doi: 10.1038/jcbfm.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenari MA, Kauppinen TM, Swanson RA. Microglial activation in stroke: therapeutic targets. Neurotherapeutics. 2010;7:378–391. doi: 10.1016/j.nurt.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- Yilmaz G, Granger DN. Leukocyte recruitment and ischemic brain injury. Neuromolecular Med. 2010;12:193–204. doi: 10.1007/s12017-009-8074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaremba J, Ilkowski J, Losy J. Serial measurements of levels of the chemokines CCL2, CCL3 and CCL5 in serum of patients with acute ischaemic stroke. Folia Neuropathol. 2006;44:282–289. [PubMed] [Google Scholar]
- Zhang M, Li W, Niu G, Leak RK, Chen J, Zhang F. ATP induces mild hypothermia in rats but has a strikingly detrimental impact on focal cerebral ischemia. J Cereb Blood Flow Metab. 2013;33:10. doi: 10.1038/jcbfm.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Chen C, Lu J, Xie M, Pan D, Luo X, Yu Z, Dong Q, Wang W. Cell cycle inhibition attenuates microglial proliferation and production of IL-1beta, MIP-1alpha, and NO after focal cerebral ischemia in the rat. Glia. 2009;57:908–920. doi: 10.1002/glia.20816. [DOI] [PubMed] [Google Scholar]
- Zhang R, Chopp M, Zhang Z, Jiang N, Powers C. The expression of P- and E-selectins in three models of middle cerebral artery occlusion. Brain Res. 1998;785:207–214. doi: 10.1016/s0006-8993(97)01343-7. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Yenari MA. Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res. 2004;26:884–892. doi: 10.1179/016164104X2357. [DOI] [PubMed] [Google Scholar]