The SKICH domain of human TAX1BP1 was successfully expressed in E. coli, purified and crystallized. The crystal diffracted to a resolution of 1.9 Å. A molecular-replacement solution was found using the structure of the SKICH domain of NDP52 as a search model.
Keywords: TAX1BP1, A20, SKICH domain, innate immune signaling, NF-κB, IRF3, autophagy receptor
Abstract
TAX1BP1 is a highly conserved, pleiotropic protein that plays many essential functions in human cells, including negative regulation of inflammatory and antimicrobial responses mediated by NF-κB and IRF3 signaling, inhibition of apoptosis, transcriptional coactivation and autophagy etc. TAX1BP1 contains a SKICH domain at the N-terminus, three coiled-coil domains in the middle and two ubiquitin-binding zinc-finger motifs at the C-terminus. The SKICH domain and the linker sequence between the SKICH domain and the coiled-coil region mediate interaction with ubiquitin-like proteins of the LC3/GABARAP family, which are autophagosome markers. For structure determination of the SKICH domain of TAX1BP1, a protein construct (amino acids 15–148) corresponding to the SKICH domain plus the linker region was expressed, purified and crystallized. A native diffraction data set has been collected to 1.9 Å resolution. A molecular-replacement solution has been found by using the structure of the SKICH domain of NDP52, a paralog of TAX1BP1.
1. Introduction
Human TAX1BP1 (also known as T6BP and TXBP151) was originally identified as a binding partner of the Tax1 protein of human T-cell leukemia virus type I (HTLV-I; Gachon et al., 1998 ▶; Verstrepen et al., 2011 ▶). TAX1BP1 is highly conserved across species. The major isoform (isoform 2) of human TAX1BP1 contains 747 amino acids with a putative domain structure as shown in Fig. 1 ▶. At the N-terminus, there is a skeletal muscle and kidney-enriched inositol phosphatase (SKIP) carboxyl homology (SKICH) domain. Within the SKICH domain, there is a potential 14-3-3 binding site (amino acids 112–117; RGASTP). Three coiled-coil and two helix–loop–helix regions are predicted in the central part of the protein (Ling & Goeddel, 2000 ▶). At the C-terminus, two C2H2-type zinc fingers are present, which are designated UBZ because they can function as novel ubiquitin-binding domains (Iha et al., 2000 ▶). Each of the UBZs contains a conserved PPXY motif which has been well established as a recognition motif for the WW domain (Sudol et al., 1995 ▶).
Figure 1.

Schematic representation of the putative domains of human TAX1BP1. The coiled-coil region contains three coiled-coil domains. UBZ1 and UBZ2: ubiquitin-binding zinc-finger motifs.
A number of important biological functions have been ascribed to TAX1BP1. The best characterized function is its role as a negative regulator of the nuclear factor-κB (NF-κB) and interferon regulatory factor 3 (IRF3) signaling (Verstrepen et al., 2011 ▶). Both NF-κB and IRF3 are transcription factors that are activated in inflammatory and antiviral responses (Kumar et al., 2011 ▶; O’Neill & Bowie, 2010 ▶). A key regulatory mechanism in both pathways is the polyubiquitination of signaling proteins, which is mediated by the action of three families of ubiquitin-handling enzymes: ubiquitin-activating enzyme E1, ubiquitin-conjugating enzyme E2 and ubiquitin ligase E3. The signal transduction cascades are initiated by binding of ligands, such as cytokines and lipopolysaccharide (LPS), to the cytokine receptors or pattern-recognition receptors (PRRs). The activated receptors recruit various adaptor proteins. The adaptor proteins function as platforms for the binding and activation of E3 enzymes, such as tumor necrosis factor (TNF) receptor-associated factors (TRAF) 2/3/5/6 and cellular inhibitor of apoptosis (cIAPs), as well as E2 enzymes such as UBC13 and UBCH5C. The E3 enzymes mediate the Lys63-linked polyubiquitination (K63-Ub) of themselves (TRAF6 and TRAF3 in the NF-κB and IRF3 pathways, respectively) or receptor-interacting protein 1 (RIP1, in the NF-κB pathway). K63-Ub-conjugated RIP1 and TRAF6 recruit the adaptor proteins TAB1/2/3 and IKKγ (also known as NF-κB essential modulator NEMO) which bind the kinases TAK1 (TGFβ activated kinase) and IKKα/IKKβ (IκB kinases α and β), respectively. TAK1 phosphorylates and activates IKKα/IKKβ. IKKα/IKKβ then phosphorylate the inhibitor of NF-κB (IκBα), leading to its Lys48-linked polyubiquitination (K48-Ub) and proteasomal degradation. The NF-κB dimers (such as p50–p65) translocate into the nucleus and activate transcription of many genes. Similarly, in the IRF3 pathway, K63-Ub-conjugated TRAF3 recruits the ubiquitin-binding adaptor protein TRAF family member-associated NF-κB activator (TANK), which binds the kinases TBK1 (TANK-binding kinase) and IKK∊. TRAF3 polyubiquitinates (K63-Ub) and activates TBK1 and IKK∊, which then phosphorylate IRF3, leading to its dimerization and nuclear translocation (Verstrepen et al., 2011 ▶).
The NF-κB and IRF3 signaling pathways are tightly regulated. Dysregulation of these pathways may lead to severe inflammatory diseases. TAX1BP1 negatively regulates these pathways by recruiting the ubiquitin-editing enzyme A20 to specific signaling proteins. A20 possesses deubiquitinating enzyme (DUB) activity at its N-terminus and E3 ubiquitin ligase activity at its C-terminus. In the NF-κB pathway, the DUB activity of A20 removes K63-Ub chains from RIP1 and TRAF6, while the E3 ligase activity conjugates K48-Ub chains to RIP1 and E2 enzymes (UBC13 and UBCH5C), leading to their proteasomal degradation. TAX1BP1 and A20 also block the interaction between the E2 and E3 enzymes. All these actions of TAX1BP1 and A20 prevent Lys63-linked polyubiquitination of RIP1 and TRAF6, leading to termination of the signaling cascades. In the IRF3 signaling pathway, TAX1BP1 recruits A20 to the kinases TBK1 and IKK∊, preventing the Lys63-linked polyubiquitylation of TBK1 and IKK∊ by TRAF3 (independent of the A20 DUB activity). As a result, the IRF3 signaling is abolished. In both of the NF-κB and IRF3 signaling pathways, TAX1BP1 functions as an essential adaptor protein that targets A20 to key signaling proteins. Working together, TAX1BP1 and A20 restrict inflammatory and antimicrobial responses. Binding of Tax1 to TAX1BP1 disrupts TAX1BP1–A20 interaction and thus evokes persistent NF-κB activation (Shembade et al., 2008 ▶).
TAX1BP1 also functions as a regulator of cell growth and apoptosis, although the mechanisms are not well understood. TAX1BP1 and A20 inhibit tumor necrosis factor (TNF)-induced apoptosis (De Valck et al., 1999 ▶). TAX1BP1 also binds to other proteins with established roles in cell-growth regulation, such as checkpoint with fork-head associated and ring finger (CHFR), an E3 ubiquitin ligase that delays entry into metaphase in response to mitotic stress (Matsusaka & Pines, 2004 ▶), and deleted in liver cancer (DLC) 2, a putative tumor suppressor that inhibits tumor-cell proliferation, migration and transformation (Nagaraja & Kandpal, 2004 ▶).
Another established function of TAX1BP1 is transcriptional coactivation. TAX1BP1 binds to and stabilizes human and bovine papilloma virus E2 proteins. Together with the transcriptional coactivator p300, TAX1BP1 enhances E2-dependent transcription (Wang et al., 2009 ▶). TAX1BP1 also forms a complex with the glucocorticoid receptor and activates the glucocorticoid response element (GRE; Chin et al., 2007 ▶). Binding of Tax1 to TAX1BP1 leads to dissociation of TAX1BP1 from the glucocorticoid receptor-containing protein complex and repression of GRE-dependent transcription.
TAX1BP1 has also been implicated in some other functions, such as autophagy (Newman et al., 2012 ▶), membrane trafficking (Morriswood et al., 2007 ▶) and neurotransmission (Ulrich et al., 2007 ▶).
As a multifunctional protein, TAX1BP1 interacts with many different proteins (Verstrepen et al., 2011 ▶). Besides those mentioned above, other TAX1BP1-interacting proteins include ubiquitin-like proteins of the LC3/GABARAP family (Newman et al., 2012 ▶), GABAC receptors ρ1 subunit (Ulrich et al., 2007 ▶), myosin VI (Morriswood et al., 2007 ▶), RhoGAP (Nagaraja & Kandpal, 2004 ▶), A20 binding inhibitor of NF-κB (ABIN) 1 (Gao et al., 2011 ▶), optineurin (Journo et al., 2009 ▶), ring finger protein 11 (RNF11; Shembade et al., 2009 ▶) and itchy E3 ubiquitin protein ligase homolog (ITCH; Shembade et al., 2008 ▶). TAX1BP1 may also mediate the formation of multi-subunit protein complexes. For example, the inhibitory activity of TAX1BP1 and A20 in the NF-κB signaling pathway also requires RNF11 and ITCH as essential components in the ubiquitin-editing complex (Verstrepen et al., 2011 ▶). Given the large number of TAX1BP1-binding proteins and the complex protein–protein interaction networks, the specificity of TAX1BP1 functions is most likely to be dependent on the peculiar TAX1BP1-containing protein complexes. It is therefore of interest to better understand the molecular mechanisms through which TAX1BP1 interacts with its protein partners.
We have initiated a structural study on TAX1BP1 and its protein interactions. Here, we report our progress on the bacterial expression, purification and crystallization of the SKICH domain of TAX1BP1.
2. Materials and methods
2.1. Cloning
A cDNA clone for the full-length human TAX1BP1 (GenBank BC050358, IMAGE clone 6055189) was purchased from DNASU Plasmid Repository. The protein product of this cDNA corresponds to the TAX1BP1 isoform 1 with 789 amino acids (GenBank NP_006015), which is identical to the 747-amino-acid isoform 2 (GenBank NP_001073333) except that amino acids 603–645 of isoform 1 are not present in isoform 2. The DNA encoding the putative SKICH domain and an extra C-terminal sequence (residues 15–148) was amplified by PCR using Pfu DNA polymerase from the plasmid with the forward primer 5′-CAAGGACCGAGCAGCCCCTCAgcccatgtcatctttcaaaatg-3′ and reverse primer 5′-ACCACGGGGAACCAACCCTTAGCCTGCTTTTGTGGTCACC-3′. Both of these primers contain 21 nt at the 5′-end as a specific sequence for ligation-independent cloning (LIC). The purified PCR product (by agarose gel electrophoresis) was processed by Pfu DNA polymerase in the presence of 2.5 mM dATP (72°C for 1 h) to generate 5′-overhangs at both ends. The cloning vector is an in-house modified LIC vector that contains DNA sequences encoding the HaloTag (Los et al., 2008 ▶), a His tag and an eight-amino-acid recognition sequence for HRV 3C protease in front of a specific LIC sequence 5′-tcaaggaccgagcagccccgggttggttccccgtggta-3′. An SmaI site is present in the middle of the LIC sequence. The vector was cut by SmaI digestion and subsequently processed by Pfu DNA polymerase in the presence of 2.5 mM dTTP (72°C for 1 h) to generate 5′-overhangs at both ends. The processed PCR insert and vector were mixed and incubated at room temperature for 20 min. The mixture was used to transform DH5α Escherichia coli competent cells. Cell cultures were grown from the colonies and plasmids were extracted. Typically, all plasmids produced by this LIC cloning procedure contain the correct insert. Protein expression was carried out using NiCo21 (DE3) E. coli cells (New England Biolabs).
2.2. Protein expression and purification
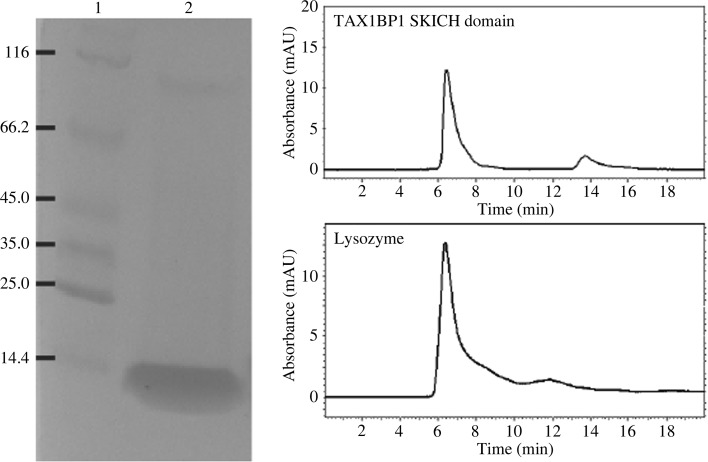
The human TAX1BP1 SKICH domain was expressed as a fusion protein in E. coli. The fusion protein contains an N-terminal HaloTag and His tag, followed by a HRV 3C protease recognition motif and the target protein. After cleavage of the tags by HRV 3C protease, the target protein contains an artificial sequence GPSSP at the N-terminus. Cell culture was grown in LB medium until it reached an OD600 of 0.6–0.8. IPTG was added to the culture (0.4 mM final concentration) to induce protein expression. After induction, the culture was allowed to grow overnight at a temperature of 10°C to facilitate production of soluble overexpressed proteins. The overexpressed proteins were purified by NTA affinity resin. After elution from the resin with 200 mM imidazole, the fusion protein was processed by HRV 3C protease in a dialysis tubing at 4°C overnight. Complete cleavage of the fusion tags was typically achieved after overnight dialysis. The cleaved tags were separated from the target protein by a reverse IMAC (immobilized metal-affinity chromatography) process with NTA resin. The purified proteins were concentrated to a concentration of ∼10 mg ml−1 in a buffer consisting of 25 mM Tris pH 7.5, 200 mM NaCl (Fig. 2 ▶).
Figure 2.
Electrophoresis and size-exclusion chromatography of the SKICH domain of human TAX1BP1. Left, SDS–PAGE of the purified crystallization construct of the SKICH domain. Lanes 1 and 2 contain molecular-weight marker (labeled in kDa) and the SKICH domain preparation, respectively. In the molecular-weight maker, the protein at 14.4 kDa is lysozyme. Right, the SKICH domain is comparable to lysozyme in terms of elution time in size-exclusion chromatography.
2.3. Crystallization, data collection and processing
To carry out the crystallization trials, we prepared seven sets of screening solutions each containing 96 different conditions. The solutions use various PEGs as the precipitants. Other variables include buffers, pHs, salts and cryoprotectants. The crystallization trials were carried out using 96-well format plates. Multiple crystallization conditions were identified within a few days. After optimizing the crystallization conditions, diffracting crystals of the TAX1BP1 SKICH domain (Fig. 3 ▶) were obtained at 22°C by sitting-drop vapor diffusion against 50 µl well solution (12% PEG 6000, 0.1 M Tris buffer pH 8.0, 0.1 M potassium acetate) using 96-well format crystallization plates. The crystallization drops consisted of 1 µl protein sample mixed with 1 µl well solution. Before flash-cooling in liquid nitrogen, the crystals were transferred to a solution consisting of 12% PEG 6000, 0.1 M Tris buffer pH 8.0, 0.1 M potassium acetate, 10% glycerol and soaked for 24 h.
Figure 3.

A crystal of the SKICH domain of human TAX1BP1.
Data collection was carried out on beamline 21ID-F of LS-CAT at the Advanced Photon Source (Argonne National Laboratory). Data were processed, integrated and scaled with iMosflm and SCALA in CCP4 (Battye et al., 2011 ▶; Winn et al., 2011 ▶). Molecular replacement was carried out using Phaser (McCoy et al., 2007 ▶).
3. Results and discussion
The human TAX1BP1 SKICH domain protein construct in our study contains 134 amino acids, spanning residues 15–148 of TAX1BP1. The protein was expressed in E. coli as a fusion protein with an N-terminal HaloTag and His tag to facilitate expression and purification of the protein. An eight-amino-acid recognition motif for HRV 3C protease is present between the tags and the target protein. The HaloTag is a 34 kDa monomeric protein derived from a bacterial haloalkane dehalogenase. It is a more recently developed fusion tag to facilitate protein expression and purification (Peterson & Kwon, 2012 ▶). In our labotatory, we have used many different fusion tags (including MBP, NusA, Trx, Sumo, trigger factor, GST, GB1 and HaloTag; Huang et al., 2013 ▶; Shang et al., 2013 ▶, 2014 ▶). The HaloTag outperforms the other tags, although how exactly the HaloTag facilitates protein expression and solubility is not known. The HaloTag is also an affinity tag which can bind to HaloLink resin covalently (Peterson & Kwon, 2012 ▶). However, this resin is very expensive; therefore, we only use His-tag affinity resin for protein purification. After purification of the fusion protein by affinity resin, the tags were cleaved by a His-tagged HRV 3C protease. The cleavage reaction is very efficient and highly specific. The tags and the added His-tagged HRV 3C protease were separated from the target protein by reverse IMAC. These procedures of protein expression and purification have become very established in our laboratory, and have been proven to be sufficient for the production of pure protein samples for crystallization (Huang et al., 2013 ▶; Shang et al., 2013 ▶, 2014 ▶). As shown in Fig. 2 ▶, the TAX1BP1 SKICH domain preparation is very pure. In solution, the protein behaves as a monomer, as indicated by size-exclusion chromatography (the protein has a molecular weight comparable to that of lysozyme). The target protein was concentrated to ∼10 mg ml−1. The target protein for crystallization contains an artificial pentapeptide sequence GPSSP at the N-terminus as a cloning artefact resulting from the 3C protease recognition sequence and the LIC sequence of the cloning vector. However, the presence of the artificial sequence did not seem to interfere with crystallization of the proteins.
A native protein sample was prepared which yielded diffraction-quality crystals. A good data set with a resolution of 1.9 Å was collected from a single crystal. Data-collection and processing statistics are shown in Table 1 ▶. The crystallization protein construct (including the N-terminal artificial sequence) has a calculated molecular weight of 15.7 kDa. The asymmetric unit is most likely to contain two molecules, with a solvent content of 47.5%.
Table 1. Data-collection and processing statistics.
Values in parentheses are for the highest resolution shell.
| Space group | P212121 |
| Unit-cell parameters (Å) | a = 45.26, b = 63.92, c = 101.67 |
| Resolution (Å) | 34.7–1.9 (2.0–1.9) |
| No. of unique reflections | 23961 |
| Completeness (%) | 99.8 (99.5) |
| R merge (%) | 12.0 (49.3) |
| 〈I/σ(I)〉 | 7.9 (2.6) |
| Multiplicity | 5.8 (5.6) |
A crystal structure of the SKICH domain of the autophagy receptor nuclear dot protein 52 (NDP52) has recently been reported (von Muhlinen et al., 2012 ▶). The crystallization protein construct of the NDP52 SKICH domain contains 123 residues, and shares 47% sequence identity (58% positive) with our 134-amino-acid protein construct of the TAX1BP1 SKICH domain. Given the reasonably high degree of sequence homology between the two SKICH domains, we tried to use the molecular-replacement method to solve the structure of the TAX1BP1 SKICH domain using the structure of the NDP52 SKICH domain (PDB entry 3vvv; von Muhlinen et al., 2012 ▶) as a search model. Using Phaser, a single solution was found, with a rotational Z-score (RFZ) of 6.6, a translational Z-score (TFZ) of 9.3 and a log-likelihood-gain (LLG) of 81.2, indicating that the solution is most likely to be correct.
The SKICH domain was first identified at the C-terminal region of SKIP and proline-rich inositol-polyphosphate 5-phosphatase (PIPP; Gurung et al., 2003 ▶). The SKICH domain is also present at the N-terminal region of TAX1BP1 and NDP52. Based on sequence alignment of the SKICH domains in these four proteins, two highly conserved motifs DWXGX 3VGX 6YX 4W and GX 3PF were identified as the signatures of the SKICH domain. It has been shown that the SKICH domain in SKIP and PIPP is important for plasma membrane localization in growth-factor-stimulated and resting cells. It was therefore proposed that the SKICH domain represented a novel membrane-targeting domain (Gurung et al., 2003 ▶). However, the SKICH domain in TAX1BP1 and NDP52 does not mediate membrane localization, although TAX1BP1 and NDP52 are involved in the regulation of membrane trafficking through their interaction with myosin VI (Morriswood et al., 2007 ▶).
The biological functions of the SKICH domain in TAX1BP1 and NDP52 are largely unknown. As a paralogous protein of TAX1BP1, NDP52 has a similar domain structure as TAX1BP1: a SKICH domain at the N-terminus, a central coiled-coil domain and a ubiquitin-binding zinc-finger motif at the C-terminus (von Muhlinen et al., 2012 ▶). NDP52 is an established autophagy receptor. NDP52 selectively binds to the ubiquitin-like protein LC3C, one of the six human AuTophaGy (ATG) 8 orthologs of the LC3/GABARAP family. The binding of ATG8 or orthologs to autophagy receptors is typically mediated by the formation of an intermolecular β-sheet which contains a single strand contributed by the so-called ATG8/LC3-interacting region (LIR) of the autophagy receptor (Noda et al., 2008 ▶; Johansen & Lamark, 2011 ▶). A consensus LIR motif (W/F-X-X-L/I/V) is present within the SKICH domain of NDP52. However, selective binding between NDP52 and LC3C is not mediated by this motif but instead by a noncanonical LIR motif (termed CLIR, comprising the tripeptide LVV) that is located in the linker region between the SKICH and coiled-coil domains. Specific interaction between NDP52 and LC3C via the CLIR motif is required for antibacterial autophagy (von Muhlinen et al., 2012 ▶).
TAX1BP1 also contains a ‘canonical LIR’ motif (W49VGI) within its SKICH domain and a ‘noncanonical LIR’ motif (L141VV) in the linker region. It has been shown that TAX1BP1 is a bona fide autophagy receptor (Newman et al., 2012 ▶). TAX1BP1 binds LC3/GABARAP proteins with varying affinities. Mutations of the ‘canonical LIR’ or ‘noncanonical LIR’ motifs (W49VGI↔AVGA and L141VV↔AAA) ablate localization of TAX1BP1 to autophagosomes, suggesting that both motifs are required for interaction with the LC3/GABARAP proteins. In non-small-cell lung cancer (NSCLC) cells, TBK1-dependent basal autophagy of the cargo receptors NDP52 and TAX1BP1 promotes noncanonical NF-κB signaling mediated by the noncanonical pathway subunit RelB (Newman et al., 2012 ▶). The results of these studies not only identify protein-binding partners for the TAX1BP1 SKICH domain and a relevant TAX1BP1 function, but also suggest a potential difference between the two SKICH domains of TAX1BP1 and NDP52 in terms of protein-binding partners and specificities. It is therefore important to determine the structure of the TAX1BP1 SKICH domain and investigate how it interacts with other proteins.
Acknowledgments
We thank staff of the LS-CAT at the Advanced Photon Source (Argonne National Laboratory) for assistance with data collection. This work was supported by the start-up fund and a seed grant from Southern Illinois University Carbondale.
References
- Battye, T. G. G., Kontogiannis, L., Johnson, O., Powell, H. R. & Leslie, A. G. W. (2011). Acta Cryst. D67, 271–281. [DOI] [PMC free article] [PubMed]
- Chin, K.-T., Chun, A. C. S., Ching, Y.-P., Jeang, K.-T. & Jin, D.-Y. (2007). Cancer Res. 67, 1072–1081. [DOI] [PubMed]
- De Valck, D., Jin, D.-Y., Heyninck, K., Van de Craen, M., Contreras, R., Fiers, W., Jeang, K.-T. & Beyaert, R. (1999). Oncogene, 18, 4182–4190. [DOI] [PubMed]
- Gachon, F., Peleraux, A., Thebault, S., Dick, J., Lemasson, I., Devaux, C. & Mesnard, J. M. (1998). J. Virol. 72, 8332–8337. [DOI] [PMC free article] [PubMed]
- Gao, L., Coope, H., Grant, S., Ma, A., Ley, S. C. & Harhaj, E. W. (2011). J. Biol. Chem. 286, 36592–36602. [DOI] [PMC free article] [PubMed]
- Gurung, R., Tan, A., Ooms, L. M., McGrath, M. J., Huysmans, R. D., Munday, A. D., Prescott, M., Whisstock, J. C. & Mitchell, C. A. (2003). J. Biol. Chem. 278, 11376–11385. [DOI] [PubMed]
- Huang, X., Wang, G., Wu, Y. & Du, Z. (2013). Acta Cryst. D69, 1598–1608. [DOI] [PubMed]
- Iha, H., Kasai, T., Kibler, K. V., Iwanaga, Y., Tsurugi, K. & Jeang, K.-T. (2000). AIDS Res. Hum. Retroviruses, 16, 1633–1638. [DOI] [PubMed]
- Johansen, T. & Lamark, T. (2011). Autophagy 7, 279–296. [DOI] [PMC free article] [PubMed]
- Journo, C., Filipe, J., About, F., Chevalier, S. A., Afonso, P. V., Brady, J. N., Flynn, D., Tangy, F., Israël, A., Vidalain, P. O., Mahieux, R. & Weil, R. (2009). PLoS Pathog. 5, e1000521. [DOI] [PMC free article] [PubMed]
- Kumar, H., Kawai, T. & Akira, S. (2011). Int. Rev. Immunol. 30, 16–34. [DOI] [PubMed]
- Ling, L. & Goeddel, D. V. (2000). Proc. Natl Acad. Sci. USA, 97, 9567–9572. [DOI] [PMC free article] [PubMed]
- Los, G. V. et al. (2008). ACS Chem. Biol. 3, 373–382. [DOI] [PubMed]
- Matsusaka, T. & Pines, J. (2004). J. Cell Biol. 166, 507–516. [DOI] [PMC free article] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- Morriswood, B., Ryzhakov, G., Puri, C., Arden, S. D., Roberts, R., Dendrou, C., Kendrick-Jones, J. & Buss, F. (2007). J. Cell Sci. 120, 2574–2585. [DOI] [PMC free article] [PubMed]
- Muhlinen, N. von, Akutsu, M., Ravenhill, B. J., Foeglein, Á., Bloor, S., Rutherford, T. J., Freund, S. M., Komander, D. & Randow, F. (2012). Mol. Cell, 48, 329–342. [DOI] [PMC free article] [PubMed]
- Nagaraja, G. M. & Kandpal, R. P. (2004). Biochem. Biophys. Res. Commun. 313, 654–665. [DOI] [PubMed]
- Newman, A. C., Scholefield, C. L., Kemp, A. J., Newman, M., McIver, E. G., Kamal, A. & Wilkinson, S. (2012). PLoS One, 7, e50672. [DOI] [PMC free article] [PubMed]
- Noda, N. N., Kumeta, H., Nakatogawa, H., Satoo, K., Adachi, W., Ishii, J., Fujioka, Y., Ohsumi, Y. & Inagaki, F. (2008). Genes Cells, 13, 1211–1218. [DOI] [PubMed]
- O’Neill, L. A. & Bowie, A. G. (2010). Curr. Biol. 20, R328–R333. [DOI] [PubMed]
- Peterson, S. N. & Kwon, K. (2012). Curr. Chem. Genomics, 6, 8–17. [DOI] [PMC free article] [PubMed]
- Shang, J., Wang, G., Yang, Y., Huang, X. & Du, Z. (2013). Acta Cryst. F69, 1097–1099. [DOI] [PMC free article] [PubMed]
- Shang, J., Wang, G., Yang, Y., Huang, X. & Du, Z. (2014). Acta Cryst. D70, 155–164. [DOI] [PubMed]
- Shembade, N., Harhaj, N. S., Parvatiyar, K., Copeland, N. G., Jenkins, N. A., Matesic, L. E. & Harhaj, E. W. (2008). Nature Immunol. 9, 254–262. [DOI] [PubMed]
- Shembade, N., Parvatiyar, K., Harhaj, N. S. & Harhaj, E. W. (2009). EMBO J. 28, 513–522. [DOI] [PMC free article] [PubMed]
- Sudol, M., Chen, H. I., Bougeret, C., Einbond, A. & Bork, P. (1995). FEBS Lett. 369, 67–71. [DOI] [PubMed]
- Ulrich, M., Seeber, S., Becker, C. M. & Enz, R. (2007). Biochem. J. 401, 429–436. [DOI] [PMC free article] [PubMed]
- Verstrepen, L., Verhelst, K., Carpentier, I. & Beyaert, R. (2011). Trends Biochem. Sci. 36, 347–354. [DOI] [PubMed]
- Wang, X., Naidu, S. R., Sverdrup, F. & Androphy, E. J. (2009). J. Virol. 83, 2274–2284. [DOI] [PMC free article] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.