Lay Summary
Invasive rose-ringed parakeets caused behavioral changes in native garden birds that reduced their feeding rates. Understanding how invasive species impact native species can be complex, especially in urban environments where many other factors are also at play. We therefore used an experiment to disentangle these factors and demonstrate that parakeets are more disruptive than a dominant native competitor.
Key words: alien, ecological impacts, foraging behavior, interspecific interference competition, parrot, ringnecked parakeet.
Abstract
Resource competition is one potential behavioral mechanism by which invasive species can impact native species, but detecting this competition can be difficult due to the interactions that variable environmental conditions can have on species behavior. This is particularly the case in urban habitats where the disturbed environment can alter natural behavior from that in undisturbed habitats. The rose-ringed parakeet (Psittacula krameri), is an increasingly common invasive species, predominantly associated with large urban centers. Using an experimental approach, we tested the behavioral responses of native garden birds in response to the presence of a rose-ringed parakeet versus the presence of a similarly sized and dominant native bird, the great spotted woodpecker (Dendrocopos major). Parakeet presence significantly reduced feeding rates and increased vigilance among native birds compared with our control treatments. Of visits made by native birds in the presence of a parakeet, feeding was more likely to occur in sites within the parakeet range compared with sites outside, suggesting some habituation of native birds has occurred following prior exposure to parakeets but overall foraging behavior is still disrupted. The results of our study suggest that nonnative species can have complex and subtle impacts on native fauna and show that a nonnative competitor can impact native species simply through their presence near resources.
INTRODUCTION
It is well documented that nonnative species can have devastating impacts on biodiversity (Mack et al. 2000; Simberloff 2005; Shochat et al. 2010; Vilà et al. 2010) but despite extensive research, a high degree of uncertainty still exists with regard to the mechanisms of these impacts on native fauna (Perrings et al. 2002; Vilà et al. 2010). Anecdotal evidence and a lack of understanding of the nature and dynamics of the invasion process have contributed to this uncertainty (Mack et al. 2000; Gurevitch and Padilla 2004). Knowledge of the behavioral impacts resulting from nonlethal interspecific competition between native and nonnative species can, therefore, give insight into the mechanisms and consequences of invasions (reviewed in Chapple et al. 2012).
It has been hypothesized that both native and nonnative species which are abundant in urban areas are those that are most adaptable and able to exploit resources in a disturbed environment (McKinney 2006). These “urban adapter” and “urban exploiter” species tend to have broad diets and high behavioral flexibility (Sol 2002). These characteristics are likely to increase their potential to compete for food with a variety of species, particularly at supplementary feeding stations (Shochat et al. 2010) and displace other species from urban habitats (e.g., Parsons et al. 2006). For instance, increased interspecific competition can result in reduced foraging success of the affected individuals, increased time spent on vigilance, displacement to less high value resources and ultimately result in nonlethal fitness consequences with the potential to indirectly affect population level changes (Cresswell 2008). Interspecific competition is widely thought to play a role in the impact of nonnative species (Probert and Litvaitis 1996; Kiesecker et al. 2001; Wauters et al. 2002; Dame et al. 2006; Soares and Serpa 2006) but may be difficult to demonstrate unambiguously (Blackburn et al. 2009) perhaps in part because the ecological impacts of nonnative species can be difficult to distinguish from other potential environmental causes (Shochat et al. 2006; Dures and Cumming 2010). It is thought that nonnative species that displace native species are likely to be better able to exploit resources and thus have strong interference effects on native species (Amarasekare 2002). There is thus a need for experimental investigation to identify how interspecific interference might lead to behavioral changes driven by nonnative species (Dame et al. 2006; Strubbe et al. 2011).
Another factor that requires consideration when studying the potential impact of invasive organisms on the behavior of native species is habituation. It has been suggested that simply the novelty of the invasive species may disrupt normal behavior in native species, by eliciting a neophobic avoidance response (McEvoy et al. 2008). Neophobic impacts can be reduced or altered by repeated exposure to the novel species, as shown in cases of habituation in predator–prey interactions (reviewed by Brown and Chivers 2005). It has also been found that bird populations, which establish in urban areas, tend to have higher tolerance for novelty due to higher behavioral flexibility and reduced neophobia (Martin and Fitzgerald 2005; Levey et al. 2009; Evans et al. 2010; Lowry et al. 2013). This could mean that birds in urban areas, which share food sources with invasive species, may not be affected as much as bird populations outside urban areas due to a preadaption to cope with an altered environment including altered species presence. However, there has been relatively little work investigating habituation of native species to invasive species and the effects of prior exposure on competitive interactions (but see Webb et al. 2008; Abril and Gómez 2009; Nelson et al. 2011). In this context, rapid ongoing range expansion by exotic populations therefore offer the opportunity to investigate whether native species do habituate to the presence of invasive species (Freidenfelds et al. 2012).
In this study, we test for evidence of interference competition with native species and for habituation in the native species to an urban population of the rose-ringed parakeet. This nonnative species is listed as one of the top 100 most invasive alien species in Europe (Vilà et al. 2009) and is a common invasive bird species around the world (Feare 1996), particularly in urban areas (Strubbe and Matthysen 2009). This study system is ideal because, although locations of establishment of invasive bird populations have been well recorded (Duncan et al. 2003), there is little quantitative evidence of their impacts on native faunas (Blackburn et al. 2009; Strubbe et al. 2011).
The high-density populations of the rose-ringed parakeet in urban centers, provides a situation in which interspecific competition for resources with native species might be expected (Tayleur 2010). Urban gardens and parks provide alternative food sources such as supplemental feed and nonnative plant species. As such they enable rapid population growth of both native and nonnative adapter species, reflected in their positive association with human population density (Daniels and Kirkpatrick 2006; McKinney 2006; Fuller et al. 2008; Strubbe and Matthysen 2009).
Previous studies on rose-ringed parakeets have been limited to investigating competition for nest sites and have suggested that the impact of this form of competition is likely to be negligible (Strubbe et al. 2010; Czajka et al. 2011; Newson et al. 2011). However, the parakeet’s highly varied diet (Feare 1996), its ability to eat nonnative plants and human-provided food sources (Koutsosms et al. 2001), as well as the fact that individuals have been found to spend half their feeding time at artificial bird feeders (Clergeau and Vergnes 2011), means that it has potential to compete with a wide variety of native bird species for these resources and particularly with species found in urban gardens.
Here, we use an experimental approach to address the following questions: 1) Does the presence of a nonnative competitor at a high value food source alter the foraging behavior of native species? 2) Does the native species’ response to the presence of the nonnative competitor differ from that of a similarly dominant native species with which they have coexisted? 3) Is the strength of response to the presence of an establishing nonnative competitor correlated with prior exposure to the nonnative species? 4) If so, is this response indicative of reinforcement of avoidance behavior of the nonnative species, or of habituation to the nonnative species?
MATERIALS AND METHODS
Experiment sites
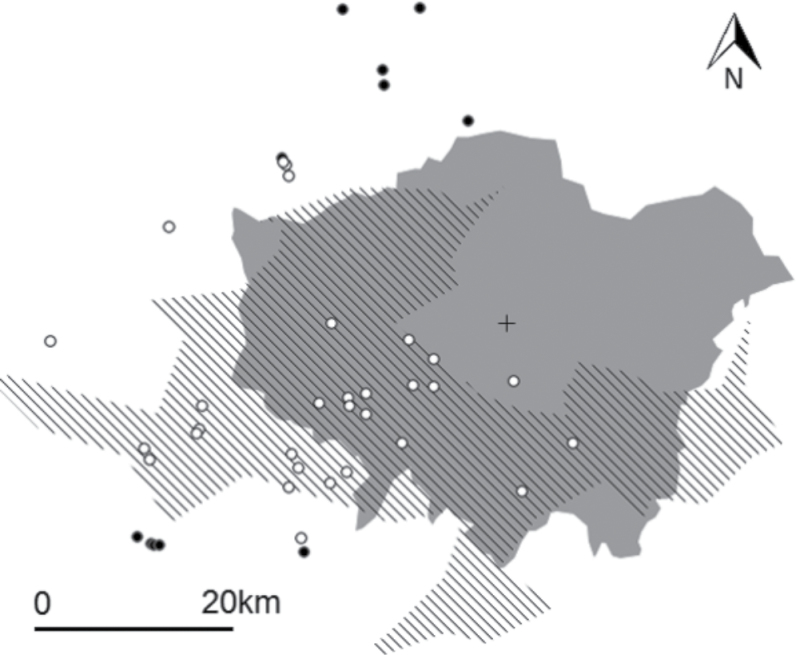
Behavioral experiments were performed at 41 sites within a 50-km radius of the center of London, UK, using the London Natural History Society method of using St. Paul’s Cathedral as an approximate for the center of London (Figure 1). Sites comprised the gardens of members of the public who responded to advertisements made through the media, project website (www.projectparakeet.co.uk no longer online), local bird watching groups, and word of mouth and therefore the location of sites used were constrained by those offered, but still provided an adequate number of sites (n = 41) spread across a broad area of London and the surrounds (Figure 1). Procedures for contacting members of the public were assessed and passed by Imperial College’s research ethics committee in April 2010. Sites were at least 200 m apart (closest distance between 2 sites 226 m, see Figure 1) to minimize the risk of repeating the experiment on same individual native birds and were gardens that used bird feeders regularly to minimize the time needed for habituation of the birds to the feeding station. To test for potential habituation, gardens were classified as being either within or outside the current range of the invasive parakeet population. This was classified based on residents’ information and verification by observations made during the experiment. Parakeet absence was also verified by British Breeding Bird Survey parakeet presence–absence data for London and the Home Counties for 2009 (see Figure 1). The range of the great spotted woodpecker covers the entire area of the study (Balmer et al. 2013) and garden owners verified that the species visited each garden used in the experiment regularly, therefore it was considered that garden birds’ exposure to great spotted woodpeckers would be similar across all sites.
Figure 1.
Experimental feeding site locations across London. Black circles represent parakeet free sites, white circles represent sites where parakeets were present, the solid gray area represents the area of Greater London, the lined polygon represents the area of the 2009 parakeet range (this is the extent of the Breeding Bird Survey 1 km2 squares where parakeets were recorded present in 2009). The cross represents the location of St. Paul’s Cathedral (sites were chosen within a 50-km radius of this).
Experimental procedure
A standardized feeding station was set up at each of the sites, with the behavior of free-living native birds being observed under a range of experimental and control conditions. Feeding stations comprised 2 squirrel proof feeders (The Nuttery NT28 and NT22 ASIN: B0007LQ3WQ, dimensions 20.3×20.3×34.3cm) hung on a steel shepherd hook pole Gardman (ASIN: B001F36RA8, height 218cm). Feeders were placed approximately 200cm above the ground as parakeets generally feed in plants and trees above ground (Clergeau and Vergnes 2011) and therefore any species affected by their presence are more likely to be species which also feed at this level rather than ground feeding species. Sunflower and peanuts were selected for use as the food sources as they are known to attract both parakeets and a variety of other birds species and therefore provided a high value food source likely to attract a variety of bird species (Lack 1986; Cowie and Simons 1991; Tvardíková and Fuchs 2010; Bonter et al. 2013). Sites were located in positions that could be viewed from a hidden location in order to be able to view the experiment without disturbing visiting birds. The feeding station was also placed in an area that had enough space for the experimental equipment (i.e., room for both the feeding station and the camera and tripod) and that was not obscured by vegetation or other structures. Where possible the feeding station was placed where the garden’s previous feeding station had been so as to limit the need for habituation to the new feeding station. To allow habituation of the local birds to the feeders before the experiment was conducted, each feeder was supplied with peanuts and sunflower seeds respectively for 2 weeks. This period was similar to acclimatization periods used for other bird studies (e.g., Sol et al. 2011; Orros and Fellowes 2012). These feeders represented a localized, high value resource that could be standardized across sites independent of season and prevented interference from gray squirrels, which are the only other nonnative vertebrate species present in the site locations that use garden bird feeders.
The behavioral experiments were conducted from May 2010 to February 2011 by H.L.P. or H.E.P., using 7 treatments that were designed to vary in the extent to which native birds were exposed to the presence of parakeets and control treatments. These consisted of 4 control treatments, which were an empty cage, a cage with a great spotted woodpecker in it, a cage with a great spotted woodpecker in it with a recording of a great spotted woodpecker call playing and an empty cage with just the call playing. In addition, there were 3 experimental treatments, these were a cage with a rose-ringed parakeet, a cage with a rose-ringed parakeet with a parakeet call playing, and an empty cage with just the parakeet call playing (see Table 1).
Table 1.
Treatments used per site, “cage” refers to whether an experimental cage was empty or contained a live woodpecker or parakeet and “call” refers to whether there was no audio recording or if a recording of a woodpecker or parakeet call was played
| Cage | Call | |||
|---|---|---|---|---|
| Control 1 | (C1) | Empty | None | |
| Control 2 | (C2) |
|
Woodpecker | None |
| Control 3 | (C3) | Woodpecker | Woodpecker | |
| Control 4 | (C4) | Empty | Woodpecker | |
| Treatment 1 | (T1) | Parakeet | None | |
| Treatment 2 | (T2) | Parakeet | Parakeet | |
| Treatment 3 | (T3) | Empty | Parakeet |
The order of the 7 treatments was randomized in each site.
Both a caged live parakeet and a recording of its call were used as treatments, as birds are known to be sensitive to both sight and sound (Sturkie 2000). In addition to the control of an empty cage with no recording it was necessary to also use native controls, making it possible to test for the strength of any effect of neophobia or habituation to the parakeet.
The great spotted woodpecker was chosen as a control species because it is the closest species in size that regularly feeds from hanging garden feeders, (rose-ringed parakeet: 400mm length, mass 120g; great spotted woodpecker: 220mm in length, mass 85g [Snow et al. 1998]) and its distribution overlaps with the study area (Balmer et al. 2013). Although smaller than the parakeet, this species has been observed to be aggressive at feeding stations in comparison to other birds and has been recorded successfully supplanting parakeets from feeding stations (Pithon 1998).
The 7 treatments were presented in a randomized order for 20min each (i.e., a total treatment time of 140min per site) generated through nonreplacement sampling in R (R Core Development Team 2009) for each individual site. For all treatments the cages (Montana KT3001, GTIN 04038374320048 46×63×53cm) were equipped with 2 metal bowls containing peanuts and water. For each treatment, the cage was placed on a stand at 0.3 m from the feeder station at the same height as the feeder for 20min. In the treatments involving sound, calls were played from a Ministry of Sound MOSMP020 MP3 player connected to 2 Skytronic 100.165 monitor speakers (frequency response 80–15000 Hz) played at full volume of an approximate amplitude of 80 dB(A), positioned on the ground directly below the cage. The great spotted woodpecker call was obtained from prerecorded material (Sample 1996), whereas the parakeet call was recorded from a series of vocalizations of an adult male rose-ringed parakeet in a garden in Richmond, Surrey, specifically for the study using a Sony Dictaphone. Both calls were general contact calls. Both calls were edited using Audacity 1.2.6 to minimize any background noise and repeated with intervals of random length (0–5 s) between calls to be of similar length (under 3min). These were played on repeat for the duration of the treatment.
The rose-ringed parakeet and great spotted woodpecker pairs (a male and female of each) used in the experimental trials were caught from the wild using a standard mist net under license from the British Trust for Ornithology and kept under Natural England (NE) licence (number 20101145) in an outside aviary between experiments. Aviaries were provisioned with nest boxes, ad libitum food and water, and provided sufficient room for flight. Each bird in a pair was used in alternate experiments in order to minimize stress and to control for any differences in behavior of visiting birds in response to differences in appearance due to sexual dimorphism. After all experiments were completed, the woodpeckers were given a 2-week soft-release at the catching site with open access to the aviary for food, water, and shelter. The parakeets were re-homed in captivity as required by the NE licence.
Data collection
The activity of native birds at the feeder was recorded using a small camcorder (Panasonic SDR-S156) mounted on a tripod 3 m from the feeders. Video recordings of each trial were watched subsequently to record each visiting bird’s species, duration of visit (in seconds), whether or not feeding occurred, and which food was eaten (sunflower seed or peanut). For sites within the current range of the parakeet population, visits made by wild parakeets to the feeding station during the trials were removed from the data before analysis (this included 136 visits altogether and occurred in 12 out of the 30 sites within the parakeet range). Data were recorded by trained volunteer research assistants, using standardized methods. Error checking was carried out by double checking a random 5-min sample of each video.
Environmental conditions of the feeder location for each trial were recorded to take into account any variables that might affect foraging behavior and visiting activity of birds within each location. These included; feeder position in the sun or shade as direct sunlight can affect perception of risk and therefore foraging (Fernández-Juricic and Tran 2007), cloud cover and rain (cloudy, rain, or clear sky) as rainfall can also affect foraging (Hilton et al. 1999), wind strength (0–3: no wind to strong wind) which has been found to affect visiting rates (Cowie and Simons 1991), time of year measured as months from May (1–10) to account for seasonal effects on feeding requirements (Chamberlain et al. 2005), time of day (AM or PM) to account for changes in foraging activity during the day (Bonter et al. 2013), and distance of the feeding station to vegetation cover (<1 m, 1–2 m, >3 m) as distance to cover is known to affect foraging in birds (Cowie and Simons 1991). Distance of the site from the center of London (measured in km from St. Paul’s Cathedral) was also measured and used to account for any between site differences due to differences in the level of urbanization in the surrounding area (Crawley 2011), which might be confounded with parakeet distribution.
Data analysis
The data on the behavior of native birds at the feeding stations were analyzed to test for differences between experimental treatments in a number of dependent variables: model 1 tested the number of visits by native birds to the feeders; model 2 tested the proportion of visits that included a bout of feeding; model 3 tested the absolute time spent feeding within a visit; model 4 tested the proportion of time spent vigilant during a feeding visit. These were chosen to test for whether parakeets inhibited birds from visiting (model 1) and then whether and how they affected foraging success (models 2 and 3) and the trade-off between foraging and vigilance (model 4). Vigilance is defined here as the proportion of time spent not feeding during feeding visits to peanuts and is an indication of risk perception. The absolute time feeding is likely to be correlated with the amount of food taken and therefore an indication of foraging success. Behaviors other than feeding and vigilance, such as aggression or preening were extremely rare and so the assumption was made that when birds are on the feeding station and not feeding then they were being vigilant. Birds visiting the feeders were not individually marked and so multiple visits by the same individual could not be accounted for but all sites were treated equally. In addition to the experimental treatments described in Table 1, we also tested for an effect of whether or not the site was within the range of the invasive parakeet population.
Data were analyzed using generalized linear models in R (R Core Development Team 2009), using the lme4 package (Bates et al. 2012). Models 1 and 3 were over dispersed so these included an observation-level random effect and were fitted using a Poisson log-normal error structure and a log link function (Elston et al. 2001). Models 2 and 4 were fitted using a binomial error structure and logit link function. Model 1 was analyzed using a data subset of the summed total visits for each treatment per site. Model 2 was analyzed using the full data set of each individual visit. Sunflower seeds were visited for such a short time per visit (median = 1 s, interquartile range [IQR] = 1, n = 2117) regardless of treatment, that it was not possible to analyze differences in time spent feeding. Therefore, only feeding visits to peanuts were analyzed for differences in time feeding, so models 3 and 4 were analyzed using a subset of only feeding visits where the bird fed on peanuts.
In models the experimental feeding site identification was added as a random effect. Variables measured in each site were added into each model as fixed effects: inside or outside the parakeet range, cloud cover and rain, wind strength, sun or shade, time of day (AM or PM), time of year (number of months from May), distance of the feeding station to vegetation, and distance (km) from central London. Inside or outside the parakeet range was categorized as binomial (absent or present), as preliminary analysis using a continuous measure of parakeet numbers in the site ranging from 0 to 5 found no difference in visits between the sites ranging from 1 to 5. The order in which each treatment took place were also added as a fixed effect to account for any interference that may result in a time lag between exposure to a treatment and resumption of foraging (Sih 1992; Evans 2003). For models 2, 3, and 4 the species visiting the feeding station were also added as a fixed effect to account for differences in behavior between species in response to the treatments. This variable could not be tested on model 1 due to it being a measure of total visits and therefore excluding details of individual visits. No explanatory variables included in the full models were highly correlated (r < 0.249 in all cases) see Supplementary Table S6.
Following Crawley (2013), we estimated minimum adequate models by entering all fixed effects and dropping them sequentially until only those that explained significant variation remained (see site variable effect results in Supplementary Table S4). At each stage the reduced model was tested against the previous model to check that a significant amount of variation had not been lost using a chi-squared statistic in an Anova.
RESULTS
In total, at the 30 sites inside the current range of the parakeet population, the feeding station was visited 6872 times (median = 96, IQR = 42–252 visits per site) and in the 11 sites outside the parakeet range the feeding station was visited 4021 times (149, 72–311). Across these visits 18 native bird species were observed on the feeding stations, which were predominantly blue tits (Cyanistes caeruleus, 42% of visits) and great tits (Parus major, 41%) (a summary of visits made by each species, Supplementary Table S5).
Sites inside the current range of the parakeet population
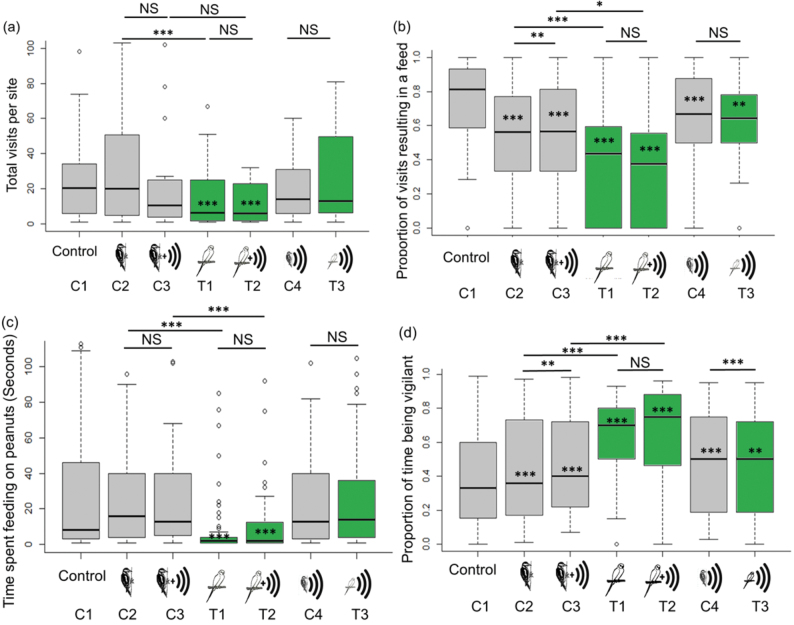
Our analyses demonstrated that at sites inside the current range of the parakeet population, experimental exposure to caged parakeets resulted in a significant reduction in the number of visits by native birds to the feeders (Figure 2a), a significant reduction in the number of visits that included a feeding bout (Figure 2b), a significant reduction in the absolute time spent feeding (Figure 2c), and a significant increase in the proportion of time spent vigilant (Figure 2d), (Supplementary Table S1). For all these behaviors, changes in the same direction were also observed in the presence of a woodpecker (Figure 2, Supplementary Table S1), but the changes in the presence of a parakeet were significantly greater than those in the presence of a woodpecker (Figure 2, Supplementary Table S2).
Figure 2.
Box and whisker plots for (a) number of visits (n = 6826), (b) proportion of visits resulting in a feeding event (n = 6826), (c) time spent feeding (seconds) on peanuts per feeding visit (n = 555), (d) vigilance (proportion of time spent not feeding [seconds] per feeding visit to peanuts) (n = 555), per treatment for sites inside the parakeet range (n = 30). Significant values within a box refer to the difference of the treatment from the control (C1), values outside a box and on a solid line refer to between treatments, (P values, ***P < 0.001, **P < 0.01, *P < 0.05). Gray boxes show controls and green boxes show treatments. C4 and T3 are both call only conditions and so are grouped together on the right of each panel to aid comparison.
A parakeet call alone reduced the proportion of visits resulting in a feeding bout and increased the proportion of time spent vigilant (Figure 2, Supplementary Table S1). This effect was significantly greater from that of the woodpecker call for the proportion of time spent vigilant. But in all cases the effect of the parakeet call alone was less pronounced than that of the presence of a caged parakeet (Figure 2, Supplementary Table S2) and the addition of a parakeet call to the presence of a parakeet had little additional effect for all response behaviors (Figure 2, Supplementary Table S2).
Sites outside the current range of the parakeet population
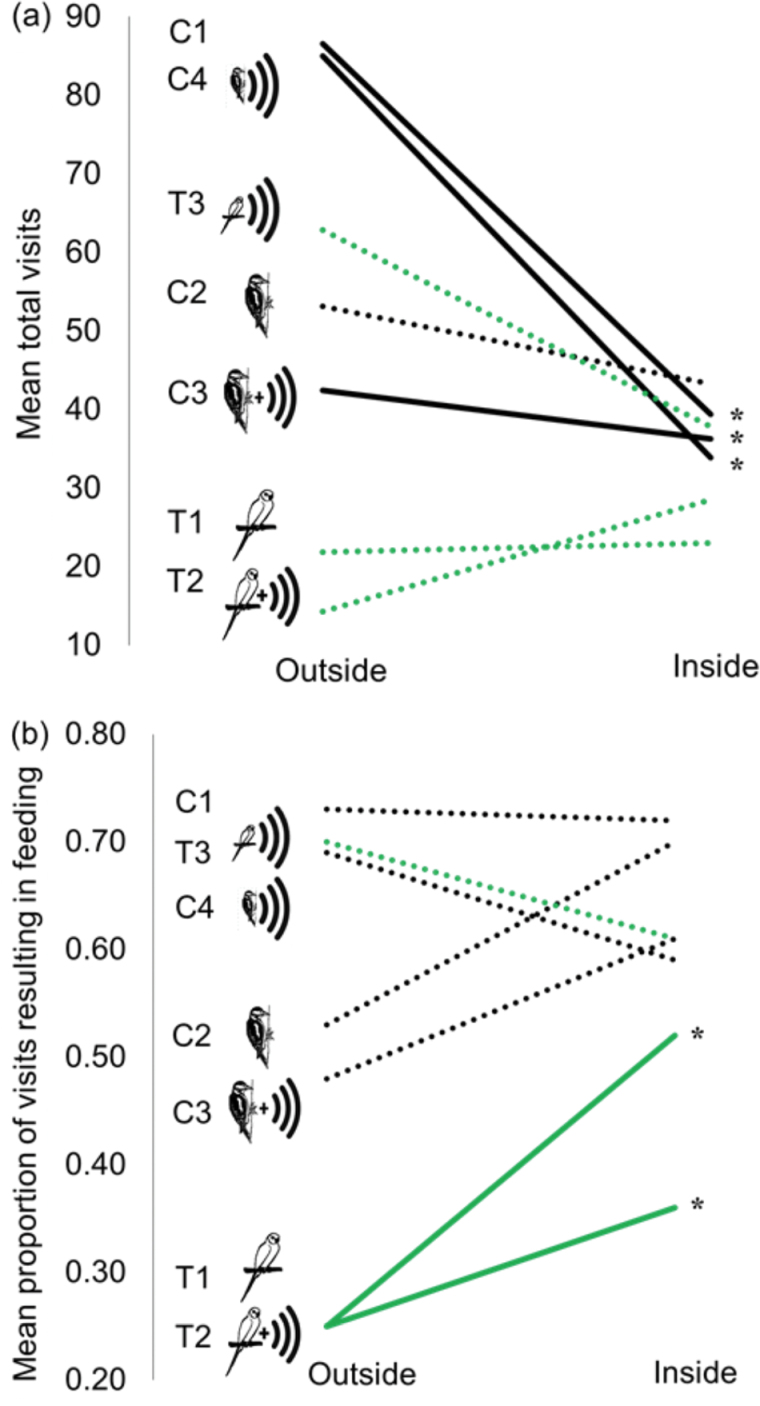
Overall the patterns in response to treatments were consistent with those inside the current parakeet range (Supplementary Tables S1 and S2). The number of total visits to feeding stations was higher for 3 out of 4 of the control treatments (C1, C3, and C4) at sites outside the parakeet range compared with sites within (Figure 3a). However, notably, parakeet treatments (T1 and T2) resulted in a lower proportion of feeding events outside of the parakeet range than inside (Figure 3b and Supplementary Table S3). During the 2 caged parakeet treatments there were so few feeding visits to peanuts, (T1, n = 7; T2 n = 3) that changes found for total feeding time and vigilance, in response to these 2 treatments could not be confidently compared (Supplementary Tables S1–S3). However, the parakeet call alone treatment (T3), which did have enough visits to compare (visits n = 95 outside, n = 63 inside), was shown to elicit a stronger vigilance response inside the parakeet range compared with outside (T3 outside range vs T3 inside range; Supplementary Table S3).
Figure 3.
Differences between sites per treatments outside and inside parakeet range for (a) mean total visits, and (b) the proportion of visits resulting in a feed (visits outside range n = 4027, inside range n = 6826). Black lines show controls and gray lines show treatments. Dotted lines denote differences between range sites within treatments which are not significant, solid lines are significant (P values, *P < 0.05). Significant difference refers to differences within treatments between sites outside and inside the parakeet range.
DISCUSSION
Our findings show that experimental exposure to parakeets influences the behavior of native birds, resulting in reduced feeding and increased vigilance. These changes in behavior are much more pronounced in the presence of a parakeet than in the presence of a dominant native species, the great spotted woodpecker. While visit rates drop significantly in the presence of a parakeet both inside and outside the current parakeet range, visits that do occur are more likely to result in feeding inside the range. Taken together, these results suggest that interference competition between a nonnative species and the native fauna does appear likely in this study system, and that some habituation may occur in the native populations.
Interference competition
Interspecific interference competition between native and nonnative fauna is a concern as it may lead to reduced energy intake, and thus potentially lower the fitness of native birds (Gustafsson 1987; Cresswell 2008). Similarly, increased vigilance induced by the presence of nonnative species can diminish the relative value of a food resource through increasing access costs (Cooper and Frederick 2007).
The majority of visits to the feeding stations were by blue tits and great tits. These species are common, ubiquitous birds in urban areas in the United Kingdom (Cowie et al. 1988; Balmer et al. 2013). Interspecific competition between tit species has been shown to cause displacement of individuals of less dominant species to lower quality food sources in coniferous forest (Alatalo et al. 1987), and spatial displacement and niche compression of blue tits in oak woodland (Herrera 1978). The presence of parakeets may simply result in temporary displacement of native species from a food source, with minimal costs. Temporal niche shift behavior has been shown in the timing of great tit dawn singing in response to supplemental feeding (Saggese et al. 2011) and also in invasive mink (Neovison vison) avoiding 2 native mustelid species (Lutra lutra and Mustela putorius) during foraging (Harrington et al. 2009). Our analyses did, however, control for time of day and found it was not a significant predictor of the number of visits to the feeding station. This suggests that parakeets may induce a spatial, rather than, temporal shift in native bird foraging behavior. Similar spatial shifts in response to environmental changes, such as loss of access to food resources, have been shown to lead to reduced population sizes (Durell et al. 2005, 2006; Stillman and Goss-Custard 2010). Consistent displacement of native birds from high-quality resources may, therefore, be expected to have long-term implications for native species’ populations.
There are very few examples in the literature of dominance over food sources by nonnative species resulting in displacement of native species. Examples of this occurring with nonnative vertebrates include an invasive gecko (Lepidodactylus lugubris) in Hawaii (Petren and Case 1996) and the invasive gray squirrel (Sciurus carolinensis) in the United Kingdom (Kenward and Holm 1993) but see (Wauters and Gurnell 1999). The subtle changes in feeding behavior seen in response to parakeet presence may represent a mechanism for displacement, which to our knowledge would be the first case of such by a nonnative avian species and therefore merits further investigation.
Habituation
Our finding of a higher likelihood of feeding in the presence of a parakeet within the parakeet range compared with sites outside, suggests habituation to parakeets following prior exposure. This is particularly evident considering the overall lower mean total number of visits in the sites within the parakeet range compared with those outside. Without data on individual behavior, it is not possible to distinguish whether this effect is due to an increased number of visits by bold individuals who have become accustomed to the parakeets, comparable to behavior seen in several other bird species exposed to a predator (Quinn and Cresswell 2005; van Oers 2005; Minderman et al. 2010; Rockwell et al. 2012), but see Couchoux and Cresswell (2011); or, if the perception of risk lowers for all individuals with continued exposure (Ellenberg et al. 2009; Rodríguez-Prieto et al. 2011). The former would suggest that some individuals may be disproportionately impacted by nonnative species’ presence, whereas the latter would indicate population-wide adjustment to the presence of a nonnative species, and potentially lower overall impact.
In contrast to habituation to the presence of the parakeets, we also found some evidence of reinforcement behavior, such that native birds within the parakeet range were more vigilant when exposed to a parakeet call, which suggests that prior exposure to parakeet calls has an influence on behavior. This finding is consistent with previous studies demonstrating this initial lack of response of prey to the calls of a novel avian predator (Reudink et al. 2007; Elmasri et al. 2012) and studies demonstrating learned association of predator cues (reviewed by Griffin et al. 2000).
It is possible differences in native bird behavior between sites within and outside the parakeet range are due to differences associated with urbanization (Lowry et al. 2013). For example, bird species in urban areas, including blue tits and great tits, have been demonstrated to have differing behavioral adaptation to predators compared with rural areas (Møller and Ibáñez-Álamo 2012) and urban populations of song sparrow (Melospiza melodia) have been found to be bolder and show greater territorial aggression compared with rural populations (Evans et al. 2010). We did control for an urbanization effect in our analysis, by testing for an effect of distance from the city center, which was not found to be of importance. Regardless of the causes for the differences in response of birds inside and outside the parakeet range, the higher proportion of feeding visits at sites inside the range in the presence of a parakeet was still lower than the control treatments and therefore foraging visits were still less successful across all sites in the presence of a parakeet at a food source, despite the prior exposure. This indicates that the inhibition of foraging in the presence of a parakeet is a permanent effect and not just a case of neophobia.
Ecological implications
It should be noted that wild rose-ringed parakeets often forage gregariously and therefore monopolize a feeding site (Pithon 1998). During our experiment, 95 out of 136 visits by wild parakeets to the experimental feeding station were when one of our captive parakeets was present in the cage, further demonstrating parakeets’ gregarious nature. Given this we would expect that the impacts demonstrated here are a conservative estimate of those that would be seen with free-living parakeets.
It has been hypothesized that optimal foraging in urban settings can alter the community structure and result in biodiversity loss (Shochat et al. 2004). It is also common for invading species to establish where competition among the resident species is low (Crawley et al. 1986) and so the dominance of nonnative species can also be increased by the provision of artificial feeding stations in gardens (Chace and Walsh 2006; McKinney 2006; Jones and Reynolds 2008; Cannon 2010). The link between garden feeding and the success of invasive species is seen directly in Chicago, where the persistence and growth of the monk parakeet (Myiopsitta monachus) population is attributed to the generalist diet of the nonnative species and its sole use of bird feeders in winter (South and Pruett-Jones 2000). Clearly garden food provisioning plays a major role in the persistence of invasive populations, which in tandem with the temporally stable climate and homogenization of the environment makes the urban landscape favorable to nonnative species (Garden et al. 2006). Given our evidence for foraging disruption at artificial food sources and the potential reliance of the parakeets on garden bird feeders (Clergeau and Vergnes 2011), the exclusion of parakeets from access to garden bird feeders might benefit native species through reducing the interference competition. In addition, exclusion of parakeets would simultaneously reduce the foraging benefits of urban areas for the parakeets, which may in turn limit the persistence of the parakeet populations. Therefore, an interesting implication of this research is that exclusion of parakeets from garden bird feeders may provide an option for mitigating impacts of parakeets on garden birds.
In conclusion, we provide evidence demonstrating interference foraging competition between a nonnative bird and 2 native birds common to urban areas. While this study does not provide proof of any population level change as a result of the disrupted foraging, it does demonstrate a mechanism by which nonnative birds could potentially impact native species at the population level and shows a need for further investigation. The increased establishment of rose-ringed parakeets in the United Kingdom (Tayleur 2010) and across Europe (Strubbe and Matthysen 2008) as well as other nonnative bird species in urban areas across the world (Peacock et al. 2007; Blackburn et al. 2009; Bonter et al. 2010), highlights the potential for widespread occurrence of similar effects of foraging behavior on urban birds.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at http://www.beheco.oxfordjournals.org/
FUNDING
This work was supported by a research studentship from the Biotechnology and Biological Sciences Research Council (reference BB/D526410/1) to H.L.P. and by The Nuttery Ltd.
Supplementary Material
Acknowledgments
The 2 parakeets and woodpeckers were kept under Natural England (NE) licence (number 20101145). We are extremely grateful for the help of all those who volunteered their gardens particularly P. Davies and J. Baker and all those who assisted with data extraction. We thank S.C. Bell, T. Coulson, and J.D. Mumford for comments on an earlier version of the manuscript. This paper is a contribution to Imperial College’s Grand Challenges in Ecosystems and the Environment initiative.
REFERENCES
- Abril S, Gómez C. 2009. Ascertaining key factors behind the coexistence of the native ant species Plagiolepis pygmaea with the invasive Argentine ant Linepithema humile (Hymenoptera: Formicidae) ant colonies and sampling. Sociobiology. 53:559–568 [Google Scholar]
- Alatalo RV, Eriksson DAG, Gustafsson L, Larsson K. 1987. Exploitation competition influences the use of foraging sites by tits: experimental evidence. Ecology. 68:284–290 [Google Scholar]
- Amarasekare P. 2002. Interference competition and species coexistence. Proc Biol Sci. 269:2541–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer D, Gillings S, Caffrey B, Swann B, Downie I, Fuller R. 2013. Bird Atlas 2007–11: the breeding and wintering birds of Britain and Ireland. British Trust for Ornithology.
- Bates D, Maechler M, Bolker B. 2012. lme4: Linear mixed-effects models using S4 classes. R package version 0.999375–42. Available from: http://CRAN.R-project.org/package=lme4
- Blackburn TM, Lockwood JL, Cassey PB. 2009. Avian Invasions: The Ecology and Evolution of Exotic Birds. Oxford: Oxford University Press [Google Scholar]
- Bonter DN, Zuckerberg B, Dickinson JL. 2010. Invasive birds in a novel landscape: habitat associations and effects on established species. Ecography 33:494–502 [Google Scholar]
- Bonter DN, Zuckerberg B, Sedgwick CW, Hochachka WM. 2013. Daily foraging patterns in free-living birds: exploring the predation-starvation trade-off. Proc Biol Sci. 280:20123087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GE, Chivers DP. 2005. Learning as an adaptive response to predation. In: Pedro B, Castellanos I, editors. Ecology of Predator-Prey Interactions. New York: Oxford University Press; p. 394 [Google Scholar]
- Cannon A. 2010. The significance of private gardens for bird conservation. Bird Conserv Int. 9:287–297 [Google Scholar]
- Chace JF, Walsh JJ. 2006. Urban effects on native avifauna: a review. Landsc Urban Plan. 74:46–69 [Google Scholar]
- Chamberlain DE, Vickery JA, Glue DE, Robinson RA, Conway GJ, Woodburn RJW, Cannon AR. 2005. Annual and seasonal trends in the use of garden feeders by birds in winter. Ibis (Lond. 1859). 147:563–575 [Google Scholar]
- Chapple DG, Simmonds SM, Wong BB. 2012. Can behavioral and personality traits influence the success of unintentional species introductions? Trends Ecol Evol. 27:57–64 [DOI] [PubMed] [Google Scholar]
- Clergeau P, Vergnes A. 2011. Bird feeders may sustain feral rose-ringed parakeets Psittacula krameri in temperate Europe. Wildlife Biol. 17:248–252 [Google Scholar]
- Cooper WE, Jr, Frederick WG. 2007. Optimal flight initiation distance. J Theor Biol. 244:59–67 [DOI] [PubMed] [Google Scholar]
- Couchoux C, Cresswell W. 2011. Personality constraints versus flexible antipredation behaviors: how important is boldness in risk management of redshanks (Tringa totanus) foraging in a natural system? Behav Ecol. 23:290–301 [Google Scholar]
- Cowie R, Hinsley S, Taylor P. 1988. The provision of food and the use of bird feeders in suburban gardens. Bird Study. 35:163–168 [Google Scholar]
- Cowie RJ, Simons JR. 1991. Factors affecting the use of feeders by garden birds: I. The positioning of feeders with respect to cover and housing. Bird Study. 38:145–150 [Google Scholar]
- Crawley MJ, Kornberg H, Lawton JH, Usher MB, Southwood R, O’Connor RJ, Gibbs A. 1986. The population biology of invaders [and discussion]. Philos Trans R Soc B Biol Sci. 314:711–731 [Google Scholar]
- Crawley MJ. 2011. London. In: Kelcey JG, Müller N, editors. Plants and Habitats of European Cities. New York: Springer; p. 207–236 [Google Scholar]
- Crawley MJ. 2013. The R Book. second. Chichester: Wiley [Google Scholar]
- Cresswell W. 2008. Non-lethal effects of predation in birds. Ibis (Lond. 1859). 150:3–17 [Google Scholar]
- Czajka C, Braun MP, Wink M. 2011. Resource use by non-native ring-necked parakeets (Psittacula krameri) and native starlings (Sturnus vulgaris) in Central Europe. Open Ornithol J. 4:17–22 [Google Scholar]
- Dame EA, Eth K, En P. 2006. Behavioural mechanisms of invasion and displacement in Pacific island geckos (Hemidactylus). Anim Behav. 71:1165–1173 [Google Scholar]
- Daniels GD, Kirkpatrick JB. 2006. Does variation in garden characteristics influence the conservation of birds in suburbia? Biol Conserv. 133:326–335 [Google Scholar]
- Duncan RP, Blackburn TM, Sol D, Duncan P. 2003. The ecology of bird introductions. Annu Rev Ecol Evol Syst. 34:71–98 [Google Scholar]
- Durell SEA, le V dit, Stillman RA, Caldow RWG, McGrorty S, West AD, Humphreys J. 2006. Modelling the effect of environmental change on shorebirds: a case study on Poole Harbour, UK. Biol Conserv. 131:459–473 [Google Scholar]
- Durell SEA, le V dit, Stillman RA, Triplet P, Aulert C, Ono dit Biot D, Bouchet A, Duhamel S, Mayot S, Gosscustard JD. 2005. Modelling the efficacy of proposed mitigation areas for shorebirds: a case study on the Seine estuary, France. Biol Conserv. 123:67–77 [Google Scholar]
- Dures SG, Cumming GS. 2010. The confounding influence of homogenising invasive species in a globally endangered and largely urban biome: does habitat quality dominate avian biodiversity? Biol Conserv. 143:768–777 [Google Scholar]
- Ellenberg U, Mattern T, Seddon PJ. 2009. Habituation potential of yellow-eyed penguins depends on sex, character and previous experience with humans. Anim Behav. 77:289–296 [Google Scholar]
- Elmasri OL, Moreno MS, Neumann CA, Daniel T. 2012. Response of brown anoles Anolis sagrei to multimodal signals from a native and novel predator. Curr Zool. 58:791–796 [Google Scholar]
- Elston DA, Moss R, Boulinier T, Arrowsmith C, Lambin X. 2001. Analysis of aggregation, a worked example: numbers of ticks on red grouse chicks. Parasitology. 122:563–569 [DOI] [PubMed] [Google Scholar]
- Evans J, Boudreau K, Hyman J. 2010. Behavioural syndromes in urban and rural populations of song sparrows. Ethology. 116:588–595 [Google Scholar]
- Evans KL. 2003. The potential for interactions between predation and habitat change to cause population declines of farmland birds. Ibis (Lond. 1859). 146:1–13 [Google Scholar]
- Feare CJ. 1996. Rose-ringed parakeet Psittacula krameri: a love-hate relationship in the making? In: Holmes JS, Simons JR, editors. The Introduction and Naturalisation of Birds. p. 107–112 [Google Scholar]
- Fernández-Juricic E, Tran E. 2007. Changes in vigilance and foraging behaviour with light intensity and their effects on food intake and predator detection in house finches. Anim Behav. 74:1381–1390 [Google Scholar]
- Freidenfelds NA, Robbins TR, Langkilde T. 2012. Evading invaders: the effectiveness of a behavioral response acquired through lifetime exposure. Behav Ecol. 23:659–664 [Google Scholar]
- Fuller RA, Warren PH, Armsworth PR, Barbosa O, Gaston KJ. 2008. Garden bird feeding predicts the structure of urban avian assemblages. Divers Distrib. 14:131–137 [Google Scholar]
- Garden J, Mcalpine C, Peterson A, Jones D, Possingham H. 2006. Review of the ecology of Australian urban fauna: a focus on spatially explicit processes. Austral Ecol. 31:126–148 [Google Scholar]
- Griffin AS, Blumstein DT, Evans CS. 2000. Training captive-bred or translocated animals to avoid predators. Conserv Biol. 14:1317–1326 [Google Scholar]
- Gurevitch J, Padilla DK. 2004. Are invasive species a major cause of extinctions? Trends Ecol Evol. 19:470–474 [DOI] [PubMed] [Google Scholar]
- Gustafsson L. 1987. Interspecific competition lowers fitness in collared flycatchers Ficedula Albicollis: an experimental demonstration. Ecology. 68:291–296 [Google Scholar]
- Harrington LA, Harrington AL, Yamaguchi N, Thom MD, Ferreras P, Windham TR, Macdonald DW. 2009. The impact of native competitors on an alien invasive: temporal niche shifts to avoid interspecific aggression? Ecology. 90:1207–1216 [DOI] [PubMed] [Google Scholar]
- Herrera CM. 1978. Niche shift in the genus Parus in Southern Spain. Ibis (Lond. 1859). 120:236–240 [Google Scholar]
- Hilton GM, Ruxton GD, Cresswell W. 1999. Choice of foraging area with respect to predation risk in redshanks: the effects of weather and predator activity. Oikos. 87:295–302 [Google Scholar]
- Jones DN, Reynolds SJ. 2008. Feeding birds in our towns and cities: a global research opportunity. J Avian Biol. 39:265–271 [Google Scholar]
- Kenward RE, Holm JL. 1993. On the replacement of the red squirrel in Britain: a phytotoxic explanation. Proc Biol Sci. 251:187–194 [DOI] [PubMed] [Google Scholar]
- Kiesecker JM, Blaustein AR, Miller CL, Iesecker JOMK, Laustein ANRB. 2001. Potential mechanisms underlying the displacement of native red-legged frogs by introduced bullfrogs. Ecology. 82:1964–1970 [Google Scholar]
- Koutsosms EA, Matsonms KD, Klasingms KC. 2001. Nutrition of birds in the order Psittaciformes: a review. J Avian Med Surg. 15:257–275 [Google Scholar]
- Lack P. 1986. The Atlas of Wintering Birds in Britain and Ireland. Academic Press Inc [Google Scholar]
- Levey DJ, Londoño GA, Ungvari-Martin J, Hiersoux MR, Jankowski JE, Poulsen JR, Stracey CM, Robinson SK. 2009. Urban mockingbirds quickly learn to identify individual humans. Proc Natl Acad Sci USA. 106:8959–8962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry H, Lill A, Wong BB. 2013. Behavioural responses of wildlife to urban environments. Biol Rev Camb Philos Soc. 88:537–549 [DOI] [PubMed] [Google Scholar]
- Mack RN, Simberloff D, Mark Lonsdale W, Evans H, Clout M, Bazzaz FA. 2000. Biotic invasions: causes, epidemiology,global consequence, and control. Ecol Appl. 10:689–710 [Google Scholar]
- Martin LB, Fitzgerald L. 2005. A taste for novelty in invading house sparrows, Passer domesticus . Behav Ecol. 16:702–707 [Google Scholar]
- McEvoy J, Sinn D, Wapstra E. 2008. Know thy enemy: behavioural response of a native mammal (Rattus lutreolus velutinus) to predators of different coexistence histories. Austral Ecol. 33:922–931 [Google Scholar]
- McKinney ML. 2006. Urbanization as a major cause of biotic homogenization. Biol Conserv. 127:247–260 [Google Scholar]
- Minderman J, Reid JM, Hughes M, Denny MJH, Hogg S, Evans PGH, Whittingham MJ. 2010. Novel environment exploration and home range size in starlings Sturnus vulgaris . Behav Ecol. 21:1321–1329 [Google Scholar]
- Møller AP, Ibáñez-Álamo JD. 2012. Escape behaviour of birds provides evidence of predation being involved in urbanization. Anim Behav. 84:341–348 [Google Scholar]
- Nelson DWM, Crossland MR, Shine R. 2011. Foraging responses of predators to novel toxic prey: effects of predator learning and relative prey abundance. Oikos. 120:152–158 [Google Scholar]
- Newson SE, Johnston A, Parrott D, Leech DI. 2011. Evaluating the population-level impact of an invasive species, ring-necked parakeet Psittacula krameri, on native avifauna. Ibis (Lond. 1859). 153:509–516 [Google Scholar]
- Van Oers K. 2005. Context dependence of personalities: risk-taking behavior in a social and a nonsocial situation. Behav Ecol. 16:716–723 [Google Scholar]
- Orros ME, Fellowes MDE. 2012. Supplementary feeding of wild birds indirectly affects the local abundance of arthropod prey. Basic Appl Ecol. 13:286–293 [Google Scholar]
- Parsons H, Major RE, French K. 2006. Species interactions and habitat associations of birds inhabiting urban areas of Sydney, Australia. Austral Ecol.:217–227 [Google Scholar]
- Peacock DS, Van Rensburg BJ, Robertson MP. 2007. The distribution and spread of the invasive alien common myna, Acridotheres tristis L. (Aves: Sturnidae), in southern Africa. S Afr J Sci. 103:465–473 [Google Scholar]
- Perrings C, Williamson M, Barbier EB, Delfino D, Dalmazzone S, Simmons P, Watkinson A. 2002. Biological invasion risks and the public good: an economic perspective. Conserv. Ecol. 6:1 Available from: http://www.consecol.org/vol6/iss1/art1/ [Google Scholar]
- Petren K, Case TJ. 1996. An experimental demonstration of exploitation competition in an ongoing invasion. Ecology .77:118–132 [Google Scholar]
- Pithon JA. 1998. The status and ecology of the ring-necked parakeet Psittacula krameri in Great Britain. Univeristy of York.
- Probert BL, Litvaitis JA. 1996. Behavioural interactions between invading and endemic lagomorphs: implications for conserving a declining species. Biol Conserv. 76:289–295 [Google Scholar]
- Quinn J, Cresswell W. 2005. Personality, anti-predation behaviour and behavioural plasticity in the chaffinch Fringilla coelebs . Behaviour. 142:1377–1402 [Google Scholar]
- R Core Development Team. 2009. R: a langauge and environment for statistical computing. Version 2.15.2. Available from: http://www.r-project.org
- Reudink MW, Nocera JJ, Curry RL. 2007. Anti-predator responses of neotropical resident and migrant birds to familiar and unfamiliar owl vocalisations on the Yucatan Peninsula. Ornitol Neotrop. 18:543–552 [Google Scholar]
- Rockwell C, Gabriel PO, Black JM. 2012. Bolder, older, and selective: factors of individual-specific foraging behaviors in Steller’s jays. Behav Ecol. 23:676–683 [Google Scholar]
- Rodríguez-Prieto I, Martín J, Fernández-Juricic E. 2011. Individual variation in behavioural plasticity: direct and indirect effects of boldness, exploration and sociability on habituation to predators in lizards. Proc Biol Sci. 278:266–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggese K, Korner-Nievergelt F, Slagsvold T, Amrhein V. 2011. Wild bird feeding delays start of dawn singing in the great tit. Anim Behav. 81:361–365 [Google Scholar]
- Sample G. 1996. Track 59: Great spotted woodpecker. Bird Songs Calls Britain North. Eur. (Disc 1). [Google Scholar]
- Shochat E, Lerman SB, Anderies JM, Warren PS, Faeth SH, Nilon CH. 2010. Invasion, competition, and biodiversity loss in urban ecosystems. Bioscience. 60:199–208 [Google Scholar]
- Shochat E, Lerman SB, Katti M, Lewis DB. 2004. Linking optimal foraging behavior to bird community structure in an urban-desert landscape: field experiments with artificial food patches. Am Nat. 164:232–243 [DOI] [PubMed] [Google Scholar]
- Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D. 2006. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol. 21:186–191 [DOI] [PubMed] [Google Scholar]
- Sih A. 1992. Prey uncertainty and the balancing of antipredator and feeding needs. Am Nat. 139:1052–1069 [Google Scholar]
- Simberloff D. 2005. Non-native species do threaten the natural environment! J Agric Environ Ethics. 18:595–607 [Google Scholar]
- Snow D, Perrins CM, Gillmor R. 1998. The birds of the western Palearctic. Concise. Oxford University Press. [Google Scholar]
- Soares AO, Serpa A. 2006. Interference competition between ladybird beetle adults (Coleoptera: Coccinellidae): effects on growth and reproductive capacity. Popul Ecol. 49:37–43 [Google Scholar]
- Sol D, Griffin AS, Bartomeus I, Boyce H. 2011. Exploring or avoiding novel food resources? The novelty conflict in an invasive bird. PLoS One. 6:e19535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol D. 2002. Behavioural flexibility and invasion success in birds. Anim Behav. 63:495–502 [Google Scholar]
- South JM, Pruett-Jones S. 2000. Patterns of flock size, diet, and vigilance of naturalized Monk Parakeets in Hyde Park, Chicago. Condor. 102:848–854 Available from: http://dx.doi.org/10.1650/0010-5422(2000)102[0848:POFSDA]2.0.CO;2 [Google Scholar]
- Stillman RA, Goss-Custard JD. 2010. Individual-based ecology of coastal birds. Biol Rev Camb Philos Soc. 85:413–434 [DOI] [PubMed] [Google Scholar]
- Strubbe D, Matthysen E, Graham CH. 2010. Assessing the potential impact of invasive ring-necked parakeets Psittacula krameri on native nuthatches Sitta europeae in Belgium. J Appl Ecol. 47:549–557 [Google Scholar]
- Strubbe D, Matthysen E. 2008. Predicting the potential distribution of invasive ring-necked parakeets Psittacula krameri in northern Belgium using an ecological niche modelling approach. Biol Invasions. 11:497–513 [Google Scholar]
- Strubbe D, Matthysen E. 2009. Establishment success of invasive ring-necked and monk parakeets in Europe. J Biogeogr. 36:2264–2278 [Google Scholar]
- Strubbe D, Shwartz A, Chiron F. 2011. Concerns regarding the scientific evidence informing impact risk assessment and management recommendations for invasive birds. Biol Conserv. 144:2112–2118 [Google Scholar]
- Sturkie PD. 2000. Sturkie’s Avian Physiology. 5th ed (Whittow G, editor). San Diego: Academic Press [Google Scholar]
- Tayleur JR. 2010. A comparison of the establishment, expansion and potential impacts of two introduced parakeets in the United Kingdom. In: BOU Proceedings - The Impacts of Non-native Species. p. 1–12 [Google Scholar]
- Tvardíková K, Fuchs R. 2010. Do birds behave according to dynamic risk assessment theory? A feeder experiment. Behav Ecol Sociobiol. 65:727–733 [Google Scholar]
- Vilà M, Basnou C, Gollasch S, Josefsson M, Pergl J, Scalera R. 2009. One hundred of the most invasive alien species in Europe. In: Handbook of Alien Species in Europe. Vol. 3 Dordrecht: Springer Netherlands; p. 265–268 [Google Scholar]
- Vilà M, Basnou C, Pyšek P, Josefsson M, Genovesi P, Gollasch S, Nentwig W, Olenin S, Roques A, Roy D, et al. 2010. How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Front Ecol Environ. 8:135–144 [Google Scholar]
- Wauters L, Tosi G, Gurnell J, Martinoli A. 2002. Interspecific competition between native Eurasian red squirrels and alien grey squirrels: does resource partitioning occur? Behav Ecol Sociobiol. 52:332–341 [Google Scholar]
- Wauters LA, Gurnell J. 1999. The mechanism of replacement of red squirrels by grey squirrels: a test of the interference competition hypothesis. Ethology. 105:1053–1071 [Google Scholar]
- Webb JK, Brown GP, Child T, Greenlees MJ, Phillips BL, Shine R. 2008. A native dasyurid predator (common planigale, Planigale maculata) rapidly learns to avoid a toxic invader. Austral Ecol. 33:821–829 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.