Abstract
Mucopolysaccharidosis type I (MPS I) is an autosomal recessive disease which leads to systemic disease, including progressive neurodegeneration, mental retardation and death before the age of 10 years. MPS I results from deficiency of α-L-iduronidase (laronidase, IDUA) in lysosomes and subsequent accumulation of glycosaminoglycans (GAG). Clinical enzyme replacement therapy (ERT) with intravenous laronidase reverses some aspects of MPS I disease (e.g., hepatomegaly, splenomegaly, glycosaminoglycanuria) and ameliorates others (e.g., pulmonary function, cardiac disease, arthropathy, exercise tolerance). However, neurologic benefits are thought to be negligible because the blood-brain barrier (BBB) blocks enzyme from reaching the central nervous system (CNS). We considered the possibility that a very high dose of intravenous laronidase might be able to transit across the BBB in small quantities, and provide some metabolic correction in the brain. To address this question, we administered high-dose laronidase (11.6 mg/kg, once per week, 4 weeks) to adult MPS I mice. IDUA enzyme activity in cortex of injected mice increased to 97% of that in wild type mice (p<0.01). GAG levels in cortex were reduced by 63% of that from untreated MPS I mice (p<0.05). Further, immunohistochemical analysis showed that the treatment reduced secondary storage material GM3-ganglioside in treated MPS I mice. Water T-maze tests showed that the learning abnormality in MPS I mice was surprisingly reduced (p<0.0001). In summary, these results indicated that repeated, high-dose ERT facilitated IDUA transit across the BBB, reduced GAG accumulation within the CNS, and rescued cognitive impairment.
Keywords: Hurler syndrome, enzyme replacement therapy, high dose, blood-brain-barrier, mucopolysaccharidosis I
1. Introduction
MPS I is an autosomal recessive disease that leads to systemic disease, including progressive neurodegeneration, mental retardation and death before the age of 10 years. Hurler syndrome (MPS IH, OMIM #67014) results from deficiency of α-L-iduronidase (IDUA, E.C.3.2.1.76), which degrades the GAG heparan sulfate and dermatan sulfate. The widespread accumulation of GAG leads to progressive cellular damage and organ dysfunction, with the central nervous system (CNS) being one of the primary sites of pathology. The CNS pathology in Hurler syndrome patients is severe, manifested by hydrocephalus, mental retardation, learning delays and dementia.
Clinical IDUA ERT ameliorates exclusively certain visceral defects [1]. The uptake of infused laronidase to affected tissues relies on the mannose 6-phosphate receptor (M6PR), which is down-regulated in the blood-brain barrier after the first two weeks of life [2]. Further, infused IDUA is rapidly cleared from the circulation by M6PR and mannose receptor (MR) [3]. As a result, neurological benefits of ERT with the conventional dose are thought to be negligible because the BBB blocks infused enzyme from reaching the CNS [4,5]. Novel therapies leading to metabolic correction in the CNS and neurological benefits are desperately needed.
High-dose ERT has been shown in several lysosomal disease animal models to ameliorate neuropathology, including mice with aspartylglycosaminuria [6], Krabbe disease [7], MPS VII [8], α-mannosidosis [9,10], arylsulfatase A deficiency [11,12], MPS II [13], and guinea pigs with α-mannosidosis [14]. However, a high-dose ERT study [15] conducted in MPS IIIA mice showed no neurological benefits, indicating a potential difference in mechanisms of enzyme uptake or that the dose required in MPS IIIA is higher than other lysosomal diseases.
In this study, a single or multiple injections of high-dose IDUA was administered in an MPS I mouse model. For the first time, this study showed that repeated, high-dose ERT allows sufficient enzyme delivery to the CNS and improves cognitive function. These results may lead to improved treatment for MPS I patients with cognitive impairment.
2. Material and Methods
2.1 MPS I mice and injection
MPS I knockout mice (idua−/−), a kind gift from Dr. Elizabeth Neufeld, UCLA, were generated in her laboratory by insertion of neomycin resistance gene into exon 6 of the 14-exon IDUA gene on the C57BL/6 background. MPS I mice (idua−/−) and heterozygotes (idua−/+) were genotyped by PCR. Aldurazyme® (BioMarin Pharmaceutics) was concentrated by centrifugation in Amicon Ultra Centrifugal Filters (Millipore) to a final concentration of ~1.2 mg/mL or 11.6 mg/mL. The preparations were diluted in 120 µL phosphate buffered saline (PBS), and injected over 10–15 seconds by the tail vein at the dose regimens detailed in the Results section. All mouse care and handling procedures were in compliance with the rules of the Institutional Animal Care and Use Committee (IACUC) of the University of Minnesota.
2.2 IDUA enzyme assay
IDUA activity was determined by a fluorometric assay using 4-methylumbelliferyl α-L-iduronide (4-MU iduronide, Glycosynth #44076) as the substrate. 4-MU iduronide was diluted with sodium formate buffer (0.4 M, pH 3.5) to different concentrations. Then, 25 µL aliquots of 4-MU substrate (180 µM) were mixed with 25 µL aliquots of tissue homogenates. The mixture was incubated at 37 °C for 30 min, and 200 µL glycine carbonate buffer (pH 10.4) was added to quench the reaction. IDUA catalyzed the cleavage of the non-fluorescent substrate (4-MU iduronide) into a fluorescent product (4-MU). 4-Methylumbelliferone (4-MU, Sigma #M1381) was used to make the standard curve. The resulting fluorescence was measured using a Bio-Tek plate reader with excitation at 355 nm and emission at 460 nm. IDUA enzyme activity was expressed in units (nmol converted to product per hour) per mg protein as determined with a Pierce protein assay kit (Fisher # PI22662). All reactions were run in triplicate.
2.3 Tissue GAG assay
Tissue GAG assays were conducted as described previously [16]. The supernatants of tissue homogenates were treated by Proteinase K (NEB #CX27439) with the ratio of 3(Pro K):1(sample), incubated at 55°C for 24 hours, and boiled for 10 min to inactivate the enzyme. Then, samples were incubated with 250 U DNase (Sigma # D4527-10KU) and 2.5 µg RNase (Sigma # R6513-10MG) at room temperature for 24 hours. After boiling for 10 min to inactivate the enzymes, GAG concentration was determined by the Blyscan Sulfated Glycosaminoglycan Assay (Biocolor Inc). Results were expressed as µg GAG/mg protein.
2.4 Water T-maze test
Water T-maze test was conducted as described previously [17]. Four days after the third injection, mice from all three groups were subjected to water T-maze testing which assessed the learning ability of mice. A pool was filled approximately 10 cm deep with water (room temperature) mixed with non-toxic white acrylic paint in order to prevent visual detection of submerged objects. A "T" shape maze was set in the water, and a platform, at the end of the left (or right) arm, was submerged 0.5–1 cm below the surface of the water. In run #1, a barrier was placed across one arm. Mice were released at the base of the "T", forced to swim to the top, and choose either the left or right arm to locate the platform. Then, in run #2, the barrier was removed and the platform was placed at the end of the other arm. The correct choice was the alternate arm from where the platform was placed in run #1. Each mouse performed 8 trials per day for 8 days.
2.5 Immunohistochemistry
Immunohistochemistry was conducted as described previously [18]. Mice were perfused with 20 mL 0.01 M PBS and sacrificed. The brain was removed, fixed overnight in paraformaldehyde and cryopreserved in 30% sucrose. Then, the brain was frozen on dry ice and sectioned coronally. After washing, free-floating sections were blocked with PBS containing 5% normal goat serum and 0.3% Triton X-100. Primary antibody against GM3-ganglioside (1:50, Abcam) was added and the sections were incubated at 4°C overnight. After washing, sections were incubated with goat-anti mouse IgM (Antibodies-online) and DAPI (Sigma) at room temperature for 1 hour. After mounting on the slides, sections were examined with a microscope (Zeiss).
2.6 Enzyme-linked immunosorbent assay (ELISA)
ELISA was conducted as described previously [19]. Aldurazyme (BioMarin Pharmaceutics) was diluted with Tris-buffered saline (pH=7.4) to a final concentration of 4 µg/mL. In a 96-well microplate, 50 µL diluted Aldurazyme was incubated at 4°C overnight. After washing and blocking with Tris-buffered saline containing 1% BSA at room temperature for 2 hours, 100 µL serum (1:50 dilutions) was incubated at room temperature for 1 hour. After washing, the wells were incubated with 100 µL anti-mouse IgG alkaline phosphatase conjugate (Abcam #97027) at room temperature for 1 hour. After washing, the wells were incubated with 200 µL of 1 mg/ml p-nitrophenyl phosphate chromogenic substrate (Sigma # P7998-100ML) at room temperature for 1h. Then, 50 µL EDTA (0.1 M, pH 8.0) was added to stop the reaction. Absorbance at 405 nm was quantified by using a Bio-tek plate reader.
2.7 Statistical analysis
Data are represented as mean±standard error. For evaluation of differences between samples, Tukey test for comparisons between paired samples and one-way or two-way analysis of variance (ANOVA) for comparisons between three or more samples were applied. Statistical significant level was set at p<0.05. Data analysis was conducted with SAS 9.3.
3. Results
3.1 Biodistribution of IDUA after single high-dose ERT
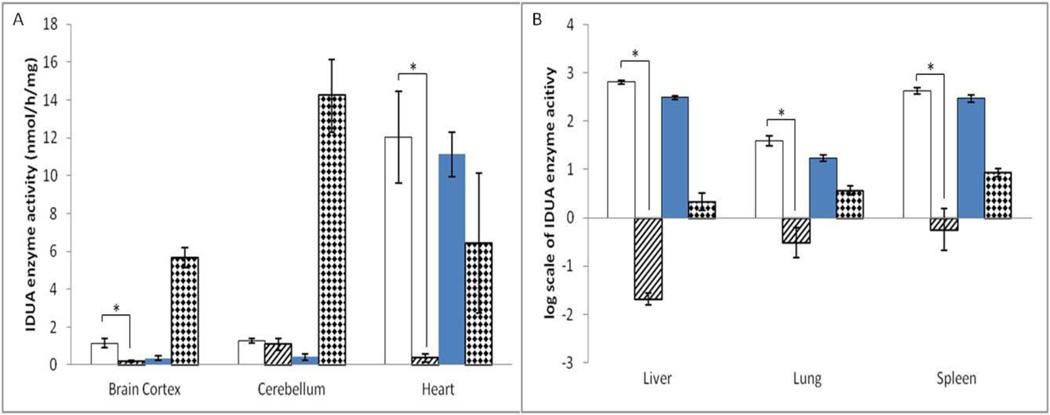
An initial experiment was designed to determine the potential effects of high-dose ERT on the transit of laronidase across the BBB. A single injection of 0.58 mg/kg (0.7×104 pmol/kg, clinical standard dose) or ~1.2 mg/kg (1.4×104 pmol/kg, approximately 2 fold the standard dose) laronidase (Aldurazyme) was intravenously administered to adult MPS I mice (idua−/−, 10 to 20 weeks old, n=6 for high-dose group, n=7 for normal-dose group) by the tail vein. Age-matched untreated MPS I mice ( n=7) and heterozygotes (idua−/+, n=6) were included as controls. It has been shown that the plasma half-life of infused IDUA in dogs is approximately 20 min [20]. Although there might be a difference in plasma half-life between dogs and mice, we presumed that 2 hours would be sufficient for infused IDUA to enter the tissues. Two hours after the injection, all mice were sacrificed, and multiple organs including brain cortex, cerebellum, heart, liver, lung and spleen were collected. IDUA enzyme activity in tissue homogenates was assessed by IDUA enzyme assay. In liver and spleen, which acquired the highest enzyme concentration, the IDUA enzyme activity in the high-dose group increased to 98-fold and 24-fold of that in wild-type mice, respectively (Figure 1). In lung and heart, the IDUA enzyme activity in the high-dose group increased to 24-fold and 93% of that in wild-type activity, respectively. Moreover, IDUA enzyme activity in brain cortex of the high-dose group mice increased to 10.1% of that in wild-type mice. There was no significant increase in enzyme activity in cerebellum. In the normal-dose group, we also observed enzyme activity increases in heart, liver, lung and spleen. However, compared with untreated MPS I mice, no significant increase was observed in brain cortex or cerebellum of the normal-dose group. Also, no statistically significant age or gender effects were found in IDUA enzyme activity.
Figure 1. IDUA enzyme activity in multiple organs increased after a single injection of ~1.2 mg/kg laronidase.
A total of 28 age-matched mice were assigned into four groups: 1) MPS I mice (idua−/−, n=6, white columns) injected with 2 fold (~1.2 mg/kg) laronidase; 2) MPS I mice (n=6, dark columns)injected with normal-dose (0.58 mg/kg) laronidase; 3) MPS I mice (n=6, hatched columns) with no injections; 4) heterozygote (idua+/−, n=5, diamond columns) with no injections. Data are mean ±standard error. * p<0.05.
Serum was collected prior to sacrifice, and high IDUA enzyme activity was observed in treated MPS I mice: 110.6±49.8 nmol/h/mL for high dose group and 78.4±25.3 nmol/h/mL for normal dose group. IDUA enzyme activity in heterozygous and untreated MPS I mice was almost non-detectable. Although perfusion was conducted, there is still a possibility that the enzyme activity increase in brain cortex can be attributed to serum within brain capillaries.
3.2 Biodistribution of IDUA after repeated, high-dose ERT
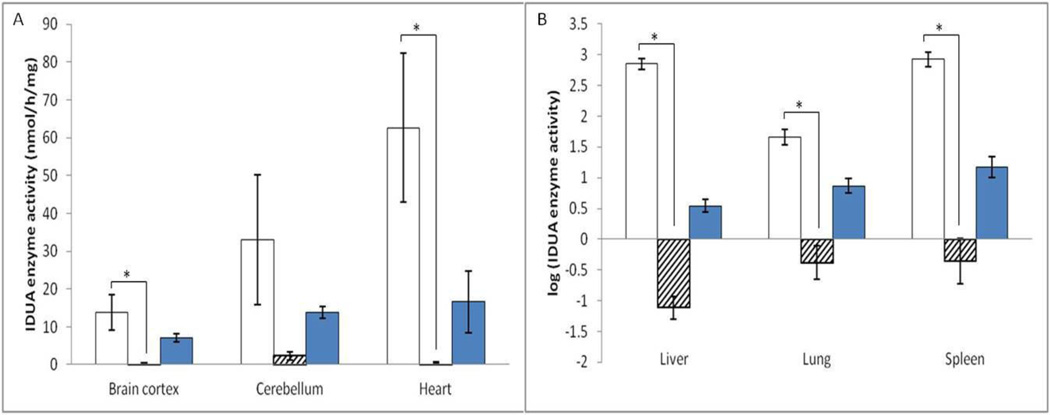
Based on the results of aforementioned single high-dose ERT and high-dose ERT studies on other lysosomal disease models, we designed a repeated ERT schedule with a 20-fold higher than the human therapeutic dose (~11.6 mg/kg body weight, 1.4 × 105 pmol/kg). Adult MPS I mice (11.7±2.7 week old, n=12) received 4 injections of 20-fold dose IDUA once a week. Age-matched untreated MPS I mice (idua−/−, n=11) and heterozygotes (idua−/+, n=11) were included as controls. All mice were sacrificed five days after the 4th injection, and multiple organs were collected.
An increase in enzyme activity was observed in heart (1.9-fold of wild-type activity), liver (95.1-fold of wild-type activity), lung (3.4-fold of wild-type activity) and spleen (21.5-fold of wild-type activity) (Figure 2A, B). IDUA enzyme activity in brain cortex and cerebellum of treated MPS I mice increased to 97% and 1.2-fold of that in wild-type mice, respectively (Figure 2A). Also, no statistically significant age or gender effects were found in IDUA enzyme activity. Serum was collected on D3, D10 and D26 (injection on D0, D7, D14, D21). Since IDUA enzyme levels in serum of treated mice were undetectable (data not shown), the enzyme activity increase in cortex and cerebellum cannot be simply explained by the presence of blood in the capillaries of the brain.
Figure 2. IDUA enzyme activity in multiple organs increased after 4 injections of ~11.6 mg/kg laronidase.
A total of 34 age-matched adult mice were assigned into four groups: 1) MPS I mice (idua−/−, n=12, white columns) injected with 20 fold (~11.6 mg/kg) laronidase; 2) MPS I mice (n=11, hatched columns) with no injections; 3) heterozygote (idua+/−, n=11, dark columns) with no injections. Data are mean ± standard error. * p<0.05.
3.3 Reduced tissue GAG accumulation after repeated, high-dose ERT
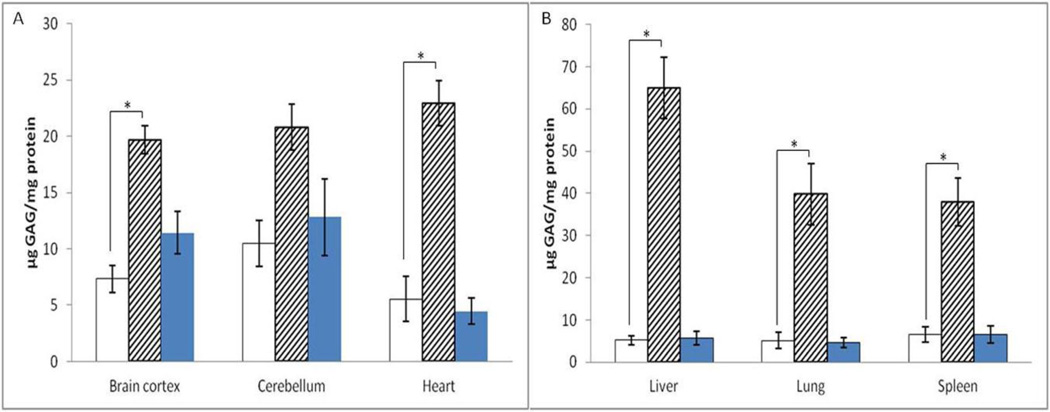
To determine the effect of repeated, high-dose ERT on GAG accumulation, GAG was quantified in tissue homogenates. Liver and spleen acquired the highest enzyme levels after injection, correspondingly, GAG accumulation was reduced the most in these two organs: by 92%, and 83%, respectively (Figure 3B). Also, GAG accumulation in heart and lung was reduced by 76% and 87%, respectively (Figure 3A, B). GAG accumulation in cortex was reduced by 63% (Figure 3A). Also, no statistically significant age or gender effects were found in GAG accumulation.
Figure 3. GAG accumulation reduced in multiple organs after 4 injections of ~11.6 mg/kg laronidase.
Data are mean ±standard error, p values are calculated by one-way ANOVA and Tukey test. * p<0.05. Treated MPS I: white columns; untreated MPS I: hatched columns; heterozygous mice: dark columns.
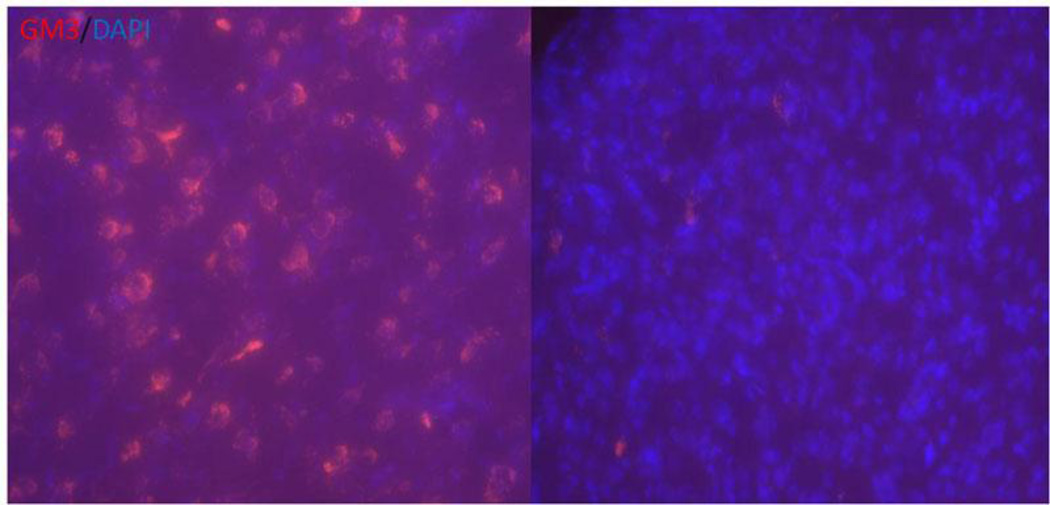
To further validate the GAG accumulation reduction within the CNS, histochemical analysis was conducted. Secondary accumulation of GM3-ganglioside was apparent in the brains of untreated MPS I mice, visualized by positive immunofluorescence staining of punctate aggregates throughout most of the brain (including thalamus, hippocampus and nuclei septi). In contrast, only a few punctate aggregates were observed in the same regions of brains from treated MPS I mice (Figure 4).
Figure 4. Secondary storage material GM3-ganglioside was reduced in the hippocampus region of treated MPS I mice.
(Red: GM3; Blue: DAPI.)
3.4 Cognitive improvements after repeated, high-dose ERT
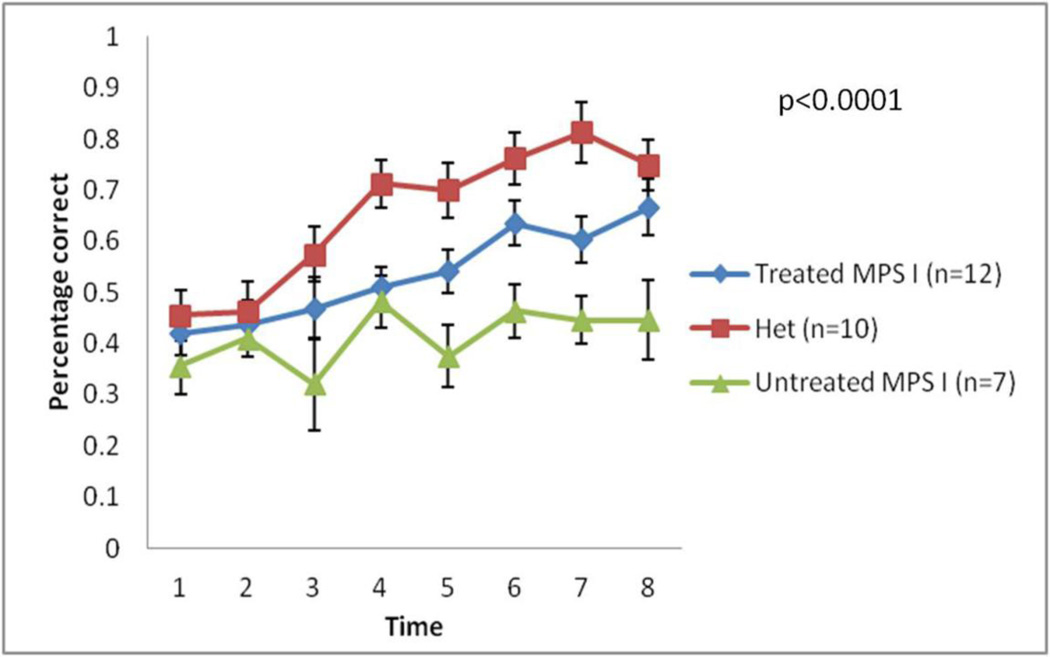
Water T-maze tests were employed to evaluate learning behavior regardless of physical performance. Five days after the third injection, mice from all three groups were subjected to 8 trials of water T-maze tests per day for a duration of 8 days. For each mouse, the percentage of finding the platform correctly was recorded. On D0, mice from all three groups had a similar correct percentage (36 to 42%). However, the heterozygote (idua−/+) group improved significantly over the testing period, reaching a final level of 75%. Treated MPS I mice also improved significantly during the trials, reaching a final level of 67%. In contrast, untreated MPS I mice only improved modestly from 36% to 45%. Because this test assesses decision-making abitlity, swimming speed was not recorded nor evaluated. Also, the platform was underneath opaque water, eye disease of MPS I mice is unlikely to be a confounding factor because corneal opacity is not detectable in the relatively young mice used in this series of experiments. There did not appear to be any physical defects in the MPS I mice that could have affected the results. Water T maze tests showed that learning abnormalities observed in MPS I mice were reduced significantly by repeated, high-dose ERT (Figure 5, p<0.0001).These results showed that by repeated, high-dose ERT, the CNS pathology in MPS I mice was functionally rescued.
Figure 5. Learning abnormality in MPS I mice was surprisingly reduced by repeated high-dose ERT.
Five days after the third injection, all three groups underwent 8-day water T maze test which investigate learning ability of mice. X axis values mean time in days. Data are mean ±standard error, p value was calculated by two-way ANOVA.
3.5 Anti-IDUA immune response after repeated, high-dose ERT
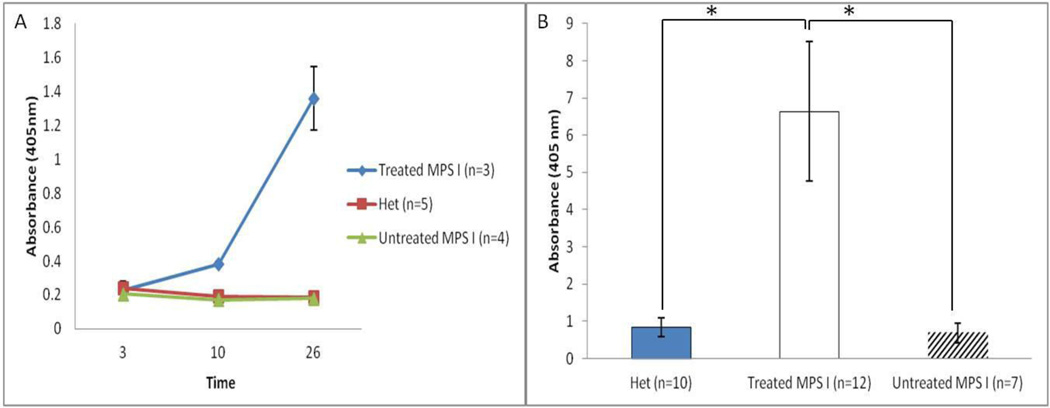
Several previous studies showed that infused IDUA elicited a humoral immune response in patients and animal models [19, 21]. Serum was collected from 12 mice of the three groups at different time-points and processed for ELISA. In treated MPS I mice, a significant increase in anti-IDUA antibody concentration was observed after the second injection (Figure 6A). Also, right before sacrifice, serum was collected from 29 mice of the three groups and processed for ELISA. In treated MPS I mice, there were significant higher antibody levels compared with the other two groups (Figure 6B).
Figure 6. Anti-IDUA immune response detected after repeated high-dose ERT.
(A) The IDUA antibody levels were measured by ELISA in serum collected at various time-points (D3, D10, D26). X axis values mean time in days. The serum was tested at 1:50 dilution. (B) Serum from a total of 29 mice were collected right before sacrifice. The IDUA antibody levels were measured by ELISA. The serum was tested at 1:50 dilution. Data are mean ±standard error. * p<0.05.
Strong adverse infusion reactions were found in some treated mice after the second injection but were alleviated by injection of 2 mg/kg dexamethasone 1 hour prior to enzyme infusion, prior to the subsequent injections. Based on ELISA data, treated mice can be divided into two groups: those with or without high anti-IDUA levels. However, no significant difference in enzyme activity, GAG accumulation or performance in behavior tests was observed between these two groups.
4. Discussion
4.1 Limitations of ERT
Progressive neurodegeneration in MPS I patients is a devastating feature leading to dramatic intellectual impairment. ERT has already been used in clinical application for treating MPS I and other lysosomal diseases. Delivery of infused enzymes to lysosomes is mediated by MR on macrophages or ubiquitously expressed M6PR. A previous study in mice has shown that M6PR-mediated transcytosis in the BBB is down-regulated after two weeks of life [2]. As a result, although ERT can achieve significant visceral storage reduction, neurological improvements are thought to be negligible. It has been a long-held dogma that the BBB prevents the transit of lysosomal enzymes from the blood to the interstitial space surrounding neuronal and glial cells of the CNS. Any means of delivering enzyme across the BBB would be of major clinical significance.
In this study of high-dose ERT, the most corrective effect was achieved in liver and spleen, which took up the majority of infused IDUA, as shown by IDUA enzyme assays. The IDUA enzyme activity in brain cortex reached 50% of that in wild-type mice. Considering the fact that only a small amount of IDUA can make a great difference in patients, this significant increase in the CNS has promise of providing therapeutic benefits [22,23,24]. Consistent with this observation, total GAG accumulation in brain cortex was reduced after 4 injections. Further, behavior analysis showed that even when the CNS impairment is clearly manifested, high-dose ERT can be effective in preventing and possibly correcting this impairment.
4.2 Analysis of Mechanism
Less than 200 µL of laronidase was administered by the tail vein, ruling out the possibility of passage of enzyme through the blood-brain barrier as the result of a hydro-dynamic effect resulting from the rapid injection of a large volume of enzyme solution. Also, blood from each mouse was collected right before sacrifice, and plasma IDUA enzyme levels in treated mice were approximately 0 (data not shown). This result excludes the possibility of blood contamination of the brain. Furthermore, although we could not directly visualize IDUA in brain sections with antibody staining (data not shown), increased enzyme activity, decreased GAG concentrations and, decreased GM3-ganglioside suggest that infused IDUA reached the CNS tissue.
The mechanism by which intravenous IDUA transits across the BBB and reaches the brain remains an open question at present. One possibility may be fluid-phase pinocytosis. This type of uptake is strictly dependent on concentrations and is relatively slow. High-dose ERT can lead to a high enzyme level in blood, which makes it possible for fluid-phase pinocytosis to be effective. Another possibility is the extracellular pathway which has been shown to allow small amounts of molecules as large as albumin to cross the BBB [25]. Also, there exists the possibility that residual M6PR or other uncharacterized receptors more efficiently facilitate the transit of IDUA in the context of high plasma enzyme levels. However, we cannot rule out the possibility that the integrity of the BBB in MPS I mice might be impaired by disease-related factors. Identifying the mechanism of delivery of IDUA to brain may suggest strategies to enhance the process and make ERT for CNS disease reduction a more realistic goal.
4.3 Implications and Future Directions
The inability of normal-dose ERT to correct pathology within the CNS may be due to two obstacles: 1) other tissues, e.g. liver and spleen, internalizing most of the infused IDUA, preventing sufficient enzyme delivery to the CNS; 2) antibody binds the infused IDUA, further reducing the efficiency of delivery to the CNS. Several high-dose ERT studies have been conducted in other lysosomal disease animal models. Most studies showed a surprising enzyme delivery to the CNS (results summarized in Table 1), indicating that the BBB is not impermeable to infused enzymes. Therefore, it is a matter of 'inefficiency' rather than an insurmountable obstacle. The inefficiency of the CNS delivery in normal-dose ERT may be circumvented by high-dose ERT.
Table 1.
Summary of outcomes from high-dose ERT studies conducted on animal models of lysosomal diseases with neurodegeneration.
| Animal model | Immuno- competent |
Doses(n) | Enzyme activity achieved (% Normal) |
Brain storage reduced (%Affected) |
Reduced Neuropathology |
Reference |
|---|---|---|---|---|---|---|
| α-mannosidosis mice | Y | 250 U/kg(2) | ND | 74 % | ND | [9] |
| α-mannosidosis mice | Y | 500 U/kg (2) | 15% | 50% | Y | [10] |
| α-mannosidosis pigs | Y | 10 mg/kg (1) | ND | ND | Y | [14] |
| ASA mice | Y | 20 mg/kg (4) | ND | 30% | Y | [11] |
| ASA mice | N | 50 mg/kg (52) | ND | 34% | Y | [12] |
| AGU mice | Y | 10 mg/kg (8) | 10% | 20% | Y | [6] |
| Krabbe mice | Y | 6 mg/kg | 7% | 18% | Y | [7] |
| MPS II mice | Y | 10 mg/kg (45) | 5% | ND | Y | [13] |
| MPS IIIA mice | Y | 20 mg/kg (4) | 22% | 0 | N | [15] |
| MPS VII mice | N | 20 mg/kg (4) | 2.5% | ND | Y | [8] |
| MPS I mice | Y | 20 mg/kg (4) | 97% | 63% | Y | This study |
Y= yes; N=no; ND = not determined.
High humoral immune response against IDUA was identified in treated mice by ELISA. In spite of high anti-IDUA antibody levels, significant increase in IDUA enzyme activity was observed and GAG accumulation was reduced. In fact, a recent study showed that development of antibodies to IDUA negatively correlates with improvement in performance on behavioral tests in murine MPS 1 [26]. It will be interesting to see whether infused IDUA can efficiently cross the BBB in immuno-tolerant or immuno-deficient animals. In addition, minimizing the immune response perhaps by modifying the epitopes of IDUA enzyme can be a promising future direction.
4.4 Significance
A recent murine study, using 1.2 mg/kg dose every 2 weeks (equivalent to clinical standard dose 0.58 mg/kg per week) achieved GAG reduction in brain and neurological improvements [26]. Similarly, a canine study showed that dogs treated from birth with ERT (0.58 to 1.57 mg/kg) also led to reduction of GAG accumulation in the brain [27]. This study, for the first time, assessed repeated high-dose ERT in MPS I mice. Using a 20-fold dose (11.6 mg/kg) compared to the generally accepted therapeutic dose in people, may not be applicable to patients considering the cost and increased immune response. However, it has been suggested that dose conversion using body surface area (BSA) instead of body weight is more appropriate [28]. According to a formula provided by the Food and Drug Administration [29], 11.6 mg/kg in this murine study would be approximately 0.94 mg/kg in adult patients, which is not a great increase from the standard dose (0.58 mg/kg). These facts make this study more meaningful in terms of potential clinical application: only a small increase in dose may make a great difference. These results provide the proof of principle: when the enzyme level is high enough, a small amount of IDUA can cross the BBB of adult animals, and provide neurological benefits. Although intranasal [30], intraventricular [18], and intrathecal [31,32] administration of enzyme or gene vector have produced neurological improvements, these strategies are limited by their invasive nature. The best therapeutic strategy might be the one that delivers enzyme uniformly to the brain. Considering the fact that each neuron is estimated to be approximately 15 µm from blood vessels, the bloodstream would be an ideal conduit for enzyme delivery.
Highlights.
We conducted high-dose ERT in MPS I mice.
High-dose ERT leads to enzyme activity increase in the CNS.
High-dose ERT leads to GAG accumulation reduction in the CNS.
High-dose ERT leads to neurological improvements.
Acknowledgements
The authors would like to thank Brenda Diethelm-Okita for revising ELISA protocol. This work was supported by NIH grant P01HD032652 .
Abbreviations
- ERT
enzyme replacement therapy
- BBB
blood-brain-barrier
- IDUA
α-L-iduronidase
- MPS
mucopolysaccharidosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giugliani R, Federhen A, Rojas MV, Vieira T, Artigalás O, Pinto LL, Azevedo AC, Acosta A, Bonfim C, Lourenço CM, Kim CA, Horovitz D, Bonfim D, Norato D, Marinho D, Palhares D, Santos ES, Ribeiro E, Valadares E, Guarany F, de Lucca GR, Pimentel H, de Souza IN, Correa J, Sr, Fraga JC, Goes JE, Cabral JM, Simionato J, Llerena J, Jr, Jardim L, Giuliani L, da Silva LC, Santos ML, Moreira MA, Kerstenetzky M, Ribeiro M, Ruas N, Barrios P, Aranda P, Honjo R, Boy R, Costa R, Souza C, Alcantara FF, Avilla SG, Fagondes S, Martins AM. Mucopolysaccharidosis I, II, and VI: Brief review and guidelines for treatment. Genet. Mol. Biol. 2010;33:589–604. doi: 10.1590/S1415-47572010005000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urayama A, Grubb JH, Sly WS, Banks WA. Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood-brain barrier. Proc. Natl. Acad. Sci. U. S. A. 2004;101:12658–12663. doi: 10.1073/pnas.0405042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao KW, Faull KF, Kakkis ED, Neufeld EF. Carbohydrate structures of recombinant human alpha-L-iduronidase secreted by Chinese hamster ovary cells. J. Biol. Chem. 1997;272:22758–22765. doi: 10.1074/jbc.272.36.22758. [DOI] [PubMed] [Google Scholar]
- 4.Begley DJ, Pontikis CC, Scarpa M. Lysosomal storage diseases and the blood-brain barrier. Curr. Pharm. Des. 2008;14:1566–1580. doi: 10.2174/138161208784705504. [DOI] [PubMed] [Google Scholar]
- 5.Enns GM, Huhn SL. Central nervous system therapy for lysosomal storage disorders. Neurosurg. Focus. 2008;24:3–4. doi: 10.3171/FOC/2008/24/3-4/E11. [DOI] [PubMed] [Google Scholar]
- 6.Dunder U, Kaartinen V, Valtonen P, Väänänen E, Kosma VM, Heisterkamp N, Groffen J, Mononen I. Enzyme replacement therapy in a mouse model of aspartylglycosaminuria. FASEB. J. 2000;14:361–367. doi: 10.1096/fasebj.14.2.361. [DOI] [PubMed] [Google Scholar]
- 7.Lee WC, Courtenay A, Troendle FJ, Stallings-Mann ML, Dickey CA, DeLucia MW, Dickson DW, Eckman CB. Enzyme replacement therapy results in substantial improvements in early clinical phenotype in a mouse model of globoid cell leukodystrophy. FASEB. J. 2005;19:1549–1551. doi: 10.1096/fj.05-3826fje. [DOI] [PubMed] [Google Scholar]
- 8.Vogler C, Levy B, Grubb JH, Galvin N, Tan Y, Kakkis E, Pavloff N, Sly WS. Overcoming the blood-brain barrier with high-dose enzyme replacement therapy in murine mucopolysaccharidosis VII. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14777–14782. doi: 10.1073/pnas.0506892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roces DP, Lüllmann-Rauch R, Peng J, Balducci C, Andersson C, Tollersrud O, Fogh J, Orlacchio A, Beccari T, Saftig P, von Figura K. Efficacy of enzyme replacement therapy in alpha-mannosidosis mice: a preclinical animal study. Hum. Mol. Genet. 2004;13:1979–1988. doi: 10.1093/hmg/ddh220. [DOI] [PubMed] [Google Scholar]
- 10.Blanz J, Stroobants S, Lüllmann-Rauch R, Morelle W, Lüdemann M, D'Hooge R, Reuterwall H, Michalski JC, Fogh J, Andersson C, Saftig P. Reversal of peripheral and central neural storage and ataxia after recombinant enzyme replacement therapy in alpha-mannosidosis mice. Hum. Mol. Genet. 2008;17:3437–3445. doi: 10.1093/hmg/ddn237. [DOI] [PubMed] [Google Scholar]
- 11.Matzner U, Herbs E, Hedayati KK, Lüllmann-Rauch R, Wessig C, Schröder S, Eistrup C, Möller C, Fogh J, Gieselmann V. Enzyme replacement improves nervous system pathology and function in a mouse model for metachromatic leukodystrophy. Hum. Mol. Genet. 2005;14:1139–1152. doi: 10.1093/hmg/ddi126. [DOI] [PubMed] [Google Scholar]
- 12.Matzner U, Lüllmann-Rauch R, Stroobants S, Andersson C, Weigelt C, Eistrup C, Fogh J, D'Hooge R, Gieselmann V. Enzyme replacement improves ataxic gait and central nervous system histopathology in a mouse model of metachromatic leukodystrophy. Mol. Ther. 2009;17:600–606. doi: 10.1038/mt.2008.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polito VA, Abbondante S, Polishchuk RS, Nusco E, Salvia R, Cosma MP. Correction of CNS defects in the MPSII mouse model via systemic enzyme replacement therapy. Hum. Mol. Genet. 2010;19:4871–4885. doi: 10.1093/hmg/ddq420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawley AC, King B, Berg T, Meikle PJ, Hopwood JJ. Enzyme replacement therapy in alpha-mannosidosis guinea-pigs. Mol. Genet. Metab. 2006;89:48–57. doi: 10.1016/j.ymgme.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Rozaklis T, Beard H, Hassiotis S, Garcia AR, Tonini M, Luck A, Pan J, Lamsa JC, Hopwood JJ, Hemsley KM. Impact of high-dose, chemically modified sulfamidase on pathology in a murine model of MPS IIIA. Exp. Neurol. 2011;230:123–130. doi: 10.1016/j.expneurol.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Rivera MF, Colvin-Wanshura LE, Nelson MS, Nan Z, Khan SA, Rogers TB, Maitra I, Low WC, Gupta P. Characterization of an immunodeficient mouse model of mucopolysaccharidosis type I suitable for preclinical testing of human stem cell and gene therapy. Brain. Res. Bull. 2007;74:429–438. doi: 10.1016/j.brainresbull.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Arco A, Segovia G, Garrido P, de Blas M, Mora F. Stress, prefrontal cortex and environmental enrichment: studies on dopamine and acetylcholine release and working memory performance in rats. Behav. Brain. Res. 2007;176:267–273. doi: 10.1016/j.bbr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Wolf DA, Lenander AW, Nan Z, Belur LR, Whitley CB, Gupta P, Low WC, McIvor RS. Direct gene transfer to the CNS prevents emergence of neurologic disease in a murine model of mucopolysaccharidosis type I. Neurobiol. Dis. 2011;43:123–133. doi: 10.1016/j.nbd.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Domenico C, Villani GR, Di Napoli D, Reyero EG, Lombardo A, Naldini L, Di Natale P. Gene therapy for a mucopolysaccharidosis type I murine model with lentiviral-IDUA vector. Hum. Gene. Ther. 2005;16:81–90. doi: 10.1089/hum.2005.16.81. [DOI] [PubMed] [Google Scholar]
- 20.Shull RM, Kakkis ED, McEntee MF, Kania SA, Jonas AJ, Neufeld EF. Enzyme replacement in a canine model of Hurler syndrome. Proc. Natl. Acad. Sci. U. S. A. 1994;91:12937–12941. doi: 10.1073/pnas.91.26.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickson P, Peinovich M, McEntee M, Lester T, Le S, Krieger A, Manuel H, Jabagat C, Passage M, Kakkis ED. Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccharidosis I. J. Clin. Invest. 2008;118:2868–2876. doi: 10.1172/JCI34676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guffon N, Souillet G, Maire I, Straczek J, Guibaud P. Follow-up of nine patients with Hurler syndrome after bone marrow transplantation. J. Pediatr. 1998;133:119–125. doi: 10.1016/s0022-3476(98)70201-x. [DOI] [PubMed] [Google Scholar]
- 23.Peters C, Balthazor M, Shapiro EG, King RJ, Kollman C, Hegland JD, Henslee-Downey J, Trigg ME, Cowan MJ, Sanders J, Bunin N, Weinstein H, Lenarsky C, Falk P, Harris R, Bowen T, Williams TE, Grayson GH, Warkentin P, Sender L, Cool VA, Crittenden M, Packman S, Kaplan P, Lockman LA, Anderson J, Krivit W, Dusenbery K, Wagner J. Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood. 1996;87:4894–4902. [PubMed] [Google Scholar]
- 24.Peters C, Shapiro EG, Anderson J, Henslee-Downey PJ, Klemperer MR, Cowan MJ, Saunders EF, de Alarcon PA, Twist C, Nachman JB, Hale GA, Harris RE, Rozans MK, Kurtzberg J, Grayson GH, Williams TE, Lenarsky C, Wagner JE, Krivit W. Hurler syndrome: II. Outcome of HLA-genotypically identical sibling and HLA-haploidentical related donor bone marrow transplantation in fifty-four children. The Storage Disease Collaborative Study Group. Blood. 1998;91:2601–2608. [PubMed] [Google Scholar]
- 25.Banks WA. Are the extracellular [correction of extracelluar] pathways a conduit for the delivery of therapeutics to the brain? Curr. Pharm. Des. 2004;10:1365–1370. doi: 10.2174/1381612043384862. [DOI] [PubMed] [Google Scholar]
- 26.Baldo G, Mayer FQ, Martinelli BZ, Carvalho TG, Meyer FS, Oliveira PG, Meurer L, Tavares A, Matte U, Giugliani R. Enzyme replacement therapy started at birth improves outcome in difficult-to-treat organs in mucopolysaccharidosis I mice. Mol. Genet. Metab. 2013;109:33–40. doi: 10.1016/j.ymgme.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Dierenfeld AD, McEntee MF, Vogler CA, Vite CH, Chen AH, Passage M, Le S, Shah S, Jens JK, Snella EM, Kline KL, Parkes JD, Ware WA, Moran LE, Fales-Williams AJ, Wengert JA, Whitley RD, Betts DM, Boal AM, Riedesel EA, Gross W, Ellinwood NM, Dickson PI. Replacing the enzyme alpha-L-iduronidase at birth ameliorates symptoms in the brain and periphery of dogs with mucopolysaccharidosis type I. Sci. Transl. Med. 2010;2:60ra89. doi: 10.1126/scitranslmed.3001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB. J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 29.Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Estimating the safe starting dose in clinical trials for therapeutics in adult healthy volunteers. Rockville, Maryland, USA: U.S. Food and Drug Administration; 2002. [Google Scholar]
- 30.Wolf DA, Hanson LR, Aronovich EL, Nan Z, Low WC, Frey WH, McIvor RS. Lysosomal enzyme can bypass the blood-brain barrier and reach the CNS following intranasal administration. Mol. Genet. Metab. 2012;106:131–134. doi: 10.1016/j.ymgme.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Dickson P, McEntee M, Vogler C, Le S, Levy B, Peinovich M, Hanson S, Passage M, Kakkis E. Intrathecal enzyme replacement therapy: successful treatment of brain disease via the cerebrospinal fluid. Mol. Genet. Metab. 2007;91:61–68. doi: 10.1016/j.ymgme.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kakkis E, McEntee M, Vogler C, Le S, Levy B, Belichenko P, Mobley W, Dickson P, Hanson S, Passage M. Intrathecal enzyme replacement therapy reduces lysosomal storage in the brain and meninges of the canine model of MPS I. Mol. Genet. Metab. 2004;83:163–174. doi: 10.1016/j.ymgme.2004.07.003. [DOI] [PubMed] [Google Scholar]