The β-l-arabinofuranosidase (HypBA1) from Bifidobacterium longum has been expressed in Escherichia coli and the purified recombinant protein crystallized.
Keywords: hydroxyproline-rich glycoproteins, glycoside hydrolase, HypBA1, Bifidobacterium longum
Abstract
The β-l-arabinofuranosidase (HypBA1) from Bifidobacterium longum JCM 1217 hydrolyzes the β-1,2-linked arabinofuranose disaccharide to release l-arabinoses. HypBA1 was classified into glycoside hydrolase family 127 (GH127) by the CAZy website (http://www.cazy.org/). The enzyme was expressed in Escherichia coli and the purified recombinant protein was crystallized. Crystals belonging to the primitive hexagonal space group P3x21, with unit-cell parameters a = b = 75.9, c = 254.0 Å, were obtained by the sitting-drop vapour-diffusion method and diffracted to 2.78 Å resolution. A BLASTP search (http://blast.ncbi.nlm.nih.gov/) of the Protein Data Bank did not reveal any similar crystal structures. Structural determination by using SeMet MAD and MIR methods is in progress.
1. Introduction
β-l-Arabinofuranosyl-linked arabinofuranosyl residues (Araf) are widely observed in extensins and solanaceous lectins. These macromolecules belong to the family of hydroxyproline (Hyp)-rich glycoproteins (HRGPs) and are broadly found as structural proteins in plant cell walls (Kieliszewski & Lamport, 1994 ▶; Kieliszewski et al., 1994 ▶). In addition, terminal β-l-Araf can be found in a large number of biopolymers from algae and plants. In spite of the abundance of β-l-Araf-containing sugars in plant cells, the degradation and metabolism of these polysaccharides remain largely unexplored owing to a lack of knowledge of the corresponding degradative enzymes.
Recently, β-l-arabinofuranosidase and β-l-arabinobiosidase (HypBA1 and HypBA2), identified from Bifidobacterium longum JCM 1217, have been found to be involved in the metabolic pathway of β-l-arabinooligosaccharides (Fujita, Takashi et al., 2011 ▶; Fujita, Sakamoto et al., 2011 ▶). In the hydrolysis of Hyp-linked β-l-arabinooligosaccharides, HypBA2 releases the β-1,2-linked Araf disaccharide (β-Ara2) from Araf-β-1,2-Araf-β-1,2-Arafβ-Hyp (Ara3-Hyp). Subsequently, HypBA1 liberates the l-arabinoses by hydrolyzing β-Ara2 (Fujita, Sakamoto et al., 2011 ▶; Fujita, Takashi et al., 2011 ▶). HypBA1 has attracted much attention because no similar crystal structure to HypBA1 has been solved and its catalytic mechanism is still unclear. BLASTP (http://blast.ncbi.nlm.nih.gov/) searches of the HypBA1 amino-acid sequence against a nonredundant protein-sequence database revealed that this enzyme is different from any other glycoside hydrolases, but has 40–100% identity to hypothetical proteins in Bifidobacterium, Metascardovia, Lactobacillus, Weissella, Clostridium, Oenococcus, Pediococcus, Coriobacterium, Bacillus, Leuconostoc, Enterococcus, Zymophilus, Butyrivibrio, Lachnobacterium, Thermobacillus, Paenibacillus, Megamonas, Thermoanaerobacter, Geobacillus, Pelosinus, Treponema, Eubacterium, Blautia, Firmicutes, Cohnella, Xanthomonas, Alicyclobacillus, Deinococcus and Caldicellulosiruptor strains. Thus, HypBA1 has recently been classified into a new glycohydrolase family GH127 (http://www.cazy.org/), and its crystal structure is important to further studies of these novel GH127-family enzymes.
2. Materials and methods
2.1. Protein preparation
The gene encoding HypBA1 (GenBank accession No. AB619598.1) from B. longum ATCC15707 (American Type Culture Collection, Manassas, Virginia, USA) was amplified by polymerase chain reaction (PCR) with forward primer 5′-CATATGAACGTTACAATCACTTCC-3′ and reverse primer 5′-GCGGCCGCTCGACGCTGGAAGACACGC-3′ (the NdeI and NotI restriction-enzyme sites are shown in bold, respectively). The PCR fragments encoding HypBA1 were digested with NdeI and NotI, and ligated with NdeI/NotI-digested pET-29a (Novagen, Madison, Wisconsin, USA). The recombinant plasmid was transformed into Escherichia coli BL21 (DE3) and gene expression was induced with 1.0 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) at 289 K for 48 h. Cell pellets were harvested by centrifugation at 6000g and resuspended in a lysis buffer consisting of 25 mM MES pH 6.5, 150 mM NaCl, 20 mM imidazole. The cell lysate was prepared with a French press instrument (JNBIO JN-3000 PLUS) and was then centrifuged at 16 000g to remove cell debris. The target protein was purified on an ÄKTA purifier 10 (GE Healthcare Life Sciences) using an Ni–NTA column. The buffer used for the Ni–NTA column was 25 mM MES pH 6.5, 150 mM NaCl, 20 mM imidazole. The target protein eluted at about 60 mM imidazole when using a 20–250 mM imidazole gradient. The protein solution was dialyzed twice against 5 l buffer consisting of 25 mM MES pH 6.5, and was then loaded onto a 20 ml DEAE Sepharose Fast Flow column (GE Healthcare Life Sciences). The buffer and gradient were 25 mM MES pH 6.5 and 0–500 mM NaCl, respectively. The eluted HypBA1 protein (73 kDa; amino acids 1–658) was then dialyzed twice against 5 l buffer (25 mM HEPES pH 7.0) and concentrated to 16 mg ml−1 using Amicon Ultra-15 Centrifugal Filter Units (Millipore). The purity was greater than 95% as judged by SDS–PAGE.
2.2. Crystallization and data collection
Initial crystallization screening was performed manually using 768 different reservoir conditions from Hampton Research crystallization screening kits (Laguna Niguel, California, USA), including Crystal Screen, Crystal Screen 2, Crystal Screen Cryo, Crystal Screen Lite, MembFac, Natrix, Index, SaltRx, SaltRx 2, PEG/Ion, PEG/Ion 2, Quick Screen and Grid Screens (Ammonium Sulfate, MPD, Sodium Chloride, Sodium Malonate, PEG 6000 and PEG/LiCl), by the sitting-drop vapour-diffusion method. All crystallization experiments were conducted at 295 K. In general, 2 µl HypBA1-containing solution (16 mg ml−1 in 20 mM HEPES pH 7.0) was mixed with 2 µl reservoir solution in 24-well Cryschem Plates (Hampton Research), and equilibrated against 300 µl reservoir solution at 295 K. Crystals of HypBA1 appeared within 5 d using PEG/Ion condition No. 30 [0.2 M ammonium acetate, 20%(w/v) polyethylene glycol 3350]. The crystallization condition was optimized to 0.4 M ammonium acetate, 18%(w/v) polyethylene glycol 3350. Within 5–6 d, the crystals reached dimensions of about 0.38 × 0.38 × 0.1 mm. Prior to data collection at 100 K, the crystal was mounted in a cryoloop and flash-cooled in liquid nitrogen. An X-ray diffraction data set was collected to 2.78 Å resolution on beamline BL13C1 of the National Synchrotron Radiation Research Center (NSRRC, Hsinchu, Taiwan). The diffraction data were processed using HKL-2000 (Otwinowski & Minor, 1997 ▶). Data-collection statistics are given in Table 1 ▶.
Table 1. Data-collection statistics for the HypBA1 crystal.
Values in parentheses are for the outer resolution shell.
| Beamline | BL-13C1, NSRRC |
| Wavelength (Å) | 0.97622 |
| Resolution (Å) | 25.00–2.78 (2.88–2.78) |
| Space group | P3x21 |
| Unit-cell parameters | |
| a (Å) | 75.9 |
| b (Å) | 75.9 |
| c (Å) | 254.0 |
| No. of measured reflections | 165433 (19602) |
| No. of unique reflections | 21797 (2178) |
| Completeness (%) | 97.9 (100.0) |
| R merge (%) | 6.8 (48.8) |
| R meas (%) | 7.3 (51.8) |
| R p.i.m. (%) | 2.7 (17.1) |
| Mean I/σ(I) | 36.9 (6.1) |
| Multiplicity | 7.6 (9.0) |
| Detector | MX300HE |
| X-ray beam size (µm) | 200 |
| Oscillation range (°) | 0.5 |
| Time of exposure (s) | 7 |
| Crystal-to-detector distance (mm) | 330 |
3. Results and discussion
As shown in Fig. 1 ▶, the single HypBA1 crystal obtained in 0.4 M ammonium acetate, 18%(w/v) polyethylene glycol 3350 was diamond-shaped. Crystals were soaked in a reservoir-based cryoprotectant for about 3 s before flash-cooling. The optimized cryoprotectant consisted of 0.5 M ammonium acetate, 25%(w/v) polyethylene glycol 3350, 5%(w/v) glycerol. Based on the diffraction pattern (Fig. 2 ▶), the HypBA1 crystal belonged to the primitive hexagonal space group P3x21, with unit-cell parameters a = b = 75.9, c = 254.0 Å. Assuming the presence of one molecule per asymmetric unit, the Matthews coefficient V M (Matthews, 1968 ▶) is 2.94 Å3 Da−1 and the estimated solvent content is 58.1%.
Figure 1.
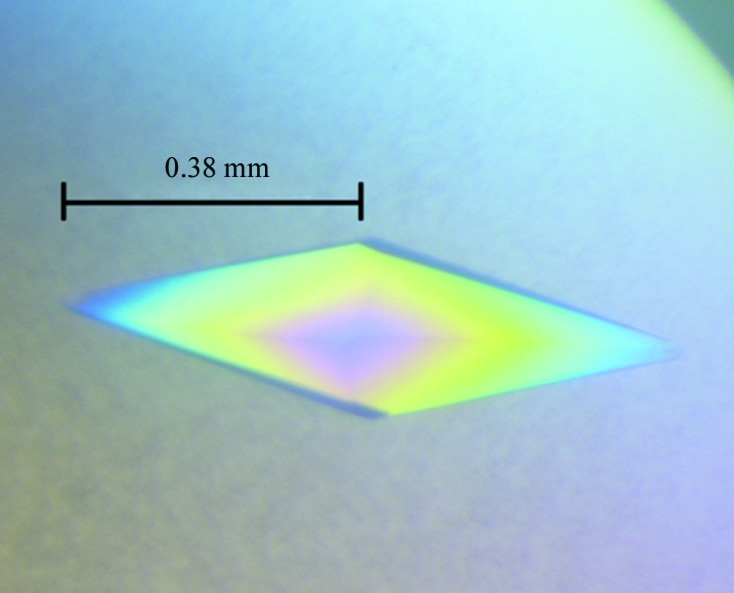
A crystal of HypBA1. The dimensions of the crystal reached 0.38 × 0.38 × 0.1 mm after 5–6 d.
Figure 2.
A typical diffraction pattern of the HypBA1 crystal.
BLASTP searches of the Protein Data Bank using the HypBA1 sequence failed to identify any similar structure. Therefore, no structure could serve as a good template for the molecular-replacement (MR) method, and structural determination of HypBA1 should be carried out using other methods. Currently, we are working on the preparation of SeMet protein for multiple-wavelength anomalous diffraction (MAD). Soaking the crystals with different heavy-atom derivatives for multiple isomorphous replacement (MIR) is also ongoing.
Acknowledgments
The synchrotron data collection was conducted on beamline BL13C1 of the NSRRC (National Synchrotron Radiation Research Center, Taiwan) supported by the National Science Council (NSC). This work was supported by grants from the National Natural Science Foundation of China (31300615) and the Tianjin Municipal Science and Technology Commission (12ZCZDSY12500).
References
- Fujita, K., Sakamoto, S., Ono, Y., Wakao, M., Suda, Y., Kitahara, K. & Suganuma, T. (2011). J. Biol. Chem. 286, 5143–5150. [DOI] [PMC free article] [PubMed]
- Fujita, K., Takashi, Y., Obuchi, E., Kitahara, K. & Suganuma, T. (2011). J. Biol. Chem. 286, 38079–38085. [DOI] [PMC free article] [PubMed] [Retracted]
- Kieliszewski, M. J. & Lamport, D. T. (1994). Plant J. 5, 157–172. [DOI] [PubMed]
- Kieliszewski, M. J., Showalter, A. M. & Leykam, J. F. (1994). Plant J. 5, 849–861. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]



